Abstract
Water deficit is one of the most limiting factors for plant growth and production. Polyamines are osmo-active compounds and have important roles in plant resistance to water limitation. A pot experiment was undertaken in a greenhouse as factorial based on complete randomized block design with three replications to assess the physiological and biochemical responses of safflower to different levels of water supply (100% and 40% field capacity) and spermine (0, 40 and 60 µM). Ascorbate peroxidase and peroxidase activities (POX), malondialdehyde (MDA), hydrogen peroxide (H2O2), anthocyanins, soluble protein, soluble sugars and proline contents in shoots increased, while total phenols, flavonoids, and photosynthetic pigments significantly decreased due to water deficit. Foliar spray of spermine mitigated the adverse effects of water deficit by increasing the catalase, superoxide dismutase, POX activities, soluble proteins and photosynthetic pigments, and by decreasing MDA and H2O2 contents. Spermine could, therefore, play an important role in protecting photosynthetic system and cellular membranes during drought stress in safflower.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Drought is an important abiotic stress that limits plant growth in arid and semiarid regions of the world (Li et al. 2013). Plant growth and development under limited water supply could be disrupted by osmotic and oxidative stresses, ion deficit and metabolic disorders (Li et al. 2013). Among other effects, drought may trigger an increased formation of reactive oxygen species (ROS) which can cause lipid peroxidation and membrane damage. ROS such as superoxide radical, hydroxyl radical and hydrogen peroxide are harmful for biological systems, because they oxidize macromolecules such as lipids and proteins and reduce photosynthetic pigments and photosynthesis (Kavas et al. 2013; Ghassemi-Golezani and Nikpour-Rashidabad 2017). Plants could remove ROS by enzymatic and non-enzymatic antioxidants (Mustafavi et al. 2016). Antioxidant enzymes including catalase (CAT), ascorbate peroxidase (APX), peroxidase (POX) and superoxide dismutase (SOD) are very effective in scavenging ROS. SOD produces H2O2 and CAT, APX and POX decompose H2O2 and convert it to O2 and H2O. Phenols and flavonoids are also important scavengers of most types of oxidizing molecules, including singlet oxygen and various free radicals; thereby improving plant resistance to different types of stress (Saeed et al. 2012). Anthocyanin also acts as an antioxidant, counteracting with free radicals and H2O2 (Zamani et al. 2013).
Peroxidation of fatty acids creates by oxidative degradation of lipids. This peroxidation leads to reactive aldehydes production such as malondialdehyde (MDA). MDA content indicates cell membrane injuries and antioxidant potential of plants under stress. Enhancing H2O2 content and subsequently lipid peroxidation under stress diminish membrane stability. Damages of cell membranes under water deficit are also measured by electrolytes leakage (Saruhan et al. 2012).
Soluble sugars and proline, proteins, glycine betaine is compatible osmolytes, which enhance under water deficit and maintain turgor and stabilize cell molecular structure (Shi et al. 2013; Farhangi-Abriz and Ghassemi-Golezani 2018). The accumulation of sugars in cytoplasm protects the membrane proteins and enhances dehydration tolerance (Guo et al. 2018). Compatible solutes protect plants from stress by cellular osmotic adjustment, ROS detoxification, maintenance of membrane integrity and enzymes/protein stabilization. High synthesis of proline from hydrolysis of proteins reduces the effect of water deficit through organic solute accumulation. Proline is a source of energy, carbon, and nitrogen for the recovering tissues. This amino acid also acts as a metal chelator, protects enzymes, activates citric acid (Krebs) cycle, participates in proteins structure, prevents electrolyte leakage (Singh et al. 1973) and regulates osmotic potential (Cvikrova et al. 2013).
Photosynthetic pigments could also be affected by drought stress. Chlorophyll is the main pigment of photosynthesis in plants. Chlorophyll content of leaves reduces as a result of water limitation mainly due to the damage to chloroplasts caused by reactive oxygen species (Farooq et al. 2009). Carotenoids operate as precursors to signaling molecules that influence abiotic stress tolerance (Havaux 2013). These pigments represent a group of lipophilic antioxidants and are able to detoxify various forms of ROS (Bartwal et al. 2013). As an antioxidant, they scavenge 1O2 to protect the photosynthetic system.
Polyamines (PAs) such as spermine (Spm) are small organic polycations found in all living creatures and have important roles in cell cycles, gene expression, signaling, growth and development of plants, and plant resistance to abiotic stresses (Ndayiragije and Lutts 2005). Several studies have shown that PAs such as spermine are involved in the acquisition of drought tolerance in plants (Saruhan et al. 2012). Exogenous PAs improve drought tolerance by increasing antioxidant enzymes activities and photosynthetic efficiency, but this effect strongly depends on PA concentration or type, and stress level (Shi et al. 2013; Shu et al. 2013; Shi and Chan 2014). PAs can also act as free radical scavengers to protect membranes from oxidative damages (Yamaguchi et al. 2007; Parvaiz et al. 2012; Shi and Chan 2014). Spermine plays an important role in drought tolerance of plants (Yamaguchi et al. 2007; Hussain et al. 2011). Comparing the effects of glycinebetaine, salicylic acid, nitrous oxide, brassinosteroid and spermine on rice plants under limited irrigation, foliar spray of spermine was the most effective in stress tolerance of these plants (Farooq et al. 2009).
Safflower (Carthamus tinctorius L.) is produced as oilseed, medicinal and industrial crop. This crop can somewhat tolerate environmental stresses such as salinity and drought (Lovelli et al. 2007). However, the productivity of safflower could be limited under severe drought and salt stresses. Since changes in water-stressed safflower plants in response to exogenous spermine is not clear, this research was laid out to investigate the effects of exogenous spermine on antioxidants, osmolytes, photosynthetic pigments and secondary metabolites of this crop (C. tinctorius L.) under different levels of water supply.
Materials and methods
Experimental conditions
Two preliminary experiments were conducted to select appropriate levels of watering and spermine spray. The final experiment was arranged as factorial based on randomized complete block design with three replications in a glass greenhouse at the University of Tabriz (Iran). Safflower seeds (cv. Goldasht) were obtained from East Azerbaijan Agricultural and Natural Resources Research Center, Tabriz, Iran. These seeds were disinfected by 5% (v/v) sodium-hypochlorite solution for 5 min and then were sufficiently washed with distilled water. In the first experiment, treatments were irrigations up to 100, 80, 60, 40, 20% field capacity after three leaves stage for 14 days. Based on some growth and physiological parameters (data are not shown), 100% and 40% FC were selected as normal watering and drought stress treatments, respectively. In another experiment, plant performance was tested in response to foliar spray of 0, 20, 40, 60, 80, 100 µM spermine, and 0, 40 and 60 µM were selected as appropriate levels of spermine.
After these preliminary experiments, seeds were sown in plastic pots (15 × 15 cm) containing perlite. The pots were irrigated up to 100% FC and then placed in a greenhouse with 25–30 °C, 60% humidity and 16/8 h light/dark conditions. The pots were weighted regularly and water loss was made up by distilled water for the first 7 days, 50% Hogland solution from day 7 to day 14, and thereafter with 100% Hogland solution until three leaves stage of plants. At this stage, the irrigation treatments (100% and 40% FC) were applied, and 2 weeks later spermine (0, 40 and 60 µM) was sprayed on plants. All plants were harvested at 60 days after sowing and physiological and biochemical parameters were measured.
Antioxidant enzymes
0.1 g fresh root and 0.1 g leaf samples were homogenized in ice-cold phosphate-buffered solution (PBS, 50 mM, pH 7), using mortar and pestle. Homogenates were centrifuged at 10,000g for 10 min at 4 °C. The supernatants were used immediately for assessment of the total soluble protein content according to a method introduced by Bradford (1976). Then, the activities of superoxide dismutase (SOD), peroxidase (POX), catalase (CAT) and ascorbate peroxidase (APX) were estimated by the following methods:
SOD activity (SOD, EC 1.15.1.1) was assayed via nitro-blue-tetrazolium (NBT) photoreduction inhibition by extracts (Winterbourn et al. 1976). Reaction mixture (3 mL) containing 2.7 mL sodium phosphate solution (1 M, pH 7.8), 100 µL NBT (1.5 mM), 100 µL ethylene diamine tetraacetic acid (EDTA) (1 M) NaCN (0.3 mM), 50 µL of riboflavin and 50 µL enzyme extract. The mixtures were illuminated at light intensity of 5000 Lux for 12 min and the absorbance of the solutions was recorded at 560 nm by a spectrophotometer (Dynamica, Halo-db-20 series, Switzerland). The amount of the enzyme causing 50% protection of NBT photoreduction was considered as one unit, and SOD activity was expressed as U mg−1 protein.
POX activity (POX, EC 1.11.1.7) was determined by recording the increase of absorbance at 470 nm during polymerization of guaicol for 3 min (Obinger et al. 1997). The reaction was initiated by adding H2O2 to the reaction mixture and POD specific activity was calculated, using the extinction coefficient of 26.6 mM−1 cm−1 for guaiacol. One unit of POX activity was considered as the enzyme amount capable of oxidizing 1 µM guaiacol to tetraguaiacol per minute. CAT activity (CAT, EC 1.11.1.6) was estimated by the method of Chance and Mealy (1955). APX activity (APX, EC 1.11.1.1) was measured by following the decrease in absorbance at 290 nm (Boominathan and Doran 2002). The reaction was initiated by adding H2O2 and APX specific activity was calculated using the extinction coefficient of 2.8 mM−1 cm−1 for ascorbic acid and one unit of enzyme activity was considered as the amount of enzyme necessary for the reduction of 1 µM ascorbic acid per minute.
Lipid peroxidation (MDA) and H2O2
The level of membrane lipid peroxidation was determined by measuring malondialdehyde (MDA) (Boominathan and Doran 2002). Plant samples (root and leaf) were homogenized using 3 mL of 0.1 trichlroacetic acid, and the crude extracts were mixed with the same volume of a 0.5% (w/v) tribarbitoric acid solution containing 20% (w/v) trichlroacetic acid. After heating for 30 min and rapid cooling, the absorbance of supernatant was measured at 530 nm. Malondialdehyde concentration was calculated in accordance with the previously prepared standard curve (using 1,1,3,3-tetraethoxy propane) and expressed as µg g−1 dry weight (DW).
The roots and leaves were harvested, immediately frozen in liquid nitrogen, ground and the powder stored at 80 °C. 100 mg of the powder was homogenized with 5 mL of the solution containing 0.25 mL trichloroacetic acid (TCA) (0.1% (w/v)), 1 mL KI (1 M) and 0.5 mL potassium phosphate buffer (10 mM) at 4 °C for 10 min. The homogenate was centrifuged at 12,000g for 15 min at 4 °C. 500 µL of supernatant from each tube were kept at room temperature (about 25 °C) for 15 min. A calibration curve of H2O2 standard solutions was prepared in 0.1% TCA. H2O2 content of the supernatant was estimated by comparing its absorbance at 390 nm with the standard calibration curve (Harinasut et al. 2003).
Total flavonoid, phenol and anthocyanin contents
Measurement of total flavonoids of root and leaf was performed by adding 1.5 mL of 80% methanol, 100 µL of 10% aluminum chloride solution, 100 µL of potassium acetate 1 M, and 2.8 mL of distilled water to 500 µL of each extract. After 40 min, absorbance of mixture was measured at 415 nm. Quercetin was used to construct the calibration curve. The total flavonoid content of the extract was reported in mg of quercetin per gram dry weight (Chang et al. 2002).
To measure the total anthocyanin content in root and leaf, 0.02 g of dry plant tissue was ground with 4 mL of 1% methanol solution of hydrochloric acid in a porcelain mortar. The resulting solution was placed in the refrigerator for 24 h. After centrifuging, the supernatant was removed and absorbance of the solutions was measured at 530 nm and 657 nm. Anthocyanin content of each extract was calculated (Mita et al. 1997) as:
where A is absorbance at appropriate wavelengths.
Total phenol measurement in root and leaf was performed by adding 2.8 mL of distilled water, 2 mL sodium carbonate 2%, 100 µL of Folin–ciocalteus phenol reagent 50% to 100 µL of each extract. After 30 min, absorbance of mixture was read at 720 nm. Galic acid (GA) was used to construct the standard curve. The total phenol content of the extract was calculated as mg galic acid per g dry weight (Meda et al. 2005).
Soluble and insoluble sugars
Soluble and insoluble sugars were assayed by phenol–sulfuric acid method (Kochert 1978). 5 mL of ethanol (70%) was added to 0.05 g of dry sample and it was kept in a refrigerator for a week. The samples were centrifuged at 10,000g for 15 min. Then, 0.5 mL of the plant extract was made up to 2 mL by adding distilled water, and then 1 mL of phenol (5%) and 5 mL concentrated sulfuric acid were added. The mixture was vortexed and kept for 30 min at room temperature. The sediment was used for determination of insoluble sugars. The absorbance of solution was recorded at 485 nm, and glucose was used to draw a standard curve. The data were expressed as mg g−1 DW.
Soluble protein and free amino acids
The supernatant of extract provided for the measurement of antioxidant enzymes activities in root and leaf was used for determination of the total soluble protein content as described by Bradford (1976). Free amino acids contents were measured by mixing 500 µL ninhydrin reagent and 100 µL of the same extract and placing it in boiling water bath for 4–7 min. After cooling, the absorbance of each sample was recorded at 570 nm and free amino acids contents were calculated using a standard curve prepared by glycine and reported as µg g−1 DW (Hwang and Ederer 1975).
Proline content
Proline was measured according to Bates et al. (1973), using 0.1 g of root or 0.1 g leaf and 2 mL of extraction medium 3% (m/v) sulfu-salicylic acid. Then samples were centrifuged at 2000g for 10 min. Proline was quantified by a spectrophotometer at 520 nm. The proline content was calculated, using a calibration curve, and expressed as µ mole proline g−1 DW.
Photosynthetic pigments
Chlorophylls a, b and total carotenoids of fresh leaves were measured according to Sukran et al. (1998). 0.1 g of fresh leaf was homogenized with 5 mL of 100% acetone and then it was placed in a refrigerator for 24 h. Thereafter, the absorbance of the extract was recorded at 645, 660 and 470 nm. The pigments contents were calculated as mg g−1 DW.
Statistical analyses
Analysis of variance (ANOVA) of the physiological and biochemical data were carried out by SAS (9.2) software. Means were compared by Duncan’s multiple range test at p ≤ 0.05. Figures were drawn by the Excel-2013 software.
Results
Antioxidant enzymes
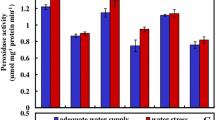
Water limitation and spermine showed a significant interaction for antioxidant enzymes activities of safflower roots including APX [F (5, 12) = 43.88, p ≤ 0.01], CAT [F (5, 12) = 25.58, p ≤ 0.01], SOD [F (5, 12) = 63.13, p ≤ 0.01], POX [F (5, 12) = 628.75, p ≤ 0.01] and leaves including APX [F (5, 12) = 91.06, p ≤ 0.01], CAT [F (5, 12) = 267.69, p ≤ 0.01], SOD [F (5, 12) = 220.93, p ≤ 0.01], POX [F (5, 12) = 191.43, p ≤ 0.01]. The APX, CAT, SOD, POX activities under drought stress and APX and POX activities under well-watering in roots were significantly decreased as a result of spermine application. In contrast, 60 µM spermine caused a significant increase in CAT and SOD activities in roots with normal water supply. Spermine application (60 µM) significantly reduced POX activity, but enhanced the activities of other enzymes under non-stress condition. However, this treatment was led to a significant decrease in APX and CAT activities and a significant increase in SOD and POX activities of leaves under stressful condition (Fig. 1).
Means ± standard deviations of a ascorbate peroxidase (APX), b catalase (CAT), c superoxide dismutase (SOD), d peroxidase (POX) of roots and leaves of safflower plants in response to water limitation and spermine application. Different letters indicate significant difference at p ≤ 0.05. I1, I2: 100% and 40% field capacity (FC), respectively
MDA and H2O2 contents
The interaction of water deficit × spermine was also significant for root MDA [F (5, 12) = 350.89, p ≤ 0.01] and H2O2 [F (5, 12) = 4287.68, p ≤ 0.01] and shoot MDA [F (5, 12) = 842.10, p ≤ 0.01], and H2O2 [F (5, 12) = 2196.73, p ≤ 0.01] contents. Application of spermine in stressed and non-stressed plants reduced MDA content of roots and shoots. The lowest amount of MDA in roots and shoots was recorded for plants treated with 60 µM spermine under water deficit. This treatment was led to H2O2 increment in roots, and its decrement in shoots under drought stress. In normal watering, spermine decreased H2O2 content in roots and enhanced it in shoots (Table 1).
Total anthocyanin, phenol and flavonoid contents
The interaction of drought stress × spermine was significant for non-enzymatic antioxidants of roots including anthocyanin [F (5, 12) = 227.47, p ≤ 0.01], phenol [F (5, 12) = 60.88, p ≤ 0.01], flavonoid [F (5, 12) = 78.48, p ≤ 0.01], and shoots including anthocyanin [F (5, 12) = 12.38, p ≤ 0.01], phenol [F (5, 12) = 102.25, p ≤ 0.01], and flavonoid [F (5, 12) = 120.38, p ≤ 0.01]. Both rates of spermine increased total anthocyanin, phenol and flavonoid contents in roots under well-watering. The 60 µM spermine also enhanced total phenol and flavonoid contents in roots under limited watering. Spray of 60 µM spermine significantly increased total anthocyanin content in shoots of stressed plants and total flavonoid in non-stressed plants (Table 1).
Soluble and insoluble sugars
The interaction of water deficit × spermine treatment was also significant for root soluble sugars [F (5, 12) = 345.59, p ≤ 0.01], soluble protein [F (5, 12) = 18,934.2, p ≤ 0.01] and proline [F (5, 12) = 614.39, p ≤ 0.01] and shoot soluble sugars [F (5, 12) = 10.51, p ≤ 0.01], soluble protein [F (5, 12) = 24,287.8, p ≤ 0.01] and proline [F (5, 12) = 89.78, p ≤ 0.01] contents. Exogenous application of 40 µM spermine in stressed and non-stressed plants improved soluble sugars of roots. Foliar spray of spermine significantly reduced soluble sugars content in shoots of stressed and non-stressed plants (Table 2). Water deficit and spermine treatment diminished insoluble sugars of shoots (Fig. 2).
Protein and free amino acids
Exogenous spermine increased protein content of roots and shoots under water deficit. However, this treatment reduced total free amino acids in shoots of stressed plants. Application of spermine in non-stressed plants significantly reduced protein content in roots and shoots (Table 2).
Proline
In roots, 40 µM spermine enhanced proline content under water deficit. In non-stressed plants, spermine improved proline in roots and reduced it in shoots. Free proline content of shoots under stress was decreased by this treatment (Table 2).
Photosynthetic pigments content
Water deficit and spermine showed a significant interaction on photosynthetic pigments including Chlorophyll a [F (5, 12) = 955.65, p ≤ 0.01], Chlorophyll b [F (5, 12) = 71.60, p ≤ 0.01], and carotenoids [F (5, 12) = 129.59, p ≤ 0.01]. Spermine significantly increased chl a, b and carotenoids contents under water deficit. Chlorophyll a, b and carotenoids were significantly higher in plants treated with 40 µM spermine. In non-stressed plants, photosynthetic pigments were significantly decreased due to spermine treatment (Fig. 3).
Discussion
Water deficit induces oxidative stress by ROS generation. Plants subjected to drought stress display various defense mechanisms such as stimulation of the antioxidant enzymes system (Kavas et al. 2013). Increasing enzymes activities in roots and leaves under drought stress by ROS scavenging is a defense process that causes plants alleviate some of the harmful effects of drought stress (Salem et al. 2014). Increasing H2O2 of roots under drought stress as a result of spermine application was related with reduction in antioxidant enzymes activities. Enhancing POX and SOD activities under water limitation with 60 µM spermine and increasing CAT activity with 40 µM spermine in leaves caused a reduction in H2O2 content (Table 1; Fig. 1). This could be attributed to free radical-scavenging and membrane protective properties of spermine. Similar results were reported for Cucumis sativus with spermidine under salinity (Duan et al. 2008), dill plants with salicylic acid under salinity (Ghassemi-Golezani and Nikpour-Rashidabad 2017) and cotton plants with putrescine under water deficit (Hanafy Ahmed et al. 2017).
ROS generated during water limitation are highly reactive and alter normal cellular metabolism through oxidative damage to membranes. The extent of oxidative injuries was estimated by the concentration of malondialdehyde. Increasing MDA content of leaves under drought stress was a direct result of lipid peroxidation and membrane damage. Polyamines stimulate the antioxidative mechanisms of defense and reduce the membrane injuries under drought stress (Saruhan et al. 2012). Spermine application diminished these damages in leaves by enhancing the antioxidant enzymes activities and reducing lipid peroxidation in membranes. Improving H2O2 contents in leaves of non-stressed plants by exogenous spermine may be resulted from the catabolism of spermine that produces H2O2.
The concentrations of non-enzymatic antioxidants such as anthocyanins, phenol and flavonoids are influenced by environmental conditions. It was reported that drought stress enhances the amounts of these secondary metabolites in some plants (Ghassemi-Golezani et al. 2013). Increasing anthocyanins in shoots and phenol and flavonoid contents of safflower roots under water deficit (Table 1) suggests that non-enzymatic mechanisms also play the role to overcome the oxidative stress under drought. The anthocyanins compounds of salt stressed red cabbage plants (Hegazi and El-Shraiy 2017) were also enhanced by low water availability. Anthocyanins were considered as an antioxidant pigment and alleviate oxidative stress. Polyamines improved drought tolerance of plants by stimulating the production of phenolic compounds (Hussain et al. 2011). Enhancing phenol and flavonoid contents in roots and anthocyanins content in leaves of drought stressed-plants by spermine spray improves plant resistance to drought by ROS detoxification (Shi et al. 2013). MDA reduction in roots of stressed-plants and in leaves of non-stressed-plants by foliar application of spermine may also be related with enhancing phenols, flavonoids (Table 1) and antioxidant enzymes activities (Fig. 1), respectively. Flavonoid and anthocyanins as antioxidants induce ROS scavenging and inhibit lipid peroxidation (Shi et al. 2013).
Plants can partly protect themselves against water limitation by accumulating of compatible solutes such as soluble protein, solute sugars and proline (Movahhedi Dehnavi et al. 2010). Increasing soluble protein in roots and shoots under water deficit (Table 2) could be associated with accumulation of proteins such as osmotin and dehydrins under low water potential (Gomathi et al. 2013). Accumulation of proteins as nitrogen resources have likely role in osmotic regulation and might be due to de novo synthesis in response to the stress (Razavizadeh et al. 2017).
Rising soluble sugars in shoots (Table 2) under water limitation is related with the increment of invertase activity and decrement of carbohydrates requirement of plants due to growth reduction. Increasing soluble sugars of roots by 40 µM spermine could improve drought tolerance of safflower plants via osmotic adjustment. Decreasing soluble sugars of shoots due to spermine application might be attributed to better water status of polyamine treated plants (Amri and Shahsavar 2010).
Proline is the most common osmolytes that increases in drought stressed-plants and maintains leaf cell turgor (Paul and Roychoudhury 2016). Enhancing shoot proline content under drought stress (Table 2) could be a mechanism for maintaining cell water potential (Movahhedi Dehnavi et al. 2010). Increment of proline could be the result of increasing pyrroline-5-carboxylate synthase (P5C5) activity (Ghassemi-Golezani and Nikpour-Rashidabad 2017). Increment of free proline in roots by 40 µM spermine under water deficit and 40 and 60 µM spermine in non-stressed plants can improve water uptake due to enhancing cell osmolytes. Proline was diminished by application of spermine in safflower shoots due to increasing chlorophyll synthesis, since proline and chlorophyll were synthesized from the same precursor (glutamate). Spermine can act as antioxidant and osmoticum (two main functions of proline) to mitigate some of the reverse effects of stressful conditions (Hanafy Ahmed et al. 2017; Farhangi-Abriz and Ghassemi-Golezani 2018).
Significant reduction of photosynthetic pigments under water deficit (Fig. 3) is mainly the result of damage to chloroplasts caused by reactive oxygen species, and chlorophyll degeneration by chlorophyllase (Paul and Roychoudhury 2016). Deduction in chlorophyll content is identified as a drought response mechanism to minimize the light absorption by chloroplasts (Pastenes et al. 2005). Photosynthetic pigments were increased in stressed-plants by spermine application in favor of decreasing proline content due to similar precursor. Exogenous polyamine improved antioxidant potential of safflower to maintain photosynthetic system (Saha et al. 2015; Mustafavi et al. 2016). In non-stressed plants, enhancing H2O2 (Table 1) reduced leaves chlorophyll and carotenoids contents (Fig. 3) as a result of oxidative stress.
Enhancing root and shoot protein contents by spermine treatment in stressed-plants (Table 2) may be related with prevention of protein degradation by this growth regulator. Polyamines act as a protective for the plasma membrane against stress damage by inhibiting protease activity (Bais and Ravishankar 2002). In addition, they protect plants from stress via stabilizing protein structure and preventing degradation of proteins (Verma and Mishra 2005). PAs covalent binding with proteins by transglutaminase (TGase) helps to stabilize cell structure and function (Campos et al. 2013). Increasing stress tolerance by polyamine was attributed to the increased levels of protein biosynthesis, antioxidant defense reaction, and energy metabolism (Li et al. 2013).
Conclusion for future biology
The beneficial impacts of spermine on safflower plants suggest that it would be worthwhile to examine the capability of this growth regulator and other polyamines in alleviating the harmful effects of environmental stresses on different plant species.
References
Amri E, Shahsavar AR (2010) Response of lime seedling (Citrus aurantifolia L.) to exogenous spermidine treatments under drought stress. Aust J Basic Appl Sci 4:4483–4489
Bais HP, Ravishankar GA (2002) Role of polyamines in the ontogeny of plants and their biotechnological applications. Plant Cell Tissue Organ Cult 69:1–34
Bartwal A, Mall R, Lohani P, Guru SK, Arora S (2013) Role of secondary metabolites and brassinosteroids in plant defense against environmental stresses. J Plant Growth Regul 32:216–232
Bates L, Waldren SP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Boominathan R, Doran PM (2002) Ni- Induced oxidative stress in roots of the Ni hyper accumulator, Alyssum bertolonii. New Phytol 156:205–215
Bradford M (1976) A rapid and sensitive method for the quantitation of quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Campos N, Castañón S, Urreta I, Santos M, Torné JM (2013) Rice transglutaminase gene: identification, protein expression, functionality, light dependence and specific cell location. Plant Sci 205:97–110
Chance B, Mealy AC (1955) Assay of catalases and peroxidases. Methods Enzymol 11:755–764
Chang C, Yang M, Wen H, Chern J (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 10:178–182
Cvikrova M, Gemperlova L, Martincova O, Vankova R (2013) Effect of drought and combined drought and heat stress on polyamine metabolism in proline—over—producing tobacco plants. Plant Physiol Biochem 73:7–15
Duan J, Li J, Guo Sh, Kang Y (2008) Exogenous spermidine effects polyamine metabolism in salinity stressed Cucumis sativus roots and enhances short-terms salinity tolerance. J Plant Physiol 165:1620–1635
Farhangi-Abriz S, Ghassemi-Golezani K (2018) How can salicylic acid and jasmonic acid mitigate salt toxicity in soybean plants? Ecotoxicol Environ Saf 147:1010–1016
Farooq M, Wahid A, Lee DJ (2009) Exogenously applied polyamines increase drought tolerance of rice by improving leaf water status, photosynthesis and membrane properties. Acta Physiol Plant 31:937–945
Ghassemi-Golezani K, Nikpour-Rashidabad N (2017) Seed pretreatment and salt tolerance of dill: osmolyte accumulation, antioxidant enzyme activities and essence production. Biocatal Agric Biotechnol 12:30–35
Ghassemi-Golezani K, Dalil B, Dastborhan S (2013) Water stress in plants, 1st edn. Jahad Daneshgahi Press, Tabriz, pp 1–80
Guo R, Shi L, Jiao Y, Li MX, Zhong X, Gu FX, Liu Q, Xia X, Li HR (2018) Metabolic responses to drought stress in the tissues of drought-tolerant and drought-sensitive wheat genotype seedlings. AoB Plants 6:1–13
Hanafy Ahmed AH, Darvish E, Alobaidy MG (2017) Impact of putrescine and 24-epibrassinolide on growth, yield and chemical constituents of cotton (Gossypium barbadense L.) plant grown under drought stress conditions. Asian J Plant Sci 16:9–23
Harinasut P, Poonsopa D, Roengmongkol K, Charoensataporn R (2003) Salinity effects on antioxidant enzymes in mulberry cultivar. Sci Asia 29:109–113
Havaux M (2013) Carotenoid oxidation products as stress signals in plants. Plant J 79:597–606
Hegazi AM, El-Shraiy AM (2017) Stimulation of photosynthetic pigments, anthocyanin, and antioxidant enzymes in salt stressed red cabbage plants by ascorbic acid and potassium silicate. Middle East J Agric Res 6:553–568
Hussain SS, Ali M, Ahmad M, Siddique KHM (2011) Polyamines: natural and engineered abiotic and biotic stress tolerance in plants. Biotechnol Adv 29:300–311
Hwang M, Ederer GM (1975) Rapid hippurate hydrolysis method for presumptive identification of group B streptococci. J Clin Microbiol 1:14–115
Kavas M, Cengiz Baloglu M, Akca O, Kose FS, Gokcay D (2013) Effect of drought stress on oxidative damage and antioxidant enzyme activity in melon seedling. Turk J Biol 37:491–498
Kochert G (1978) Carbohydrate determination by the phenol sulfuric acid method. In: Helebust JA, Craig JS (eds) Handbook physiological methods. Cambridge University Press, Cambridge, p 9697
Li Z, Peng Y, Ma X (2013) Different response on drought tolerance and post-drought recovery between the small-leafed and the large-leafed white clover (Trifolium repens L.) associated with antioxidative enzyme protection and lignin metabolism. Acta Physiol Plant 35:213–222
Lovelli S, Perniola M, Ferrara A, Di Tommaso T (2007) Yield response factor to water and water use efficiency of Carthamus tinctorius L. and Solanum melongena L. Agric Water Manag 92:73–80
Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG (2005) Determination of the total phenolic, flavonoid and proline contents in BurkinaFasan honey, as well as their radical scavenging activity. Food Chem 91:571–577
Mita S, Murano N, Akaike M, Nakamura K (1997) Mutants of Arabidopsis thaliana with pleiotropic effects on the expression of the gen for beta-amylase and on the accumulation of anthocyanin that is inducible by sugars. Plant J 11:841–851
Movahhedi Dehnavi M, Ranjbar M, Yadavi A, Kavoosi B (2010) Effect of cycocel on proline, soluble carbohydrate, protein, oil content and fatty acids of Linum usitatissimum under drought stress in greenhouse condition. Environ Stress Crop Sci 3:129–138
Mustafavi SH, Shekari F, Hatam Maleki H (2016) Influence of exogenous polyamines on antioxidant defence and essential oil production in valerian (Valeriana officinalis L.) plants under drought stress. Acta Agric Slov 107:81–91
Ndayiragije A, Lutts S (2005) Do exogenous polyamines have an impact on the response of a salt sensitive rice cultivar to NaCl. J Plant Physiol 163:506–516
Obinger C, Maj M, Nicholls P, Loewen P (1997) Activity, peroxide compound formation, and heme d synthesis in Escherichia coli HPII catalase. Arch Biochem Biophys 342:58–67
Parvaiz A, Kumar A, Gupta A, Sharma S (2012) Polyamines, role in plants under abiotic stress. Crop Prod Agric Improv 19:492–512
Pastenes C, Pimentel P, Lillo J (2005) Leaf movements and photo inhibition in relation to water stress in field-grown beans. J Exp Bot 56:425–433
Paul S, Roychoudhury A (2016) Seed priming with spermine ameliorates salinity stress in germinated seedlings of two rice cultivars differing in their level of salt tolerance. Trop Plant Res 3:616–633
Razavizadeh R, Adabavazeh F, Rostami F, Teimouri A (2017) Comparative study of osmotic stress effects on the defense mechanisms and secondary metabolites in Carum copticum seedling and callus. J Plant Proc Funct 18:23–33
Saeed N, Khan MR, Shabbir M (2012) Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement Altern Med 12:1–12
Saha J, Brauer EK, Sengupta A, Popescu SC, Gupt K, Gupta B (2015) Polyamines as redox homeostasis regulators during salt stress in plants. Front Environ Sci 3:1–13
Salem N, Msaada K, Elkahoui S, Aaeiz S, Slimen IB, Kefi S, Pintore G, Limam F, Marzouk B (2014) Evaluation of antibacterial, antifungal, and antioxidant activities of safflower natural dyes during flowering. Biomed Res Int 2014:1–10
Saruhan N, Turgut Terzi R, Kadioglu A (2012) The effects of exogenous polyamines on some biochemical changes during drought stress in Ctenanthe setosa (Rosc.) Eichler. Acta Biol Hung 57:221–229
Shi H, Chan Z (2014) Improvement of plant abiotic stress tolerance through modulation of the polyamine pathway. J Integr Plant Biol 56:114–121
Shi J, Fu XZ, Peng T, Huang XS, Fan QJ, Liu JH (2013) Spermine pretreatment confers dehydration tolerance of citrus in vitro plants via modulation of antioxidative capacity and stomatal response. Tree Physiol 30:914–922
Shu S, Yuan LY, Guo SR, Sun J, Yuan YH (2013) Effects of exogenous spermine on chlorophyll fluorescence, antioxidant system and ultrastructure of chloroplasts in Cucumis sativus L. under salt stress. Plant Physiol Biochem 63:209–216
Singh TN, Paleg LG, Aspinall D (1973) Stress metabolism. III. Variations in response to water deficit in the barley plant. Aust J Biol Sci 26:65–76
Sukran D, Gunes T, Sivaci R (1998) Spectrophotometric determination of chlorophyll A, B and total carotenoid contents of some algae species using different solvents. Turk J Bot 22:13–18
Verma S, Mishra SN (2005) Putrescine alleviation of growth in salt stressed Brassica juncea by inducing antioxidative defense system. J Plant Physiol 162:669–677
Winterbourn CC, Mc Grath BW, Carrell RW (1976) Reactions involving superoxide and normal unstable hemoglobins. Biochem J 155:493–502
Yamaguchi K, Takahashi Y, Berberich T, Imai A, Takahashi T, Michael AJ (2007) A protective role for the polyamine of spermine against drought stress in Arabidopsis. Biochem Biophys Res Commun 352:486–490
Zamani Z, Niakan M, Gorbanly M (2013) Effect of exogenous putrescine in phenolic composition, antioxidant enzymes and nitrate reductase of Hyosyamus niger under drought stress. J Iran Plant Ecophysiol Res 3:78–90
Acknowledgements
We appreciate the financial support of this work by the University of Tabriz.
Author information
Authors and Affiliations
Contributions
Z.T.K.: Experimental work, data analysis and writing. K.G.-G.: Experimental design, supervision and writing. S.Y.S.-L.: Experimental work. R.M.: Experimental work.
Corresponding author
Ethics declarations
Conflict of interest
We have no competing interests.
Ethical statement
The researchers have acknowledged and upheld all human, environment and animal rights.
Rights and permissions
About this article
Cite this article
Khosrowshahi, Z.T., Ghassemi-Golezani, K., Salehi-Lisar, S.Y. et al. Changes in antioxidants and leaf pigments of safflower (Carthamus tinctorius L.) affected by exogenous spermine under water deficit. BIOLOGIA FUTURA 71, 313–321 (2020). https://doi.org/10.1007/s42977-020-00039-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42977-020-00039-z