Abstract
Background
Renal blood flow (RBF) decreases with exercise, but this change is only temporary, and habitual exercise may be an effective method to improve renal function. The kidney shows structural and functional changes with aging, but it is unclear how aging affects the hemodynamic response of the kidneys to exercise. Therefore, we evaluated the differences in the hemodynamic response of the kidneys to high-intensity exercise between younger and older men.
Methods
Sixteen men (8 young and 8 older) underwent an incremental exercise test using a cycle ergometer with a 1-min warm up followed by exercise at 10–20 W/min until the discontinuation criteria were met. Renal hemodynamics were assessed before exercise, immediately after exercise, and at 60-min after exercise using ultrasound echo.
Results
High-intensity exercise significantly reduced RBF in both groups (younger: ∆ – 53 ± 16%, p = 0.0005; older: ∆ – 53 ± 19%, p = 0.0004). In the younger group, RBF returned to the pre-exercise level 60-min after exercise (∆ – 0.4 ± 5.7%, p > 0.9999). In contrast, RBF 60-min after exercise was significantly lower than that before exercise in the older group (∆ – 24 ± 19%, p = 0.0006). The older group had significantly lower RBF than younger adults 60-min after exercise (423 ± 32 vs. 301 ± 98 mL/min, p = 0.0283).
Conclusions
Our findings demonstrate that RBF following high-intensity exercise recovered 60-min after exercise in younger group, whereas RBF recovery was delayed in the older group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is known that exercise increases blood flow to the skin and skeletal muscles, and that blood flow is reduced to the internal organs, including the kidney. Decreased renal blood flow (RBF) during exercise enhances the glomerular permeability and the filtration rate [1], resulting in a decrease in glomerular filtration rate (GFR) and transient proteinuria [1, 2]. Post-exercise proteinuria is one of the most common signs observed after strenuous exercise, proteinuria indicates kidney damage and is used to predict progression to chronic kidney disease (CKD) [3]. Moreover, elevated biomarkers of kidney damage following exercise could be caused by higher intensity and prolonged duration of exercise, resulting in reduced RBF via enhanced sympathetic-nervous-system and body temperature, hypohydration [4]. Among them, it is speculated that the decrease in RBF play a central role against the elevated biomarkers of kidney damage after exercise. Therefore, we considered that the assessment of renal hemodynamic response to exercise is important.
In healthy individuals, all bodily functions decline in a linear fashion starting from the age of 30 years [5], and kidney function is no exception [6]. In the kidneys, estimated GFR (eGFR), which is an indicator of renal function, decreases by 0.41% per year with aging [7]. This age-related decrease in eGFR is associated with changes in renal hemodynamics. Aging is associated with a decrease in resting RBF [8]. In fact, RBF decreases by approximately 10% per decade after the age of 30 years [9]. Furthermore, there is the potential that aging can affect renal hemodynamics during exercise as well as at rest [10]. For the relationship between aging and exercise-induced changes in renal hemodynamics, previous study [7] reported that the older individual, the greater the impairment in renal function after exercise. In contrast, another study reported that the effects of exercise on the kidney decrease with aging [11], the effect of aging on the recovery of renal hemodynamics after high-intensity exercise is not well understood.
The traditional method used to assess RBF is para-aminohippuric clearance (CPAH) [1, 2, 12]. Although this method provides useful information, it is invasive and requires blood and urine sampling. Therefore, we used ultrasound echo [13,14,15], which is non-invasive and can quickly evaluate RBF. Ultrasound echo also places less strain on participants than the traditional method. Additionally, ultrasound echo allows for changes in other renal hemodynamic parameters, such as blood flow velocity (BFV) and cross-sectional area (CSA), to be assessed following exercise. It would be of considerable clinical importance to determine the changes in renal hemodynamic parameters following exercise using ultrasound echo.
Therefore, we hypothesized that older adults would have delayed recovery of RBF. Hence, we aimed to determine whether renal hemodynamics following high-intensity exercise differs between younger and older adults using ultrasound echo.
Materials and methods
Participants
Eight younger and eight older men were enrolled in this study (Table 1). Each participant met all eligibility requirements and underwent a complete medical examination, including electrocardiography. The inclusion and exclusion criteria are whether the presence of serious or progressive disorders, relatively preserved renal function (eGFR ≥ 60 ml/min/1.73 m2), and age 18 years or older. We approached those who have previously participated in studies conducted by our laboratory and who met above criteria. Participants avoided strenuous exercise the day before testing and fasted for eight hours prior to testing (water consumption was permitted). Sample size was calculated using G × power version 3.1.9 software (Dusseldorf University, Düsseldorf, Germany) considering the effects of aging on renal hemodynamics during high-intensity exercise based on a previous study [10] to generate a power of 80% and an alpha risk of 5%. A sample size of eight (younger) and eight (older) participants was estimated to be statistically appropriate. Although the older group included subjects from our previous study [14], the purpose of that study was to examine the relationship between RBF and exercise-intensity, whereas the purpose of this study was to identify differences in renal hemodynamic responses following exercise between younger and older adults. Therefore, this does not constitute duplicate publication.
Experimental trial
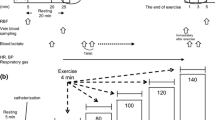
All participants underwent an incremental exercise test using the ramp load method and a cycle ergometer (Lode; Corival, The Netherlands), as described previously (Fig. 1) [13, 14]. After a 1-min warm up, the exercise load was gradually increased by 10–20 watts per minute. Considering the safety of the participants, the exercise ended according to the exercise discontinuation criteria outlined in the American College of Sports Medicine guidelines [16]. Respiratory gas was measured using the mixing chamber method and mass spectrometry equipment for biogas analysis (ARCO-2000 MET; Arco System, Kashima, Japan). Peak oxygen uptake was calculated from the data obtained during exercise. Renal hemodynamics were assessed before (Pre) and immediately after (Post 0) exercise, 60-min after exercise (Post 60), using ultrasound echo (Aplio 300; Toshiba Medical Systems, Tokyo, Japan). Blood samples were collected at Pre and Post 0.
Experimental protocol. All participants performed an incremental exercise test using the ramp load method and a cycle ergometer. The assessment of renal hemodynamic was conducted in Pre, Post 0 and Post 60. Blood samples were collected in Pre and Post 0. Pre, before exercise; Post 0, immediately after exercise; Post 60, 60-min after exercise
Assessment of renal hemodynamics
Renal hemodynamics were assessed using pulsed Doppler with a 3.5-MHz convex electronic scanning probe from an ultrasound system, as described previously [13]. We measured RBF, BFV, CSA, peak systolic flow velocity (PSV) and end-diastolic flow velocity (EDV) using the pulse Doppler method. Furthermore, Renal pulsatility index (rPI) and resistive index (rRI) were calculated as follow [17, 18]:
Sample analysis
Blood samples were collected from the antecubital vein on the morning after the participants had fasted for eight hours, and adrenaline, noradrenaline, cystatin-C, and creatinine concentrations were measured. Each blood sample was centrifuged for 10-min at 1750 × g at 4 °C. The samples were stored at – 80 °C until analysis. The blood analysis was performed by a commercial blood analysis company (LSI Medience Corp., Tokyo, Japan, SRL Inc., Tokyo, Japan). Additionally, eGFR with serum cystatin-C or creatinine and filtration fraction (FF) as an indicator of renal function and hemodynamics were calculated, as described previously [15].
Statistical analysis
The results are presented as the mean ± standard deviation. The participant characteristics were evaluated using the unpaired Student’s t test. A two-way linear mixed model was used to examine any differences in the dependent variables over time (Pre, Post 0, Post 60) and between groups (younger or older) with random effects to control for participant in the repeated measures design. From the results of the linear mixed models, estimated marginal means using Bonferroni’s adjustment for multiple comparisons were used to test for differences between younger and older groups at the measurement time points. To determine the factors that affected the change in RBF following exercise, the associations between the changes in RBF and renal hemodynamic parameters from Pre to Post 60 were evaluated using the linear regression analysis. Statistical analyses were performed using Prism version 9.5.1 (GraphPad Software, San Diego, CA, USA), and a p value of < 0.05 was considered statistically significant.
Results
In both groups, there was no significant difference in RBF at Pre, RBF at Post 0 decreased significantly from Pre (Fig. 2A, p = 0.0004). In the younger group, RBF returned to its pre-exercise level at Post 60, and there was no significant difference compared with the value at Pre (p > 0.9999). RBF at Post 60 was significantly lower than the Pre value in the older group (p = 0.0006). Furthermore, RBF in the older group was significantly lower than in the younger group at Post 60 (p = 0.0283) and the reduction in RBF in the older group was greater than younger group (Fig. 2D, p = 0.0288). Regarding CSA that constitutes RBF, there was no significant difference in the CSA between the younger and older groups at Pre, although maximal exercise significantly reduced the CSA in both groups (Fig. 2B, p < 0.0001). At Post 60, the CSA in both groups remained significantly lower than at Pre (p < 0.05), although the reduction in CSA was more pronounced in the older group than in the younger group (Fig. 2E). Maximal exercise had no effect on the BFV, which is another component of RBF in both groups. Furthermore, the BFV in the older group was significantly lower than in the younger group (Fig. 2C, F).
Changes in renal blood flow (A), cross-sectional area (B), and blood flow velocity (C) in Pre and Post 0, Post 60 in younger (■) and older (□) groups. The change from Pre to Post 0 and to Post 60 in renal blood flow (D), cross-sectional area (E), and blood flow velocity (F) in younger (●) and older (○) groups. Data are the mean ± standard deviation. **p < 0.01 vs. Pre. # p < 0.05 vs. younger group. Younger group: Pre, n = 8; Post 0, n = 8; Post 60, n = 8; older group: Pre, n = 8; Post 0, n = 8; Post 60, n = 8. Pre before exercise, Post 0 immediately after exercise, Post 60 60-min after exercise. Open circles represent individual data (Fig. A–C). Thick lines indicate the mean (Fig. D–F). Multiple pairwise comparisons were corrected using the Bonferroni method
There were no significant differences in PSV between the younger and older groups (Fig. 3A, p = 0.248), and maximal exercise had no effect on PSV in both groups (p = 0.199). In the older group, EDV was significantly lower than in the younger group (Fig. 3B, p = 0.011), and maximal exercise had no effect on EDV in both groups (p = 0.129). There was no significant difference in rPI between the younger and older groups (Fig. 3C, p = 0.104), although rPI in both groups decreased at Post 60 compared with at Pre (p = 0.033). In the older group, rRI was significantly higher than in the younger group (Fig. 3D, p = 0.024). The rRI in both groups decreased at Post 60 compared with at Pre (p = 0.001).
Changes in peak systolic flow velocity (A), end-diastolic flow velocity (B), renal pulsatility index (C), renal resistive index (D) in Pre and Post 0, Post 60 in younger (■) and older (□) groups. Data are the mean ± standard deviation. * p < 0.05, ** p < 0.01 vs. Pre. Younger group: Pre, n = 8; Post 0, n = 8; Post 60, n = 8; older group: Pre, n = 8; Post 0, n = 8; Post 60, n = 8. Pre before exercise, Post 0 immediately after exercise, Post 60 60-min after exercise. Renal pulsatility index (peak systolic flow velocity – end-diastolic flow velocity) / blood flow velocity, Renal resistive index (peak systolic flow velocity – end-diastolic flow velocity) / peak systolic flow velocity. Open circles represent individual data. Multiple pairwise comparisons were corrected using the Bonferroni method
In both groups, maximal exercise increased the catecholamine concentration (Fig. 4A, p = 0.002; Fig. 4B, p < 0.001) and the FF (Fig. 4D, p < 0.001), and decreased the eGFR (Fig. 4C, p < 0.001).
Changes in adrenaline (A), noradrenaline (B), estimated GFR (C), filtration fraction (D) in Pre and Post 0 in younger (■) and older (□) groups. Data are the mean ± standard deviation. Younger group: Pre, n = 8; Post 0, n = 8; older group: Pre, n = 7; Post 0, n = 7. Pre before exercise, Post 0 immediately after exercise. GFR glomerular filtration rate. Open circles represent individual data. Multiple pairwise comparisons were corrected using the Bonferroni method
In the younger group, ∆RBF showed no associations with ∆BFV (p = 0.4107), ∆CSA (p = 0.4745), ∆PSV (p = 0.9702), ∆EDV (p = 0.329), ∆rPI (p = 0.542), or ∆rRI (p = 0.4651) (Fig. 5A-F). However, ∆RBF was positively associated with ∆BFV (p = 0.0298), ∆EDV (p = 0.0166), and negatively associated with ∆rPI (p = 0.0163) and ∆rRI (p = 0.0694), but it did not show an obvious association with ∆CSA (p = 0.7961) or ∆PSV (p = 0.0919) in the older group.
The associations between the change of RBF (∆RBF) from Pre to Post 60 and the changes of renal hemodynamic parameters (A: ∆BFV, B: ∆CSA, C: ∆PSV, D: ∆EDV, E: ∆rPI, F: ∆rRI) from Pre to Post 60 in younger (filled circle, n = 8) and older groups (open circle, n = 8). BFV blood flow velocity, CSA cross-sectional area, PSV peak systolic flow velocity, EDV end-diastolic flow velocity, rPI renal pulsatility index, rRI renal resistive index. Renal pulsatility index (peak systolic flow velocity – end-diastolic flow velocity) / blood flow velocity, Renal resistive index (peak systolic flow velocity – end-diastolic flow velocity) / peak systolic flow velocity
A condition-by-time interaction effect was observed regarding systolic blood pressure (SBP) (p = 0.0095). SBP at Post 0 increased significantly from Pre in both groups (p < 0.0001). SBP at Pre was lower in the younger group than in the older group (p = 0.0005); however, SBP at Post 0 did not differ between groups. There was a condition-by-time interaction effect regarding heart rate (HR) (p = 0.0052). HR at Pre did not differ between groups; however, HR at Post 0 was higher in the younger group than in the older group (p < 0.0001). HR at Post 0 increased significantly from Pre in both groups (p < 0.0001).
Discussion
This study was conducted to examine the differences in the renal hemodynamic response to exercise between younger and older adults using ultrasound echo. The most noteworthy finding of this study was that the decrease in RBF immediately after exercise was not different across groups, but that 60-min after exercise, RBF remained significantly decreased from baseline only in older adults, not in younger adults. High-intensity exercise cannot be recommended for older adults according to our finding. However, our study indicated moderate-intensity exercise causes no decrease in RBF [15] and physical activity associated with renal function decline [19], suggesting exercise can be recommended for older adults. However, the exercise-intensity should be considered.
The reduction in RBF with exercise is generally considered a physiological response [2]. To perform and sustain exercise, active skeletal muscles need to be supplied with oxygen, which increases cardiac output. However, as there is a limit to the increase in cardiac output, blood flow is increased to active skeletal muscles, which have a higher demand for oxygen, and is decreased to other inactive skeletal muscles and internal organs, especially the kidneys, rather than the blood flow being equally distributed to the organs and tissues. Therefore, the reduction in RBF with exercise is a transient physiological response that recovers sometime after exercise. However, few studies have focused on the recovery in RBF following exercise [12, 20]. One study [12] determined CPAH as an indicator of RBF, and examined the variations in CPAH during heavy exercise on a bicycle ergometer and during recovery. CPAH decreased significantly after heavy exercise and returned to near pre-exercise levels 30-min after exercise. Moreover, Suzuki and colleagues repeatedly measured RBF following rigorous cycling using radionuclide angiography with technetium-99 m phytate. Relative to the pre-exercise value, RBF decreased by 53.4% immediately after rigorous cycling, and remained 17.5% and 21.1% at 30- and 60-min into the recovery period, respectively [20]. These previous studies were conducted in healthy young males and reported that the reduction in RBF was restored to pre-exercise levels 60-min after exercise, which is consistent with our findings and suggests that RBF decreases immediately after exhaustive exercise but returns to the pre-exercise level 60-min after exercise in younger adults. Conversely, in this study, RBF in the older group did not return to the pre-exercise level 60-min after exercise, which was different to the younger group. Furthermore, previous studies have evaluated RBF following exercise using CPAH and technetium-99 m phytate, but the influence of exercise on renal hemodynamics has not yet been clarified. In this study, we evaluated not only RBF, but also other renal hemodynamic parameters using ultrasound echo, and determined the detailed renal hemodynamic response to exercise. Focusing on the changes in BFV and CSA, the older group consistently showed a lower BFV than the younger group. By contrast, the CSA showed similar changes and remained significantly lower at Post 60 than at Pre in both groups. However, CSA recovery was more delayed in the older group than the younger group (Fig. 2E). These observations imply that the differential response of CSA to exercise may influence renal hemodynamics in the recovery phase.
The constriction and dilation of resistance vessels in various parts of the body regulate blood flow during exercise. The degree of constriction and dilation of resistance vessels during exercise is determined by vasoactive substances, such as vasoconstrictors and vasodilators. Several factors influence the peripheral circulation during exercise, one of which is aging [10]. Previous investigation on changes in the responsiveness of vasoactive substance with aging demonstrated that the renal vasoconstrictor response to angiotensin II was not modified by age, although the vasodilator response to acetylcholine administration worsened with age [21]. Aging is also associated with a reduction in the endothelium-dependent vasodilatory response [22] mediated by endothelium-derived nitric oxide, which is a critical vasodilator that regulates vascular resistance and tissue perfusion [23]. Previous study [24] demonstrated that intravenous administration of an amino acid with a vasodilatory effect produced an increase in RBF, but the effect of intravenous amino acid administration on RBF disappeared in the impaired kidney [25]. Other study [26] examined the renal hemodynamic response to amino acid infusion in younger and older adults. Renal vascular resistance decreased after amino acid infusion in the younger adults, but the same was not consistently observed in the older adults, suggesting that the response to vasodilators is reduced in older adults. According to these observations, it is possible that the delayed recovery of RBF in the older group is caused by a diminished vasodilator response leading to a sustained regression of the CSA following exercise.
rRI [17] and rPI [18] are used as indicators of pulsatility and vascular compliance [27]. Detailed renal hemodynamic parameters were evaluated to examine other factors associated with changes in RBF following high-intensity exercise. In this study, there were no associations between ∆RBF and renal hemodynamic parameters in younger adults, whereas ∆RBF was positively associated with ∆BFV and ∆EDV, and negatively associated with ∆rPI and ∆rRI in older adults, suggesting that older adults with a lower ∆BFV or ∆EDV, or a higher ∆rPI or ∆rRI, demonstrate more delayed recovery in RBF. It has been indicated that the factors that reduce pulsatility exert almost all of their effects downstream of where rRI and rPI are usually measured [27]. Moreover, any changes in velocity in the larger arteries primarily reflect changes in the total arteriolar conductance. Therefore, an elevation in interstitial pressure leads to a decrease in arteriolar conductance and a reduction in the mean velocity of the large arteries. Specifically, the reduction in velocity is most marked at diastole. The EDV value depends on the diastolic arterial pressure and arteriolar conductance, and previous study showed that renal capillary wedge pressure (the combination of renal interstitial and venous pressure) and peripheral resistance contribute to EDV and rRI [28]. Moreover, Halimi et al. assessed the influence of PSV and EDV on rRI. They found that EDV (but not PSV) was associated with rRI [29]. Therefore, it is suggested that older adults with higher rRI (or rPI) via a slower BFV (especially EDV) following exercise have lower vascular compliance, resulting in delayed RBF recovery.
This study has several limitations that should be noted. Firstly, one of the main limitations of this study is the relatively small sample size, which should be considered when interpreting the results. Second, there was a lack of blood indicators in the recovery phase, so the mechanisms that control the renal hemodynamics in the recovery phase remain unclear. Third, we did not measure albuminuria or acute kidney injury (AKI) biomarker. Thus, future research should evaluate albuminuria and AKI biomarker. Finally, we included only younger and older adults with relatively well-maintained renal function. It is considered that the response to exercise could differ between individuals with normal renal function and those with reduced renal function. Therefore, similar research should be conducted in non-dialysis CKD patients in the future.
Conclusions
This study is the first to show differences in the renal hemodynamic response following high-intensity exercise. We utilized ultrasound echo to understand the influence of exercise on renal hemodynamics and provided novel evidence that RBF recovers 60-min after exercise in younger adults, whereas RBF recovery was delayed in older adults. The use of ultrasound echo allowed detailed information on the intrarenal hemodynamic response to exercise to be obtained, while minimizing the burden on the subjects. Therefore, non-invasive observation of renal hemodynamic changes following exercise using ultrasound echo may be clinically useful.
References
Poortmans JR, Mathieu N, de Plaen P. Influence of running different distances on renal glomerular and tubular impairment in humans. Eur J Appl Physiol Occup Physiol. 1996;72:522–7.
Grimby G. Renal clearances during prolonged supine exercise at different loads. J Appl Physiol. 1965;20:1294–8.
Ene-Iordache B, Perico N, Bikbov B, Carminati S, Remuzzi A, Perna A, et al. Chronic kidney disease and cardiovascular risk in six regions of the world (ISN-KDDC): a cross-sectional study. Lancet Glob Health. 2016;4:e307–19.
Juett LA, James LJ, Mears SA. Effects of exercise on acute kidney injury biomarkers and the potential influence of fluid intake. Ann Nutr Metab. 2020;76:53–9.
Sehl ME, Yates FE. Kinetics of human aging: I. Rates of senescence between ages 30 and 70 years in healthy people. J Gerontol A Biol Sci Med Sci. 2001;56:B198–208.
Rule AD, Amer H, Cornell LD, Taler SJ, Cosio FG, Kremers WK, et al. The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann Intern Med. 2010;152:561–7.
Poortmans JR, Ouchinsky M. Glomerular filtration rate and albumin excretion after maximal exercise in aging sedentary and active men. J Gerontol A Biol Sci Med Sci. 2006;61:1181–5.
Davies DF, Shock NW. Age changes in glomerular filtration rate, effective renal plasma flow, and tubular excretory capacity in adult males. J Clin Invest. 1950;29:496–507.
Berg UB. Differences in decline in GFR with age between males and females. Reference data on clearances of inulin and PAH in potential kidney donors. Nephrol Dial Transplant. 2006;21:2577–82.
Kenney WL, Zappe DH. Effect of age on renal blood flow during exercise. Aging Clin Exp Res. 1994;6:293–302.
Suzuki M, Ishiyama I, Seino T. Age-associated changes in renal function after exhaustive exercise in healthy males ranging in age from 8–80 years old. Adv Exerc Sport Physiol. 2006;12:65–72.
Castenfors J. Renal clearances and urinary sodium and potassium excretion during supine exercise in normal subjects. Acta Physiol Scand. 1967;70:207–14.
Kawakami S, Yasuno T, Matsuda T, Fujimi K, Ito A, Yoshimura S, et al. Association between exercise intensity and renal blood flow evaluated using ultrasound echo. Clin Exp Nephrol. 2018;22:1061–8.
Kotoku K, Yasuno T, Kawakami S, Fujimi K, Matsuda T, Nakashima S, et al. Effect of exercise intensity on renal blood flow in patients with chronic kidney disease stage 2. Clin Exp Nephrol. 2019;23:621–8.
Kawakami S, Yasuno T, Kawakami S, Ito A, Fujimi K, Matsuda T, et al. The moderate-intensity continuous exercise maintains renal blood flow and does not impair the renal function. Physiol Rep. 2022;10: e15420.
Liguori G, Feito Y, Fountaine C, Roy B. ACSM’s guidelines for exercise testing and prescription. 11th ed. Philadelphia: Lippincott Williams & Wilkins; 2021.
Arbeille PH, Roncin A, Berson M, Patat F, Pourcelot L. Exploration of the fetal cerebral blood flow by duplex doppler—linear array system in normal and pathological pregnancies. Ultrasound Med Biol. 1987;13:329–37.
Gosling RG, Dunbar G, King DH, Newman DL, Side CD, Woodcock JP, et al. The Quantitative analysis of occlusive peripheral arterial disease by a non-intrusive ultrasonic technique. Angiology. 1971;22:52–5.
Hiraki K, Otobe Y, Izawa KP, Sakurada T, Shibagaki Y. Physical activity in patients with pre-dialysis chronic kidney disease is associated with decreased renal function. Clin Exp Nephrol. 2021;25:683–4.
Suzuki M, Sudoh M, Matsubara S, Kawakami K, Shiota M, Ikawa S. Changes in renal blood flow measured by radionuclide angiography following exhausting exercise in humans. Eur J Appl Physiol Occup Physiol. 1996;74:1–7.
Hollenberg NK, Adams DF, Solomon HS, Rashid A, Abrams HL, Merrill JP. Senescence and the renal vasculature in normal man. Circ Res. 1974;34:309–16.
Ungvari Z, Tarantini S, Kiss T, Wren JD, Giles CB, Griffin CT, et al. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat Rev Cardiol. 2018;15:555–65.
Reckelhoff JF, Kellum JA, Blanchard EJ, Bacon EE, Wesley AJ, Kruckeberg WC. Changes in nitric oxide precursor, L-arginine, and metabolites, nitrate and nitrite, with aging. Life Sci. 1994;55:1895–902.
Graf H, Stummvoll H, Luger A, Prager R. Effect of amino acid infusion on glomerular filtration rate. N Engl J Med. 1983;308:159–60.
ter Wee PM, Rosman JB, van der Geest S, Sluiter WJ, Donker AJM. Renal hemodynamics during separate and combined infusion of amino acids and dopamine. Kidney Int. 1986;29:870–4.
Fliser D, Zeier M, Nowack R, Ritz E. Renal functional reserve in healthy elderly subjects. J Am Soc Nephrol. 1993;3:1371–7.
Bude RO, Rubin JM. Relationship between the resistive index and vascular compliance and resistance. Radiology. 1999;211:411–7.
Di Nicolò P, Granata A. Renal resistive index: not only kidney. Clin Exp Nephrol. 2017;21:359–66.
Halimi J-M, Vernier L-M, Gueguen J, Goin N, Gatault P, Sautenet B, et al. End-diastolic velocity mediates the relationship between renal resistive index and the risk of death. J Hypertens. 2023;41:27–34.
Acknowledgements
The authors acknowledge the contribution of the staff at Fukuoka University, who helped with the recruitment of participants. The authors thank the volunteers who participated in this study.
Funding
This work was supported by Japan Society for the Promotion of Science (JSPS) (Grant numbers: 22K11489 to S. K., and 15H03082 to Y.H.) and Fukuoka University Institute for Physical Activity, Fukuoka, Japan.
Author information
Authors and Affiliations
Contributions
SK, TY, KK, SK, and AI performed experiments; SK analyzed data; SK, TY, KK, YH, and RM. interpreted results of experiments; SK prepared figures; SK Writing – original draft; SK, TY, KK, SK, AI, KF, TM, SN, KM, YU, YH, and RM Writing – review and editing; SK, TY, KK, SK, AI, KF, TM, SN, KM, YU, YH, and RM approved final version of manuscript and SK, TY, YH, and RM conceived and designed research.
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest, financial or otherwise, are declared by the authors.
Ethical approval
The study protocol in this study complied with the Declaration of Helsinki and the principles of Good Clinical Practice and was approved by the Ethics Committee of Fukuoka University (21-07-M1, 16-4-01).
Informed consent
All potential risks and procedures were explained to the participants, who provided written informed consent in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Kawakami, S., Yasuno, T., Kotoku, K. et al. The differences in renal hemodynamic response following high-intensity exercise between younger and older males. Clin Exp Nephrol 27, 972–980 (2023). https://doi.org/10.1007/s10157-023-02381-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-023-02381-7