Abstract
Purpose
Polycystic ovary syndrome (PCOS) has been associated with Hashimoto’s thyroiditis (HT) and 4 phenotypes have been described in this syndrome. The aim of this work was to investigate the frequency of anti-thyroid antibodies (TAb) and thyroid function in the 4 phenotypes of PCOS.
Patients
This study included 448 patients with PCOS: 260 (58.0%) with phenotype A, 119 (26.6%) with phenotype B, 38 (8.5%) with phenotype C and 31 (6.9%) with phenotype D.
Results
TAb positivity was detected in 90/448 patients (20.1%) and was statistically significant higher (p = 0.03) in the grouped phenotypes A-B (83/379, 21.9%) than in phenotypes C-D (7/69, 10.1%). Positive anti-thyroglobulin antibodies (TgAb) were detected in 74/448 (16.5%) patients and positive anti-thyroperoxidase antibodies (TPOAb) in 66/448 (14.7%) patients. Both TgAb and TPOAb positivity was higher but not statistically significant in phenotype A-B than phenotype C-D. High titer TgAb (> 100 UI/ml) frequency was significantly higher (p = 0.005) in grouped phenotypes A-B (39/379, 10.3%) than in phenotypes C-D (0/69, 0.0%), while no significant difference was observed for low titer TgAb (≤ 100 UI/ml). According to a binary logistic regression analysis hypothyroidism was significantly associated with TAb positivity (OR 4.19; CI 2.25–7.79; p < 0.01) but not with PCOS phenotype. Androgen profile was not associated with TAb positivity.
Conclusion
A higher frequency of positive TAb and of high titer TgAb and TPOAb have been detected in PCOS women with phenotypes A and B, probably in relation to the greater imbalances between estrogen and progesterone levels present in these phenotypes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polycystic ovary syndrome (PCOS) is the most common female endocrine disorder, with a prevalence of about 5–10% in women of reproductive age [1,2,3]. PCOS is characterized by hyperandrogenism, hirsutism, acne, and alopecia, menstrual irregularity, infertility and polycystic ovaries [1,2,3]. Metabolic alterations, such as insulin resistance and central obesity, are also described [1,2,3]. All these are risk factors for impaired glucose tolerance, type 2 diabetes, metabolic syndrome and increased risk for cardiovascular disease [4, 5].
After the first description of PCOS by Stein and Leventhal [6], the diagnostic criteria of PCOS have evolved during the years. The diagnosis of PCOS is usually made according to the ESHRE/ASRM criteria. In 2012, during an Evidence-Based Methodology PCOS Workshop at NIH a panel of experts recommended the use of the ESHRE/ASRM 2003 criteria but suggested that a detailed description of the PCOS phenotype should be provided [7].
The association between autoimmunity and PCOS has been extensively discussed in recent years [8]. An association has been reported between PCOS and autoimmune diseases [9, 10] and autoimmune thyroid diseases (AITD) [11,12,13]. Autoimmune thyroiditis is the most common autoimmune pathology of women of childbearing age and is characterized by the presence of antithyroid autoantibodies (TAb) and by lymphocytic infiltration of the thyroid leading to a progressive destruction of the glandular parenchyma and in development of hypothyroidism. A recent meta-analysis [14] has shown that the presence of subclinical hypothyroidism in PCOS women is associated with mild metabolic abnormalities, affecting the levels of high-density lipoprotein (HDL), triglyceride and homeostatic model assessment for insulin resistance (HOMA-IR).
The aim of this work was to evaluate the frequency of thyroid autoimmunity and hypothyroidism in PCOS patients and to verify any possible difference among the different 4 phenotypes of PCOS.
Patients and Methods
Patients
In this retrospective study, 448 consecutive patients with PCOS were included, all recruited at the Department of Endocrinology of Pisa from 2014 to 2022. Clinical evaluation included measurement of weight and height, body mass index [BMI, weight (kg)/height2 (m2)]. Patients were grouped according to BMI levels as follows: normal weight (BMI 18–24.9 kg/m2); overweight (BMI 25–29.9 kg/m2); obesity (BMI > 30 kg/m2). Anamnestic data about menstrual cycle irregularities were collected, and clinical exam, including the evaluation of any signs of hyperandrogenism such as acne, alopecia, hirsutism, was performed. The modified Ferriman–Gallwey score [15] was used to evaluate the hair growth in nine body sites: upper lip, chin, chest, back, upper abdomen, lower abdomen, arms, thighs, and buttocks. In each of these areas, a score ranging from 0 (absence of terminal hairs) to 4 (extensive terminal hair growth) was assigned. A total score higher than 8 was indicative of hirsutism.
Acne was assessed according to the Global Acne Grading System [16] and alopecia was evaluated by Ludwig’s stage [17].
The diagnosis of PCOS was made following the Rotterdam diagnostic criteria (ESHRE-ASRM 2003 [18]), including in the study the patients who presented at least two among:
-
Clinical (acne, seborrhea, alopecia, hirsutism) and/or biochemical hyperandrogenism. Biochemical hyperandrogenism was assessed by measurement of total testosterone, free testosterone calculated as a function of total testosterone and sex hormone-binding globulin (SHBG) levels, androstenedione, and dehydroepiandrosterone sulfate (DHEA-S).
-
Amenorrhea (lack of menstruation > 3 months) or Oligo-anovulation (menstrual cycle > 35 days).
-
Polycystic ovarian morphology (PCOM) at pelvic ultrasound scan (at least one ovary with 12 or more follicles measuring 2 and 9 mm, regardless of their arrangement and/or ovarian volume > 10 ml or cm3) [19]. Ovarian morphology was preferentially assessed by transvaginal ultrasound using a transducer frequency of 5–13 MHz. When the transvaginal route was not feasible, the examination was performed abdominally using a transducer frequency of 2–8 MHz. The same machine (Voluson E8 Expert, General Electric) was used for both US.
The concentration of 17-OH-progesterone (17-OH-P) was measured in all patients. In patients with basal 17-OH-P levels were higher than 3 ng/ml, an ACTH test was performed to exclude congenital adrenal hyperplasia [20].
We performed the overnight 1-mg dexamethasone suppression test to exclude Cushing’s syndrome. A suppressed cortisol values < 1.8 mcg/dl was considered normal [21].
Patients with idiopathic hyperandrogenism (clinical and/or biochemical hyperandrogenism, without oligo-anovulation and PCOM at pelvic ultrasound) or idiopathic hirsutism (clinical hyperandrogenism without biochemical hyperandrogenism, oligo-anovulation, PCOM at pelvic ultrasound) were excluded.
Patients with ovarian or adrenal tumors diagnosed according to laboratory and imaging data and patients with hyperprolactinemia defined as the presence of serum prolactin (PRL) levels above the normal range were also excluded.
None of the patients took any medication during a period of at least 3 months before the study.
PCOS patients were subdivided in 4 phenotypes according to the recommendation of the 2012 NIH consensus panel [7]:
-
Phenotype A: “complete phenotype”, with signs and symptoms of clinical and/or biochemical hyperandrogenism and oligo-anovulation with ovaries having a micropolycystic appearance on ultrasound.
-
Phenotype B: “classic phenotype”, characterized by the presence of clinical and/or biochemical hyperandrogenism and oligo-anovulation.
-
Phenotype C: “ovulatory phenotype”, with clinical and/or biochemical hyperandrogenism and ovaries with a micropolycystic ultrasound appearance.
-
Phenotype D: “non-hyperandrogenic phenotype”, characterized only by the presence of oligo-anovulation and PCO-like ovaries on ultrasound, and by a milder clinical presentation than the previous ones [22].
Laboratory tests
In all patients, fasting blood samples were collected during the follicular phase of the menstrual cycle. In amenorrhoeic woman blood samples were obtained after Medroxy-Progesterone Acetate (MPA) test. Serum levels of plasma luteinizing hormone (LH), follicle-stimulating hormone (FSH), and estradiol (E2) concentrations were determined by immunometric assays (Johnson & Johnson S. p. A-Ortho Clinical Inc., Rochester, NY). Serum levels of 17-OH-Progesterone concentrations were determined by Elisa (DRG International, INC USA) and Androstenedione (A4) levels by a radioimmunoassay (Diasource Europe S. A., Nivelles, Belgium). Total testosterone (TT) concentration was evaluated by using a competitive immunoassay (Johnson & Johnson S. p. A-Ortho Clinical Inc.). Dehydroepiandrosterone sulfate (DHEAS) concentrations were determined with a radioimmunoassay (Orion Diagnostica, Espoo, Finland). Sex hormone binding globulin (SHBG) levels were detected by immunoassay (Access Immunoassay System, SHBG, Beckman Coulter, Brea, CA, USA). The free androgen index (FAI) was calculated with the formula: FAI = T (nmol/L) × 100/SHBG (nmol/L) [23]. The sensitivity and inter- and intra-assay variation of each hormone had been described in detail in a previous paper [24].
Levels of free thyroxine (FT4) and free triiodothyronine (FT3) in serum were determined by chemiluminescent immunoassay (CLIA) using the VITROS Eci/ECiQ (Vitro System, Ortho-Clinical Diagnostic, Rochester, NY, USA). Thyroid stimulating hormone (TSH) in serum was determined by immunochemiluminescent assays (ICMA) using the VITROS Eci/ECiQ Immunodiagnostic Systems. The reference values of TSH were 0.400 mU/L–4.000 mU/L. Quantitative determination of TgAb and TPOAb was performed by immunofluorimetric assay (IFMA) using the two-step enzyme immunoassay system (Tosoh Corp., Tokyo, Japan). The reference values of TgAb and TPOAb are respectively < 30 IU/ml (NIBSC 65/093) and < 10 IU/ml (NIBSC 66/387).
Hypothyroidism was defined by the presence of high levels of TSH and normal or low levels of thyroid hormones. We considered PCOS patients with hypothyroidism if they had serum level of TSH > 4 mU/L or if under hormone replacement therapy with L-thyroxine (L-T4).
Statistical analysis
Variables were preliminarily tested for normal distribution with the Shapiro–Wilk test and since all variables did not show a normal distribution they were expressed as median (interquartile range [IQR]). Continuous variables were compared by the Mann–Whitney-U-test and Kruskall–Wallis test. Categorical variables were compared by the chi-square test or Fisher exact tests. A forward stepwise binary logistic regression was performed to identify the significant predictors of hypothyroidism.
Results
PCOS phenotypes
A total of 448 patients with PCOS were recruited and were divided into the 4 PCOS phenotypes, according to the ESHRE-ASMR 2003 diagnostic criteria: 260 patients (58.0%) were included in Phenotype A (Complete PCOS), 119 patient (26.6%) were included in Phenotype B (Classic PCOS), 38 patients (8.5%) in Phenotype C (Ovulatory PCOS), 31 patients (6.9%) in Phenotype D (Non-Hyperandrogenic PCOS).
The patients’ age was not significantly different among the 4 subgroups.
The patients were further subdivided according to their BMI as shown in Fig. 1. In phenotype A 36.5% (95/260) of patients were normal weight, 21.1% (55/260) overweight and 42.4% (110 /260) obese. In phenotype B, 29.4% (35/119) of patients were normal weight, 26.1% (31/119) overweight and 44.5% (53/119) obese. In phenotype C, 50% (19/38) of patients were normal weight, 15,7% (6/38) overweight and 34.2% (13/38) obese. In phenotype D, 67.7% (21/31) of patients were normal weight, 25.8% (8/31) overweight and 6.4% (2/31) obese. Phenotype D included a significantly higher frequency of normal weight and lower frequency of obese compared to the other subgroups. Weight distribution does not differ in the other three phenotypes (Fig. 1).
Weight distribution in different PCOS phenotypes. A progressive reduction of frequency of obese patients and an increase of frequency of normal weight subjects were observed moving from phenotype A to D. Phenotype D included a significantly higher frequency of normal weight and lower frequency of obese compared to the other subgroups. Weight distribution does not differ in the other three phenotypes
Serum levels of androgens in the different PCOS phenotypes are shown in Table 1. Serum levels of testosterone, Free T, androstenedione and 17-OH-P were significantly different among the 4 phenotypes (Kruscal–Wallis test p < 0.05). In particular:
-
Testosterone, Free T and androstenedione values were significantly lower (Mann–Whitney test p < 0.05) in phenotype D (non-androgenic) compared to phenotypes A, B and C;
-
SHBG values were significantly higher (Mann–Whitney test p < 0.05) in phenotype D versus phenotypes A, D and C and also in phenotype C versus phenotypes A and B;
-
FAI values were significantly lower (Mann–Whitney test p < 0.05) in phenotype D versus phenotypes A, B and C, but also in phenotype C versus A and B;
-
17OH P values were significantly higher (Mann–Whitney test p < 0.05) in phenotype C vs phenotypes A, B and D
-
DHEAs did not show significant differences
We also evaluated serum level of prolactin (PRL) in the different phenotypes of PCOS and no significant difference was observed.
PCOS phenotypes and thyroid autoimmunity
Antithyroid antibodies
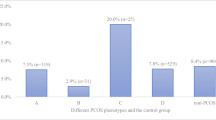
Positive Tab were detected in 90/448 (20.1%) patients. TAb positivity was more frequent in phenotype A (59/260, 22.7%) and phenotype B (24/119, 20.2%) compared to phenotype C (5/38, 13.2%) and phenotype D (2/31, 6–5%), albeit no statistical significance was found. The patients were grouped in phenotype A-B (including patients with phenotype A and phenotype B) and in phenotype C-D (including patients with phenotype C and phenotype D). TAb positivity was significantly more frequent in grouped phenotypes A-B (83/379, 21.9%) than grouped phenotypes C-D (7/69, 10.1%) (chi square p = 0.03) (Fig. 2).
Frequency of positive TAb in grouped phenotypes. When patients were grouped in phenotype A-B (including phenotype A and phenotype B) and phenotype C-D (including phenotype C and phenotype D) a significant higher frequency of positivity TAb was observed in grouped phenotypes A-B than in grouped phenotypes C-D (chi-square p = 0.03)
Anti-thyroglobulin antibodies
Positive TgAb were detected in 74/448 (16.5%) patients. TgAb frequency was higher in phenotype A (49/260, 18.8%) and phenotype B (19/119, 16.0%) than phenotype C (4/38, 10.5%) and phenotype D (2/31, 6.5%), even though those differences were not statistically significant. We then grouped the patients as indicated before we found that TgAb frequency was higher in grouped phenotypes A-B (68/379, 17.9%) than grouped phenotypes C-D (6/69, 8.7%), although these results were not significant (Chi square p = 0.07) (Fig. 3a).
Frequency of positive TgAb in grouped phenotypes. Panel A: the frequency of positive TgAb was higher in grouped phenotypes A-B than grouped phenotypes C-D although these results were not significative (chi square p = 0.07). Panel B: the frequency of low titer TgAb (< 100 U/ml) was not significantly different phenotypes A-B and C-D. The frequency of high titer TgAb (> 100 U/ml) was significantly higher in phenotypes A-B than in phenotypes C-D (Fisher exact test p < 0.01)
We subsequently subdivided the patients according to the serum level of TgAb. We found that low titer TgAb (≤ 100 UI/ml) frequency did not differ significantly among the 4 phenotypes (17/260, 6.5% in phenotype A; 12/119, 10.1% in phenotype B; 4/38, 10.5% in phenotype C; 2/31, 6.5% in phenotype D). High titer TgAb (> 100 UI/ml) were instead significantly more frequent in phenotype A (32/260, 12.3%) and phenotype B (7/119, 5.9%) compared to phenotype C (0/38, 0.0%) and phenotype D (0/31, 0.0%) (Fisher exact tests p < 0.01).
Low titer TgAb frequency did not differ significantly among the grouped phenotypes A-B (6/69, 7.7%) and C-D (29/379, 8.7%). High titer TgAb frequency was instead significantly more frequent in grouped phenotypes A-B (39/379, 10.3%) than grouped phenotypes C-D (0/69, 0.0%) (Fisher exact tests p < 0.01) (Fig. 3b).
Anti-thyroperoxidase antibodies
Positive TPO were found in 66/448 (14.7%) patients. TPOAb frequency was higher in phenotype A (43/260, 16.5%) and phenotype B (18/119, 15.1%) than phenotype C (4/38, 10.5%) and phenotype D (1/31, 3.2%), although the differences were not statistically significant.
TPOAb frequency was higher in regrouped phenotypes A-B (61/379, 16.1%) than regrouped phenotypes C-D (5/69, 7.2%), although the results were on borderline statistical significance (Fisher exact tests p = 0.06) (Fig. 4a). Considering different TPOAb titer levels, low titer TPOAb (≤ 100 UI/ml) frequency did not differ significantly among the 4 phenotypes (16/260, 6.2% in phenotype A; 6/119, 5.0% in phenotype B; 3/38, 7.9% in phenotype C; 0/31, 0.0% in phenotype D). High titer TPOAb (> 100 UI/ml) frequency was higher in phenotype A (27/260, 10.4%) and phenotype B (12/119, 10.1%) than phenotype C (1/38, 2.6%) and phenotype D (1/31, 3.2%), without statistical significance (p = 0.43). Low titer TPOAb frequency did not differ significantly between regrouped phenotypes A-B (22/379, 5.8%) and C-D (3/69, 4.3%). High titer TPOAb frequency was instead higher in regrouped phenotypes A-B (39/379, 10.3%) than regrouped phenotypes C-D (2/69, 2.9%), although no statistical significance was found (Fig. 4b).
Frequency of positive TPOAb in grouped phenotypes. Panel A: the frequency of positive TPOAb was higher but not significantly different in phenotypes A-B compared to phenotypes C-D. Panel B: the frequency of low titer TPOAb (< 100 U/ml) was not significantly different in phenotypes A-B and C-D. The frequency of high titer TPOAb (> 100 U/ml) was higher but not significantly different in phenotypes A-B compared to phenotypes C-D
PCOS phenotypes and thyroid function
Thyroid function was evaluated in 448 PCOS patients. Hypothyroidism was found in 53/448 patients (11.8%). Out of these 53 patients 21 had serum TSH levels > 4 mU/L and 32 were hypothyroid under treatment with L-T4.
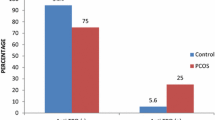
Hypothyroidism frequency was significantly higher in phenotype B (24/119, 20.7%) than phenotype A (22/260, 8.6%), phenotype C (5/36, 13.9%) and phenotype D (2/31, 6.5%) (p = 0.009).
Hypothyroidism was significantly more frequent in patients with TAb positivity (23/87, 26.4%) rather than patients with TAb negativity (30/352, 8.5%) (p < 0,01) (Chi square) (Fig. 5).
A binary logistic regression was performed to assess whether the differences in hypothyroidism frequency were influenced only by TAb presence or also by metabolic differences among the phenotypes. Presence of hypothyroidism resulted to be significantly associated to TAb presence (OR 4.19; CI 2,25–7.79; p < 0,01) but not associated to phenotype (OR 1.29; CI 0.97–1.76; p = 0.109) (Table 2).
Thyroid autoimmunity and hormonal profile
No significant difference was observed in serum levels of androgen hormones and 17-OH progesterone levels among TAb positive and TAb negative patients, as shown in Table 3.
Discussion
PCOS is a heterogeneous disease whose pathophysiology and etiology are still debated. Several epidemiological studies have reported a prevalence of PCOS is 5–10% in women of reproductive age [7, 25], however the frequency of these disease depends on the criteria used for its diagnosis.
PCOS phenotype changes with age. Clinical and biochemical hyperandrogenism are a major concern in young PCOS women, while metabolic burden tends to increase with aging [24].
This study included 448 patients with PCOS, all recruited at the Endocrinology Unit I by the Pisan University Hospital. Out of the 448 patients 260 (58.0%) were included in Phenotype A, 119 (26.6%) in Phenotype B, 38(8.5%) in Phenotype C and 31 (6.9%) in Phenotype D.
In this study we evaluated the frequency of thyroid autoimmunity in PCOS patients subdivided in the four phenotypes described. The association between autoimmunity and PCOS has been widely discussed in recent years [8, 26, 27] and several studies have investigated the prevalence of Hashimoto’s thyroiditis in PCOS patients in different geographic areas. In a recent meta-analysis [28] the relationship between AITD and PCOS was evaluated in 13 studies including a total of 1210 women diagnosed with PCOS and 987 healthy controls. AITD was observed in 26.03% of PCOS patients and 9.72% of controls. Overall, there was a significant association between PCOS and the possibility of AITD (OR = 3.27, 95% CI 2.32–4.63).
In this study, positive TAb (including TgAb and/or TPOAb positivity) were detected in 90/448 patients (20.1%). In particular, positive TgAb were found in 74/448 (16.5%) patients and TPOAb in 66/448 (14.7%). These frequencies are comparable to those reported in the majority of studies on this topic. In the study of Arduc et al. [29] a higher prevalence of AITD was observed in women with PCOS (22.1%) compared to non-PCOS controls (5%), with positive TPOAb detected in 26.7% of PCOS patients and 6.6% of controls and TgAb in 16,2% of PCOS and 5% of controls. A higher prevalence of HT in women with PCOS has also been reported in other studies conducted in Europe. In the work of Janssen et al. of of 2004 in Germany HT was diagnosed in 20.6% of PCOS patients and 6.5% of controls [30] and in the study of Garelli et al. of 2013 in Italy and 27% and 8% respectively [31].
The reasons for the association between thyroid autoimmunity and PCOS are not known. Genetic factors are involved in the pathogenesis of both autoimmune thyroiditis and PCOS but a common background between these two diseases has not been established.
Sex hormones are involved in the pathogenesis of autoimmunity as demonstrated by the higher frequency of autoimmune disease in women. Estrogen levels have a stimulating action on the immune system [30, 32], while androgens have an inhibitory effect on most elements of the immune system [33]. Progesterone can also inhibit the proliferation of macrophages, IL6 synthesis and peripheral antibody production [32]. Furthermore, in vivo and in vitro data indicate that progesterone has some ability to suppress CD4+T cell proliferation and TH1 response [34].
Women with PCOS usually have similar estrogen levels, higher testosterone levels, and lower progesterone levels than healthy controls. In patients with oligo-anovulatory cycles, periods characterized by a high concentration of estrogen and a low concentration of progesterone frequently occur, which could therefore increase the susceptibility to the development of autoimmune diseases. Androgens, on the other hand, have an immunosuppressive activity, which however does not seem to be sufficiently powerful to be able to be in contrast the combined action of estrogen and progesterone [35]. Consequently, the imbalance between estrogens, progesterone, and androgens typical of PCOS could be a predisposing factor to the development of HT.
Mariotti et al. first described that female predominance of autoimmune thyroiditis strongly increases during puberty, suggesting a major role for sex hormones in this phenomenon [36].
In this work we evaluated the frequency of thyroid autoimmunity in different PCOS phenotypes.
Positive TAb were significantly more frequent (Fig. 2) in phenotypes A-B (83/379, 21.9%) than in phenotypes C-D (7/69, 10.1%). Positive TgAb were more frequent in grouped phenotypes A-B (68/379, 17.9%) than in C-D phenotypes (6/69, 8.7%) even if this difference was not significant (p = 0.07) (Fig. 3a). However, when TgAb were subdivided according to their serum level (Fig. 2b) high titer TgAb were significantly more frequent (p = < 0.01) in grouped phenotypes A-B (39/379, 10.3%) than in phenotypes C-D (0/69, 0.0%) while low titer positive TgAb showed a similar frequency in the regrouped phenotypes A-B (6/69, 7.7%) and C-D (29/379, 8.7%) (Fig. 3b). A similar result was observed when only TPOAb were taken into account even if the differences were not statistically significant (Fig. 4a and b).
Taken together, these results show that the frequency and serum levels of TAb are different in the different PCOS phenotypes. In particular, a higher frequency of TAb and higher titer of TgAb and TPOAb are mainly found in phenotypes A (complete PCOS) and B (classic PCOS), characterized by the presence of hyperandrogenism and oligo-anovulation, conditions in which the imbalance between estrogen, androgen and progesterone hormones may induce the onset of autoimmunity. Phenotype C (ovulatory PCOS) is characterized by the presence of ovulatory cycles in which progesterone can balance the action of estrogens in the induction of thyroid autoimmunity. Phenotype D (non-hyperandrogenic PCOS) is characterized by a clinical and hormonal picture different from that found in phenotypes A and B. In phenotypes C and D, we found not only a lower frequency of TAb, but also the absence of high titer TgAb and the presence of high titer TPOAb only in 1/ 38 patients with phenotype C and in 1/31 patients with phenotype D.
Different results have been reported by Gawron [37] who found a lower incidence of TAb (12%) and no significant association between the different PCOS phenotypes and the presence of TAb.
The discrepancy between these results and our findings may be due to different characteristics of the population studied and diagnostic criteria adopted; in the study of Gawron autoimmune thyroiditis was diagnosed only in 4.4% of patients, a frequency much lower compared to the data reported in the majority of the studies in the literature.
We have analyzed the hormonal profile of the women with PCOS evaluating the serum levels of androgens and 17OH progesterone of the patients divided according to the presence of positive TAb. We did not find any statistically significant hormonal difference between the TAb positive and TAb negative patients (Table 3). Our findings are in agreement with the results reported in other studies that didn’t find any relationship between androgens and the presence of anti-thyroid autoantibodies in PCOS patients [29, 37].
Finally, we evaluated the frequency of hypothyroidism in patients with PCOS subdivided in the 4 phenotypes described. Hypothyroidism has been found in 10–36% of women with PCOS [14, 37,38,39,40,41,42,43,44] and the differences may depend on the geographical area, ethnicity, and age of the reference population, but also on the diagnostic criteria adopted by each study. In a meta-analysis conducted by Ding et al. the results of 6 studies were analyzed, including a total of 692 women diagnosed with PCOS and 540 controls, and a significant association between PCOS and hypothyroidism was found (OR = 2.87, CI 1.82–9. 92; p < 0.01) [14, 37,38,39,40,41,42,43,44].
In our study, hypothyroidism was present in 53/439 (12.1%) patients (21 untreated with serum TSH level > 4 mU/L and 32 in treatment with L-T4). These results are in agreement with the frequency of hypothyroidism in PCOS patients reported in literature [14, 37,38,39,40,41,42,43,44].
We have also evaluated the prevalence of hypothyroidism in the 4 PCOS phenotypes. Hypothyroidism was significantly more frequent in phenotype B (24/119, 20.7%) than in phenotype A (22/256, 8.6%), C (5/36, 13.9%) and D (2/31, 6.5%).
Unfortunately, the majority of our hypothyroid patients were under treatment with L-thyroxine and therefore it was not possible to study the relationship between hypothyroidism and metabolic features.
Furthermore, subdividing the patients according to the presence of TAb, hypothyroidism was significantly more frequent in patients with TAb positive (23/87, 26.4%) than in patients with TAb negative (30/352, 8.5%) (Fig. 5). A binary logistic regression was performed showing a significant association between hypothyroidism only with presence of TAb (OR 4.19; IC 2.25–7.79; p < 0.01), but not with the PCOS phenotype.
One limitation of this study is the relatively small size of the group of patients included. For this reason, we observed in phenotypes A-B a significant higher frequency only of positive TgAb, while the frequency of positive TPOAb was higher but not statistically significant. In order to confirm these observations, further and possibly multicentric studies will be required. A further limitation of this study is that it is a cross-sectional study, being thyroid function evaluated with a single measurement of TSH. As the majority of hypothyroid patients were under treatment with L-T4 we were not able to evaluate the possible relationship between hypothyroidism and metabolic features in these PCOS patients. Further prospective studies are needed in order to define the role of hypothyroidism in the different phenotypes of PCOS.
In conclusion, in this study we observed that the frequency of positive TAb is different in the different PCOS. This difference is probably related to the imbalances between estrogens, androgens, and progesterone typical of the syndrome and more evident in phenotypes A and B, in which we found a higher frequency of positive TAb and higher serum levels of TgAb and TPOAb.
References
Escobar-Morreale HF (2018) Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. https://doi.org/10.1038/nrendo.2018.24
Azziz R, Carmina E, Chen Z et al (2016) Polycystic ovary syndrome. Nat Rev Dis Prim. https://doi.org/10.1038/nrdp.2016.57
Sirmans SM, Pate KA (2013) Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol. https://doi.org/10.2147/clep.s37559
Lo JC, Feigenbaum SL, Yang J, Pressman AR, Selby JV, Go AS (2006) Epidemiology and adverse cardiovascular risk profile of diagnosed polycystic ovary syndrome. J Clin Endocrinol Metab. https://doi.org/10.1210/jc.2005-2430
Wild RA, Carmina E, Diamanti-Kandarakis E et al (2010) Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the androgen excess and polycystic ovary syndrome (AE-PCOS) society. J Clin Endocrinol Metab. https://doi.org/10.1210/jc.2009-2724
Stein IF, Leventhal ML (1935) Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. https://doi.org/10.1016/s0002-9378(15)30642-6
Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, Azziz R (2016) Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril 106(1):6–15. https://doi.org/10.1016/j.fertnstert.2016.05.003
Sen A, Kushnir VA, Barad DH, Gleicher N (2014) Endocrine autoimmune diseases and female infertility. Nat Rev Endocrinol 10(1):37–50. https://doi.org/10.1038/nrendo.2013.212
Kaltsas GA, Korbonits M, Isidori AM et al (2000) How common are polycystic ovaries and the polycystic ovarian syndrome in women with Cushing’s syndrome? Clin Endocrinol (Oxf) 53(4):493–500. https://doi.org/10.1046/j.1365-2265.2000.01117.x
Moro F, De Simone C, Morciano A et al (2013) Psoriatic patients have an increased risk of polycystic ovary syndrome: results of a cross-sectional analysis. Fertil Steril 99(3):936–942. https://doi.org/10.1016/j.fertnstert.2012.10.040
Jacobson DL, Gange SJ, Rose NR, Graham NMH (1997) Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol 84(3):223–243. https://doi.org/10.1006/clin.1997.4412
Vanderpump MPJ, Tunbrldge WMG, French JM et al (1995) The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf) 43(1):55–68. https://doi.org/10.1111/j.1365-2265.1995.tb01894.x
McGrogan A, Seaman HE, Wright JW, de Vries CS (2008) The incidence of autoimmune thyroid disease: a systematic review of the literature. Clin Endocrinol (Oxf) 69(5):687–696. https://doi.org/10.1111/j.1365-2265.2008.03338.x
Huang R, Zheng J, Li S, Tao T, Liu W (2014) Subclinical hypothyroidism in patients with polycystic ovary syndrome: distribution and its association with lipid profiles. Eur J Obstet Gynecol Reprod Biol 177:52–56. https://doi.org/10.1016/j.ejogrb.2014.04.013
Hatch R, Rosenfield RL, Kim MH, Tredway D (1981) Hirsutism: implications, etiology, and management. Am J Obstet Gynecol. https://doi.org/10.1016/0002-9378(81)90746-8
Doshi A, Zaheer A, Stiller MJ (1997) A comparison of current acne grading systems and proposal of a novel system. Int J Dermatol 36(6):416–418. https://doi.org/10.1046/j.1365-4362.1997.00099.x
Piraccini BM, Alessandrini A (2014) Androgenetic alopecia. G Ital Dermatol Venereol 149(1):15–24 (PMID: 24566563)
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 81(1):19–25. https://doi.org/10.1016/j.fertnstert.2003.10.004
Moro F, Scavello I, Maseroli E et al (2023) The physiological sonographic features of the ovary in healthy subjects: a joint systematic review and meta-analysis by the Italian Society of Gynecology and Obstetrics (SIGO) and the Italian Society of Endocrinology (SIE). J Endocrinol Invest 46(3):439–456. https://doi.org/10.1007/s40618-022-01939-8
Carmina E, Dewailly D, Escobar-Morreale HF et al (2017) Non-classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency revisited: an update with a special focus on adolescent and adult women. Hum Reprod Updat. https://doi.org/10.1093/humupd/dmx014
Nieman LK, Biller BMK, Findling JW et al (2008) The diagnosis of Cushing’s syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. https://doi.org/10.1210/jc.2008-0125
Azziz R, Carmina E, Dewailly D et al (2006) Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an androgen excess society guideline. J Clin Endocrinol Metab 91(11):4237–4245. https://doi.org/10.1210/jc.2006-0178
Robinson S, Rodin DA, Deacon A, Wheeler MJ, Clayton RN (1992) Which hormone tests for the diagnosis of polycystic ovary syndrome? BJOG. https://doi.org/10.1111/j.1471-0528.1992.tb14505.x
Falcetta P, Benelli E, Molinaro A et al (2021) Effect of aging on clinical features and metabolic complications of women with polycystic ovary syndrome. J Endocrinol Invest 44(12):2725–2733. https://doi.org/10.1007/s40618-021-01594-5
Norman RJ, Dewailly D, Legro RS, Hickey TE (2007) Polycystic ovary syndrome. Lancet 370(9588):685–697. https://doi.org/10.1016/S0140-6736(07)61345-2
Qu J, Li B, Qiu M et al (2022) Discovery of immune-related diagnostic biomarkers and construction of diagnostic model in varies polycystic ovary syndrome. Arch Gynecol Obstet. https://doi.org/10.1007/s00404-022-06686-y
Van Gelderen CJ, Gomes dos Santos ML (1993) Polycystic ovarian syndrome. Evidence for an autoimmune mechanism in some cases. J Reprod Med 38(5):381–386
Romitti M, Fabris VC, Ziegelmann PK, Maia AL, Spritzer PM (2018) Association between PCOS and autoimmune thyroid disease: a systematic review and meta-analysis. Endocr Connect 7(11):1158–1167. https://doi.org/10.1530/EC-18-0309
Arduc A, Aycicek Dogan B, Bilmez S et al (2015) High prevalence of Hashimoto’s thyroiditis in patients with polycystic ovary syndrome: does the imbalance between estradiol and progesterone play a role? Endocr Res 40(4):204–210. https://doi.org/10.3109/07435800.2015.1015730
Janssen O, Mehlmauer N, Hahn S, Offner A, Gartner R (2004) High prevalence of autoimmune thyroiditis in patients with polycystic ovary syndrome. Eur J Endocrinol. https://doi.org/10.1530/eje.0.1500363
Garelli S, Masiero S, Plebani M et al (2013) High prevalence of chronic thyroiditis in patients with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol 169(2):248–251. https://doi.org/10.1016/j.ejogrb.2013.03.003
Petríková J, Lazúrová I, Yehuda S (2010) Polycystic ovary syndrome and autoimmunity. Eur J Intern Med 21(5):369–371. https://doi.org/10.1016/j.ejim.2010.06.008
Quintero OL, Amador-Patarroyo MJ, Montoya-Ortiz G, Rojas-Villarraga A, Anaya JM (2012) Autoimmune disease and gender: plausible mechanisms for the female predominance of autoimmunity. J Autoimmun 38(2–3):J109–J119. https://doi.org/10.1016/j.jaut.2011.10.003
Hughes GC (2012) Progesterone and autoimmune disease. Autoimmun Rev 11(6–7):A502–A514. https://doi.org/10.1016/j.autrev.2011.12.003
Gaberšček S, Zaletel K, Schwetz V, Pieber T, Obermayer-Pietsch B, Lerchbaum E (2015) Mechanisms in endocrinology: thyroid and polycystic ovary syndrome. Eur J Endocrinol 172(1):R9–R21. https://doi.org/10.1530/EJE-14-0295
Mariotti S, Prinzis A, Ghiani M et al (2009) puberty is associated with a marked increase of the female sex predominance in chronic autoimmune thyroiditis. Horm Res Paediatr 72(1):52–56. https://doi.org/10.1159/000224341
Gawron IM, Baran R, Derbisz K, Jach R (2022) Association of subclinical hypothyroidism with present and absent anti-thyroid antibodies with PCOS phenotypes and metabolic profile. J Clin Med 11(6):1547. https://doi.org/10.3390/jcm11061547
Yu Q, Wang JB (2016) Subclinical hypothyroidism in PCOS: impact on presentation, insulin resistance, and cardiovascular risk. Biomed Res Int 2016:1–7. https://doi.org/10.1155/2016/2067087
Novais JDSM, Benetti-Pinto CL, Garmes HM, Menezes Jales R, Juliato CRT (2015) Polycystic ovary syndrome and chronic autoimmune thyroiditis. Gynecol Endocrinol 31(1):48–51. https://doi.org/10.3109/09513590.2014.958990
Singla R, Gupta Y, Khemani M, Aggarwal S (2015) Thyroid disorders and polycystic ovary syndrome: an emerging relationship. Indian J Endocrinol Metab 19(1):25. https://doi.org/10.4103/2230-8210.146860
Benetti-Pinto CL, Berini Piccolo VRS, Garmes HM, Teatin Juliato CR (2013) Subclinical hypothyroidism in young women with polycystic ovary syndrome: an analysis of clinical, hormonal, and metabolic parameters. Fertil Steril 99(2):588–592. https://doi.org/10.1016/j.fertnstert.2012.10.006
Morgante G, Musacchio MC, Orvieto R, Massaro MG, De Leo V (2013) Alterations in thyroid function among the different polycystic ovary syndrome phenotypes. Gynecol Endocrinol 29(11):967–969. https://doi.org/10.3109/09513590.2013.829445
Tagliaferri V, Romualdi D, Guido M et al (2016) The link between metabolic features and TSH levels in polycystic ovary syndrome is modulated by the body weight: an euglycaemic–hyperinsulinaemic clamp study. Eur J Endocrinol 175(5):433–441. https://doi.org/10.1530/EJE-16-0358
Fatima M, Amjad S, Sharaf Ali H et al (2020) Correlation of subclinical hypothyroidism with polycystic ovary syndrome (PCOS). Cureus. https://doi.org/10.7759/cureus.8142
Funding
The author(s) received no specific funding for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
Ethical approval was waived by the local Ethics Committee of University of Pisa in view of the retrospective nature of the study and all the procedures being performed were part of the routine care.
Research involving human participants and/or animals
This study does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Benelli, E., Marradi, M., Sciarroni, E. et al. Thyroid autoimmunity in different phenotypes of polycystic ovary syndrome: a single-center experience. J Endocrinol Invest (2024). https://doi.org/10.1007/s40618-024-02404-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40618-024-02404-4