Abstract
Dysfunction of extravillous trophoblasts (EVTs) might cause early pregnancy failure by interfering with embryo implantation and/or placentation. We previously reported that the villus miR-3074-5p expression level was increased, whereas the peripheral level of GDF15, a predict target gene of miR-3074-5p, was decreased in recurrent miscarriages (RM) patients, and miR-3074-5p could enhance apoptosis but reduce invasion of human extravillous trophoblast cells (EVTs). The aim of this study was to further explore roles of miR-3074-5p/GDF15 pathway in regulation of EVTs function. It was validated that GDF15 was not the direct target of miR-3074-5p, whereas EIF2S1, an upstream regulator of GDF15 maturation and secretion, was the direct target of miR-3074-5p. The villus expression levels of GDF15 and EIF2S1 were significantly decreased in RM patients. Knockdown of GDF15 expression presented inhibitory effects on proliferation, migration, and invasion of HTR8/SVneo cells. Up-regulated miR-3074-5p expression led to the significant decreased GDF15 expression in HTR8/SVneo cells, and this effect could be efficiently reversed by the overexpression of EIF2S1. Meanwhile, the suppressive effects of miR-3074-5p on proliferation, migration, and invasion of HTR8/SVneo cells could be intercepted by the treatment of recombinant human GDF15 protein. Collectively, these data suggested that miR-3074-5p could reduce GDF15 production via targeting inhibition of EIF2S1 expression, and the deficiency in GDF15 function might lead to the early pregnancy loss by attenuating proliferation and invasion of EVTs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recurrent miscarriage (RM) is defined as two or more clinical pregnancy losses occurring before 20 weeks of gestation, with a population prevalence of 2.6% [1]. Although the causes of nearly 50% RM cases have not yet been well understood presently, abnormalities in embryo implantation and placentation are undoubtedly involved in pathogenesis of early pregnancy loss. Extravillous trophoblast (EVTs), differentiated from the trophectoderm, could attach and invade into the uterine decidua basalis to participate in the processes of embryo implantation and placentation, including the remodeling of spiral arterioles at the maternal–fetal interface [2, 3]. Thus, dysfunction of EVTs might lead to adverse pregnancy outcomes, such as RM and pre-eclampsia (PE), by interfering with embryo implantation or placentation [4, 5]. However, the molecular mechanisms underlying the dysfunction of EVTs are still largely unknown.
MicroRNAs (miRNAs), a kind of endogenous non-coding small RNA molecules, approximately 22 nucleotides in length, are involved in post-transcriptional regulation of target gene expression [6]. There have been accumulating evidences that suggest the involvement of dysregulated miRNAs expression in human reproductive disorders [7]. In our previous studies, the significantly increased miR-3074-5p expression levels were observed both in villus and decidual tissues of RM patients [8, 9], and it was found that miR-3074-5p not only promoted apoptosis and inhibit invasion of HTR8/SVneo cells, a human EVT cell line [10], but also inhibit proliferation and decidualization of T-HESC cells, a human endometrial stromal cell (ESC) line [9], indicating that the up-regulated miR-3074-5p expression at the maternal–fetal interface might lead to early pregnancy loss by impairing normal activities of EVTs and ESCs. The molecular pathway mediating the adverse effects of miR-3074-5p on EVTs activities, however, still need to be revealed.
Growth differentiation factor 15 (GDF15), a member of the transforming growth factor-beta superfamily, is a cellular stress–induced secretory protein, and its expression increases in response to stressors such as tumors, chemotherapy, infection, and inflammation [11,12,13]. GDF15 is highly expressed in human placenta and necessary for the maintenance of pregnancy [14]. The peripheral blood GDF15 level increases significantly along with gestation in normal pregnancy, and decreased circulating GDF15 level is association with RM [15, 16] or PE [17]. Interestingly, our previous results showed that the expression of GDF15 in human endometrial stromal cells significantly decreased after miR-3074-5p overexpression, and peripheral plasma GDF15 level was observed to be significantly decreased in RM patients [9], suggesting that miR-3074-5p might impede cell activities by inhibiting GDF15 expression.
Meanwhile, eukaryotic translation initiation factor 2 subunit 1 alpha (EIF2S1/eIF2a), a transcription factor, was also identified to be a predicted target of miR-3074-5p by Tagetscan analysis. Given it was reported that expression and secretion of GDF15 could be regulated by EIF2S1 [18, 19], and the deficiency of EIF2S1 could lead to the reduced trophectoderm cell number and the delay of fetal development, as well as an increased incidence of early pregnancy failure in mice [20], we speculated that miR-3074-5p might inhibit GDF15 expression and secretion via targeting EIF2S1 expression if GDF15 is not the direct target of miR-3074-5p. Thus, the objective of this study was to determine the villus GDF15 expression level in RM patients, to validate the directly targeting inhibition of GDF15 or EIF2S1 expression by miR-3074-5p, and to observe the effects of down-regulated GDF15 expression on HTR8/SVneo cells activities, as well as the reverse effects of EIF2S1 overexpression or treatment of recombinant GDF15 protein on impaired activities of HTR8/SVneo cells caused by miR-3074-5p, with a view to explore the role of miR-3074-5p/EIF2S1/GDF15 pathway in regulation of EVTs activities.
Materials and Methods
Collection of Human Placental Villus Samples
Human placental villus tissues of RM patients (RV) and normal pregnant women who had no history of miscarriage and were undergoing legal elective abortions at 6–10 weeks of gestation (CV) were collected from April to October of 2017 at the Department of Gynecology and Obstetrics, the Second Hospital of Tianjin Medical University, Tianjin, China. No significant differences in clinical characteristic between the two groups except for pregnancy history and miscarriage history (Table S1). All patients were excluded from classical risk factors for early pregnancy losses, including abnormal parental karyotypes, uterine anatomical abnormalities, infectious diseases, and thyroid dysfunction. The collected tissues were washed twice with ice-cold PBS. Then, approximately 0.5 ml of each specimen was procured for RNA isolation and subsequent RT-qPCR. About 0.3 ml of tissue per specimen was utilized for protein extraction and Western blot analysis. The remaining tissues were promptly frozen in liquid nitrogen for subsequent tissue sectioning and immunofluorescence assay. All the experiments involving humans were approved by the Medical Ethics Committees of The Second Hospital of Tianjin Medical University (KY2017K002) and Shanghai Institute of Planned Parenthood Research (PJ2018-06). Written consent has been obtained from each patient or subject after full explanation of the purpose and nature of all procedures used.
Cell Line Culture, Transfection, and Gene Silencing
The human immortalized first-trimester extravillous trophoblasts (EVTs) line, HTR8/SVneo, was a gift from Professor Hongmei Wang, Institute of Zoology, Chinese Academy of Sciences, China. HTR8/SVneo cells were cultured in RPMI-1640 (Gibco, Thermo Fisher Scientific, Waltham, MA) containing 10% fetal bovine serum (FBS; Gibco-BRL, Carlsbad, CA). The cells were incubated at 37 °C in a humidified incubator in an atmosphere of 5% CO2.
For transfection, Lipofectamine 2000 (Invitrogen, Waltham, MA) was mixed with miR-3074-5p mimics (GUUCCUGCUGAACUGAGCCAG; RiboBio, Guangzhou, China), GDF15-specific siRNA (siR-GDF15: 5′-CCAAACAGCUGUAUUUAUATT-3′; GenePharma, Shanghai, China), and pCDNA3.0-Flag-EIF2S1 recombinant plasmids (Genechem, Shanghai, China) according to the manufacturer’s protocols. Corresponding negative control fragment (NC) and pCDNA3.0-Flag vector plasmids (Genechem, Shanghai, China) were used as controls for these reactions. HTR8/SVneo cells were added to each well at a density of 80% confluence well in 6-well plates, and the transfection solutions were placed into each well. After incubation for 24 h or 48 h, we performed functional analyses of HTR8/SVneo cells. At the same time, the total protein was analyzed by Western blotting, and the RNA was analyzed by real-time PCR to verify the transfection efficiency. We also constructed and packaged a lentivirus (GenePharma, Shanghai, China) to infect HTR8/SVneo cells for the generation of polyclonal cell lines stably overexpressing miR-3074-5p or negative control.
RNA Isolation, Reverse Transcription, and Quantitative Real-Time PCR
The total RNA was extracted from HTR8/SVneo cells or villus tissues using TRIzol Reagent (Invitrogen, Waltham, MA), and 1 µg RNA from each sample was reverse transcribed to cDNA (TOYOBO Ltd., Osaka, Japan) according to the manufacturer’s instructions. The synthesized cDNA was amplified with specific primers (Table S2) and TB Green (TOYOBO Ltd, Osaka, Japan) using a LightCycler 480 II real-time fluorescence quantitative PCR system (Roche, Basel, Switzerland). Triplicate samples were examined for each condition. The relative mRNA expression level was calculated using the 2 − ΔΔCt method.
Real-Time PCR for miR-3074-5p
Real-time PCR for miR-3074-5p was carried out using the FastStart Universal SYBR Green Master kit (Roche Diagnostics) and analyzed using LightCycler 480 system (Roche) according to our previously reported method. Briefly, the primers used for has-miR-3074-5p were supplied by Ribobio, which is a Bulge-Loop TM miRNA PCR Primer Set (RiboBio). Primer efficiencies were determined by the standard curve method. The relative expression level of miR-3074-5p was calculated by the efficiency-corrected ΔCt method and normalized to the endogenous control snRNA U6 [9].
Protein Extraction and Western Blot Analysis
Total lysates were extracted from cells and tissues with pre-configured RIPA lysis buffer (Sangon Biotech, Shanghai, China) containing 1 mM PMSF and protease inhibitor cocktail (Selleck, Shanghai, China). Equal quantities of proteins were separated by SDS-PAGE and blotted onto nitrocellulose membranes (Merck Millipore, Darmstadt, Germany). The blots were blocked with Tris-buffered saline (TBS) containing 5% skimmed milk powder for 1 h at room temperature and incubated with the appropriate primary antibodies (Table S3) overnight at 4 °C. Then, the blots were washed with 1 × TBS/Tween 20 buffer three times, incubated with appropriate secondary antibodies (Table S3) for 1 h at room temperature and detected using an Odyssey CLx Imaging System (LI-COR, Lincoln, Nebraska).
Immunofluorescence Staining Assays
The villus tissues were retrieved from liquid nitrogen and pre-embedded with OCT compound (Leica REF14020108926). And prepare 10 μm cryosections using Cryo-Ultramicrotomes (Leica CM1950). The sections were fixed using 4% PFA for 5 min, permeabilized with 0.1% Triton X-100 (Sigma-Aldrich, St. Louis, Missouri), and blocked with 2% BSA (Amresco, Solon, OH) in PBS for 1 h at room temperature. Sections were incubated with anti-GDF15 antibody (15 μg/ml, ab206414; Abcam) and Pan-Keratin antibody (10 μg/ml, Cat#4545; Cell Signaling Technology) overnight at 4 ℃ in a humidified chamber. HTR8/SVneo cells were placed on coverslips and fixed with 4% PFA in PBS after miR-3074-5p mimics and negative control transfection for 48 h. And 0.1% Triton X-100 and 2% BSA in PBS were used to block and permeabilize cells. The cells were incubated overnight at 4 °C using primary antibodies (Table S3) diluted in PBS with 2% BSA. The sections of OCT-embedded first-trimester human villus and the coverslips plated with HTR8/SVneo cells were stained with DAPI (Invitrogen, Waltham, MA) for 5 min avoiding light at 37 °C after incubated with the appropriate secondary antibodies (Table S3) for 30 min. Next, Confocal images were captured with a Nikon A1R confocal system.
CCK-8 and EdU Staining Assay
The viability of HTR8/SVneo cells was measured using Cell Counting Kit-8 (Cat. No.: C0037, Beyotime, Shanghai, China) and BeyoClick™ EdU Cell Proliferation Kit with Alexa Fluor 555 (Cat. No.: C0075S, Beyotime, Shanghai, China). HTR8/SVneo cells were seeded into a 96-well plate at a density of 1000 cells per well after 24 h of transfection. CCK-8 solution was added to the culture medium at 48 h or 72 h after transfection of HTR8/SVneo cells and then incubated for 1 h. Next, the absorbance of samples at 450 nm wavelength was measured with an ultraviolet spectrophotometer. HTR8/SVneo cells were transfected for 48 h in 6-well plates at a density of 1 × 105 cells per well, following incubated with 10 μM EdU for 2 h at 37 °C, fixed with 4% PFA for 30 min, and permeabilized with 0.1% Triton X-100 for 10 min. Next, 500 μl Click Additive Solution was added into a well for 30-min incubation and DAPI was used to stained DNA for 5 min in light-free condition. All samples were observed by fluorescence microscopy. The experiment was repeated at least three times independently.
Cell Cycle Assay and Flow Cytometry
The 24-h transfected HTR8/SVneo cells were harvested, washed twice with cold PBS, and then fixed in 75% cold ethanol overnight. HTR8/SVneo cells were stained with propidium iodide using Cell Cycle and Apoptosis Analysis Kit (Cat. No.: C1052, Beyotime, Shanghai, China) according to the manufacturer’s protocols and analyzed by flow cytometry on a BD LSRFortessa system (BD Biosciences, San Jose, CA). FlowJo version 7.6.1 software was used to analyze the data of cell cycle profiles. This experiment was independently repeated at least three times.
Wound Healing Assay
HTR8/SVneo cells were plated in 6-well plates at a density of 3 × 105 cells per well. After miRNA mimics/NC and siRNA/NC transfection for 24 h, linear scratch wounds were made in the confluent cell monolayers with a 200-μl pipette tip. The scratch wounds were visualized with bright-field microscopy (Nikon Instruments Inc., Melville, NY). Images were captured at 0 h, 24 h, and 48 h. The area of each scratch wound was traced using ImageJ software.
Transwell Assays
Transwell matrigel invasion assay in 24-well Transwell units (Corning® BioCoat™ Matrigel® Invasion Chamber 354480) was used to determine cell invasion. Following rehydration for 2 h with culture medium added to top and lower chambers, 500 μl RPMI-1640 containing 1 × 105 cells was transferred to matrigel-coated top chambers and 750 μl RPMI-1640 supplemented with 25% FBS was added to the lower chambers to induce invasion. After incubation at 37 °C with 5% CO2 for 24 h, the non-invading cells still on the upper matrigel were removed with cotton swabs. The invaded cells were fixed with 4% paraformaldehyde, stained with 0.1% crystal violet (Sangon Biotech Ltd., Shanghai, China), and then captured in five random fields for each group under a light microscope. In addition, the whole membranes containing all invaded cells were dissolved in methanol on ice for 10 min, and the absorbance at 560 nm was measured with an ultraviolet spectrophotometer. The difference of OD560 values represent the strength of cell invasion ability.
Formation of Trophoblast Spheroids and Co-culture Invasion Assay
Co-culture invasion assay was applied to investigate the invasion of decidualized cells by HTR8/SVneo spheroids. One milliliter culture medium containing 500,000 cells (20 μl per drop) was plated onto the lid of 60-mm Petri dish in regular arrays. The lid was inverted over the bottom of the PBS-filled Petri dish. The dish with the hanging drops were incubated at 37 ℃ with 5% CO2 for 24 h to form cell spheres with a diameter of 150 nm. Subsequently, spheres were transferred to round-bottom glass shaker flasks containing 5 ml of complete medium and cultured on the shaker (130 rpm/min) at 37 °C with 5% CO2 for 48 h to form 300 nm spheroids. To recreate the structure of the endometrium, human endometrial stromal cells (HESC, ATCC® CRL-4003™) were seeded into 35-mm glass bottom dish (Corning Incorporated, Corning, NY) at a density of 3 × 105 cells per dish for 24 h. The in vitro decidualization of HESC was formed as described previously [9]. Briefly, 0.5 nM Dibutyryl-cAMP (cAMP; MCE, Monmouth Junction, NJ), 10 nM β-estradiol (E2; Sigma-Aldrich, St. Louis, MO), and 1 mM medroxyprogesterone 17-acetate (MPA; Sigma-Aldrich, St. Louis, MO) in phenol red-free DMEM/F-12 containing 2% charcoal-stripped fetal bovine serum (CS-FBS; Biological Industries, Cromwell, CT) were applied for the in vitro decidualization. PKH26 cell linker kit (MINI26-1KT; Sigma-Aldrich, St. Louis, Missouri) was used to label the membrane of HESC before co-culture. HTR8/SVneo spheroids were transferred into the decidualized HESC. The invasion of spheroids was observed under 10 × objective at 0 h and 24 h. And the infiltration area of spheres was measured and analyzed by ImageJ.
Dual Luciferase Reporter Assays
A double luciferase reporter gene was used to investigate whether miR-3074-5p regulates the expression of EIF2S1 gene. Both the wild-type and mutated 3′UTRs of EIF2S1 gene were cloned into the GV272 vector (Genechem, Shanghai, China) after firefly Luciferase site. The constructed recombinant plasmids were confirmed by sequencing. 5 × 104 of HEK293 cells were plated per well in 24-well plates. Twenty-four hours after seeding, Firefly luciferase reporter constructs were transiently co-transfected with miR-3074-5p mimic or control and CV045 Renilla luciferase vector (Genechem, Shanghai, China) per well, using Lipofectamine 2000 (Invitrogen, Waltham, MA). Twenty-four hours after transfection, cells were lysed in cell lysis buffer (Yeasen Biotech Ltd., Shanghai, China). The Firefly luciferase gene was used as the reporter fluorescence, whereas the Renilla luciferase gene was used as the internal reference gene in the reporter vector. Luciferase activity in cell lysates was measured using Dual Luciferase Reporter Gene Assay Kit (Yeasen Biotech Ltd., Shanghai, China). The regulatory effect of miR-3074-5p on EIF2S1 was determined based on the relative changes in Firefly luciferase activity.
Cytokine Rescue Experiment
Recombinant human GDF15 protein (rhGDF15; Peprotech, Rocky Hill, NJ) was dissolved in 0.1% BSA containing PBS and stored as recommended. HTR8/SVneo cells were plated in a 35-mm culture dish at a density of 2.5 × 105 cells per dish and incubated 24 h at 37 °C with 5% CO2. After adhesion, HTR8/SVneos were transfected with GDF15-specific siRNA and control. After 24 h, the culture media was refreshed and HTR8/SVneos were stimulated with 20 ng/ml rh-GDF15 for 24 h. Then, the cell proliferation evaluation and invasiveness/migration measurement as described above were repeated.
Statistical Analysis
The detailed information of statistical analysis could be found in the “Results” and Figure Legends. Data are expressed as the means ± SD. Analysis of variance (ANOVA) and Student’s t-test were chosen for comparison among groups. A two-tailed value of P < 0.05 was considered statistically significant. All the statistical analysis were performed by SPSS 20.0 software (SPSS Inc. Chicago, IL).
Results
Villus Expression Level of GDF15 Was Significantly Decreased in RM Patients
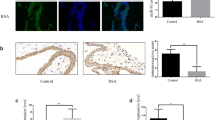
We recruited 10 RM patients who had experienced at least two consecutive spontaneous miscarriages at first-trimester pregnancy and 10 well-matched early pregnant women (control group) between which no significant differences in age and gestational week were observed (Table S1). The villus GDF15 expression level in RM patients and control group was detected by real-time PCR and Western blot analyses. The results showed that the villus GDF15 expression level of RM patients (RV) was significantly lower than that of control group (CV) (Fig. 1A–C). Pan-Keratin signals were detected as marker of trophoblast cells. Immunofluorescence staining showed that GDF15 protein expression was observed in Pan-Keratin-positive trophoblasts in human villus tissues. And the average fluorescence intensity of GDF15 (red) in the villus of RM patients (CV) was obviously weaker than that of control group (CV) (Fig. 1D). These data indicated that GDF15 might play critical roles in trophoblasts, and deficiency in GDF15 might lead to early pregnancy loss.
Villus expressions of GDF15 in RM patients and the normal early pregnant women. A Western blotting of GDF15 expression in villus of RM patients (n = 10) and the control group (n = 10). B Quantification of GDF15 protein levels normalized Tubulin (**P < 0.01, n = 10). C Comparison of the villus expression levels of GDF15 between RM patients (n = 7) and the control group (n = 7) by real-time quantitative PCR analysis (*P < 0.05). D Confocal images of double immunofluorescence staining showing the GDF15 (red) and Pan-Keratin (green) expression in villus of RM patients and the control group (scale bar, 50 μm). CV, villus of control group; RV, villus of RM patients
Knockdown of GDF15 Expression Inhibited Proliferation of HTR8/SVneo Cells
As extravillous trophoblasts (EVTs), an elemental cell type of villus tissues, are involved in embryo implantation and placenta development, and dysfunction of EVTs could cause the adverse pregnancy outcomes including RM, we therefore observed effects of down-regulated GDF15 expression on activities of HTR8/SVneo cells, the human immortalized first-trimester EVTs. HTR8/SVneo cells were respectively transfected with GDF15-siRNA (GDF15-KD) or the negative control fragment (NC), and the significantly reduced GDF15 expression was validated by Western blot and qPCR analyses (Fig. 2A). Then, CCK8 assay and EdU incorporation assay were performed to detect the proliferative activity of HTR8/SVneo cells after 24 h or 48 h of transfection with GDF15-siRNA (GDF15-KD) or NC. The results of the CCK8 assay (Fig. 2B) and the EdU incorporation assay (Fig. 2C) showed that, compared to NC-transfected cells (NC), the proliferative ability of GDF15-siRNA-transfected cells (GDF15-KD) was significantly reduced. Meanwhile, the HTR8/SVneo cells were transfected with miR-3074-5p mimics to overexpressed miR-3074-5p. And it was found by the flow cytometry analysis that, compared to the NC-transfected cells (NC), either down-regulation of GDF15 (GDF15 KD) or overexpression of miR-3074-5p (3074-OE) could inhibit cell cycles by blocking the transformation from G0/G1 to G2/M phase in HTR8/SVneo cells (Fig. 2D).
Knockdown of GDF15 inhibits proliferation, migration, and invasion of HTR8/SVneo cells. A Validation of RNA interference in GDF15 by Western blotting (*P < 0.05) and real-time quantitative PCR (****P < 0.001). B The proliferation ability of HTR8/SVneo cells transfected with either NC or siRNA-GDF15 were measured by CCK-8 assay (**P < 0.01, ns, not significantly different). C EdU incorporation in HTR8/SVneo and purple signal in merge pictures indicate cells capable of DNA synthesis (scale bar, 100 px; n = 3; *P < 0.05). D The cell cycle of HTR8/SVneo cells with miR-3074-5p overexpression, GDF15 knockdown, and control group was analyzed by flow cytometric analysis (n = 3; *P < 0.05, **P < 0.01, ***P < 0.001, ns, not significantly different). E Representative pictures and quantification statistical results of scratch wound assays after 24 h and 48 h from HTR8/SVneo cells (scale bar, 250 μm; n = 5; ****P < 0.001). F Representative pictures and quantitation of transwell assays were applied to show the invasive activity of HTR8/SVneo cells (scale bar, 250 μm; n = 5; **P < 0.01). G Schematic diagram of formation of HTR8/SVneo spheroids and co-culture model of spheroids and decidualized HESC. H HTR8/SVneo spheroids migrated and spread between HESC monolayer, followed by analysis photographs taken at 0 h and 24 h adhesion to monolayer (scale bar, 200 μm; n = 3; *P < 0.05). NC, negative control; GDF15-KD, GDF15 knockdown; 3074-OE, miR-3074-5p overexpression
Knockdown of GDF15 Expression Attenuated Migration and Invasion of HTR8/SVneo Cells
The scratch wound healing assay and the transwell assay were performed to determine the migration and invasion of HTR8/SVneo cells. It was observed that, compared to NC-transfected cells (NC), the migration (Fig. 2E) and invasion (Fig. 2F) of GDF15-siRNA-transfected (GDF15-KD) HTR8/SVneo cells were significantly reduced, indicating a suppressive effect of down-regulated GDF15 expression on migration and invasion of EVTs. Furthermore, the model of co-cultured HTR8/SVneo cells and T-HESCs, the human endometrial stromal cell (ESC) line, was also applied to detect the invasive ability of EVTs on ESCs (Fig. 2G). It was found that, compared to NC-transfected HTR8/SVneo cells (NC), the outgrowth of GDF15-siRNA-transfected (GDF15-KD) HTR8/SVneo cells on T-HESCs was significantly inhibited after 24 h of adhesion (Fig. 2H), suggesting an inhibitory effect of down-regulated GDF15 expression in EVTs on the invasion of EVTs into the maternal endometrial cells.
GDF15 and EIF2S1 Expression Were Down-Regulated by miR-3074-5p in HTR8/SVneo Cells
For we supposed that miR-3074-5p might disturb normal activities of EVTs by directly targeting GDF15, we observed the effect of up-regulated miR-3074-5p expression on GDF15 expression level in HTR8/SVneo cells. Cells were respectively transfected with miR-3074-5p mimic or NC, and the GDF15 expression level was determined by Western blot and qPCR analyses. The results showed that GDF15 expression level in miR-3074-5p mimic-transfected HTR8/SVneo cells (3074-OE) was significantly decreased compared to that in NC-transfected cells (NC) (Fig. 3A and B), indicating an inhibitory effect of miR-3074-5p on GDF15 expression in EVTs. Consistently, the results of immunofluorescence staining assay also showed that GDF15 protein signals in miR-3074-5p mimic-transfected HTR8/SVneo cells (3074-OE) were obviously weakened compared to that in NC-transfected cells (NC) (Fig. 3C). However, as unexpected, the results of luciferase reporter assays showed that GDF15 was not the direct target gene of miR-3074-5p, suggesting an indirectly inhibitory effect of miR-3074-5p on GDF15 expression in EVTs. Given GDF15 expression could be regulated by EIF2S1, another predicted target of miR-3074-5p, we speculated that miR-3074-5p might inhibit GDF15 expression via direct targeting EIF2S1 expression in EVTs. As expected, the results of qPCR and Western blot analyses showed that expression levels of EIF2S1 and ATF4 in HTR8/SVneo cells transfected with miR-3074-5p mimic (3074-OE) were significantly decreased compared to that in cells transfected with NC (NC) (Fig. 3D–F), indicating miR-3074-5p might inhibit expression of EIF2S1 and ATF4 in EVTs. And the results of immunofluorescence assays also showed that EIF2S1 protein signals in miR-3074-5p mimic-transfected cells (3074-OE) were significantly attenuated compared to that in NC-transfected cells (NC) (Fig. 3G).
GDF15 and EIF2S1 are down-regulated by miR-3074-5p. A Real-time PCR analyses of GDF15 and miR-3074-5p mRNAs expression in HTR8/SVneo cells after miR-3074-5p overexpression (***P < 0.001, ****P < 0.001). B Western blot and densitometric analysis of GDF15 protein expression in HTR8/SVneo cells (*P < 0.05). C Immunofluorescence staining of GDF15 (red) in HTR8/SVneo cells transfected by miR-3074-5p mimic or NC (scale bar, 200 μm). D Real-time PCR analysis of EIF2S1 mRNAs expression in HTR8/SVneo cells after miR-3074-5p overexpression (*P < 0.05). E and F Western blot and densitometric analyses of EIF2S1 and ATF4 protein expression in HTR8/SVneo cells after miR-3074-5p overexpression (*P < 0.05, **P < 0.01). G Immunofluorescence staining of EIF2S1 (red) in HTR8/SVneo cells transfected by miR-3074-5p mimic or NC (scale bar, 100 μm)
miR-3074-5p Inhibited GDF15 Expression via Direct Targeting EIF2S1 in HTR8/SVneo Cells
Subsequently, the results of luciferase reporter assay showed that the relative luciferase activity was decreased significantly when the 3′UTR construct with wild-type (WT) sequence of EIF2S1 co-transfected with miR-3074-5p mimic in 293 T cells, whereas no differences were observed when EIF2S1 target sequence mutated (MUT) (Fig. 4A), indicating that miR-3074-5p could inhibit EIF2S1 expression by direct targeting the 3′UTR of EIF2S1 mRNA. We supposed that miR-3074-5p might inhibit GDF15 expression by targeting EIF2S1 in EVTs. Thus, we observed whether or not EIF2S1 overexpression could reverse the inhibitory effect of miR-3074-5p on GDF15 expression in EVTs. And the results of qPCR and Western blot analyses showed that EIF2S1 expression level in HTR8/SVneo cells co-transfected with pCDNA3.0-Flag-EIF2S1 recombinant plasmid and NC (NC-EIF2S1) or miR-3074-5p mimic (3074-EIF2S1) was significantly increased respectively compared to that in cells co-transfected with pCDNA3.0-Flag vector plasmid and NC (NC-vector) or miR-3074-5p mimic (3074-vector) (Fig. 4B–D), indicating that the EIF2S1 expression was significantly up-regulated in cells transfected with pCDNA3.0-Flag-EIF2S1 recombinant plasmid. And as expected, GDF15 expression level in cells co-transfected with recombinant plasmid and miR-3074-5p mimic (3074-EIF2S1) was significantly up-regulated compared to that in cells co-transfected with vector plasmid and miR-3074-5p mimic (Fig. 4B–D), indicating that the overexpression of EIF2S1 could reverse the inhibitory effect of miR-3074-5p on GDF15 expression in EVTs. Furthermore, the villus EIF2S1 expression level in RM patients (RV) was significantly decreased compared to that in control early pregnant women (CV) (Fig. S1), suggesting that miR-3074-5p might be involved in early pregnancy loss by inhibiting GDF15 expression via EIF2S1 pathway in EVTs.
MiR-3074-5p down-regulates GDF15 expression by targeting EIF2S1. A Luciferase reporter assay to validate EIF2S1 as a direct target gene of miR-3074-5p. Upper: Sequence alignment found in bioinformatics analysis between miR-3074-5p and its predicted putative and mutated site in the EIF2S1 3’UTR (mutations in blue). Below: The relative luciferase activity was reduced when the EIF2S1 3'UTR reporter construct with a wild-type target site was co-transfected with a miR-3074-5p mimic. When the target sequence of EIF2S1 was mutated, luciferase activity did not significantly change (WT, wild type; Mut, mutated type; ***P < 0.001). B Real-time PCR analyses of EIF2S1 and GDF15 mRNAs expression levels in HTR8/SVneo cells (*P < 0.05, **P < 0.01, ns, not significantly different). C and D Western blot and densitometric analyses of EIF2S1 and GDF15 protein expression levels in HTR8/SVneo cells (*P < 0.05, **P < 0.01, ns, not significantly different). NC, control group; EIF2S1, EIF2S1 overexpression; 3074/3074-OE, miR-3074-5p overexpression; 3074 + EIF2S1, miR-3074-5p and EIF2S1 overexpression
GDF15 Efficiently Reversed the Effects of miR-3074-5p on Activities of HTR8/SVneo Cells
In order to explore whether or not miR-3074-5p impairs EVTs activities by inhibiting GDF15 secretion, we observed the effect of exogenous GDF15 protein on impairments caused by miR-3074-5p-overexpression in HTR8/SVneo cells. Recombinant human GDF15 protein (rhGDF15) was added into the culture media of HTR8/SVneo cells pre-treated with miR-3074-5p mimic or NC, and the cell proliferation, migration, and invasion were determined respectively by CCK8 (Fig. 5A), EdU staining (Fig. 5B), cell cycle (Fig. 5C), wound healing (Fig. 5D), transwell (Fig. 5E), and co-culture invasion assays (Fig. 5F). The results showed that the proliferation, migration, and invasion of cells transfected by miR-3074-5p mimic (3074-OE) were significantly reduced compared to that of cells transfected by NC (NC), whereas the treatment by rhGDF15 (3074 + GDF15) could efficiently enhance the proliferation, migration, and invasion of HTR8/SVneo cells, suggesting that miR-3074-5p might impair normal activities of EVTs at least partially through the inhibition of GDF15 protein secretion.
GDF15 rescue HTR8/Svneo dysfunction caused by miR-3074-5p. A CCK-8 assay measured the proliferation of HTR8/SVneo cells (**P < 0.01, ***P < 0.001, ****P < 0.001). B EdU assay was used to detect DNA synthesis of HTR-8/Svneo cells (scale bar, 100 px; n = 3; **P < 0.01, ***P < 0.001). C The cell cycle of HTR8/SVneo cells were measured by flow cytometric analysis (n = 3; *P < 0.05, **P < 0.01, ***P < 0.001, ns, not significantly different). D Representative pictures and quantification statistical results of scratch wound assay after 24 h and 48 h from HTR8/SVneo cells (scale bar, 250 μm; n = 5; **P < 0.01, ***P < 0.001, ****P < 0.001). E Transwell assays were applied to show the invasive activity of HTR8/SVneo cells (scale bar, 250 μm; n = 5; ***P < 0.001). F Co-culture invasion assay was applied to investigate the migrated and spread of HTR8/SVneo spheroids on HESC monolayer (scale bar, 200 μm; n = 3; *P < 0.05, ***P < 0.001). NC, negative control; 3074-OE, miR-3074-5p overexpression; 3074 + GDF15, miR-3074-5p and GDF15 overexpression
Discussion
The present study observed not only the significantly decreased villus expression levels of GDF15 and its upstream regulator EIF2S1 in RM patient, and remarkably reduced proliferative and invasive activities of HTR8/SVneo cells followed by the knockdown of GDF15 expression, but also the suppressive effects of miR-3074-5p on GDF15 expression levels in HTR8/SVneo cells, of which could be efficiently reversed by overexpression of EIF2S1, a direct targe gene of miR-3074-5p, as well as the remediation effects of exogenous GDF15 protein on impaired activities of HTR8/SVneo cells caused by the up-regulated miR-3074-5p expression.
During the embryo implantation and placenta development, embryonic trophectoderm cells (TEs) differentiate into cytotrophoblasts (CTBs), and CTBs differentiate into syncytiotrophoblasts (STBs) and extravillous trophoblasts (EVTs) subsequently. STBs are bathed in maternal blood to regulate maternal–fetal material exchange and endocrine function, and EVTs invade into maternal decidua to participate in the remodeling of spiral arterioles and interaction with decidual immune cells (DICs) [21, 22]. And the aberrant activities of trophoblasts could lead to several pregnancy complications, including RM [2, 23]. GDF15 (also known as MIC-1, PDF, PLAB, PTGF-β, and NAG-1) is a highly expressed secretory protein at the maternal–fetal interface, of which mainly consists of trophoblasts, decidual stromal cells (DSCs), and DICs, since the early stage of pregnancy [24,25,26].
It was reported that the production of GDF15 was significantly increased during syncytialization of CTBs to STBs to induce and maintain the immune tolerance at the maternal–fetal interface [27]. However, the roles of GDF15 in EVTs are still largely unclear. Here, we observed that the down-regulated GDF15 expression led to the reduced proliferation, migration, and invasion of HTR8/SVneo cells, indicating that GDF15 might play vital roles in regulation of EVTs function during implantation and placentation, and deficiency in GDF15 function could lead to early pregnancy failure by interfering with activities of EVTs.
In consistent with reported data [15, 16], it was observed in our previous research that the peripheral plasma GDF15 level of RM patients was remarkably lower than that of normal early pregnant (NP) women [9]. And in this study, an obviously decreased villus GDF15 expression level was also detected in RM patients, further indicating that deficiency in GDF15 function might be involved in the pathogenesis of RM. But, we have noticed that, it was recently reported that villus GDF15 expression was increased in RM patients compared with NP women, and knockdown of GDF15 promoted migration, whereas overexpression of GDF15 inhibited migration of HTR8 cells [28]. We are unable to explain such a contradictory result presently, but it was reported that the increased GDF15 expression induced by activated glutaminolysis in decidual NK cells (dNKs), the largest group of DICs at the mater-fetal interface, promotes trophoblast invasion, and the inactivated glutamine metabolism in dNKs could impair trophoblast invasion and lead to pregnancy loss [29], indicating that an appropriately high level of GDF15 at the mater-fetal interface should be beneficial to the successful pregnancy. In our preliminary research works on GDF15, we had carried out the experiments to observe the GDF15 expression in human decidua tissues of early pregnancy by WB analysis, and results showed that the decidual GDF15 expression level of RM patients was not notably different compared to that of Control women (Fig. S2). Nonetheless, it is imperative to corroborate this finding through the examination of more extensive sample sizes.
In addition, GDF15 could promote the anti-inflammatory M2 polarization of macrophages, and GDF15 deficient macrophages retain pro-inflammatory M1 polarization [30]. Decidual macrophages (dMϕs), the second largest group of DICs, participate in the immune modulation and spiral artery remodeling at the maternal–fetal interface [31], and the enhanced M1 polarization is associated with RM [32]. Thus, the increased GDF15 expression and secretion by EVTs might contribute to the establishment and maintenance of pregnancy by inducing M2 polarization of dMϕs. Namely, aberrantly decreased GDF15 expression in EVTs might lead to miscarriage not only by inhibiting proliferation and invasion of EVTs, but also by promoting M1 polarization of dMϕs via paracrine pathway, but this speculation should be further authenticated in our next investigation.
Given it was found in our previous studies that villus and decidual miR-3074-5p expression levels of RM patients were significantly decreased compared to NP women, the up-regulated miR-3074-5p expression could attenuate invasive activity of HTR8/SVneo cells, and GDF15 was a potential downstream regulating gene of miR-3074-5p [8,9,10]; we hypothesized that miR-3074-5p might dysregulate trophoblasts activities by regulating GDF15. Consistently, the significantly decreased GDF15 expression was observed in miR-3074-5p overexpressed HTR8/SVneo cells. However, GDF15 was confirmed not to be a target gene of miR-3074-5p by bioinformatics analysis, suggesting that inhibitory effect of miR-3074-5p on GDF15 expression is mediated by an intermediary regulator. And then, the upstream regulator of GDF15, EIF2S1 comes into our sight, as it was identified as a predict target gene of miR-3074-5p.
It has been demonstrated that endoplasmic reticulum (ER) stress presents adverse effects on placental development, and higher ER stress in blastocysts leads to a reduced amount of trophectoderm cells [20]. ER stress activates the phosphorylation of EIF2S1, and phosphorylated EIF2S1 induces the activation of activating transcription factor 4 (ATF4), which forms a heterodimer with the C/EBP homologous protein (CHOP) to control GDF15 transcription [18, 33]. The deficiency in EIF2S1 function caused by mutation of EIF2S1 gene led to early pregnancy loss and intrauterine growth restriction in mice [20, 34]. Encouragingly, it was observed here that villus EIF2S1 expression level in RM patients was significantly decreased, miR-3074-5p could inhibit the expression of EIF2S1 and ATF4 in HTR8/SVneo cells, and EIF2S1 is a direct downstream target gene of miR-3074-5p. These data indicated that miR-3074-5p might suppress GDF15 production by the targeting inhibition of EIF2S1 expression. At the same time, we also noticed that EIF2S1 can partially rescue the inhibitory effect of miR-3074-5p on GDF15 expression, despite being more than 70%, and we speculated that miR-3074-5p may potentially influence GDF15 through other pathways.
More convincingly, it was also witnessed here that not only the inhibitory effects of miR-3074-5p on GDF15 expression in HTR8/SVneo cells could be efficiently blocked by overexpression of EIF2S1, but also the impaired proliferative and invasive activities of HTR8/SVneo cells caused by miR-3074-5p could be significantly improved by the treatment with exogenous GDF15 protein, furtherly indicating that miR-3074-5p might disturb normal activities of EVTs via EIF2S1/GDF15 pathway. However, we understood that data of this study are not sufficient enough, for the lack of in vivo data about roles of miR-3074-5p/EIF2S1/GDF15 pathway at maternal–fetal interface. The miR-3074-5p knock-in mouse model should be established and applied in our next investigation to explore the effects of dysregulated miR-3074-5p/EIF2S1/GDF15 pathway on pregnancy outcomes in vivo.
In summary, it was demonstrated in the present study that villus expression levels of GDF15 and EIF2S1 were significantly decreased in RM patients, EIF2S1 was a direct target gene of miR-3074-5p, and miR-3074-5p could suppress the GDF15 expression via the inhibition of EIF2S1 expression in EVTs, leading to the reduced proliferation, migration, and invasion abilities of EVTs (Fig. 6). These data indicated that abnormally increased miR-3074-5p in EVTs might lead to early pregnancy loss by impairing proliferative and invasive activities of EVTs via EIF2S1/GDF15 pathway, providing a potential biomarker or/and therapeutic target for recurrent miscarriages.
A working model for the role of miR-3074-5p/EIF2S1/GDF15 axis in recurrent miscarriage disease. A decreased GDF15 expression might cause recurrent miscarriage by disturbing the biological function of trophoblast cells, and GDF15 expression is regulated via miR-3074-5p/EIF2S1 axis, and these may serve as potential therapeutic targets for recurrent miscarriage treatments
Data Availability
The data for this study are available by contacting the corresponding author upon reasonable request.
Code Availability
Not applicable.
References
Quenby S, Gallos ID, Dhillon-Smith RK, Podesek M, Stephenson MD, Fisher J, Brosens JJ, Brewin J, Ramhorst R, Lucas ES, et al. Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet. 2021;397(10285):1658–67.
Abbas Y, Turco MY, Burton GJ, Moffett A. Investigation of human trophoblast invasion in vitro. Hum Reprod Update. 2020;26(4):501–13.
Turco MY, Moffett A. Development of the human placenta. Development. 2019;146(22):1–14. https://doi.org/10.1242/dev.163428.
Liu X, Fei H, Yang C, Wang J, Zhu X, Yang A, Shi Z, Jin X, Yang F, Wu D, et al. Trophoblast-derived extracellular vesicles promote preeclampsia by regulating macrophage polarization. Hypertension. 2022;79(10):2274–87.
Pan Y, Yan L, Chen Q, Wei C, Dai Y, Tong X, Zhu H, Lu M, Zhang Y, Jin X, et al. Dysfunction of Shh signaling activates autophagy to inhibit trophoblast motility in recurrent miscarriage. Exp Mol Med. 2021;53(1):52–66.
Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350–5.
Reza A, Choi YJ, Han SG, Song H, Park C, Hong K, Kim JH. Roles of microRNAs in mammalian reproduction: from the commitment of germ cells to peri-implantation embryos. Biol Rev Camb Philos Soc. 2019;94(2):415–38.
Gu Y, Zhang X, Yang Q, Wang J, He Y, Sun Z, Zhang H, Wang J. Aberrant placental villus expression of miR-486–3p and miR-3074–5p in recurrent miscarriage patients and uterine expression of these microRNAs during early pregnancy in mice. Gynecol Obstet Invest. 2016;81(2):112–7. https://doi.org/10.1159/000435879.
Meng N, Wang X, Shi Y, Mao Y, Yang Q, Ju B, Zhu Q, Zhang T, Gu Y, Zhang X. miR-3074-5p/CLN8 pathway regulates decidualization in recurrent miscarriage. Reproduction. 2021;162(1):33–45.
Gu Y, Shi Y, Yang Q, Gu WW, He YP, Zheng HJ, Zhang X, Wang JM, Wang J. miR-3074-5p promotes the apoptosis but inhibits the invasiveness of human extravillous trophoblast-derived HTR8/SVneo cells in vitro. Reprod Sci. 2018;25(5):690–9.
Myojin Y, Hikita H, Sugiyama M, Sasaki Y, Fukumoto K, Sakane S, Makino Y, Takemura N, Yamada R, Shigekawa M, et al. Hepatic stellate cells in hepatocellular carcinoma promote tumor growth via growth differentiation factor 15 production. Gastroenterology. 2021;160(5):1741-1754 e1716.
Yang CZ, Ma J, Zhu DW, Liu Y, Montgomery B, Wang LZ, Li J, Zhang ZY, Zhang CP, Zhong LP. GDF15 is a potential predictive biomarker for TPF induction chemotherapy and promotes tumorigenesis and progression in oral squamous cell carcinoma. Ann Oncol. 2014;25(6):1215–22.
Luan HH, Wang A, Hilliard BK, Carvalho F, Rosen CE, Ahasic AM, Herzog EL, Kang I, Pisani MA, Yu S, et al. GDF15 Is an inflammation-induced central mediator of tissue tolerance. Cell. 2019;178(5):1231-1244 e1211.
Yokoyama-Kobayashi M, Saeki M, Sekine S, Kato S. Human cDNA encoding a novel TGF-beta superfamily protein highly expressed in placenta. J Biochem. 1997;122(3):622–6.
Kaitu’u-Lino TJ, Bambang K, Onwude J, Hiscock R, Konje J, Tong S. Plasma MIC-1 and PAPP-a levels are decreased among women presenting to an early pregnancy assessment unit, have fetal viability confirmed but later miscarry. PLoS ONE. 2013;8(9):e72437.
Tong S, Marjono B, Brown DA, Mulvey S, Breit SN, Manuelpillai U, Wallace EM. Serum concentrations of macrophage inhibitory cytokine 1 (MIC 1) as a predictor of miscarriage. Lancet. 2004;363(9403):129–30.
Chen Q, Wang Y, Zhao M, Hyett J, da Silva CF, Nie G. Serum levels of GDF15 are reduced in preeclampsia and the reduction is more profound in late-onset than early-onset cases. Cytokine. 2016;83:226–30.
L'homme L, Sermikli BP, Staels B, Piette J, Legrand-Poels S, Dombrowicz D. Saturated fatty acids promote GDF15 expression in human macrophages through the PERK/eIF2/CHOP signaling pathway. Nutrients. 2020;12(12):3771. https://doi.org/10.3390/nu12123771.
Wang D, Day EA, Townsend LK, Djordjevic D, Jørgensen SB, Steinberg GR. GDF15: emerging biology and therapeutic applications for obesity and cardiometabolic disease. Nat Rev Endocrinol. 2021;17(10):592–607.
Capatina N, Hemberger M, Burton GJ, Watson ED, Yung HW. Excessive endoplasmic reticulum stress drives aberrant mouse trophoblast differentiation and placental development leading to pregnancy loss. J Physiol. 2021;599(17):4153–81.
Gude NM, Roberts CT, Kalionis B, King RG. Growth and function of the normal human placenta. Thromb Res. 2004;114(5–6):397–407.
Hemberger M, Hanna CW, Dean W. Mechanisms of early placental development in mouse and humans. Nat Rev Genet. 2020;21(1):27–43.
Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol. 2006;6(8):584–94.
Fejzo MS, Trovik J, Grooten IJ, Sridharan K, Roseboom TJ, Vikanes A, Painter RC, Mullin PM. Nausea and vomiting of pregnancy and hyperemesis gravidarum. Nat Rev Dis Primers. 2019;5(1):62.
Turco MY, Gardner L, Kay RG, Hamilton RS, Prater M, Hollinshead MS, McWhinnie A, Esposito L, Fernando R, Skelton H, et al. Trophoblast organoids as a model for maternal-fetal interactions during human placentation. Nature. 2018;564(7735):263–7.
Segerer SE, Rieger L, Kapp M, Dombrowski Y, Muller N, Dietl J, Kammerer U. MIC-1 (a multifunctional modulator of dendritic cell phenotype and function) is produced by decidual stromal cells and trophoblasts. Hum Reprod. 2012;27(1):200–9.
Hong K, Muralimanoharan S, Kwak YT, Mendelson CR. NRF2 serves a critical role in regulation of immune checkpoint proteins (ICPs) during trophoblast differentiation. Endocrinology. 2022;163(7):1–15. https://doi.org/10.1210/endocr/bqac070.
Hong W, Chen JH, Ma HJ, Li L, Li XC. Fragile X-related protein 1 (FXR1) promotes trophoblast migration at early pregnancy via downregulation of GDF-15 expression. Reprod Sci. 2022;29(1):110–21.
Yang SL, Tan HX, Lai ZZ, Peng HY, Yang HL, Fu Q, Wang HY, Li DJ, Li MQ. An active glutamine/α-ketoglutarate/HIF-1α axis prevents pregnancy loss by triggering decidual IGF1(+)GDF15(+)NK cell differentiation. Cell Mol Life Sci. 2022;79(12):611.
Zhang X, Dong S. Protective effect of growth differentiation factor 15 in sepsis by regulating macrophage polarization and its mechanism. Bioengineered. 2022;13(4):9687–707.
Pollheimer J, Vondra S, Baltayeva J, Beristain AG, Knöfler M. Regulation of placental extravillous trophoblasts by the maternal uterine environment. Front Immunol. 2018;9:2597.
Wang WJ, Hao CF, Lin QD. Dysregulation of macrophage activation by decidual regulatory T cells in unexplained recurrent miscarriage patients. J Reprod Immunol. 2011;92(1–2):97–102.
Donnelly N, Gorman AM, Gupta S, Samali A. The eIF2α kinases: their structures and functions. Cell Mol Life Sci. 2013;70(19):3493–511.
Yung HW, Hemberger M, Watson ED, Senner CE, Jones CP, Kaufman RJ, Charnock-Jones DS, Burton GJ. Endoplasmic reticulum stress disrupts placental morphogenesis: implications for human intrauterine growth restriction. J Pathol. 2012;228(4):554–64.
Acknowledgements
We acknowledge all the authors for their contribution to the study.
Funding
This study was kindly supported by grants from the following: National Natural Science Foundation of China (No. 82371686); the Natural Science Foundation of Shanghai (No. 20ZR1447900).
Author information
Authors and Affiliations
Contributions
JS and LY contributed equally to this work. JW and XZ conceived and designed the study. JS, LY, and WG contributed to experiment and data analysis. YG and YS contributed to sample collection and clinical diagnosis. JG, HJ, HX, and SY provided useful advice to the analyses of the data. JS and LY wrote the manuscript. All authors critically revised the manuscript and approved the final version.
Corresponding authors
Ethics declarations
Ethics Approval
All experiments involving human were approved by the Medical Ethics Committees of The Second Hospital of Tianjin Medical University (KY2017K002) and the Shanghai Institute for Biomedical and Pharmaceutical Technologies (PJ2018-06).
Consent for Publication
Data have been approved for publication.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, JX., Yang, L., Gan, J. et al. MiR-3074-5p Regulates Trophoblasts Function via EIF2S1/GDF15 Pathway in Recurrent Miscarriage. Reprod. Sci. 31, 1290–1302 (2024). https://doi.org/10.1007/s43032-023-01436-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-023-01436-0