Abstract
Purpose
High-intensity exercise reduces renal artery blood flow (RBF) compared to other forms of exercise. However, it is unclear whether moderate-intensity exercise, including those at the ventilation threshold (VT), decreases RBF. Additionally, attenuated renal autoregulation and associated blood flow can cause renal injury in patients with underlying disease. Therefore, this study aimed to confirm the changes in RBF after moderate-level exercise in healthy subjects, which will have implications for the study of renal arterial blood flow in patients with renal failure.
Methods
Cardiopulmonary exercise tests were performed by 10 healthy male participants (mean age, 31 ± 8 years): 3 min constant work-rate exercise tests, varying in exercise intensity 1 min before VT (pre-VT), after VT (post-VT), and after the respiratory compensation point (RCP). The RBF was measured using ultrasonic inspection equipment following each exercise. The VT was determined using the ventilatory equivalent method (VEQ method), while the RBF was calculated from the time-averaged flow velocity (TAV) and cross-sectional area (CSA).
Results
At baseline (resting phase), RBF was 461 ± 142 mL/min. While RBFs at pre-VT were not significantly different from those at baseline (482 ± 142 mL/min; P = 0.82), significant differences were observed at post-VT (289 ± 111 mL/min; P < 0.01 vs. baseline). RBFs at the RCP were also different from those at the baseline (212 ± 56 mL/min; P < 0.01 vs. baseline).
Conclusions
In healthy individuals, exercises varying in intensity up to the vicinity of the VT can be performed without any significant decrease in RBF.
Trial Registration Number and Registration Date
The trial was approved by an independent ethics committee at the Asahi University Hospital (approval No. 1/May/2018) and was registered (Name of the registry: Changes of renal blood flow with exercise load. Consideration using ultrasonic inspection equipment. UMIN000035598, https://center6.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000040561, 24/January/2019).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The kidney processes 20%–25% of the blood volume of the entire cardiac output and is one of the organs most affected by exercise-induced blood redistribution [13, 17]. Renal autoregulatory mechanisms maintain renal blood flow (RBF) and glomerular filtration rate (GFR) independent of renal perfusion pressure (RPP) over a defined range (80–180 mmHg). However, attenuated renal autoregulation contributes to renal injury in many models of renal, diabetic, and hypertensive diseases [21]. Furthermore, renal autoregulatory functions have been reported to be reduced in type 2 diabetes mellitus [4]. Diabetes and hypertension are major causes of chronic kidney disease and are both associated with impaired autoregulation of RBF and increased transmission of arterial pressure variations to the glomerular capillaries [20]. Particularly, attenuated renal autoregulation and associated blood flow can cause renal injury in patients with underlying diseases. Guidelines for renal rehabilitation propose that patients should perform aerobic exercises (moderate-intensity exercises) [33]. While moderate-intensity exercises are safe, high-intensity exercises can increase the biomarkers of muscle and kidney damages, including serum creatine kinase (CK, urine myoglobin, and creatinine levels [15, 28]). Several studies have reported a decrease in RBF in healthy subjects after high-intensity exercises, like bicycle exercise and running [23]. However, whether diseased or healthy subjects can maintain RBF with moderate-intensity exercise [ventilation threshold (VT)] is unclear. It is crucial to confirm this in healthy subjects before confirming it in patients with kidney disease. Therefore, this study aims to confirm the changes in RBF in healthy subjects after moderat level exercise, which will have implications for the study of renal arterial blood flow in patients with renal failure.
Moderate-intensity exercises are often used based on the VT. We determined the VT value by measuring carbon dioxide excretion, ventilatory volume exhalation, and physiological autonomic turning point examination [27, 30]. Autonomic changes result in heart rate (HR) fluctuation, vascular changes, and increased sympathetic nerve activity, which causes constriction of renal arteries [26].
Therefore, the two hypotheses of this study are (1) RBF is maintained until VT and (2) RBF decreases after VT.
Methods
Participants and Procedure
Healthy participants without any underlying disease were recruited between November 2018 and January 2020. All test participants volunteered to participate in the study, were informed about the details of the study, and provided written consent to participate before enrollment. The trial was approved by the institutional review board of the Asahi University Hospital (approval No. 2018-05-01), and the trial was registered on 2019/1/24 (UMIN 000035598) [32].
Eligibility criteria included healthy volunteers aged 18 years or older without hypertension, pulmonary and cardiac disorders, cerebrovascular disease, other pre-existing diseases, and obesity, while exclusion criteria included the lack of respiratory cooperation and large amounts of intestinal gases [29].
Protocol and Assessment
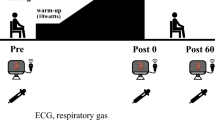
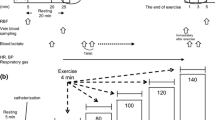
A cardiopulmonary exercise test (CPX) was conducted on the first visit before VT (pre-VT), after VT (post-VT), and after the respiratory compensation point (RCP). On the second visit, participants performed a constant work-rate exercise test at three exercise intensities—pre-VT, post-VT, and RCP—for 3 min each. The RBF was measured immediately after each load (Fig. 1). CPX was performed using a cycle ergometer (STB-3400 Nihon Kohden). Oxygen uptake (\(\dot{V}\)O2), carbon dioxide production (\(\dot{V}\)CO2), and minute ventilation (\(\dot{V}\)E) were measured using an expired gas analyzer (Ae-310 Minato). The HR of the participants was monitored throughout the test using the stress system STS 2100 (Nihon Kohden). Systolic blood pressure (SBP) was monitored every minute using a stress test monitor by Sun Tech (Tango M2).
VT [30] is a value determined by the amount of carbon dioxide expelled during exhalation and ventilation. It acts as a turning point for the autonomic nervous system [34], and sympathetic nerve responses are increased post-VT.
We determined the ventilatory threshold using the ventilatory equivalent method (VEQ method) [30]. The exercise intensity examined in this study leads to an increase in the ventilatory equivalent of oxygen (\(\dot{V}\)E/\(\dot{V}\)O2) without a concurrent increase in the ventilatory equivalent of carbon dioxide (\(\dot{V}\)E/\(\dot{V}\)CO2) [30]. The RCP can be calculated from the increase in the elevation of the slope when \(\dot{V}\)E is plotted against \(\dot{V}\)CO2.
Ultrasonography for RBF Measurement
Recent advances in ultrasonography allow simultaneous assessments of blood flow velocity and the cross-sectional area (CSA) of blood vessels [18]. Several studies have also evaluated the value of color-coded duplex ultrasonography [18, 22] and the reproducibility of blood flow measurements [3]. RBF was determined using the formula: RBF (mL/min) = time-averaged flow velocity (TAV) (cm/s) × CSA (cm2) × 60 (beats/min) [18]. Ultrasound visualization of the renal arteries was performed using the HITACHI ALOKA Noblus (Hitachi Aloka, Tokyo, Japan) flank view approach.
Statistical Analysis and Sample Size
Analyses of HR, SBP, TAV, CSA, and RBF were performed for all participants and analyzed immediately after CPX. A normality test (Shapiro–Wilk test) was performed to confirm normality. Data are presented as mean ± SE (standard error). Bonferroni’s test was used to compare the variables to the baseline data. Statistical significance was set at P < 0.05. All analyses were performed using IBM SPSS Statistics ver. 24 (IBM Corp., Armonk, NY, USA). The sample size was calculated to generate 80% power and 5% alpha risk using G power version 3.1.9 software (Dusseldolf University, Düsseldorf, Germany), considering the number of cases based on previous studies. A sample size of ten participants was estimated to be statistically adequate, since changes in blood flow had been measured in 10–11 cases by previous studies [8, 18].
Results
The clinical characteristics and CPX results of the ten participants are presented in Table 1. The mean age of the participants was 31 years. All participants displayed normal ECG records during CPX. The peak \(\dot{V}\)O2 was 30.4 ± 3.3 mL/kg/min. The VT point was 66.8 ± 11.1 W (Table 1).
Change in HR and SBP
The HR of the participants increased linearly at pre-VT, post-VT, and RCP, respectively. There was a significant increase in HR pre-VT, post-VT, and RCP compared to the baseline (pre-VT 97.8 ± 10.8 beats/min, post-VT115 ± 12.6 bearts/min, RCP 147.9 ± 20.1 beats/min, P < 0.01 vs. baseline). The SBP did not show any significant change (pre-VT145 ± 4.8 mmHg vs. baseline131 ± 5.6 mmHg, P = 0.06) at pre-VT, but showed a significant increase at post-VT and RCP (post-VT166 ± 7.2 mmHg, RCP 207 ± 7.5 mmHg, P < 0.05 and P < 0.01 vs. baseline, respectively) (Fig. 2).
Ultrasonography Data
The TAV at the baseline was 29.0 ± 7.8 cm/s. The TAV at pre-VT, post-VT, and RCP did not show any significant difference compared to the values at the baseline. In addition, significant differences were found in RCP (pre-VT, 30.0 ± 7.7 cm/s, P = 0.44; post-VT, 24.0 ± 5.5 cm/s, P = 0.50; RCP, 22 ± 3.8 cm/s, P < 0.05 vs. baseline) (Fig. 3). Moreover, CSA at pre-VT, post-VT, and RCP did not show significant differences compared to the CSA at baseline (pre-VT, 0.27 ± 0.06 cm2, P = 0.67; post-VT, 0.19 ± 0.05 cm2, P = 0.19; RCP, 0.15 ± 0.03 cm2, P = 0.05 vs. baseline) (Fig. 4).
The RBF measured at baseline was 461 ± 142 mL/min. While the RBF at pre-VT was not significantly different from that at baseline (482 ± 142 mL/min; P = 0.82 vs. Baseline), significant differences were observed in the RBFs at post-VT when compared with baseline (289 ± 111 mL/min; P < 0.01). Similar trends were also seen for RBFs at the RCP (212 ± 56 mL/min; P < 0.01 vs. baseline) (Fig. 5). The RBF at post-VT and RCP was approximately 37% and 54% lower, respectively, than that at the baseline.
Discussion
We verified our hypothesis that VT-based moderate-intensity exercise lowers RBF in healthy subjects. Our findings showed that during exercise, RBF decreased immediately after VT and confirmed that RBF fluctuates at pre-VT, post-VT, and RCP. We found that (1) there was no change in the RBF at pre-VT, and (2) the RBF was significantly lower at post-VT and RCP. Compared with the resting phase (baseline), RBF decreased by approximately 63% at post-VT and 46% at RCP. According to Suzuki et al. [31], RBF decreases by 53.4% after exercise, and their values at RCP coincide with the findings of earlier studies.
Exercise-induced proteinuria is strictly related to exercise intensity, not exercise duration [2]. While some reports indicate that high-intensity loading does not cause renal dysfunction in young diabetic patients [16], high-intensity loading is not recommended as it leads to organ damage, including increased renal damage; while exercise, including aerobic exercise, improves glomerular filtration and reduces risk factors of cardiovascular disease in patients with kidney disease [14]. In addition, oxygen transport in patients with renal disease is proportional to total RBF, and it directly depends on blood flow regulation. In some patients with renal diseases, high-intensity training exacerbates hypoxia in the renal medulla. In contrast, a report has shown that low/moderate intensity is not harmful [6]. Particularly, when autoregulation is impaired due to disease, reduced blood flow during exercise leads to renal hypoxia. Hypoxia is a vital factor in the development and progression of chronic kidney disease (CKD) [12].
The present study confirms that RBF was maintained at pre-VT exercise intensity, including aerobic exercise. Aerobic exercise can improve renal function tests and lipid profiles and slow the progression of stages 3 and 4 CKD [24]. In addition, more than half (6/10) of all studies conducted at moderate intensity tended to show a decrease in proteinuria [19]. Moderate exercise does not increase sympathetic activity due to the cardiopulmonary baroreflex, thus, suppressing the rise in sympathetic activity [19]. However, when exercise is above moderate intensity, increased central and peripheral nerve activity stimulates ventilation, cardiovascular function, and other functions [1].
Changes in autonomic nerves affect the HR and blood vessels. Sympathetic hyperactivity causes renal blood vessels to constrict [10]. In addition, angiotensin II (Ang II) has a significant effect on the contraction of blood vessels. The vasopressor action of Ang II involves an increase in peripheral vascular resistance due to the vasoconstriction of vascular smooth muscles, which is associated with the release of noradrenaline (NA) from sympathetic nerve endings. Furthermore, a high correlation was observed between the plasma NA value and lactate threshold [29]. The activation of these NA, norepinephrine, and epinephrine causes a decrease in the glomerular filtration rate and the RBF [5]. Our findings at pre-VT showed an increase in HR, but no changes in blood pressure, TAV, or CSA. Based on this, we concur that changes did not occur in the pre-VT stage. Consequently, exercise at pre-VT was capable of maintaining the RBF. Notably, exercising at 50%–60% of \(\dot{\text V}\)O2 max exceeding O2 max causes renal vasoconstriction with a detectable increase in renal nerve activity, circulating catecholamines, and plasma renin activity [9]. Renal arterial blood flow is affected by renal sympathetic nerve activity (RSNA) over its entire range, thereby inducing contraction and dilation of the renal vessels [25]. Therefore, we believe that the contraction of these systemic blood vessels and renal arteries contributed to the decrease in RBF at post-VT. Additionally, post-VT showed a decrease in RBF to 289 ± 111 mL/min from 461 ± 142 mL/min at baseline. The HR was 115 ± 12.6 beats/min, which was approximately twice of that at rest. The SBP significantly increased at post-VT, and the CSA also changed to 0.19 cm2 from 0.27 cm2 at baseline. Therefore, blood flow changes during the contraction of systemic blood vessels and renal arteries.
In this study, a decrease in TAV was observed at this load intensity, and a significant change in CSA was also confirmed. This result is similar to that of a previous study [15]. Furthermore, HR and SBP were significantly elevated post-VT, suggesting that vascular reactivity was enhanced after VT, corroborating the changes in RBF. In addition, since the HR and SBP significantly increased at RCP, we concur that they were affected by changes in the autonomic nerves, as seen during the post-VT stage.
The risk of kidney injury only increases when exercise intensity exceeds the lactate threshold [19]. This reduced renal arterial blood flow response after VT has important implications in renal disease patients with renal autoregulation disorders. However, patients with heart failure or renal failure with heart failure do not necessarily have a coincident decrease in blood flow due to abnormal cardiac pump function. At least in patients with the appearance of oscillatory ventilation on CPX, they do not necessarily have a concordant decrease in perfusion due to the difficulty in determining VT. The same is true for patients whose VT is difficult to determine, regardless of disease. Patients with renal failure have lower exercise tolerance than healthy subjects [7]. However, in patients with clearly identifiable VT, the exercise physiological response is similar [11] and may produce similar RBF changes. Therefore, a future research question is to confirm the blood flow changes associated with different diseases.
This study has some important limitations. First, ultrasonography did not accurately measure the RBF during CPX using the cycle ergometer. Therefore, it was used immediately after the participants completed the test. Second, the ultrasonography probe could not be used in a ventral approach. Third, participants with obesity could not provide stable measurements and had to be excluded from the study. This study observed that the exercise intensity that maintains RBF was that at pre-VT. However, it is unclear whether this applies to patients with comorbidities; in particular, the effect of moderate-intensity loading on renal artery blood flow in patients with cardiac disease is a topic for future research. Finally, if the blood distribution or volume of a patient differs from that of healthy subjects, the blood flow response to exercise may diverge from that of healthy subjects. Furthermore, there is insufficient evidence for blood distribution around the VT in patients with kidney disease, which cannot be substantiated by studies in healthy subjects, and this is a major limitation of this study.
Conclusions
This study focused on RBF measured using ultrasonography immediately after the participants used the cycle ergometer. The findings showed that exercises with intensity up to the vicinity of the VT could be performed without decreasing the RBF in healthy people.
Data Availability Statement
Upon a reasonable request, derived data supporting the findings of this study are available from the corresponding author.
References
Ana BP, Jesús JR, Francisco JC, Nicola M. Responses to increasing exercise upon reaching the anaerobic threshold, and their control by the central nervous system. BMC Sports Sci Med Rehabil. 2014;6:17. https://doi.org/10.1186/2052-1847-6-17.
Bellinghieri G, Savica V, Santoro D. Renal alterations during exercise. J Ren Nutr. 2008;18(1):158–64. https://doi.org/10.1007/BF00376487.
Chapman CL, Johnson BD, Hostler D, Lema PC, Schlader ZJ. Reliability and agreement of human renal and segmental artery hemodynamics measured using Doppler ultrasound. J Appl Physiol (1985). 2020;128(3):627–36. https://doi.org/10.1152/japplphysiol.00813.2019.
Christensen PK, Akram K, Kønig KB, Parving HH. Autoregulation of glomerular filtration rate in patients with type 2 diabetes during isradipine therapy. Diabetes Care. 2003;26(1):156–62. https://doi.org/10.2337/diacare.26.1.156.
Colombo R, Santos RA, Rech LS, Tonolli BT, Farina GS, Gerhardt GJL. An educational tool to improve understanding of angiotensin II function and the adrenergic system in renal circulation. Adv Physiol Educ. 2019;43(4):529–33. https://doi.org/10.1152/advan.00080.2018.
Daniel DS, Melinda S, Luigi P, Eleonora D, Devaki SS. Association of high-intensity exercise with renal medullary carcinoma in individuals with sickle cell trait: clinical observations and experimental animal studies. Cancers (Basel). 2021;13(23):6022. https://doi.org/10.3390/cancers13236022PMCID:PMC8656882. (PMID: 34885132).
Danielle LK, Bryce JM, Joseph MS, Raymond RT, David GE. Cardiopulmonary exercise testing reveals subclinical abnormalities in chronic kidney disease. Eur J Prev CardiolCardiol. 2018;25(16):1717–24. https://doi.org/10.1177/2047487318777777.
Davi AS, Jacques RP, Dórea EL, de Juliana BAM, Alan LF. Acute exercise does not impair renal function in nondialysis chronic kidney disease patients regardless of disease stage. Am J Physiol Renal Physiol. 2017;313:F547–52. https://doi.org/10.1152/ajprenal.00131.2017.
David RH, Catherine M, Kenneth M. The athletic horse: principles and practice of equine sports med. St. Louis: Elsevier/Saunders; 2014.
Edward JJ, Ulla CK, Gerald FD. Neural control of renal function. Compr Physiol. 2011;1(2):731–67. https://doi.org/10.1002/cphy.c100043.
Eva P, Afroditi B, Aristi B, Christodoulos EP, Aikaterini P, Pantelis S. Cardiopulmonary exercise testing in patients with end-stage kidney disease: principles, methodology and clinical applications of the optimal tool for exercise tolerance evaluation. Nephrol Dial Transplant. 2021. https://doi.org/10.1093/ndt/gfab150.
Fine LG, Norman JT. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: from hypothesis to novel therapeutics. Kidney Int. 2008;74(7):867–72. https://doi.org/10.1038/ki.2008.350. (Epub 2008 Jul 16).
Koçer G, Kuru O, Gündüz F, Bayram Z, Ozdem S, Aksoy D, Sentürk UK. The effect of exercise training on the responsiveness of renal resistance arteries in rats. Ren Fail. 2011;33(6):587–92. https://doi.org/10.3109/0886022X.2011.585000.
Hamid A, Majid M, Payam S, Akram F, Katsuhiko S. Effects of different types of exercise on kidney diseases. Sports (Basel). 2022;10(3):42. https://doi.org/10.3390/sports10030042.
Honda S, Kawasaki T, kamitani T, Kiyota K. Rhabdomyolysis after high intensity resistance training. Intern Med. 2017;56(10):1175–8. https://doi.org/10.2169/internalmedicine.56.7636.9.
Jacques R, Jacques PV. Kidney function during exercise in healthy and diseased humans. Sports Med. 1994;18(6):419–37.
Kaufman D, Basit H, Knohl SJ. Physiology, glomerular filtration rate. Treasure Island (FL): StatPearls; 2022.
Kawakami S, Yasuno T, Matsuda T, Fujimi K, Ito A, Yoshimura S, Uehara Y, Tanaka H, Saito T, Higaki Y. Association between exercise intensity and renal blood flow evaluated using ultrasound echo. Clin Exp Nephrol. 2018;22(5):1061–8. https://doi.org/10.1007/s10157-018-1559-1.
Lei Y, Xiaoxia W, Ying W, Chunfeng W, Rong H. Effects of exercise training on proteinuria in adult patients with chronic kidney disease: a systematic review and meta-analysis. BMC Nephrol. 2020;21(1):172.
Marilyn B, Mallikarjuna RP, Jerry F, Richard JR. Molecular mechanisms of renal blood flow autoregulation. Curr Vasc Pharmacol. 2014;12(6):845–58.
Mattias C, Christopher SW, Arendshorst WJ. Renal autoregulation in health and disease. Physiol Rev. 2015;95(2):405–511. https://doi.org/10.1152/physrev.00042.2012.
Meola M, Petrucci I. Color Doppler sonography in the study of chronic ischemic nephropathy. J Ultrasound. 2008;11(2):55–73. https://doi.org/10.1016/j.jus.2008.03.006.
Poortmans JR. Exercise and renal function. Sports Med. 1984;1(2):125–53. https://doi.org/10.2165/00007256-198401020-00003.
Rahmy AF, Afifi WM, Ghorab AA, Mostafa HA. Effect of moderate aerobic exercises on kidney function and lipid profile in chronic kidney disease patients. JESNT. 2016;16(3):97–105.
Malpas SC, Leonard BL. Neural regulation of renal blood flow: a re-examination. Rev Clin Exp Pharmacol Physiol. 2000;27(12):956–64.
Sata Y, Head GA, Denton K, May CN, Schlaich MP. Role of the sympathetic nervous system and Its modulation in renal hypertension. Front Med (Lausanne). 2018;5:82. https://doi.org/10.3389/fmed.2018.00082.
Shiraishi Y, Katsumata Y, Sadahiro T, Azuma K, Akita K, Isobe S, Yashima F, Miyamoto K, Nishiyama T, Tamura Y, Kimura T, Nishiyama N, Aizawa Y, Fukuda K, Takatsuki S. Real-time analysis of the heart rate variability during incremental exercise for the detection of the ventilatory threshold. J Am Heart Assoc. 2018;7(1):e006612. https://doi.org/10.1161/JAHA.117.006612.
Spada TC, Silva JMRD, Francisco LS, Marçal LJ, Antonangelo L, Zanetta DMT, Yu L, Burdmann EA. High intensity resistance training causes muscle damage and increases biomarkers of acute kidney injury in healthy individuals. PLoS ONE. 2018;13(11): e0205791. https://doi.org/10.1371/journal.pone.0205791.
Sparks MA, Crowley SD, Gurley SB, Mirotsou M, Coffman TM. Classical renin-angiotensin system in kidney physiology. Compr Physiol. 2014;4(3):1201–28. https://doi.org/10.1002/cphy.c130040.
Steven E, Brent C, Ruby J, Sanchez A, Serfass C, Leon AS. Validity and reliability of combining three methods to determine ventilatory threshold. Med Sci Sports Exer. 2001;33(11):1841–8. https://doi.org/10.1097/00005768-200111000-00007.
Suzuki M, Sudoh M, Matsubara S, Kawakami K, Shiota M, Ikawa S. Changes in renal blood flow measured by radionuclide angiography following exhausting exercise in humans. Eur J Appl Physiol Occup Physiol. 1996;74(1–):1–7.
University hospital Medical Information Network (UMIN) Center. UMIN 000035598 - UMIN Clinical Trials. http://www.umin.ac.jp/.
Yamagata K, Hoshino J, Sugiyama H, Hanafusa N, Shibagaki Y, Komatsu Y, Konta T, Fujii N, Kanda E, Sofue T, Ishizuka K, Kitagawa M, Kono K, Hinamoto N, Miyai T, Koike K, Toda S, Hasegawa J, Yamanouchi M, Yoshimura R, Ishii R, Goto S, Kawarazaki H, Takase K, Taki F, Matsumura M, Raita Y, Sakurai S, Shimizu T, Yamanoto S, Kawaguchi T, Oguchi H, Tsujita M, Yazawa M, Uchida A, Ando Y, Kaneko S, Matsunaga A, Harada T, Ito O, Kohzuki M. Clinical practice guideline for renal rehabilitation: systematic reviews and recommendations of exercise therapies in patients with kidney diseases. Ren Replace Ther. 2019;5:28.
Yamamoto Y, Hughson RL, Nakamura Y. Autonomic nervous system responses to exercise in Relation to ventilatory threshold. Chest. 1992;101(Suppl):206S-S210. https://doi.org/10.1378/chest.101.5_supplement.206s.
Acknowledgements
The authors thank all members of the Department of Cardiology, Asahi University Hospital, and Shinichi Arizono.
Funding
The authors declare that no support, financial or otherwise, was received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
YF contributed to literature research, study design, data analysis, manuscript preparation, and manuscript review. SA contributed to study design, data analysis, and manuscript preparation. ST contributed to study design and data collection. TK, NT, TF, and JM contributed to data collection. YT contributed to data collection and study design. TS contributed to data collection. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Shinichi Arizono reports grants from Hoshi Iryo-Sanki Co. Ltd and NPO Central Japan Lung Study Group outside the submitted work. All other authors declare that they have no competing interests.
Ethics Approval and Consent to Participate
This trial was approved by an independent ethics committee at the Asahi University Hospital (Approval No. 1/May/2018) and was registered (Name of the registry: Changes of renal blood flow with exercise load. Consideration using ultrasonic inspection equipment.UMIN 000035598, https://center6.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000040561, 24/January/2019). All test subjects were volunteers who were informed about the details of the study. Written consent to participate was obtained from all the participants before enrollment in the study.
Consent for Publication
Not applicable.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fukuta, Y., Arizono, S., Tanaka, S. et al. Effects of Exercise Around the Ventilation Threshold on Renal Blood Flow in Healthy Individuals. J. of SCI. IN SPORT AND EXERCISE 6, 44–51 (2024). https://doi.org/10.1007/s42978-022-00216-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42978-022-00216-7