Abstract
The order Gobiiformes is made up of more than 2200 species, representing one of the most diverse groups among teleost fishes. The biological causes for the tachytelic karyotype evolution of the gobies have not yet been fully studied. Here we expanded cytogenetic data for the Eleotridae family, analyzing the neotropical species Dormitator maculatus, Eleotris pisonis, Erotelis smaragdus, and Guavina guavina. In addition, a meta-analytical approach was followed for elucidating the karyotype diversification versus biological aspects (habitat and egg type) of the Gobiiformes. The species E. smaragdus and E. pisonis present 2n = 46 acrocentric chromosomes (NF = 46), D. maculatus 2n = 46 (36sm + 4st + 6a; NF = 86), and G. guavina, the most divergent karyotype, with 2n = 52 acrocentric chromosomes (NF = 52). Besides numeric and structural diversification in the karyotypes, the mapping of rDNAs and microsatellites also showed noticeable numerical and positional variation, supporting the high chromosomal evolutionary dynamism of these species. In Gobiiformes, karyotype patterns which are more divergent from the basal karyotype (2n = 46a) are associated with characteristics less effective to dispersion, such as the benthic habit. These adaptive characteristics, connected with the organization of the repetitive DNA content in the chromosomes, likely play a synergistic role in the remarkable karyotype diversification of this group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gobiiformes (Osteichthyes, Teleostei) constitute one of the most diverse groups among vertebrates, encompassing nine families, 268 genera, and notably 2211 fish species (Betancur-R et al. 2017; Eschmeyer and Fong 2020; Nelson et al. 2016). Its wide geographic distribution covers the areas of Oceania, Asia, Europe, North America, and Latin America, inhabiting marine, brackish and freshwater environments. Some species live in hypersaline waters or even great oceanic depths (Muus and Nielsen 1999; Oto et al. 2017; Suzuki et al. 2015), however they generally occur in estuaries, rocky marine coasts, or are associated with coral reefs (Baensch and Riehl 1991; Kottelat and Freyhof 2007; Patzner et al. 2012). Their reproductive strategies include (1) parental care for eggs and larvae, (2) internal or external fertilization and (3) males’ sex change under certain environmental conditions (Nakashima et al. 1996; Skóra et al. 1999).
The percomorph fish clade Gobiiformes, despite its great diversity, is a monophyletic group (Thacker 2003). Its origin dates from the Paleocene (~ 65 Ma), and now has representatives in marine, estuarine, and continental waters of vast areas of tropical and subtropical regions (Fanta 1997; Rocha et al. 2005; Ruber et al. 2003). In general, they represent a fish model of rapid and intense karyoevolutionary divergences (Lima-Filho et al. 2012; Molina 2005). A wide range of chromosomal rearrangements is associated with the karyotype diversification of this group, in which pericentric inversions and Robertsonian fusions stand out, in addition to others such as tandem fusions and fission events on a smaller scale (Amores et al. 1990; Caputo et al. 1997; Prazdnikov et al. 2013). Moreover, chromosome polymorphisms are also frequent in Gobiiformes populations (Caputo et al. 1997; Ene 2003; Nishikawa et al. 1974; Webb 1986), indicating continuous processes of karyotypic changes. In this scenario, meta-analysis can provide patterns under a phylogenetic perspective, or correlate the rapid and intense chromosomal changes with the biological characteristics of the species.
Cytogenetic data for Gobiiformes are restricted to five out of nine families, representing less than 10% of the valid species present in this order. Nevertheless, even though most of the chromosomal data are restricted to Giemsa-stained karyotypes (Arai 2011), they point to a high numerical and structural chromosome diversity within this group (Fanta 1997; Lima-Filho et al. 2012; Rocha et al. 2005; Ruber et al. 2003). Such high karyotype diversity in some marine fishes are punctually attributed to the diversity of habitats, limited dispersive capacity, and rich behavioral repertoire of the species (Lima-Filho et al. 2016; Molina et al. 2014a; Rocha et al. 2005). On the basis of its greatest frequency, it has been suggested that the karyotype composed by 46 acrocentric chromosomes corresponds to the basal one for Gobiiformes (Vasil’ev and Gregorian 1994). However, this suggestion needs to be confirmed, since it is based on a small set of cytogenetic data available for some families, without considering the phylogenetic extent within the order.
The cytogenetic diversification in Gobiiformes shows a very extensive panel of chromosome changes (Amores et al. 1990; Caputo et al. 1997; Lima-Filho et al. 2014a, b; Prazdnikov et al. 2013). In Eleotridae, popularly known as "sleepers" (the name given due to their mode of life, hiding in dens in the substrate and low vagility), karyotype divergences among biogeographic regions (Molina 2005), chromosome polymorphisms (Uribe-Alcocer and Ramirez-Escamilla 1989), and differentiated sex chromosomes (Oliveira and Almeida-Toledo 2006) have already been reported despite the limited cytogenetic data available for this group.
Extensive cytogenomic analyses, associated with phylogenetic meta-analyses of biological traits, have led to increased understanding of the macro and microstructural reorganization levels of the chromosomes. Therefore, to correlate the biological characteristics of the Gobiiformes groups with the possible basal karyotype for the order, we performed a detailed analysis of the karyotypes by applying standard and advanced molecular cytogenetic techniques in four Gobiiformes species belonging to different genera. In addition to conventional chromosomal methods, base-specific fluorochromes Mithramycin A (MM), DAPI (4′,6-diamidino-2-phenylindole) and fluorescence in situ hybridization (FISH) using the repetitive sequences of 18S rDNA, 5S rDNA and microsatellite sequences [(CA)15 and (CAA)10] as probes were performed.
Results
Karyotypes, C-, Ag- and DAPI/MM banding
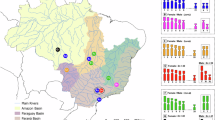
The species E. smaragdus and E. pisonis have karyotypes with 2n = 46 acrocentric chromosomes (NF = 46), D. maculatus has 2n = 46 composed of 36sm + 4st + 6a (NF = 86), and G. guavina has 2n = 52 acrocentric chromosomes (NF = 52) (Fig. 1).
Karyotypes of E. smaragdus, E. pisonis, D. maculatus and G. guavina, after Giemsa staining, C-banding, and fluorescence in situ hybridization with 18S rDNA (red) and 5S rDNA (green) probes. The Ag-NORs and MM+/DAPI− regions (green) are showed in the boxes of the first column. The two rDNA arrays in the chromosomes of D. maculatus are highlighted in the larger box. Scale bar = 5 μm
The C-positive heterochromatin shows a diversified distribution and content among the species. In E. smaragdus, it occurs as conspicuous centromeric and terminal blocks in the chromosomes, in E. pisonis as small centromeric segments and in D. maculatus and G. guavina with an irregular distribution in centromeric, interstitial, and terminal blocks. In all species, some heterochromatic blocks occupy the interstitial regions or the entire arms of two-armed chromosomes (Fig. 1).
Ag-NORs sites are located on a single pair of chromosomes and are the only regions in the karyotypes exhibiting a MM+/DAPI− pattern (Fig. 1; in the boxes). These sites are localized in the terminal position on the long arms of pair 9 in E. smaragdus, in the interstitial position of the long arms of pair 21 in E. pisonis, in the terminal position of the short arms of pair 4 in D. maculatus, and in the interstitial region of the long arms of pair 19 in G. guavina (Fig. 1; in the boxes).
FISH mapping
The 18S rDNA sites are congruent with the Ag-NORs signals in all species but located in non-homologous chromosomes. The 5S rDNA sites, in addition to numerical variation, also show large interspecific divergences in their chromosomal location (Fig. 1). In E. smaragdus, they have a proximal location on the chromosome pairs 7 and 14; in E. pisonis, they are interstitially co-located with the 18S rDNA site in pair 21; in G. guavina, they occupy an interstitial position on pair 4. In addition, D. maculatus exhibits a structural polymorphism. In this case, some individuals have 18S and 5S rDNA sites on the short arms of pairs 4 and 5, respectively, while others have only one homologue of pair 4 carrying an 18S rDNA site, the other homologue of this same pair carrying co-located 18S rDNA/5S rDNA sites, and a single homologue of pair 5 carrying a 5S rDNA site (Fig. 1).
The mapping of microsatellites (CA)15 and (CAA)10 was performed for E. pisonis, D. maculatus and G. guavina. The results show the distribution of these motifs in both heterochromatic and euchromatic regions (Fig. 2). The (CA)15 repeats occur in all chromosomes of the three species, mainly in the terminal region of their long arms. In E. pisonis, this motif additionally occurs in both arms of pairs 15 and 21. In D. maculatus, these sequences occupy the terminal regions of both arms of most chromosomes. On the other hand, in G. guavina, they have a very variable distribution, occurring exclusively in the terminal position of the short or long arms, in both arms of the chromosomes, or in the interstitial regions of a few chromosomal pairs (Fig. 2). In contrast, microsatellite sequences (CAA)10, do not occur in all chromosomes of any given species. In E. pisonis, they are mainly located in the terminal regions of the long arms in most of the chromosomes. However, in D. maculatus and in G. guavina, they occur in the terminal region of only one or both chromosome arms, but with a different distribution pattern along the chromosomes in each species (Fig. 2).
Meta-analysis
The cytogenetic survey on Gobiiformes covered 139 species, and showed a diploid variation from 2n = 30 to 56 chromosomes, where 2n = 46 represents the most frequent condition, followed by 2n = 44 chromosomes, also present in high frequency and prevalent in some clades. Oxudercidae is the most representative family, with cytogenetic data available for 69 spp. Of these, 37% (25 spp.) have 2n = 44 chromosomes, 31% (21 spp.) have 2n = 46 chromosomes, and the remaining 32% (23 spp.) have diploid numbers varying from 2n = 38 to 2n = 56 chromosomes. The NF in this group ranges from 40 to 92. Gobiidae is the second group with the largest number of accessible cytogenetic data (50 spp.), among which, 42% (21 spp.) have 2n = 46 chromosomes, 24% (12 spp.) have 2n = 44 chromosomes, and the remaining 34% (17 spp.) have 2n = 30 to 2n = 50 chromosomes. The NF variation was shown to be extensive in this family, ranging from 38 to 98. Cytogenetic data for Eleotridae encompassed 11 species: 64% (7 spp.) with 2n = 46 chromosomes, and the others with 2n = 48 or 52 chromosomes. The NF ranges from 46 to 90, with NF = 46 being the most frequent. The Butiidae and Odontobutidae families are the least investigated: the former with five species analyzed exhibiting 2n = 46 or 2n = 48, NF from 48 to 58, and the only four species of the second having 2n = 44 acrocentric chromosomes.
The association between karyotype diversification and biological factors affecting the dispersive potential in Gobiiformes species revealed a greater divergence in 2n and NF in the benthic species (66% and 73%, respectively, of the karyotypes different from the basal pattern), while species with pelagic and bentho-pelagic habits share more conservative karyotype patterns. Similar trends occur in the Gobiidae and Oxudercidae families with the greatest samples (Table 1).
In another comparison, the 2n and NF data of the karyotypes of 124 Gobiiformes species were analyzed with respect to their preferential environments. Species with more than one habitat were divided into grouped categories. The results show that the chromosome variation is not precisely related to environmental categories (Table 2). However, the 2n of species from freshwater and freshwater/estuarine habitats are mainly equal to the considered basal karyotype (2n = 46) for the order. Similar results were obtained for the families Gobiidae and Oxudercidae.
Discussion
The four Eleotridae species exhibited a conspicuous karyotype diversification regarding their fundamental number, chromosome morphology (Table 3), and organization of repetitive sequences on the chromosomes. These data are consistent with a wider evaluation of the karyotype evolution among Gobiiformes (Molina et al. 2014b).
The evolutionary history of some Eleotridae groups, such as Dormitator in the Atlantic, is recent (0.19–0.35 Ma) and linked with population fragmentation derived from some major geological and ecological events, such as the uplift of Central American Isthmus and regional isolation by climate and oceanographic changes (Galván-Quesada et al. 2016). These processes, on macro- or micro-scales, apparently had direct evolutionary effects on genomic diversification and on the fixation of chromosome rearrangements alongside their distribution limits (Molina 2005). D. maculatus has different regional karyotypes, such as in the Brazilian northeast (2n = 46; NF = 86) (Molina 2005, present data), and southeastern (2n = 46; NF = 90) (Oliveira and Almeida-Toledo 2006) coasts, in Western Atlantic and Caribbean (2n = 46; NF = 80) (Maldonado-Monroy et al. 1985). These karyotype divergences highlight a cryptic macroevolution pattern and support an under perceived scenario of profuse allopatric speciation in the Dormitator maculatus complex.
Similarly, karyotype divergences also occur among E. pisonis populations from the Brazilian (2n = 46; NF = 46) (Molina 2005, present data) and Caribbean coasts (2n = 44; NF = 46) (Uribe-Alcocer and Diaz-Jaimes 1996). As a whole, such karyotype variations also suggest the occurrence of cryptic species within the Eleotridae family (Molina 2005). However, despite exhibiting 2n variations (2n = 44–52), Eleotridae species most often have 2n = 46 chromosomes, a condition also found in E. pisonis, E. smaragdus and D. maculatus, suggesting that it may represent a basal trait for this family. Karyotypes with 2n > 46, as in G. guavina (2n = 52; the highest diploid value in the group), and NF > 46 (Table 3), indicate the importance of fission events, as well as pericentric inversions in the karyotype evolution of this fish group. Such rearrangements are also frequent in large marine groups as Percomorpha (Galetti et al. 2000).
Besides karyotype variations, marked intra- and interspecific heterogeneities in the amount and location of heterochromatin occur among the Eleotridae species. While a reduced and centromeric heterochromatic pattern occurs in G. guavina and E. pisonis, the C-positive heterochromatin is present in the interstitial and terminal regions of chromosomes of E. smaragdus and D. maculatus. This diversified heterochromatic organization is phylogenetically wide and has been recognized in several gobiiform groups (Caputo et al. 1997; Lima-Filho et al. 2012, 2014a), indicating an intense inner chromosomal reorganization of repetitive DNAs, probably associated with changes in the macrostructure of the Eleotridae chromosomes.
The mapping of rDNA sequences has shown a wide variation at both population and interspecific levels in Gobiiformes (Lima-Filho et al. 2012, 2014a, b; Ocalewicz and Sapota 2011). In Eleotridae, although only two Ag-NORs/18S rDNA sites occur, they show distinct size and location in conspicuously different chromosomal pairs among the species, thus suggesting the occurrence of disruptive events of the syntenic order in these chromosomes.
Evidence of significant internal reorganizations in the Eleotridae chromosomes is also provided by the differentiated distribution that the 5S rDNA sites have in this group. Location of the 18S and 5S rDNA sites in different chromosomes, like in G. guavina and E. smaragdus, is a common condition in several fish groups (Gornung 2013). However, syntenic arrays such as in E. pisonis, hitherto uncommon in Gobiiformes, constitute a derived condition. Indeed, collectively the rDNA sites create very exclusive species-specific patterns. The set of diversifications related to rDNA sequences and the bearing chromosome indicates that microstructural changes are frequent in Eleotridae and probably extend to other chromosomes of the species. Interestingly, D. maculatus exhibits a rDNA polymorphism related to the 18S and 5S sequences on pairs 4 and 5 of the karyotype comprising different arrangements which include a syntenic 18S/5S state in only one homologue of pair 4. This polymorphism reinforces the dynamic condition of the ribosomal DNAs among Eleotridae species and suggests a transient stage toward the colocalization of the 18S/5S sequences in the same chromosome pair.
Like the rDNA, microsatellite sequences are also evolutionarily dynamic, susceptible to high mutational rates in the genome (Oliveira et al. 2006), and can present independent evolutionary paths in chromosomes (Xu et al. 2017). In E. pisonis, D. maculatus and G. guavina, the (CA)15 and (CAA)10 microsatellites are clustered on different regions of the chromosomes, presenting an incomplete overlap with the C-banding regions. In these species, the heterogeneity of heterochromatin is identified by the heterochromatic and euchromatic regions harboring both, one or neither (CA)15 and (CAA)10 repeats. This level of heterogeneity suggests that these regions are evolutionarily less stable and potentially associated with the high karyotype changes in Eleotridae.
As a whole, the inter- and intraspecific diversification of the karyotypes, and the great potential for population fragmentation, make Eleotridae a target group for deeper taxonomic approaches in the search for the real meaning of its biodiversity.
Additional remarks on karyoevolution, biological features and geographic dispersion of Gobiiformes
The significant diversification of chromosomal numbers and karyotypic formulas (Arai 2011), distinguishes Gobiiformes from other large groups of marine fish with a clear 2n = 48 conservatism (Motta-Neto et al. 2019). Phylogenetic relationships (Betancur-R et al. 2013; Thacker 2009) indicate a higher frequency of karyotypes with 2n = 46 acrocentric chromosomes distributed from basal clades to recent lineages of this order. While in families Eleotridae and Butiidae 2n = 46 acrocentric chromosomes (NF = 46) is a prevalent condition, Oxudercidae shows a greater frequency of 2n = 46 chromosomes, but with NF > 46. Apart from the Odontobutidae, which possess 2n = 44 chromosomes, other families of Gobiiformes, with ancient or recent divergence, have some species with 2n = 46 chromosomes. The presence of a high incidence of karyotypes with 2n = 46 chromosomes in Apogonidae (Araújo et al. 2010), a family closely related to Gobiiformes (Betancur-R et al. 2017), suggests that 2n = 44 chromosomes is a homoplasic and recurrent trait in some groups of Gobiiformes. In addition, Gobiiformes also include variations in intraspecific diploid number (Caputo et al. 1999; Prazdnikov et al. 2013), in 5S rDNA sites (Lima-Filho et al. 2012; present data), in karyotypes of congeneric species (Caputo et al. 1997; Grigoryan and Vasiliev 1993; Thode et al. 1988), and in the emergence of sex chromosomes (Lima-Filho et al. 2014b; Pezold 1984).
This diversified scenario is also supported by the high evolutionary variation of the ribosomal sequences, indicating a massive internal reorganization in the chromosomes. Although generally present on a single pair of chromosomes, the present study shows that 18S rDNAs can be found in different positions and on different chromosomes among gobiiform species, which is consistent with the findings of Lima-Filho et al. (2012) and Ocalewicz and Sapota (2011). Similar reorganizations are also found for 5S sites in parallel to large numerical variations. In addition, syntenic arrangements such as those in E. smaragdus, or complex polymorphic arrangements showed in D. maculatus, along with their location on the sex chromosomes (Lima-Filho et al. 2014b), complement the evolutionary dynamism of these sequences.
Some biological characteristics of Gobiiformes, such as particular habitats and reproductive strategies, seem to act on the dispersive potential of the species, thus supporting population stratifications and the fixation of chromosomal rearrangements. Some divergent cytogenetic patterns are found in marine species, contrasting with the more obvious biogeographic stratification of freshwater species. This is in accordance with to the patterns of genetic variability in Gobiiformes, whose pelagic species have a more homogeneous genetic structure than the benthic ones (Giovannotti et al. 2009).
The extensive variation in NF values among the Eleotridae species (NF = 46–90; Table 4), and in Gobiiformes generally (NF = 40–96; Arai 2011) indicates a significant participation of pericentric inversions in the karyotype evolution of these groups. Genomic-based studies revealed that large inversions are common in fishes and keep favorable allelic combination involved in local environmental adaptations (Kess et al. 2020; Kirubakaran et al. 2016; Pearse et al. 2014). Inversions are central to the evolution of many species (Faria et al. 2019), which the eco-evolutionary effects are extensive, encompassing morphological, physiological, behavioral adaptations and phyletic diversification (Ayala et al. 2017; Berg et al. 2016, 2017; Wellenreuther and Bernatchez 2018). In the order Gobiiformes, the reorganization of genomic architecture promoted by inversions possibly favored fine‐scale adaptation to the several environments and salinity gradients occupied, and it is likely that such mechanisms have played an equally important role in the evolution of the lineages within this group. Despite offering an apparent chance for greater gene flow among populations, marine environments are large and subdivided by extensive ecosystems that become progressively occupied during species colonization. The available data illustrate the unusual chromosomal diversity found in Eleotridae and other Gobiiformes fishes, offering a new example of congruence of phyletic and karyotype diversification within the marine ichthyofauna.
Materials and methods
Sampling
The collection sites, numbers, and sex of the individuals investigated are presented in Fig. 1 and Table 4. All the specimens were collected under the appropriate authorization of the Brazilian environmental agency ICMBIO/SISBIO (License number 19135-4).
Chromosome preparations, C-, Ag- and DAPI/MM banding
The specimens were subjected to in vivo mitotic stimulation with bacterial and fungal antigen complexes (Molina et al. 2010). Mitotic chromosomes were obtained from cell suspensions of fragments of the anterior kidney (Gold et al. 1990) and stained with Giemsa 5% diluted in phosphate buffer (pH 6.8).
The nucleolus organizing regions (NORs) and the C-positive heterochromatic regions were identified following the method described by Howell and Black (1980) and Sumner (1972), respectively. Additionally, the chromosomes were also stained with the base-specific fluorochromes DAPI and MM (Schweizer 1976).
Fluorescence in situ hybridization (FISH) for repetitive DNA mapping
The location of the rDNA sites on chromosomes were determined using fluorescence in situ hybridization with 5S and 18S rDNA probes, containing approximately 200 bp and 1400 bp, respectively. The probes were amplified by PCR from the nuclear DNA of Rachycentron canadum (Teleostei, Rachycentridae), using primers NS1 5′-GTA GTC ATA TGC TTG TCT C-3′ and NS8 5′-TCC GGT GCA TCA CCT ACG GA-3′ (White et al. 1990) and A 5′ (5′-TAC GCC CGA TCT CGT CCG ATC-3′ and B 5′-CAG GCT GGT ATG GCC GTA AGC-3′ (Pendás et al. 1994), respectively. The 18S rDNA probe was labeled with digoxigenin-dUTP-11, and the 5S rDNA probe with biotin-14-dATP using nick translation according to the manufacturer's specifications (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA). The hybridization signals were detected using anti-digoxigenin rhodamine (Roche, Mannheim, Germany) for the 18S rDNA probe, and streptavidin-FITC (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) for the 5S rDNA probe.
Simple sequence repeats (SSRs) were mapped by in situ hybridization (Kubat et al. 2008) using the oligonucleotides (CA)15 and (CAA)10 labeled with AlexaFluor 555, at the 5′ terminal position during the synthesis process (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA). The chromosomes were counterstained with Vectashield/DAPI (1.5 μg/ml).
Image analysis and processing
At least 30 metaphase spreads per individual were analyzed to confirm the 2n, karyotype structure, and FISH results. Images were captured using an Olympus BX51 microscope (Olympus Corporation, Ishikawa, Japan) with CoolSNAP and the images were processed using the Image Pro Plus 4.1 software (Media Cybernetics, Silver Spring, MD, USA). Chromosomes were classified as metacentric (m), submetacentric (sm), subtelocentric (st), or acrocentric (a), according to their arm ratios (Levan et al. 1964).
Meta-analysis
Searches for associations among karyotype, biological and ecological features were performed using several scientific web portals. Diploid numbers (2n < 46; 2n = 46, 2n > 46) and chromosome arm numbers (NF < 46; NF = 46, NF > 46) comprising 139 species, 54 genera and five families of Gobiiformes were associated with their biological and ecological parameters, including their habitat types (benthic, pelagic or benthic-pelagic; freshwater, estuarine, or marine environments). For the chromosome arm number (Nombre fundamental, NF) determination, the m/sm chromosomes were considered bi-armed whereas the st/a chromosomes were considered to have a single arm. The karyotypes of the homogametic sex were considered as the standard for the species when sex chromosome systems were present.
References
Amores A, Giles V, Thode G (1990) Adaptive character of a Robertsonian fusion in chromosomes of the fish Gobius paganellus (Pisces, Perciformes). Heredity 65:151–155
Arai R (2011) Fish karyotypes: a check list. Springer, Japan
Arai R, Sawada Y (1974) Chromosomes of Japanese gobioid fishes. Bull Natl Sci Mus [Tokyo] 17:97–102
Araújo WC, Martínez PA, Molina WF (2010) Mapping of ribosomal DNA by FISH, EcoRI digestion and replication bands in the cardinalfish Apogon americanus (Perciformes). Cytologia 75:109–117
Ayala D, Acevedo P, Pombi M, Dia I, Boccolini D, Costantini C, Simard F, Fontenille D (2017) Chromosome inversions and ecological plasticity in the main African malaria mosquitoes. Evolution 71:686–701
Baensch HA, Riehl R (1991) Aquarien Atlas. Band. 3. Melle: Mergus, Verlag für Natur-und Heimtierkunde, Germany, p 992
Berg P, Star B, Pampoulie C, Sodeland M, Barth JMI, Knutsen H, Jakobsen KS, Jentoft S (2016) Three chromosomal rearrangements promote genomic divergence between migratory and stationary ecotypes of Atlantic cod. Sci Rep 6:23246
Berg P, Star B, Pampoulie C, Bradbury IR, Bentzen P, Hutchings JA, Jentoft S, Jakobsen KS (2017) Trans-oceanic genomic divergence of Atlantic cod ecotypes is associated with large inversions. Heredity 119:418–428
Betancur-R R, Broughton RE, Wiley EO, Carpenter K, Lopez JA, Li C, Holcroft NI, Arcila D, Sanciangco M, Cureton JC II, Zhang F, Buser T, Campbell MA, Ballesteros JA, Roa-Varon A, Willis S, Borden WC, Rowley T, Reneau PC, Hough DJ et al (2013) The tree of life and a new classification of bony fishes. PLoS Curr 5:ecurrents.tol.53ba26640df0ccaee75bb165c8c26288
Betancur-R R, Wiley EO, Arratia G, Acero A, Bailly N, Miya M, Lecointre G, Orti G (2017) Phylogenetic classification of bony fishes. BMC Evol Biol 17:162
Caputo V, Marchegiani F, Sorice M, Olmo E (1997) Heterochromatin heterogeneity and chromosome variability in four species of gobiid fishes (Perciformes: Gobiidae). Cytogenet Cell Genet 79:266–271
Caputo V, Caniglia ML, Machella N (1999) The chromosomal complement of Aphia minuta, a paedomorphic goby. J Fish Biol 55:455–458
Ene AC (2003) Chromosomal polymorphism in the goby Neogobius eurycephalus (Perciformes: Gobiidae). Mar Biol 142:583–588
Eschmeyer WN, Fong JD (2020) Species by family/subfamily. http://researcharchive.calacademy.org/research/ichthyology/catalog/SpeciesByFamily.asp. Accessed 30 Jun 2020.
Fanta E (1997) Behaviour and circadian rhythm of the fish Bathygobius soporator Valenciennes (Gobiidae) under the influence of environmental salinity and temperature. Rev Bras Zool 1:221–244
Faria R, Johannesson K, Butlin RK, Westram AM (2019) Evolving inversions. Trends Ecol Evol 34:239–248
Galetti PM Jr, Aguilar CT, Molina WF (2000) An overview of marine fish cytogenetics. Hydrobiologia 420:55–62
Galván-Quesada S, Doadrio I, Alda F, Perdices A, Reina RG, Varela MG, Hernández N, Mendoza AC, Bermingham E, Domínguez-Domínguez O (2016) Molecular phylogeny and biogeography of the amphidromous fish genus Dormitator Gill 1861 (Teleostei: Eleotridae). PLoS ONE 11:e0153538
Giovannotti M, La Mesa M, Caputo V (2009) Life style and genetic variation in teleosts: the case of pelagic (Aphia minuta) and benthic (Gobius niger) gobies (Perciformes: Gobiidae). Mar Biol 156:239–252
Gold JR, Li C, Shipley NS, Powers PK (1990) Improved methods for working with fish chromosomes with a review of metaphase chromosome banding. J Fish Biol 37:563–575
Gornung E (2013) Twenty years of physical mapping of major ribosomal RNA genes across the teleosts: a review of research. Cytogenet Genome Res 141:90–102
Grigoryan KA, Vasiliev VP (1993) Karyotypes of five species of goby (Gobiidae) from the basins of the Black and Caspian seas. J Ichthyol 33:137–143
Howell WM, Black DA (1980) Controlled silver staining of nucleolus organizer region with protective colloidal developer: a 1-step method. Experientia 36:1014–1015
Kess T, Bentzen P, Lehnert SJ, Sylvester EVA, Lien S, Kent MP, Sinclair-Waters M, Morris C, Wringe B, Fairweather R, Bradbury IR (2020) Modular chromosome rearrangements reveal parallel and nonparallel adaptation in a marine fish. Ecol Evol 10:638–653
Kirubakaran TG, Grove H, Kent MP, Sandve SR, Baranski M, Nome T, De Rosa MC, Righino B, Johansen T, Otterå H, Sonesson A, Lien S, Andersen Ø (2016) Two adjacent inversions maintain genomic differentiation between migratory and stationary eco-types of Atlantic cod. Mol Ecol 25:2130–2143
Kottelat M, Freyhof J (2007) Handbook of European freshwater fishes. Publications Kottelat, Cornol and Freyhof, Berlin, p 646
Kubat Z, Hobza R, Vyskot B, Kejnovsky E (2008) Microsatellite accumulation on the Y chromosome in Silene latifolia. Genome 51:350–356
Levan A, Fredga K, Sandeberg AA (1964) Nomenclature for centromeric position on chromosomes. Heredity 52:201–220
Lima-Filho PA, Cioffi MB, Bertollo LAC, Molina WF (2012) Chromosomal and morphological divergences in Atlantic populations of the frillfin goby Bathygobius soporator (Gobiidae, Perciformes). J Exp Mar Biol Ecol 434:63–70
Lima-Filho PA, Bertollo LAC, Cioffi MB, Costa GWWF, Molina WF (2014a) Karyotype divergence and spreading of 5S rDNA sequences between genomes of two species: darter and emerald gobies (Ctenogobius, Gobiidae). Cytogenet Genome Res 3:197–203
Lima-Filho PA, Amorim KDJ, Cioffi MB, Bertollo LAC, Molina WF (2014b) Chromosomal mapping of repetitive DNAs in Gobionellus oceanicus and G. stomatus (Gobiidae; Perciformes): a shared XX/XY system and an unusual distribution of 5S rDNA sites on the Y chromosome. Cytogenet Genome Res 144:333–340
Lima-Filho PA, Rosa RS, Souza AS, Costa GWWF, Oliveira C, Molina WF (2016) Evolutionary diversification of Western Atlantic Bathygobius species based on cytogenetic, morphologic and DNA barcode data. Rev Fish Biol Fisher 26:109–121
Maldonado-Monroy MC, Uribe-Alcocer M, Arreguin-Espinosa J, Castro-Perez A (1985) Karyological studies on Dormitator maculatus Bloch and Gobiomorus dormitor Lacépède (Gobiidae: Perciformes). Cytologia 50:663–669
Molina WF (2005) Intraspecific karyotypical diversity in brackish water fishes of the Eleotridae family (Pisces, Perciformes). Cytologia 70:39–45
Molina WF, Alves DEO, Araújo WC, Martinez PA, Silva MFM, Costa GWWF (2010) Performance of human immunostimulating agents in the improvement of fish cytogenetic preparations. Genet Mol Res 9:1807–1814
Molina WF, Martinez PA, Bertollo LAC, Bidau CJ (2014a) Evidence for meiotic drive as an explanation for karyotype changes in fishes. Mar Genomics 15:29–34
Molina WF, Martinez PA, Bertollo LAC, Bidau CJ (2014b) Preferential accumulation of sex and Bs chromosomes in biarmed karyotypes by meiotic drive and rates of chromosomal changes in fishes. An Acad Bras Ciênc 86:1801–1812
Motta-Neto CC, Cioffi MB, Costa GWWF, Amorim KDJ, Bertollo LAC, Artoni RF, Molina WF (2019) Overview on karyotype stasis in Atlantic grunts (Eupercaria, Haemulidae) and the evolutionary extensions for other marine fish groups. Front Mar Sci 6:628
Muus BJ, Nielsen JG (1999) Sea fish. Scandinavian Fishing Year Book, Hedehusene, p 340
Nakashima Y, Kuwamura T, Yogo Y (1996) Both-ways sex change in monogamous coral gobies, Gobiodon spp. Environ Biol Fishes 3:281–288
Nelson JS, Grande TC, Wilson MVH (2016) Fishes of the world, 5th edn. Wiley, Hoboken, p 752
Nishikawa S, Amaoka K, Nakanishi K (1974) A comparative study of chromosomes of twelve species of gobioid fishes in Japan. Jpn J Ichthyol 21:61–71
Ocalewicz K, Sapota MR (2011) Cytogenetic characteristics of the round goby Neogobius melanostomus (Pallas, 1814) (Teleostei: Gobiidae: Benthophilinae). Mar Biol Res 7:195–201
Oliveira C, Almeida-Toledo LF (2006) Evidence of an XX/XY sex chromosome system in the fish Dormitator maculatus (Teleostei, Eleotrididae). Genet Mol Biol 4:635–655
Oliveira EJ, Pádua JG, Zucchi MI, Vencovsky R, Vieira MLC (2006) Origin, evolution and genome distribution of microsatellites. Genet Mol Biol 29:294–307
Oto Y, Nakamura M, Murakami H, Masuda R (2017) Inconsistency between salinity preference and habitat salinity in euryhaline gobiid fishes in the Isazu River, northern Kyoto Prefecture. J Ethol 35:203–211
Patzner RA, Tassell JLV, Kovacic M, Kapoor BG (2012) The biology of gobies. CRC Press, Taylor & Francis Group, New York
Pearse DE, Miller MR, Abadía-Cardoso A, Garza JC (2014) Rapid parallel evolution of standing variation in a single, complex, genomic region is associated with life history in steelhead/rainbow trout. Proc R Soc B 281:20140012
Pendás AM, Morán P, García-Vázquez E (1994) Organization and chromosomal location of the major histone cluster in brown trout, Atlantic salmon and rainbow trout. Chromosoma 103:147–152
Pezold F (1984) Evidence for multiple sex chromosomes in the freshwater goby, Gobionellus shufeldti (Pisces: Gobiidae). Copeia 1984:235–238
Prazdnikov DV, Vasil’ev VP, Vasil’eva ED (2013) Polymorphism and interpopulation variability of karyotype of Caspian bighead goby Neogobius gorlap (Gobiidae, Perciformes). J Ichthyol 53:425–430
Rocha LA, Robertson DR, Rocha CR, Van-Tassell JL, Craig MT, Bowen BW (2005) Recent invasion of the tropical Atlantic by an Indo-Pacific coral reef fish. Mol Ecol 14:3921–3928
Ruber L, Vantassell JL, Zardoya R (2003) Rapid speciation and ecological divergence in the American seven-spined gobies (Gobiidae, Gobiosomatini) inferred from a molecular phylogeny. Evolution 57:1584–1598
Schweizer D (1976) Reverse fluorescent chromosome banding with chromomycin and DAPI. Chromosome 58:307–224
Skóra K, Olenin S, Gollasch S (1999) Neogobius melanostomus (Pallas, 1811) In: Gollasch S, Michin D, Rosenthal H, Voight M (eds) Exotics across the ocean. Case histories on introduced species: their general biology, distribution, range expansion and impact: prepared by members of the European Union Concerted Action on testing monitoring systems for risk assessment of harmful introductions by ships to European waters (MAS-CT-97-0111), Department of Fishery Biology, Institute for Marine Science, University of Kiel, Germany, pp 69–73
Sumner AT (1972) A simple technique for demonstrating centromeric heterochromatin. Exp Cell Res 75:304–306
Suzuki T (1996) Hypseleotris cyprinoides. In: Fisheries Agency of Japan (ed) Basic data on rare Japanese wild aquatic species. (III). Nihonsuisanshigenhogokyokai, Tokyo, pp 215–221.
Suzuki T, Shibukawa K, Senou H, Chen I (2015) Redescription of Rhinogobius similis Gill 1859 (Gobiidae: Gobionellinae), the type species of the genus Rhinogobius Gill 1859, with designation of the neotype. Ichthyol Res 63:227–238
Thacker CE (2003) Molecular phylogeny of the gobioid fishes (Teleostei: Perciformes: Gobioidei). Mol Phylogenet Evol 26:354–368
Thacker CE (2009) Phylogeny of Gobioidei and placement within Acanthomorpha, with a new classification and investigation of diversification and character evolution. Copeia 2009:93–104
Thode G, Martinez G, Ruiz JL, Lopez JR (1988) A complex chromosomal polymorphism in Gobius fallax (Gobiidae, Perciformes). Genetica 76:65–71
Uribe-Alcocer M, Diaz-Jaimes P (1996) Chromosome complements of Gobionellus microdon and Eleotris picta collected in México. J Fish Biol 48:796–798
Uribe-Alcocer M, Ramirez-Escamilla A (1989) Comparacion citogenetica entre las especies del genero Dormitator (Pisces: Gobiidae). An Inst Cienc del Mary Limnol Univ Nal Auton México 16:75–80
Uribe-Alcocer M, Espinosa JA, Padilla AT, Pérez AC (1983) Los cromosomas de Dormitator latifrons (Pisces: Gobiidae). An Inst Cienc del Mary Limnol Univ Nal Auton México 10:23–30
Vasilev VP, Grigoryan KA (1994) Chromosome polymorphism and karyological relationships in the group of gobies Neogobius cephalarges Pallas-Neogobius platyrostris Pallas (Gobiidae). Russ J Genet 30:1251–1259
Webb CJ (1986) Karyology of the Indo-Pacific Parioglossus raoi (Herre) (Teleostei: Gobioidei) from Fiji. Mar Freshwater Res 37:347–351
Wellenreuther M, Bernatchez L (2018) Eco-evolutionary genomics of chromosomal inversions. Trends Ecol Evol 33:427–440
White TJ, Bruns TD, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols, a guide to methods and applications. Academic Press, San Diego, pp 315–322
Xu D, Molina WF, Yano CF, Zhang Y, De OEA, Lou B, Cioffi MB (2017) Comparative cytogenetics in three sciaenid species (Teleostei, Perciformes): evidence of interspecific chromosomal diversification. Mol Cytogenet 10:37
Yu X, Zhou T, Li K, Zhou M (1987) On the karyosystematics of cyprinid fishes and a summary of fish chromosome studies in China. Genetica 72:225–235
Acknowledgements
The authors thank to the ICMBio/SISBIO (#19135-4) for the authorization in collecting specimens. We are also grateful to Dr. José Garcia Júnior for the taxonomic identification of specimens utilized in the study. This work was supported by the Conselho Nacional de Desenvolvimento Cient.ico e Tecnol.gico (CNPq) [#442664/2015-0; #442626/2019-3].
Author information
Authors and Affiliations
Contributions
SASS: conceptualization, methodology, writing- original draft preparation, data curation. WFM: conceptualization, methodology, writing- original draft preparation, funding acquisition, project administration, writing—reviewing and editing. GWWFC: investigation, validation. PAL-F, CCM-N: supervision, visualization. MBC, LACB: writing—reviewing and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Animal and human rights statement
The experimental work fulfilled all ethical guidelines regarding the handling of specimens. The experiments followed ethical and anesthesia conducts in accordance with the Ethics Committee on the Use of Animals (#044/2015) at the Federal University of Rio Grande do Norte (UFRN). The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Additional information
Edited by Jiamei Li.
Rights and permissions
About this article
Cite this article
da Silva, S.A.S., de Lima-Filho, P.A., da Motta-Neto, C.C. et al. High chromosomal evolutionary dynamics in sleeper gobies (Eleotridae) and notes on disruptive biological factors in Gobiiformes karyotypes (Osteichthyes, Teleostei). Mar Life Sci Technol 3, 293–302 (2021). https://doi.org/10.1007/s42995-020-00084-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42995-020-00084-6