Abstract
The functional unit within mammalian ovaries is the ovarian follicle. The development of the ovarian follicle is a lengthy process beginning from the time of embryogenesis, passing through multiple different stages of maturation. The purpose of this review is to describe the most basic events in the journey of ovarian follicle development, discussing the importance of ovarian reserve and highlighting the role of several factors that affect oocyte quality and quantity during aging including hormonal, genetic and epigenetic factors. Novel, promising anti-aging strategies are also discussed.
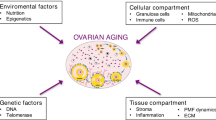
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian follicles are the basic units of the female reproductive biology characterised by a long period of development in the female gonad, the ovary. The human premenopausal ovary contains follicles at different stages of development, from the primordial to the preovulatory size [1]. By the time of ovulation, the mature ovarian follicle ruptures and discharges an oocyte which travels through the fallopian tubes. The remnants of the ruptured follicle, form the corpus luteum which remains in the ovary and in case of fertilization secretes progesterone, a steroid hormone essential for establishing and maintaining pregnancy. The whole process of ovarian follicle maturation and ovulation is complex involving multiple endocrine events controlled by a well-balanced interaction between the hypothalamus, the anterior lobe of the pituitary gland of the brain and the ovary (HPO axis) [2]. The initial pool of ovarian follicles with which a woman is born represents the ovarian reserve, which is an important determinant of the reproductive capacity of a female, as during the normal process of reproductive aging this pool of oocytes progressively declines, being exhausted at menopause [3]. This review disentangles the different stages of ovarian follicle development, discussing the importance of factors that control and determine the reproductive capacity and lifespan of a female.
Ovarian Follicle Formation
Ovarian follicle formation is a lengthy process beginning from the time of embryogenesis. Ovarian follicles are first formed as primordial follicles, which represent a follicle assembly that can stay in a quiescent state for a long period. Of this pool of follicles, only a few can be recruited to form primary follicles which thereafter can either gradually grow to the stage of antral follicles and eventually ovulate or can undergo apoptosis and follicular atresia [4].
Primordial Germ Cells: The Origin of Oocytes
During embryogenesis, the mammalian gonad first develops as an “indifferent” gonad. Cells that give rise to gametes in both males and females can be identified before gastrulation, at around the 3rd week of gestation in humans. During this period, primordial germ cells (PGCs), the founder cell population of the gametes, become specified in the proximal part of the epiblast. PGCs subsequently migrate to the endoderm of the dorsal wall of the yolk sac near the allantois [5]. Thereafter, PGCs are incorporated into the epithelium of the hindgut, from which they migrate at around the 6th week in humans, into the genital ridges where they rapidly proliferate [5]. The key factor that initiates the differentiation of the indifferent gonad to a testis or an ovary is the sex-determining region of the Y chromosome (SRY) [6] which presence determines a XY embryo, whereas its absence a XX embryo. Thus, in humans, sex determination is initiated just before 6 weeks post conception (wpc), when XY gonads start expressing SRY; in the ovary, ovarian cords start to form around 8 wpc [7].
In a female embryo, PGCs are called oogonia by the time they reach the gonads. Oogonia are characterised by increased mitotic activity. Their number increases from merely 10,000 at the 6th week of gestation, to 600,000 and 6 million at the 8th and the 20th week of gestation, respectively; thereafter the rate of oogonial mitosis declines, whereas the rate of oogonial atresia is increased. Of the 1 million germ cells present in a newborn ovary and 300,000 at menarche, approximately 300 will reach the ovulatory state before menopause [8]. During the last mitotic divisions, clusters of oogonia connected with cytoplasmic bridges are formed. Meiosis begins simultaneously in the connected clusters of oogonia between the 8th and 13th weeks of gestation and before follicle formation, which is accomplished by the break down of these syncytia. Entry into meiosis marks the transition of oogonia to oocytes, which are at this stage called primary oocytes. The primary oocytes are arrested at the early diplotene phase of meiotic prophase I [germinal vesicle (GV) stage] and are surrounded by a single layer of flattened pregranulosa cells, forming the primordial follicles [9].
From Primordial to Antral Follicles
Primordial follicles appear in the human fetus at the 15th week of gestation [9] and remain quiescent, with only a few of them proceeding to the next stage, the formation of primary follicles. During this transition, flattened granulosa cells of primordial follicles become cuboidal, whereas the oocyte increases in diameter and gets surrounded by a new layer called zona pellucida [9]. The whole process is complicated, controlled by multiple signals between different cell types of the primordial follicles. After the formation of the primary follicles, the follicular development gradually proceeds up to the stage of antral follicles with a mitotic expansion of granulosa cells; at the beginning of this growth process, primary follicles are surrounded by only one layer of cuboidal granulosa cells, secondary follicles have two to four layers of granulosa cells, large preantral follicles have four to six layers of granulosa cells and antral follicles have more than five layers of granulosa cells. In an antral follicle, a fluid-filled cavity called the antrum is formed adjacent to the oocyte. Growing follicles are also characterized by an increase of oocyte size and the production a new somatic cell type, known as theca cells which form a layer around the granulosa–oocyte structure after the formation of the secondary follicle. Theca cells surround the follicle’s outermost layer, the basal lamina, and undergo cytodifferentiation to become the theca externa and theca interna [10]. In the stage of antral follicle, both granulosa and theca cells continue to undergo mitotis, concomitant with an increase in antrum volume and with granulosa cells differentiating themselves into four distinct subtypes (i) corona radiata that surrounds the zona pellucida (ii) membrana that’s interior to the basal lamina (iii) periantral that’s adjacent to the antrum, and (iv) cumulus oophorous that connects the membrana and corona radiata granulosa cells together [11].
The majority of the antral follicles undergoes atresia and only a few of them continue to grow to reach the preovulatory stage. In fact, each month, only one follicle, in most cases, will complete growth and ovulate to release the mature oocyte. This follicle is the dominant Graafian follicle [12]. After ovulation, the remnants of the ruptured follicle undergo luteinization and differentiation into the corpus luteum (Fig. 1), whereas the mature oocyte, completes meiosis I and is arrested at metaphase of meiosis II. The second meiotic division will be completed by the time of fertilization.
The Role of the Hypothalamus-Anterior Pituary Gland-Ovary (HPO) Axis in Ovarian Follicle Formation
The development of the ovarian follicle is not a simple process. Multiple endocrine events need to be highly orchestrated by an axis including, the hypothalamus, the anterior pituary gland and the ovary. Under the control of pulses of gonadotropin-releasing hormone (GnRH) from the hypothalamus, the anterior pituitary gland secretes two hormones, follicle-stimulating hormone (FSH) and luteinizing hormone (LH), also called gonadotropins. Early in a woman’s menstrual cycle, FSH and to a less extend LH, support follicle growth. An LH surge, just before mid-cycle, is responsible for ovulation and the formation of the corpus luteum. In the ovary, the production of estrogen, by the cells of the developing follicle, controls feedback mechanisms to the hypothalamus and pituitary gland, affecting GnRH production and gonadotropin fluctuations [2].
Although gonadotropins stimulate growth and differentiation of preantral follicles, follicles can develop up to the antral stage in the absence of gonadotropins [12] (Fig. 1). In fact, the transition of primordial to primary follicles is controlled by multiple signals between the oocyte, surrounding somatic cells and certain extra-cellular matrix components and growth factors, such as insulin-like growth factor 1 (IGF-1), epidermal growth factor (EGF), transforming growth factor-α (TGF-α), and TGF-β, growth differentiation factor-9 (GDF-9) and bone morphogenetic protein-15 (BMP-15) [13]. Antimullerian hormone (AMH) has also an important dual role in this process (i) AMH inhibits the transition of follicles from primordial into maturation stages, regulating the number of follicles that remain in the primordial pool (ii) AMH has an inhibitory effect on follicular sensitivity to FSH, probably affecting the process of dominant follicle selection. In line with this, AMH is expressed in follicles that have undergone recruitment from the primordial follicle pool and have not been selected for dominance [14].
Unlike the early stages of follicle development, follicle maturation, including the luteal–follicular transition and the selection of the dominant follicle, is gonadotropin-dependent and requires the fine collaboration of FSH and LH [12]. According to the two-cell, two-gonadotropin theory, LH acts on the LH receptors of theca cells and stimulate the production of androgens. Androgens, in turn, are transferred to the granulosa cells, where they are aromatized into estrogens, under the influence of FSH. This process is mediated by P450 aromatase, which is produced under the influence of FSH [15]. Inhibin, produced by the granulosa cells, further enhances the action of LH on the theca cells, thus the production of androgens, whereas activin antagonizes this action. Activin also augments the effect of FSH on the aromatization of androgens into estrogens [16], facilitating the estrogenization of the follicular environment, which is of vital importance for the survival of the follicle. Other factors, such as IGFs, have also been found to promote estrogenization [17].
Follicles with fewer FSH-receptors are not able to develop further, show retardation of their growth rate and become atretic. Eventually, only one follicle will be viable. This remaining follicle, called the dominant follicle, produces estradiol more quickly from other follicles, leading to a decline in FSH concentrations, due to negative feedback [18]. However, the dominant follicle is able to grow further as, on the one hand, intra-follicular substances increase sensitivity of granulosa cells to the decreased concentration of FSH and on the other hand, follicle growth becomes gradually less dependent on FSH and more on LH [19]. Additionally, during the second half of the follicular phase, LH receptors appear on the granulosa cells under the influence of FSH, thus LH can directly stimulate the production of estrogens and the proliferation of the granulosa cells [20]. After sufficient degree of estrogenic stimulation, an LH surge, just before mid-cycle, results to ovulation.
To sum up, the orchestration of folliculogenesis requires precise regulation and coordination, with complicated interactions between the oocyte, granulosa cells, and surrounding ovarian tissues. A large body of novel factors involved in regulation of folliculogenesis has been identified in the recent years [21]. Among them, microRNAs (miRNAs), short non-coding RNA molecules, have attracted a lot of attention, due to the fact that they regulate all stages of ovarian follicle development—from growth to regression (atresia) to ovulation [22,23,24]. An increased understanding of ovarian miRNAs, as well as of other regulators of folliculogenesis, is awaited to have great impact in the field of reproduction (Table 1).
Interestingly, important landmarks of ovarian follicle development are similar in different species, however with some differences. (Table 2) [7, 25].
Ovarian Reserve
Females are born with a specific total pool of oocytes, representing the total ovarian reserve, an important determinant of women’s reproductive capacity. This reproductive “ammunition” of the ovary is consisted of several million primordial-non growing follicles (NGFs) at around five months of gestational age, however even before birth, this number gradually decreases. Several factors contribute to the progressive decline of NGFs, such as follicle death via apoptosis, as well as the process of “initial recruitment” by which primordial cells (NGFs) are activated from a quiescent state to a follicle maturation state. The recruited NGFs, thus the growing follicles (GFs), represent the functional ovarian reserve [26]. Initial oocyte numbers at birth and the pace of follicular recruitment actually determines the reproductive capacity of women, at all ages. Interestingly, with aging, along with the decrease in follicle number, there is a decrease in the quality of oocytes, as well [27]. The time by which the pool of primordial follicles will be exhausted and the female reproductive axis will reach a senescent state is different in every woman, thus the normal process of reproductive aging varies considerably among women. Some women remain highly fertile until the fifth decade of their life, whereas others face the loss of natural fertility already in their mid-thirties and are characterized by premature ovarian failure (POF) [28].
Main Mechanisms of Ovarian Aging
Much of the reproductive capacity of a woman is associated with factors affecting the quality and the quantity of the oocytes, such as mitochondrial dysfunction, genetic factors, epigenetic factors and telomere shortening.
Mitochondria Dysfunction
Mitochondria is the energy power house in the cell. They consist of an outer membrane, inner membrane, crista, and matrix. The human mitochondrial DNA (mtDNA) is circular, double-stranded ~ 16.6 kb and lacks histones. It contains 37 genes encoding 2 mitochondrial rRNAs, 22 tRNAs and 13 oxidative phosphorylase complex protein subunits. Mitochondria are the most abundant organelles in the egg cytoplasm. The quantity of mitochondria in oocytes affects reproduction. The number of mitochondria increases during meiosis in oocytes, and the mid-ovulation MII egg reaches its peak, with 100,000 mitochondria and 50,000 to 550,000 copies of mtDNA. Mature oocytes have the highest mitochondrial content in human cells, probably due to the huge energy demands [29].
Aging is associated with mtDNA instability and accumulation of mtDNA mutations in the oocytes, leading to mitochondrial swelling, vacuolization, and fragmentation [30, 31]. Reactive oxygen species (ROS) are the leading cause of mtDNA mutations [32] and aging is closely related to increased ROS production. Interestingly, in the MII phase, the mtDNA content is negatively correlated with the age of women, and the increase in women’s age leads to a decrease in the number of mtDNA in oocytes. Additionally, in elderly women, the mutation rate of mtDNA in oocyte granule cells is higher, and the evaluation of their mtDNA content can be used to predict embryo quality [33, 34]. Thus, mitochondrial dysfunction in oocytes due to aging has been found to accelerate ovarian insufficiency.
Aneuploidy
Aneuploidy and maternal aging are in close relationship, too. Importantly, aneuploidy occurs in more than 50% of eggs from women over 35 years old [35, 36]. Cohesion complexes are essential for the correct segregation of chromosomes during meiotic divisions. Deficiencies in chromosomal cohesin affect chromosome segregation in oocytes, causing an increase in the proportion of aneuploidy [37]. Thus, decreased cohesion results in a greater probability of chromosome mis-segregation, premature chromatid separation, and aneuploidy [38,39,40]. Moreover, during sister chromatid separation, the centromere is subjected to opposite traction forces from spindle fibers. Unequivalent forces can cause faulty separation and oocyte aneuploidy [41]. Additionally, defects in the spindle assembly checkpoint can also lead to a significant increase in aneuploidy rate associated with aging [42, 43].
DNA Damage
Endogenous and exogenous stressors can cause DNA damage in the developing oocytes. Cellular mechanisms that repair DNA damage become less effective during aging. In oocytes, deceased efficacy of DNA repair mechanisms promotes cell death, resulting in depleted oocyte reserve. A recent genome-wide association study of approximately 200,000 women of European ancestry identified 290 genetic determinants of ovarian aging [44], highlighting a significant association between DNA damage response and ovarian aging. Notably, Breast cancer-associated 1 (BRCA1) and BRCA2, which participate in homologous recombination repair, are associated with depleted oocyte reserve [45]. Importantly, women with BRCA mutations have lower serum AMH, a hormone which indirectly measures ovarian reserve [46,47,48,49,50,51,52]. Moreover, women with BRCA mutations experience menopause at earlier age [53,54,55].
FMR1
Recent evidence suggests that the fragile X mental retardation 1 (FMR1) gene exerts controlling functions on follicle recruitment and ovarian reserve. FMR1 is mapped on the X chromosome at position q27.3, spans approximately 40 kb and contains 17 exons [56]. Of them, exons 12, 14, 15 and 17 can be alternatively spliced, resulting in different mRNAs and protein isoforms. FMR1 cloning revealed that the fragile site of the X chromosome contains a CGG repeat in the 5’-untranslated region (UTR) of the gene, which is unstable and thus polymorphic. In the normal population, the repeat length varies from 6 to 55 repeats. Individuals with 55 to 200 CGG repeat length are considered as premutation carriers who are characterised by increased FMR1 mRNA transcription and decreased FMR1 expression. A full mutation repeat (over 200) leads to FMR1 silencing and lack of FMR1 expression. The CGG repeat length has been associated with different clinical outcomes, mostly with increased risk towards certain neuro-psychiatric conditions [57].
Interestingly, variations in the number of FMR1 CGG repeats have recently been implicated in different ovarian aging patterns and variable declines in ovarian reserve [58, 59]. The normal range of CGG repeats in regard to ovarian reserve differs from repeat ranges used to assess neuro/psychiatric risks. In regard to ovarian function, 26 to 34 CGG nucleotide repeats represent normal (median 30), independent of ethnicity/race [60]; according to this normal range, women can be defined as norm (both alleles in normal range), het (1 allele outside normal) and hom (both alleles outside normal). At young ages, norm women have been found to possess a better ovarian reserve compared to het and hom women, who have a poorer initial pool of oocytes [26]. The molecular background of this association is currently unknown. According to a toxic RNA gain-of-function hypothesis, the abnormal-range repeat lengths result in highly increased mRNA expression in the ovaries, thus RNA toxicity, which may lead to elevated follicle atresia. Moreover, due to the toxic effect attributed to the gain of function of mRNA (i) specific cellular proteins may fail to perform certain cellular functions and (ii) others may not be able to be degraded and accumulate, leading to the formation of cellular inclusions [57]. Interestingly, although norm women begin with a larger “ovarian ammunition”, this advantage is lost in the long run, as the rates of decline in ovarian reserve follow a different pattern. According to the observations of recent studies, young norm women are characterized by an increased rate of ovarian aging, whereas het and hom women are characterized by a slower rate of follicular recruitment. Consequently, norm women deplete their ovarian reserve much quicker than het or hom women; thus at more advanced ages, het and hom women maintain a larger follicle pool compared to norm women [26]. Interestingly, het and hom women are characterized by a slower and steadier decline of AMH levels; measuring AMH hormone is currently the most accurate method to estimate the functional ovarian reserve [61]. Overall, these findings support a more robust ovarian reserve for het and hom women compared to norm women. The better fertility potential provided by het and hom women, could actually provide a substantial explanation for the preservation of a “bad” gene like FMR1 which has been associated with multiple severe neurophychiatric disorders [26].
Telomere Attrition
One of the most important parameters for the reproductive lifespan of females and a surrogate indicator for biological age is telomeres. Telomeres are specialized structures at the ends of all eukaryotic chromosomes, which comprise extended arrays of a few thousand tandem repeats of TTAGGG. They undergo progressive shortening as cells divide without complete DNA replication at the extreme ends of chromosomes [62]. In fact, with each round of chromosome replication, approximately 100–200 bp of telomeric DNA are lost. When the telomeres are critically short, cells exit the cell division cycle, a characteristic of cell senescence [62]. According to this process, the length of telomeres indirectly depicts the proliferative capacity and thus the survival prospect of cells. Counteracting to this aging process, an evolutionarily conserved ribonucleioprotein enzyme, called telomerase, exists in highly proliferative and renewing tissues, enabling these cells to escape senescence and to proliferate at a high rate. Telomerase prohibits the cell division-dependent telomere shortening via binding and elongating telomeric DNA [63].
Telomerase has a pivotal role during oocyte development, acting actually as a “mitotic clock” in the ovary. In the smallest preantral follicles, thus in early maturation stages, where intense cell division is important for the development of ovarian follicles, high levels of telomerase activity are present, suggesting that the high proliferative activity of granulosa cells much depends on telomerase activity and its role to sustain telomere length. During the transition from small- to medium-sized follicles, thus in the late maturation stages, telomerase activity gradually declines, whereas the rate of apoptosis of granulosa cells is increased. Importantly, estrogen has been suggested to have a regulating role in telomerase activity, as administration of estrogen in rats prevented the inhibition of telomerase [64]. Recently, in an estrogen-deficient aromatase-knockout mouse model, telomerase activity in the ovary was inhibited [65]. On the contrary, prolonged exposure of the ovary to high levels of estrogen has been associated with ovarian cancer and high levels of telomerase activity [65], highlighting the importance of telomere homeostasis for normal and pathological functions of the ovary.
Epigenetic Modifications
Epigenetic modifications are also very important in the regulation of oocyte aging. DNA methylation is one of the epigenetic mechanisms and plays a crucial role in the regulation of gene expression in the oocytes and granulosa cells during folliculogenesis, as well as in other ovarian cells. In general, global DNA methylation gradually increases during oocyte growth and reaches the highest level in MII oocytes [66]. During oocyte maturation from GV to MII stages, genomic imprints are also established [67]. Also, granulosa cells undergo DNA methylation to regulate repression or activation of the genes required for proper follicular development [68] The DNA methyltransferases (DNMTs) regulate methylation of genes during oogenesis and establish genomic imprints, too. Thus, the lack of DNMT results in abnormal global methylation or defects in the imprinting genes [69]. With age, the levels of DNMTs decrease in oocytes and DNA demethylases increase, hence overall DNA methylation levels decrease [70].
Histone modifications like methylation, acetylation, ubiquitination and non-coding RNA-regulated modifications are also important in oocyte aging. During meiosis, histone is deacetylated globally at the MI and MII stages by histone deacetylases (HDAC). With increasing age, the acetylation levels of H4K12 and H4K16 in aged GV oocytes decrease, but the deacetylation of H4K12 is impaired in MII oocytes. The histone methylation in mouse GV oocytes has also been found affected by advanced maternal age [71]. Moreover, the GV and MII oocytes of older females lack H3K9me3, H3K36me2, H3K79me2 and H4K20me2 compared with the GV and MII oocytes of younger females. The expression of the histone methylation related factors is also changed in ageing GV oocytes [72]. Ubiquitination may be affected by age in human oocytes [73], too.
RNA interference is a biological process according to which double-stranded RNA (dsRNAs) can be used for post-transcriptional gene silencing. miRNA is a kind of small non-coding RNA which functions in post-transcriptional regulation of gene expression. In a recent study, approximately 400 small RNAs were identified in the ovary and of them 122 miRNAs were expressed only in reproductive organs, highlighting their importance in reproduction. Different patterns of small RNA expression were found in different stages of ovarian follicle development as well as according to the ovarian state, normal or pathological [74]. In humans, miRNAs are abundant in MII oocytes and cumulus cells and they may be essential for follicle development [75]. Moreover, the miRNA expression profile in follicular fluid of women with premature ovarian failure is different from that in follicular fluid of unaffected women [76]. In addition, miRNAs expression profiling of the follicular fluid of younger and older women has also been found to be different. Additionally, in a recent genome-wide screen, a number of small RNAs were found to affect multiple different cell functions in the ovary including proliferation, apoptosis, ovarian secretory activity, luteogenesis, oocyte maturation or ovarian tumorgenesis [77], highlighting their importance in reproduction.
Promising Therapeutic Interventions
Female reproductive aging is a major unsolved challenge in the field of reproductive medicine. A number of cutting-edge strategies are currently investigated targeting both oocyte number and quality that are both diminished during aging.
Stem cell-based therapy appears to be quite promising. Preclinical studies have shown that ovarian failure can be recovered by the transplantation of mesenchymal stem cells (MSCs) from different sources, such as bone marrow, adipose tissue, amniotic fluid, umbilical cord, menstrual blood, etc [78,79,80,81].. Among different types of stem cells autologous mesenchymal stem cells are the most popular, as they are abundant, accessible, with low immunogenicity and stability. Umbilical cord-derived MSC (UCMSC) was found to improve ovarian function in 61 patients with primary ovarian insufficiency (POI), following transvaginal intra-ovarian allogenic stem cell infusion of UCMS isolated from donor healthy full-term human placental samples. There was an increased AFC and overall pregnancy rate was reported to be 6.6% [82]. One other promising strategy is the autologous stem cell ovarian transplantation (ASCOT). This procedure involves (i) bone marrow derived stem cell (BMDSC) mobilization to peripheral blood after granulocyte-colony stimulating factor (G-CSF) treatment, (ii) collection by apheresis and (iii) infusion into the ovarian artery. ASCOT has been described to increase AMH, AFC levels and the number of retrieved oocytes after in vitro fertilization [83, 84]. Interestingly, the ASCOT technique has recently been found to modify the signature of the human plasma proteome, having beneficial effects on the ovary and regulating crucial factors of ovarian aging [85].
Platelet-rich plasma (PRP) therapy is another innovative anti-aging approach that takes advantage of the inherent healing capabilities of platelets and growth factors derived from an individual’s blood. According to this process, blood sample from the patient is collected and thereafter centrifugated to segregate concentrated platelet-rich plasma which is then activated and administered into the target organ, such as the ovary. This regenerative methodology has been proposed to have anti-inflammatory effects, modulate extracellular matrix remodeling, promote angiogenesis and direct cellular recruitment to the injection site, thus promoting differentiation and reparative procedures. In a study conducted by Sills et al. in 2018, 4 patients diagnosed with diminished ovarian reserve (average age of 42 ± 4 years) were included. Autologous PRP injections were administered bilaterally into the ovaries. An increase in serum AMH levels and a decrease in FSH levels was reported in all women and a mean retrieval of 5.3 mature oocytes was accomplished. One woman achieved pregnancy and the other three women decided to freeze their embryos for future use [86]. Another study reported a 15.8% pregnancy rate after bilateral PRP infusion, in 19 patients with diminished ovarian reserve [87]. A large non-randomized clinical trial of 83 women with low ovarian reserve studied two different groups; 46 received PRP injections for three consecutive cycles, and in the rest no intervention was made. A significant decrease in FSH and increase in AMH and AFC was reported after 3 months. The number of live birth rates was significantly higher compared to the control group [88]. However, a number of studies have also been published with more negative or contradictory results, as for the efficacy of PRP on the rejuvenation of ovarian function [88]. Thus, PRP as an alternative therapy should be further examined.
Strategies to target oocyte quality which also significantly declines with age are also increasingly investigated. Mitochondrial enrichment has been proposed as a potential therapy option to improve oocyte quality [89]. Heterologous or autologous sources of mitochondria have been used to rejuvenate and improve oocyte health. In the heterologous approach, mitochondrial enrichment is accomplished by transferring a healthy cytoplasm into the patient’s oocyte; this approach has many ethical and safety concerns due to uncertain degree of mitochondrial heteroplasmy. Therefore, attention has turned to the autologous approach in order to improve oocyte quality. Mitochondrial autologous sources include immature oocytes, granulosa cells, germline stem cells and adipose-derived stem cells. However, the autologous germline mitochondrial energy transfer (AUGMENT) has not yielded as many beneficial outcomes as initially expected [90]. Pharmacological methods have also been observed to improve oocyte quality by increasing mtDNA copy numbers or mitochondrial function, especially due to decreasing oxidative stress. Senotheraputic interventions and pharmacological approaches promoting telomere lengthening, differentiation of stem cell types into oocytes, as well as better understanding and targeting of aging pathways, are some additional and undoubtedly intriguing challenges of future research [88].
Conclusion
Women are believed to be born with a finite number of follicles set around the time of birth. This initial pool of oocytes represents the ovarian reserve. The human premenopausal ovary contains follicles at different stages of development, from the primordial to the preovulatory size. The process of folliculogenesis is coordinated by the HPO axis. As maternal age advances, the number of follicles that can be successfully recruited for a possible pregnancy steadily declines until menopause. Much of the reproductive capacity of a female depends on the initial pool of oocytes, as well as the rate of follicular apoptosis and “recruitment” plus the quality of oocytes. Many factors, such as mtDNA mutations, DNA damage, aneuploidy, genetic and epigenetic alterations and telomere attrition lead to ovarian aging. New therapeutic strategies and interventions are investigated in order to delay ovarian aging; the use of antioxidants, heterologous or autologous sources of mitochondria to rejuvenate and improve oocyte health or injections of platelet-rich plasma and of stem cells are considered as promising alternatives to stimulate ovarian rejuvenation; however, a lot of research is essential in order to be able to disentangle the mystery of ovarian aging. Rapid advances in high-throughput technologies in fields such as epigenetics, transcriptomics, proteomics, and metabolomics, are awaited to drastically increase our knowledge regarding the lifespan of human oocytes. Undoubtedly, the journey of ovarian development is really fascinating with many mysterious pathways to be further investigated.
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Gougeon A. Dynamics of follicular growth in the human: a model from preliminary results. Hum Reprod. 1986;1:81–7.
Gougeon A. Human ovarian follicular development: from activation of resting follicles to preovulatory maturation. Ann Endocrinol (Paris). 2010;71:132–43.
Zhu Q, Ma H, Wang J, Liang X. Understanding the mechanisms of diminished Ovarian Reserve: insights from genetic variants and Regulatory factors. Reprod Sci. 2024 Feb;12. https://doi.org/10.1007/s43032-024-01467-1. Online ahead of print.
McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;2:200–14.
Mc KD, Hertig AT, Adams EC, Danziger S. Histochemical observations on the germ cells of human embryos. Anat Rec. 1953;2:201–19.
Sinclair AH, Berta P, Palmer MS, et al. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990;346:240–4.
Nicol L, Bishop SC, Pong-Wong R, Bendixen C, Holm LE, Rhind SM, et al. Homozygosity for a single base-pair mutation in the oocyte-specific GDF9 gene results in sterility in Thoka sheep. Reproduction. 2009;138:921–33.
Oktem O, Oktay K. The ovary: anatomy and function throughout human life. Ann NY Acad Sci. 2008;1127:1–9.
Maheshwari A, Fowler PA. Primordial follicular assembly in humans—revisited. Zygote. 2008;4:285–96.
Young JM, McNeilly AS. Theca: the forgotten cell of the ovarian follicle. Reproduction. 2010;140:489–504. Review.
Fontana J, Martínková S, Petr J, Žalmanová T, Trnka J. Metabolic cooperation in the ovarian follicle. Physiol Res. 2020;69:33–48.
Erickson GF. The graafian follicle: a functional definition. In: Adashi EY, editor. Ovulation: evolving scientific and clinical concepts. New York: Springer-Verlaag; 2000. pp. 31–48.
Erickson GF, Shimasaki S. The physiology of folliculogenesis: the role of novel growth factors. Fertil Steril. 2001;76:943–9.
Visser JA, Themmen AP. Anti-mullerian hormone and folliculogenesis. Mol Cell Endocrinol. 2005;234:81–6.
Hillier SG, Whitelaw PF, Smyth CD. Follicular oestrogen synthesis: the ‘two-cell, two-gonadotrophin’ model revisited. Mol Cell Endocrinol. 1994;100:51–4.
Hillier SG. Gonadotropic control of ovarian follicular growth and development. Mol Cell Endocrinol. 2001;179:39–46.
Spicer LJ. Proteolytic degradation of insulin-like growth factor binding proteins by ovarian follicles: a control mechanism for selection of dominant follicles. Biol Reprod. 2004;70:1223–30.
van Santbrink EJ, Hop WC, van Dessel TJ, de Jong FH, Fauser BC. Decremental follicle-stimulating hormone and dominant follicle development during the normalmenstrual cycle. Fertil Steril. 1995;64:37–43.
Mihm M, Baker PJ, Ireland JL, et al. Molecular evidence that growth of dominant follicles involves a reduction in follicle-stimulating hormone dependence and an increase in luteinizing hormone dependence in cattle. Biol Reprod. 2006;74:1051–9.
Lindeberg M, Carlstr¨om K, Ritvos O, Hovatta O. Gonadotrophin stimulation of non-luteinized granulosa cells increases steroid production and the expression of enzymes involved in estrogen and progesterone synthesis. Hum Reprod. 2007;22:401–6.
Gershon E, Dekel N. Newly identified regulators of ovarian folliculogenesis and ovulation. Int J Mol Sci. 2020;21:4565.
Kusumaningtyas I, Dasuki D, Mubarika Harjana S, Hamim Sadewa A, Cempaka Sweety M, Septiani L. Unraveling the microRNAs, key players in folliculogenesis and ovarian diseases. Middle East Fertil Soc J 2024; 29:13.
Alexandri C, Daniel A, Bruylants G, Demeestere I. The role of microRNAs in ovarian function and the transition toward novel therapeutic strategies in fertility preservation: from bench to future clinical application. Hum Reprod Update. 2020;26:174–96.
Donadeu FX, Schauer SN, Sontakke SD. Involvement of miRNAs in ovarian follicular and luteal development. J Endocrinol. 2012;215:323–34.
Jiang Y, He Y, Pan X, Wang P, Yuan X, Ma B. Advances in oocyte maturation in Vivo and in Vitro in mammals. Int J Mol Sci. 2023;24:9059.
Gleicher N, Weghofer A, Barad DH. Defining ovarian reserve to better understand ovarian aging. Reprod Biol Endocrinol. 2011;9:23.
Macklon NS, Fauser BC. Ovarian reserve. Semin Reprod Med. 2005; 23: 248– 56. Review.
Goswami D, Conway GS. Premature ovarian failure. Hum Reprod Update. 2005;11:391–410.
May-Panloup P, Chretien MF, Jacques C, Vasseur C, Malthiery Y, Reynier P. Low oocyte mitochondrial DNA content in ovarian insufficiency. Hum Reprod. 2005;20:593–7.
Wilding M, Dale B, Marino M, et al. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum Reprod. 2001;16:909–17.
Ben-Meir A, Burstein E, Borrego-Alvarez A, et al. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell. 2015;14:887–95.
Babayev E, Wang T, Szigeti-Buck K, et al. Reproductive aging is associated with changes in oocyte mit dynamics, function, and mtDNA quantity. Maturitas. 2016;93:121–30.
Ogino M, Tsubamoto H, Sakata K, et al. Mitochondrial DNA copy number in cumulus cells is a strong predictor of obtaining good-quality embryos after IVF. J Assist Reprod Genet. 2016;33:367–71.
Desquiret-Dumas V, Clement A, Seegers V, et al. The mitochondrial DNA content of cumulus granulosa cells is linked to embryo quality. Hum Reprod. 2017;32:607–14.
Gruhn JR, Zielinska AP, Shukla V, et al. Chromosome errors in human eggs shape natural fertility over reproductive life span. Science. 2019;365:1466–9.
Tyc KM, McCoy RC, Schindler K, Xing J. Mathematical modeling of human oocyte aneuploidy. Proc Natl Acad Sci U S A. 2020;117:10455–64.
Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet. 2001;2:280–91.
Chiang T, Duncan FE, Schindler K, Schultz RM, Lampson MA. Evidence that weakened centromere cohesion is a leading cause of age-related aneuploidy in oocytes. Curr Biol. 2010;20:1522–8.
Chiang T, Schultz RM, Lampson MA. Meiotic origins of maternal age-related aneuploidy. Biol Reprod. 2012;86:1–7.
Holton RA, Harris AM, Mukerji B, Singh T, Dia F, Berkowitz KM. CHTF18 ensures the quantity and quality of the ovarian reserve. Biol Reprod. 2020;103:24–35.
Shomper M, Lappa C, FitzHarris G. Kinetochore microtubule establishment is defective in oocytes from aged mice. Cell Cycle. 2014;13:1171–9.
Yun Y, Holt JE, Lane SI, McLaughlin EA, Merriman JA, Jones KT. Reduced ability to recover from spindle disruption and loss of kinetochore spindle assembly checkpoint proteins in oocytes from aged mice. Cell Cycle. 2014;13:1938–47.
Polanski Z. Spindle assembly checkpoint regulation of chromosome segregation in mammalian oocytes. Reprod Fertil Dev. 2013;25:472–83.
Ruth KS, Day FR, Hussain J, et al. Genetic insights into biological mechanisms governing human ovarian ageing. Nature. 2021;596:393–7.
Li Q, Engebrecht J. BRCA1 and BRCA2 tumor suppressor function in meiosis. Front Cell Dev Biol. 2021;9:668309.
Titus S, Li F, Stobezki R, et al. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci Transl Med. 2013;5:172ra21.
Michaelson-Cohen R, Mor P, Srebnik N, Beller U, Levy-Lahad E, Eldar-Geva T. BRCA mutation carriers do not have compromised ovarian reserve. Int J Gynecol Cancer. 2014;24:233–7.
Wang ET, Pisarska MD, Bresee C, et al. BRCA1 germline mutations may be associated with reduced ovarian reserve. Fertil Steril. 2014;102:1723–8.
Giordano S, Garrett-Mayer E, Mittal N, et al. Association of BRCA1 mutations with impaired ovarian reserve: connection between infertility and breast/ovarian cancer risk. J Adolesc Young Adult Oncol. 2016;5:337–43.
van Tilborg TC, Derks-Smeets IA, Bos AM, et al. Serum AMH levels in healthy women from BRCA1/2 mutated families: are they reduced? Hum Reprod. 2016;31:2651–9.
Lambertini M, Goldrat O, Ferreira AR, et al. Reproductive potential and performance of fertility preservation strategies in BRCA-mutated breast cancer patients. Ann Oncol. 2018;29:237–43.
Gunnala V, Fields J, Irani M, et al. BRCA carriers have similar reproductive potential at baseline to noncarriers: comparisons in cancer and cancer-free cohorts undergoing fertility preservation. Fertil Steril. 2019;111:363–71.
Rzepka-Gorska I, Tarnowski B, Chudecka-Glaz A, Gorski B, Zielinska D, Toloczko-Grabarek A. Premature menopause in patients with BRCA1 gene mutation. Breast Cancer Res Treat. 2006;100:59–63.
Finch A, Valentini A, Greenblatt E, et al. Frequency of premature menopause in women who carry a BRCA1 or BRCA2 mutation. Fertil Steril. 2013;99:1724–8.
Lin WT, Beattie M, Chen LM, et al. Comparison of age at natural menopause in BRCA1/2 mutation carriers with a non-clinic-based sample of women in northern California. Cancer. 2013;119:1652–9.
Verkerk AJ, Pieretti M, Sutcliffe JS, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–14.
Willemsen R, Levenga J, Oostra B. CGG repeat in the FMR1 gene: size matters. Clin Genet. 2011;80:214–25.
Wittenberger MD, Hagerman RJ, Sherman Sl, et al. The FMR1 premutation and reproduction. Fertil Steril. 2007;87:456–65.
Gleicher N, Weghofer A, Barad DH. Ovarian reserve determinations suggest new function of FMR1 (fragile X gene) in regulating ovarian ageing. Reprod Biomed Online. 2010;20:768–75.
Gleicher N, Weghofer A, Oktay K, Barad DH. Can the FMR1 (fragile X) gene serve as predictor of response to ovarian stimulation? Reprod Sci. 2009;16:462–7.
Domingues TS, Rocha AM, Serafini PC. Tests for ovarian reserve: reliability and utility. Curr Opin Obstet Gynecol. 2010;22:271–6. Review.
Shay JW, Wright WE. Hallmarks of telomeres in ageing research. J Pathol. 2007;211:114–23.
Liu JP, Li H. Telomerase in the ovary. Reproduction. 2010;140:215–22. Review.
Li H, Simpson ER, Liu JP. Oestrogen, telomerase, ovarian ageing and cancer. Clin Exp Pharmacol Physiol. 2010;37:78–82.
Bayne S, Li H, Jones ME, et al. Estrogen deficiency reversibly induces telomere shortening in mouse granulosa cells and ovarian aging in vivo. Protein Cell. 2011;2:333–46.
Smallwood SA, Kelsey G. De novo DNA methylation: a germ cell perspective. Trends Genet. 2012;28:33–42.
Yang X, Smith SL, Tian XC, Lewin HA, Renard JP, Wakayama T. Nuclear reprogramming of cloned embryos and its implications for therapeutic cloning. Nat Genet. 2007;39:295–302.
Huntriss J, Hinkins M, Oliver B, et al. Expression of mRNAs for DNA methyltransferases and methyl-CpG-binding proteins in the human female germ line, preimplantation embryos, and embryonic stem cells. Mol Reprod Dev. 2004;67:323–36.
Uysal F, Ozturk S. DNA methyltransferases in mammalian oocytes. Results Probl Cell Differ. 2017;63:211–22.
Yue MX, Fu XW, Zhou GB, et al. Abnormal DNA methylation in oocytes could be associated with a decrease in reproductive potential in old mice. J Assist Reprod Genet. 2012;29:643–50.
Manosalva I, González A. Aging changes the chromatin configuration and histone methylation of mouse oocytes at germinal vesicle stage. Theriogenology. 2010;74:1539–47.
Shao GB, Wang J, Zhang LP, et al. Aging alters histone H3 lysine 4 methylation in mouse germinal vesicle stage oocytes. Reprod Fertil Dev. 2015;27:419–26.
Steuerwald NM, Bermúdez MG, Wells D, Munné S, Cohen J. Maternal age-related differential global expression profiles observed in human oocytes. Reprod Biomed Online. 2007;14:700–8.
Ro S, Song R, Park C, Zheng H, Sanders KM, Yan W. Cloning and expression profiling of small RNAs expressed in the mouse ovary. RNA. 2007;13:2366–80.
Assou S, Al-edani T, Haouzi D, et al. MicroRNAs: new candidates for the regulation of the human cumulus-oocyte complex. Hum Reprod. 2013;28:3038–49.
Roth LW, McCallie B, Alvero R, Schoolcraft WB, Minjarez D, Katz-Jaffe MG. Altered microRNA and gene expression in the follicular fluid of women with polycystic ovary syndrome. J Assist Reprod Genet. 2014;31:355–62.
Sirotkin AV, Lauková M, Ovcharenko D, Brenaut P, Mlyncek M. Identification of microRNAs controlling human ovarian cell proliferation and apoptosis. J Cell Physiol. 2010;223:49–56.
Cho J, Kim TH, Seok J, Jun JH, Park H, Kweon M, et al. Vascular remodeling by placenta-derived mesenchymal stem cells restores ovarian function in ovariectomized rat model via the VEGF pathway. Lab Invest. 2021;101:304–17.
Ding C, Zou Q, Wang F, Wu H, Wang W, Li H, et al. HGF and BFGF secretion by human adipose-derived stem cells improves ovarian function during natural aging via activation of the SIRT1/FOXO1 signaling pathway. Cell Physiol Biochem. 2018;45:1316–32.
Li J, Mao Q, He J, She H, Zhang Z, Yin C. Human umbilical cord mesenchymal stem cells improve the reserve function of perimenopausal ovary via a paracrine mechanism. Stem Cell Res Ther. 2017;8:55.
Feng P, Li P, Tan J. Human menstrual blood-derived stromal cells promote recovery of premature ovarian insufficiency via regulating the ECM dependent FAK/AKT signaling. Stem Cell Rev Rep. 2019;15:241–55.
Yan L, Wu Y, Li L, Wu J, Zhao F, Gao Z, Liu W, Li T, Fan Y, Hao J, Liu J, Wang H. Clinical analysis of human umbilical cord mesenchymal stem cell allotransplantation in patients with premature ovarian insufficiency. Cell Prolif. 2020;53:e12938.
Herraiz S, Romeu M, Buigues A, Martínez S, DíazGarcía C, Gómez-Seguí I, et al. Autologous stem cell ovarian transplantation to increase reproductive potential in patients who are poor responders. Fertil Steril. 2018;110:496–e5051.
Pellicer N, Herraiz S, Romeu M, Martínez S, Buigues A, Gómez-Seguí I et al. Bone Marrow derived stem cells restore ovarian function and fertility in premature ovarian insufficiency women. Interim report of a randomized trial: mobilization versus ovarian injection. 36th Virtual Annual Meeting of the European Society of Human Reproduction and Embryology. Hum Reprod. 2020 (Suppl 1); 35: i38–9.
Buigues A, Ramírez-Martin N, Martínez J, Pellicer N, Meseguer M, Pellicer A, et al. Systemic changes induced by autologous stem cell ovarian transplant in plasma proteome of women with impaired ovarian reserves. Aging. 2023;15:14553–73.
Sills ES, Wood SH. Autologous activated platelet-rich plasma injection into adult human ovary tissue: molecular mechanism, analysis, and discussion of reproductive response. Biosci Rep. 2019;39:1–15.
Farimani M, Heshmati S, Poorolajal J, Bahmanzadeh M. A report on three live births in women with poor ovarian response following intra-ovarian injection of platelet-rich plasma (PRP). Mol Biol Rep. 2019;46:1611–6.
Pellicer N, Cozzolino M, Diaz-García C, Galliano D, Cobo A, Pellicer A, et al. Ovarian rescue in women with premature ovarian insufficiency: facts and fiction. Reprod Biomed Online. 2023;46:543–65.
Jiang Z, Shen H. Mitochondria: emerging therapeutic strategies for oocyte rescue. Reprod Sci. 2022;29:711–22.
Labarta E, de Los Santos MJ, Herraiz S, Escribá MJ, Marzal A, Buigues A, et al. Autologous mitochondrial transfer as a complementary technique to intracytoplasmic sperm injection to improve embryo quality in patients undergoing in vitro fertilization-a randomized pilot study. Fertil Steril. 2019;111:86–96.
Acknowledgements
We thank all authors for their contributions.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Kallirhoe Kalinderi had the idea for the article, Kallirhoe Kalinderi and Michail Kalinderis performed the literature search and analysis, and drafted the manuscript. Vasileios Papaliagkas and Liana Fidani critically revised the work. All the authors read and approved the final manuscript for submission.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kalinderi, K., Kalinderis, M., Papaliagkas, V. et al. The Reproductive Lifespan of Ovarian Follicle. Reprod. Sci. 31, 2604–2614 (2024). https://doi.org/10.1007/s43032-024-01606-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-024-01606-8