Abstract
Drought is a common abiotic stress that influences crop production to a large extent all over the world. Wheat crop experiences drought at main stages during its life cycle, which induces oxidative stress in the plants. The antioxidant mechanisms of the plant have a significant role in providing tolerance against the water stress. The objective of this work was to investigate the antioxidant activity and transcript profile of antioxidant enzyme related genes in three wheat genotypes to be TN4228 (drought tolerant), TN1399 (moderately tolerant) and TN3737 (susceptible) at control [80% Field capacity (FC)] medium (40% FC) and severe stress (25% FC). Water-limited conditions led to a decrease in relative water content (RWC). However, drought increased malondialdehyde (MDA), hydrogen peroxide (H2O2), proline content, and antioxidant enzyme activities [superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX)]. In addition, we observed the enhancement of the delta-1-pyrroline-5-carboxylate synthase (P5CS), CAT, APX and SOD gene expressions at drought stress conditions. Both moderate and severe stresses caused genotype-specific responses, which were dependent on stress severity. According to drought stress and defense systems in TN4228, it was obtained that the engaged genes and enzymes play significant roles in defense responses and could be viable targets to determine the level of drought tolerance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Drought is a major abiotic stress that adversely influences crop production and leads to economic losses in the world (Dolferus et al. 2019). Wheat (Triticum aestivum L.) is an economically important food for about 1/3 of the world’s population (Dudziak et al. 2019). Recently, the importance of drought-resistant cultivars is more highlighted due to increasing the adverse impact of climate change particularly in arid and semiarid areas such as most parts of Iran (Fang and Xiong 2015). Genotypic variations for resistance to drought stress are known to exist in different crops. The development of resistant cultivars, however, is hampered by low heritability for drought tolerance and lack of effective selection strategies (Sheoran et al. 2015). The response of resistant and susceptible wheat genotypes to water deficit-induced oxidative stress and antioxidant management at a particular growth stage and under controlled growth conditions has been reported in the literature (Lascano et al. 2001; Sheoran et al. 2015; Rahimi et al. 2019).
Drought stress induces morpho-physiological and molecular variability in plants (Shinozaki and Yamaguchi-Shinozaki 2007). Moderate to severe stress significantly influences physiological and biochemical status in wheat such as relative water content (RWC) and antioxidant enzyme activities. Upon exposure to drought, reactive oxygen species (ROS) levels significantly increase, which is because of their higher generations than detoxifications, leading to oxidative burst (Sheoran et al. 2015). Excessive ROS concentration at any form induces the damages in DNA, protein and membrane lipid or even cell death (Mattos and Moretti 2015). To minimize the ROS concentration and preserve plant cells from oxidative damage, there are enzymatic and non-enzymatic antioxidant defense systems that are able to scavenge the ROS efficiently. In addition to antioxidant system, non-antioxidant mechanisms such as proline synthesized from glutamate through delta-1-pyrroline-5-carboxylate synthase (P5CS) is required as an osmolyte for maintaining cell turgidity (Yanlei et al. 2017) and even has an essential role as a ROS scavenger. Malondialdehyde (MDA) can be used as an index of membrane damage because of being ultimate product of lipid peroxidation (Dudziak et al. 2019).
The strategies combining physiological, molecular and genetic techniques have been increasing to elucidate plant growth and tolerance to stresses (Sheoran et al. 2015). Understanding the association of antioxidant enzyme activity and gene expression with genetic variation in drought tolerance is important for further deciphering the factors controlling antioxidant defense. However, most studies evaluating the effect of water stress in wheat have been restricted to morphological, physiological and biochemical responses of antioxidant genes at the seedling stage only. Therefore, the present work was designed (a) to identify the sensitive and tolerate genotypes of wheat under drought, (b) to assess the effect of oxidative stress on physiological traits and antioxidant responses, integrating physiological, biochemical and molecular approaches at anthesis stage of the plants. Our hypothesis is that the genotypes have different responses to drought stress in terms of physiological and biochemical properties and also the expression of several stress response-related genes including delta-1-pyrroline-5-carboxylate synthase (P5CS), CAT, APX, and SOD genes is different under drought levels.
Materials and methods
Plant materials and growth conditions
Seeds of three wheat genotypes including highly tolerant (TN4228), moderately tolerant (TN1399) and susceptible (TN3737) were obtained from National Plant Gene Bank, Iran. The pot experiment was conducted in Karaj, Iran as a complete randomized block design (CRBD) with three replications. Five seeds of each genotype were sown in plastic pots with 5 L size in a mixture of coco peat, peat moss, and perlite (2:2:1). The average temperature was between 18 and 28 °C, and the relative humidity varied from 55 to 60%. Until anthesis stage (BBCH-61), all plants were evenly irrigated. At anthesis stage (on July 17, 2018), the plants were subjected to drought by deficit irrigation, in a way that soil moisture content declined gradually and reached 40% field capacity (FC) as moderate and 25% FC as severe drought treatments, 20 days after the last normal irrigation. Control plants were irrigated normally. The sampling of leaves was done in dough developmental stage. To measure antioxidant enzyme activities, lipid peroxidation and gene transcription levels, harvested flag leaves were transferred in liquid nitrogen and kept at − 80 °C until they were used in the laboratory.
Relative water content (RWC) measurement
The RWC of leaves was calculated as a percentage according to the method of Heath and Packer (1968) as follows:
where FW is fresh weight, SW is leaf weight after soaking for 24 h at room temperature and DW is leaf dry weight after drying for 24 h at 75 °C.
Malondialdehyde (MDA) concentration
To determine the MDA content, the samples were extracted with phosphate buffer and centrifuged at 14,000 rpm for 30 min. After that, the thiobarbituric acid (0.5% w/v) containing 20% w/v trichloroacetic acid was added to the mixture. The samples were placed in a hot water bath for 30 min and then were immediately cooled with ice and finally centrifuged at 10,000 rpm for 10 min. Samples were read at 532 and 600 nm wavelengths (Alexieva et al. 2001).
Measurements of H2O2 production
H2O2 was determined by 3,3′-diaminobenzidine (DAB) staining as described by Guan and Scandalios (2000). Wheat leaf samples were incubated in DAB solution (1 mg/ml, pH 3.8; Sigma-Aldrich) for 8 h in dark at 28 °C, and then dipped into boiling 80% (v/v) ethanol for 10 min. The samples were extracted with 80% (v/v) ethanol after cooling, and photographed.
Proline concentration
To measure proline content, 0.5 g fresh leaf sample was mixed with 10 ml of sulfosalicylic acid (3% w/v). The mixture containing the sample were centrifuged at 4000×g for 20 min, and then 2 ml of ninhydrin acid and 2 ml of glacial acetic acid were added. Simultaneously, 2 ml of standard 0, 4, 8, 12, 16, 20 mg l−1 proline and 2 ml ninhydrin acid and 2 ml acetic acid were mixed. All samples were heated in a hot water bath for 60 min and then placed on ice to be cooled completely. Four milliliters of toluene was added to the solution and stirred for 20 s. Using 0, 4, 8, 12, 16, and 20 mg l−1 proline standards, the standard curve was determined spectrophotometrically at 520 nm. The toluene-soluble proline was sufficiently was measured at 520 nm expressed as μmol proline g−1 FW (Bates et al. 1973).
Enzyme essay
The extraction procedures were carried out at 0–4 °C. Fresh samples (0.5 g) were homogenized in a Heidolph, Diax 900 homogenizer in 5 ml 100 mM potassium phosphate buffer (pH 7.6) containing 1 mM EDTA-Na2 and 0.5 mM ascorbate. Because ascorbate peroxidase (APX) is labile in the absence of ascorbate, 0.5 mM ascorbate was included for the extraction of this enzyme with the above procedure. The homogenized shoot samples were centrifuged at 10,000 g for 5 min. The supernatant was used as a crude enzyme extract. All colorimetric measurements were made at room temperature in a Shimadzu UV/VIS 1201 spectrophotometer.
Catalase (CAT) activity was measured with initial rate for H2O2 disappearance (Dhindsa et al. 1981). The reaction mixture contained 3% H2O2 and 0.1 mM ethylenediaminetetraacetic acid (EDTA) in 0.05 M Na-phosphate buffer (pH 7). The reduction in H2O2 concentration was determined as a decline in optical density at 240 nm, and the activity was measured as μmol H2O2 decomposed per minute.
Superoxide dismutase (SOD) activity was determined as described by Beyer and Fridovich (1987). The reaction mixture consisted of 1.17 × 10−6 M riboflavin, 0.1 M methionine, 2 × 10−5 M potassium cyanide (KCN) and 5.6 × 10−5 M nitroblue tetrazolium salt (NBT), which was dissolved in 3 ml of 0.05 M sodium phosphate buffer (pH 7.8). 1 ml of enzyme extract was enriched with 3 ml of the reaction. Illumination was started to initiate the reaction at 30 °C for 60 min. The blanks were identical solutions that were kept under dark condition. The absorbance was determined at 560 nm with the spectrophotometer against the blank.
Ascorbate peroxidase (APX) activity was calculated by following the decrease of ascorbate and measuring the change in absorbance at 290 nm for 1 min in 2 ml of a reaction mixture containing 50 mM potassium phosphate buffer (pH 7.0), 1 mM EDTA-Na2, 0.5 mM ascorbic acid, 0.1 mM H2O2 and 50 µl of crude enzyme extract (Nakano and Asada 1981). The activity was calculated from the extinction coefficient (2.8 mM−1 cm−1) for the ascorbate.
Gene expression analysis
Total RNA was extracted using GeneAll® Ribospin™ kit (BioFrontier, Korea). RNA quality and quantity were measured by 1% agarose gel electrophoresis and NanoDrop spectrophotometry (Thermoscientific 2000c, USA), respectively. 3 µg of RNA was treated by DNaseI (Thermofisher, USA) and 1 µg of treated RNA subjected to complementary DNA (cDNA) Synthesis (Fermentas, Revert Aid™ First Strand cDNA, USA) in total volume 20 µl containing 1 mM dNTPs, 0.5 µM dT primer and 1 µl reverse transcriptase enzyme. Primer designing was carried out by online PrimerQuest software (Thornton and Basu 2011) based on cDNA sequence (Table 1). Wheat actin gene (AB181991.1) was used as an internal standard for gene expression. qRT-PCR was done in StepOne Plus Real Time PCR (ABI, USA) in total volume 20 µl consisting of 0.2 U/µl Taq DNA polymerase, 0.2 mM dNTPs, 0.25 µM of each primer and 200 ng cDNA under thermal program of initial denaturation 95 °C for 15 min, followed by 40 cycles of 95 °C for 15 s, 58 °C for 30 s and 72 °C for 20 s. Finally, melt curve analysis was implemented to assess the PCR specificity for all reactions. Every sample was replicated three times independently.
Statistical analysis
Analysis of variance was conducted using SAS software. Tukey's test at P < 0.05 was utilized for identifying the significant difference among mean values. Data of gene expressions were analyzed by Relative Expression Software Tool (REST).
Results
RWC, H2O2, MDA, and proline concentrations
Drought, especially severe stress, resulted in a significant decline of RWC. Under both severe and moderate stresses, the rate of RWC reduction was similar in drought-tolerant (TN4228) and intermediate (TN1399) genotypes, but it was lower when compared to susceptible (TN3737) genotype (Fig. 1a). Compared to normal irrigation, the severe and moderate stress caused higher accumulation of H2O2 and MDA in flag leaves of all plants. However, TN3737 genotype contained higher H2O2 and MDA content than the other ones, and no significant difference was observed between TN4228 and TN1399 (Fig. 1b, c). Severe drought led to higher proline accumulation compared to moderate stress. Under moderate drought stress, both TN4228 and TN1399 genotypes showed a similar level of proline content (Fig. 1d).
Relative water content (RWC, a), hydrogen peroxide (H2O2, b), malondialdehyde (MDA, c), and proline content (d) in three wheat genotypes of drought-tolerant (TN4228), intermediate (TN1399), and susceptible (TN3737) under drought stress at anthesis stage for a 20-day period. The values are represented with mean ± SE, n = 3
Enzyme activity
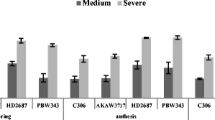
Drought stress increased the antioxidant enzyme activities. In TN4228, moderate stress increased CAT activity by 73% compared to control (Fig. 2a). For SOD, we obtained a significant enhancement when plants were treated with both moderate and severe stresses. In TN1399, it was found twofold enhancement of SOD activity under moderate stress in comparison with control (Fig. 2b). TN4228 and TN3737 represented the highest and lowest APX activity, respectively (Fig. 2c).
The activity of catalase (CAT, a), superoxide dismutase (SOD, b), and ascorbate peroxidase (APX, c) in three wheat genotypes of drought-tolerant (TN4228), intermediate (TN1399) and susceptible (TN3737) under drought stress at anthesis stage for a 20-day period. The values are represented with mean ± SE, n = 3
Gene expression
Severe drought increased P5CS gene expression up to 16, 6, and fourfold in TN4228, TN1399, and TN3737, respectively, and moderate stress upregulated P5CS gene expression up to fivefold in TN4228 and TN1399 (Fig. 3a). The antioxidant enzymes exhibited activity variations under severe stress compared to moderate drought conditions, and TN4228 and TN1399 genotypes indicated the highest CAT, APX, and SOD activity under both drought conditions. In TN3737, APX activity remained unchanged at severe stress, while decreased at moderate drought (Fig. 2c). Different patterns of gene expression were detected for SOD, CAT and APX during exposure to drought treatments (Fig. 3b–d). In TN4228, CAT gene was highly overexpressed under drought treatments, while in TN1399, only upregulated under severe stress condition. In TN3737, CAT transcript level increased highly under moderate drought, but slightly under severe drought. TN4228 and TN1399 indicated a higher increase in APX transcript level under moderate drought as compared to severe stress. In TN3737, APX gene was upregulated to twofold at moderate stress conditions, whereas downregulated in severe drought-stressed plants. The transcript level of SOD gene increased significantly in TN4228 and TN1399 as they were exposed to severe drought. In TN1399, SOD gene was upregulated up to threefold under moderate drought, but its expression was not affected by severe drought stress. In TN3737, SOD gene was slightly downregulated at moderate stress condition, whereas upregulated to threefold under severe stress.
Gene expression of delta-1-pyrroline-5-carboxylate synthase (P5CS, a), catalase (CAT, b), superoxide dismutase (SOD, c), and ascorbate peroxidase (APX, d) in three wheat genotypes of drought-tolerant (TN4228), intermediate (TN1399) and susceptible (TN3737) under drought stress at anthesis stage for a 20-day period. The values are represented with mean ± SE, n = 3
Discussion
RWC as an important indicator for plant water condition is associated with metabolic activities and physiological processes (Huang et al. 2013). In this research, RWC was found dependent on drought intensity in all genotypes. Higher tolerance in TN4228 and TN1399 could be attributed to their higher RWC and their abilities in preserving water content under drought conditions, which is necessary for the maintenance of plants optimum physiological and metabolic functions (Eftekhari et al. 2017). In line with our findings, several studies have demonstrated the connections between the maintenance of higher RWC under drought and the higher drought tolerance in wheat (Marcek et al. 2019; Eftekhari et al. 2017).
The lower H2O2 and MDA quantity, as stress indexes, in tolerant and intermediate genotypes could be contributed to their higher activities of antioxidant enzymes. It could be suggested that morefhtolerant genotypes possess high efficient antioxidant defense system at the cellular level to protect them against oxidative stress. Similar to our results, different studies on wheat reported that lower soil water content resulted in higher H2O2 and MDA concentrations in leaf cells (Marcek et al. 2019; Dudziak et al. 2019).
Proline accumulation has a defensive function in oxidative stress due to its important role in osmotic adjustment, withstanding cellular dehydration and ROS scavenging (Yanlei et al. 2017). Our results indicated higher proline content under drought treatments, which can be explained by the restriction of its oxidation or/and the stimulation of its biosynthesis. In fact, TN4228 and TN1399 tolerant genotypes had higher proline content and P5CS expression when compared to TN3737 susceptible genotype. Thus, it could be suggested that under drought, the upregulation of P5CS gene can enhance drought tolerance by causing higher proline concentration. Similar investigations also approve this result (Wang et al. 2018; Bandurska et al. 2017; Chakraborty and Pradhan 2012). In addition, higher P5CS expression and more tolerance under drought conditions have been reported in wheat (Dudziak et al. 2019) and barley (Bandurska et al. 2017). The elevated level of P5CS transcripts under osmotic stress can be attributed to H2O2-derived signals and ABA-dependent pathways (Bandurska et al. 2017).
Lower CAT, APX, and SOD activities under severe drought compared to moderate drought implies that severe drought weakened the capability of genotypes in H2O2 scavenging, which may be a result of synthesis inhibition and/or degradation of CAT, APX and SOD enzymes. Similar to our results, Sofo et al. (2015) observed that the CAT and APX activity is associated with the degree of drought. The elevated level of CAT, APX, and SOD activity under water-limited conditions has been attributed to a higher drought tolerance in previous researches on rice (Wang et al. 2019), barley (Marok et al. 2013), ramie (Huang et al. 2013), and wheat (Chakraborty and Pradhan 2012). Down regulation of APX gene under water shortage has been also reported in studies on Arachis hypogaea L. (Furlan et al. 2014) and P. pratensis (Bian and Jiang 2009 ). Harb et al. (2015) reported that the drought-induced pattern of SOD gene expression was specific for each genotype in barley, which is in agreement with our findings. Furthermore, indicated a significant increase in transcript levels of SOD gene in more drought-tolerant orchard grass genotypes. In this study, it was observed that in drought-tolerant genotype, the activities of studied antioxidant enzymes had a better relative consistency with their corresponding genes in comparison with intermediate and susceptible genotypes under both drought treatments. Luna (2004) reported a correlation between CAT gene expression and CAT enzyme activity in wheat cultivars under water deficit.
In summary, both drought stress conditions caused genotype-specific responses, which were dependent on drought severity. Considering all studied drought-induced responses together, superior upregulation of defense systems in tolerant TN4228 indicated that the studied genes and enzymes play meaningful roles in drought defense responses and could be viable targets to identify drought tolerance level.
Abbreviations
- CAT:
-
Catalase
- SOD:
-
Superoxide dismutase
- APX:
-
Ascorbate peroxidase
- MDA:
-
Malondialdehyde
- H2O2 :
-
Hydrogen peroxide
- P5CS:
-
Delta-1-pyrroline-5-carboxylate synthase
- RWC:
-
Relative water content
- FC:
-
Field capacity
- ROS:
-
Reactive oxygen species
References
Alexieva V, Sergiev I, Mapelli S, Karanov E (2001) The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ 24:1337–1344
Bandurska H, Niedziela J, Pietrowska-Borek M, Nuc K, Chadzinikolau T, Radzikowska D (2017) Regulation of proline biosynthesis and resistance to drought stress in two barley (Hordeum vulgare L.) genotypes of different origin. Plant Physiol Biochem 118:427–437
Bates LS, Waldern RP, Teave ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Beyer WF, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161:559–566
Bian S, Jiang Y (2009) Reactive oxygen species, antioxidant enzyme activities and gene expression patterns in leaves and roots of Kentucky bluegrass in response to drought stress and recovery. Sci Hortic 120:264–270
Chakraborty U, Pradhan B (2012) Oxidative stress in five wheat varieties (Triticum aestivum L.) exposed to water stress and study of their antioxidant enzyme defense system, water stress responsive metabolites and H2O2 accumulation. Braz J Plant Physiol 24:2–11
Dhindsa RS, Plumb-Dhindsa PL, Reid DM (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Dolferus R, Thavamanikumar S, Sangma H, Kleven S, Wallace X, Forrest K, Rebetzke G (2019) Determining the genetic architecture of reproductive stage drought tolerance in wheat using a correlated trait and correlated marker effect model. G3: Genes Genom Genet 9:473–489
Dudziak K, Zapalska M, Börner A, Szczerba H, Kowalczyk K, Nowak M (2019) Analysis of wheat gene expression related to the oxidative stress response and signal transduction under short-term osmotic stress. Sci Rep 9:2743
Eftekhari A, Baghizadeh A, Yaghoobi MM, Abdolshahi R (2017) Differences in the drought stress response of DREB2 and CAT1 genes and evaluation of related physiological parameters in some bread wheat cultivars. Biotechnol Biotechnol Equip 31(4):709–716
Fang Y, Xiong L (2015) General mechanisms of drought response and their application in drought resistance improvement in plants. Cell Mol Life Sci 72:673–689
Furlan A, Bianucci E, Tordable M, Castro S, Dietz KJ (2014) Antioxidant enzyme activities and gene expression patterns in peanut nodules during a drought and rehydration cycle. Funct Plant Biol 41:704–713
Guan LM, Scandalios JG (2000) Hydrogen-peroxide-mediated catalase gene expression in response to wounding. Free Radic Biol Med 28:1182–1190
Harb A, Awad D, Samarah N (2015) Gene expression and activity of antioxidant enzymes in barley under controlled severe drought. J Plant Interact 10:109–116
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Huang CJ, Zhao SY, Wang LC, Shakeel AA, Chen M, Zhou HF (2013) Alteration in chlorophyll fluorescence, lipid peroxidation and antioxidant enzymes activities in hybrid ramie (Boehmeria nivea L.) under drought stress. Aust J Crop Sci 7:594–599
Lascano HR, Antonicelli GE, Luna CM, Melchiorre MN, Gomez LD, Racca RW, Trippi VS, Casano LM (2001) Antioxidant system response of different wheat cultivars under drought: field and in vitro studies. Funct Plant Biol 28:1095–1102
Luna CM (2004) Drought controls on H2O2 accumulation, catalase (CAT) activity and CAT gene expression in wheat. J Exp Bot 56:417–423.
Marcek T, Hamow KA, Vegh B, Janda T, Darko E (2019) Metabolic response to drought in six winter wheat genotypes. PLoS ONE 14:e0212411
Marok M, Tarrago L, Ksas B, Henri P, Abrous-Belbachir O, Havaux M, Rey P (2013) A drought-sensitive barley variety displays oxidative stress and strongly increased contents in low-molecular weight antioxidant compounds during water deficit compared to a tolerant variety. J Plant Physiol 170:633–645
Mattos LM, Moretti CL (2015) Oxidative stress in plants under drought conditions and the role of different enzymes. Enzyme Eng 5:136
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Rahimi Y, Bihamta MR, Taleei A, Alipour H, Ingvarsson PK (2019) Applying an artificial neural network approach for drought tolerance screening among Iranian wheat landraces and cultivars grown under well-watered and rain-fed conditions. Acta Physiol Plant 41:156–165
Sheoran S, Thakur V, Narwal S, Turan R, Mamrutha HM, Singh V, Sharma I (2015) Differential activity and expression profile of antioxidant enzymes and physiological changes in wheat (Triticum aestivum L.) under drought. Appl Biochem Biotechnol 177:1282–1298
Shinozaki K, Yamaguchi-Shinozaki K (2007) Gene networks involved in drought stress response and tolerance. J Exp Bot 58:221–227
Sofo A, Scopa A, Nuzzaci M, Vitti A (2015) Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int J Mol Sci 16:13561–13578
Thornton B, Basu C (2011) Real-time PCR (qPCR) primer design using free online software. Biochem Mol Biol Educ 39(2):145–154
Wang X, Yanhua X, Jingjing L, Ren Y, Wang Z, Xin Z, Lin T (2018) Identification of two novel wheat drought tolerance-related proteins by comparative proteomic analysis combined with virus-induced gene silencing. Int J Mol Sci 19:4020
Wang XP, Liu HL, Yu FL, Hu BW, Jia Y, Sha HJ (2019) Differential activity of the antioxidant defence system and alterations in the accumulation of osmolyte and reactive oxygen species under drought stress and recovery in rice (Oryza sativa L.) tillering. Sci Rep 9:8543
Yanlei F, Hailing M, Siying C, Tianyu G, Jiming G (2017) Control of proline accumulation under drought via a novel pathway comprising the histone methylase CAU1 and the transcription factor ANAC055. J Exp Bot 69:579–588
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nasirzadeh, L., Sorkhilaleloo, B., Majidi Hervan, E. et al. Changes in antioxidant enzyme activities and gene expression profiles under drought stress in tolerant, intermediate, and susceptible wheat genotypes. CEREAL RESEARCH COMMUNICATIONS 49, 83–89 (2021). https://doi.org/10.1007/s42976-020-00085-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42976-020-00085-2