Abstract
Wheat crop may experience water deficit at crucial stages during its life cycle, which induces oxidative stress in the plants. The antioxidant status of the plant plays an important role in providing tolerance against the water stress. The objective of this study was to investigate the impact of water stress on physiological traits, antioxidant activity and transcript profile of antioxidant enzyme related genes in four wheat genotypes (C306, AKAW3717, HD2687, PBW343) at three crucial stages of plants under medium (75 % of field capacity) and severe stress (45 % of field capacity) in pots. Drought was applied by withholding water for 10 days at a particular growth stage viz. tillering, anthesis and 15 days after anthesis (15DAA). For physiological traits, a highly significant effect of water stress at a particular stage and genotypic variations for resistance to drought tolerance was observed. Under severe water stress, the malondialdehyde (MDA) content increased while the relative water content (RWC) and chlorophyll index decreased significantly in all the genotypes. The drought susceptibility index (DSI) of the genotypes varied from 0.18 to 1.9. The drought treatment at the tillering and anthesis stages was found more sensitive in terms of reduction in thousand grain weight (TGW) and grain yield. Antioxidant enzyme activities [superoxide dismutase (SOD) and peroxidase (POX)] increased with the decrease in osmotic potential in drought tolerant genotypes C306 and AKAW3717. Moreover, the transcript profile of Mn-SOD upregulated significantly and was consistent with the trend of the variation in SOD activity, which suggests that Mn-SOD might play an important role in drought tolerance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Drought limits plant growth and field crop production more than any other environmental stresses [1, 2]. Out of 29 mha of wheat area in India, about 9–13 mha is prone to high temperature stress and 3–5 mha prone to drought. Genotypic variations for resistance to drought stress are known to exist in different crops, e.g. in maize [3], wheat [4] and triticale [5]. The development of resistant cultivars, however, is hampered by low heritability for drought tolerance and lack of effective selection strategies [1]. Plants usually experience a fluctuating water supply during their life cycle due to continuously changing climatic factors [6]. The drought stress can occur at any growth stage and may lead to complete crop failure if it happens at a sensitive growth stage. Wheat is reported to be sensitive to drought during the planting–jointing stage [7, 8] and booting–heading stage [9]. The response of resistant and susceptible wheat genotypes to water deficit-induced oxidative stress and antioxidant management at a particular growth stage and under controlled growth conditions has been reported in the literature [10, 11].
Drought stress responses are multigenic as the tolerance mechanism is altered by changes in the expression level of various compatible solutes/osmolytes and the reactive oxygen species (ROS), and these changes adversely affect all the levels of plant organization including morphological, physiological, biochemical and molecular levels [12, 13]. Moderate to severe stress drastically affects various physiological traits in wheat such as relative water content (RWC), chlorophyll content and chlorophyll fluorescence. It has been reported that the downregulation of photosynthesis due to mild drought stress is mainly the result of a reduction in stomatal conductance, while the photosynthetic apparatus is not significantly affected [14, 15]. As a consequence of severe drought stress events, both stomatal and non-stomatal limitations lead to a decline in photosynthesis [16]. Therefore, chances are there that genotypes may respond differentially under moderate to severe water stress at a similar growth stage.
During drought stress, the plant water relations play a key role in the activation and/or modulation of the antioxidant defence mechanism [17]. The removal of O2 − by superoxide dismutase (SOD, EC 1.15.1.1) generates H2O2, which is removed by catalase (CAT, EC 1.111.1.6) and peroxidase (POX, EC 1.111.1.7) [18]. A number of studies have indicated that higher activity levels of antioxidant enzymes may contribute to better drought tolerance in wheat by increasing the protection capacity against oxidative damage [19, 20]. However, change in activities of antioxidant enzymes under drought stress is dependent on plant species, cultivar and stress intensity and duration [21].
The use of approaches combining genetic, physiological and molecular techniques has been a promising strategy in elucidating the plant stress response mechanism [22]. Understanding the association of antioxidant enzyme activity and gene expression with genetic variation in drought tolerance is important for further deciphering the factors controlling antioxidant defence. However, most studies evaluating the effect of water stress in wheat have been restricted to morphological, physiological and biochemical responses of antioxidant genes at the seedling stage only. Keeping this in view, the present study was conducted to identify the sensitive stage of wheat under water stress and to evaluate the effect of oxidative stress on physiological traits and antioxidant responses, integrating physiological, biochemical and molecular approaches at different stages of the plant. Our hypothesis is that water stress imposed at different stages of plant growth in genotypes’ response is different and antioxidative enzymes and some of the genes related to them will display more in contrasting genotypes.
Materials and Methods
Plant Materials
Four wheat genotypes, two drought tolerant (C306 and AKAW3717) and two drought susceptible (HD2687 and PBW343), were used in the present study (Table 1).
Experimental Design and Sampling
A pot experiment was conducted in completely randomized block design. The pots were filled with appropriate potting mixture of soil, sand and FYM in the ratio 2:1:1 (v/v). The seeds of the above mentioned four wheat genotypes were grown in two levels of moisture stress, i.e. medium [75 % FC (field capacity)] and severe (45 % FC), induced by the gravimetric method along with the control treatment. In three different treatments, drought stress was imposed at three growth stages individually, i.e. at tillering (35 days after sowing), anthesis and 15 days after anthesis (15DAA), and at each stage, stress treatment was maintained for 10 days continuously by withholding the water, later, watering was allowed for plant recovery. Four replications were maintained for each genotype for each level of drought stress, with three plants in each pot. The pots were maintained in the natural condition with a precaution to avoid rainfall during the stress induction period.
Physiological Traits
Determination of Relative Water Content
The relative water content in flag leaves was measured from three randomly chosen fully expanded leaves early in the morning (9 a.m.) after stress treatment. A 10 cm long leaf was excised from the middle portion of the flag leaf in the early morning and cut into two equal halves, fresh weight (FW) was recorded and the leaf segments were immediately immersed in distilled water in tarson tubes overnight at room temperature. The leaf segments were blotted properly and turgid weight (TW) was recorded. Then, the samples were placed in a paper bag and dried in a hot air oven (FISHER isotemperature oven) at 80 °C till constant weight was obtained, and later, the dry weight (DW) was recorded. A precision analytical balance (Afcoset, ER-182A) was used for all weight measurements. The relative water content of a leaf was calculated as RWC (%) = [(FW–DW) (TW–DW)] × 100 [25].
Chlorophyll Content
Leaf chlorophyll content indirectly measures the photosynthetic ability of the plants. The chlorophyll content index (CCI) was estimated using a chlorophyll content meter (atLEAF+). The measurements were done in a fully expanded leaf after each stress treatment, and three biological measurements were taken from each pot as replications.
Phenotypic and Yield Traits
The height of the plant was recorded using a scale from the soil surface to the tip of the spike, excluding awns in the plant. The harvested plants were threshed using a single plant thresher and grain weight was recorded. Plants harvested at maturity and 1000 grain weight, grain yield/plant were recorded. One thousand grains were counted using a seed counter (WAVER, Daidex Co. Ltd.) and weighed on an electric balance. The drought susceptibility index (DSI) was calculated using the formula:
Y D and Y are the grain yield for each genotype under water stress and control respectively.
X D and X are the mean grain yield of all genotypes under water stress and control respectively.
Extraction and Determination of Enzyme Activities
Enzyme extract for each sample (collected on day 1 (1d) and day 10 (10d) of stress treatment at a particular stage) was prepared by grinding 0.1 g leaf in an ice-cold pestle and mortar with cold extraction buffer (100 mM potassium phosphate buffer, pH 7.5) containing 0.5 mM EDTA. The extract was then centrifuged at 15,000×g at 4 °C for 20 min. The supernatant was used for the spectrophotometric assay of different antioxidant enzymes.
Superoxide Dismutase
Superoxide dismutase (SOD) activity was determined by using the method SPCYO01 of Sigma [26]. A 3.0 ml reaction mixture contained 50 mM potassium phosphate, 0.1 mM EDTA, 0.01 mM cytochrome C, 0.05 mM xanthine, 0.005 unit xanthine oxidase and 10 μl enzyme extract. One unit of SOD activity was defined as the amount of enzyme necessary for 50 % inhibition of cytochrome C in a coupled system with xanthine and xanthine oxidase at pH 7.8 at 25 °C in 3.0 ml reaction volume. SOD activity was expressed in units per gram fresh weight.
Peroxidase
Peroxidase (POX) activity was determined by using the method described by Jebara et al. [27]. The final assay volume of 3.0 ml contained 50 mM potassium phosphate buffer (pH 7.0), 9 mM guaiacol, 19 mM H2O2 and 0.1 ml of enzyme extract. Absorbance was recorded at 1 min interval up to 5 min at 470 nm. Peroxidase activity was calculated using the extinction coefficient of 26.6 mM−1 cm−1.
Catalase
Catalase (CAT) activity was determined by following the consumption of H2O2 at 240 nm for 5 min [28]. The reaction mixture contained 100 mM potassium phosphate buffer (pH 7.0) and plant extract (25 μl) in a 3.0 ml reaction volume. The reaction was initiated by adding 10 μl of 6 mM H2O2. The enzyme activity was calculated using the extinction coefficient of H2O2 39.4 mM−1 cm−1 and expressed as units/gram fresh weight.
Protein content was measured by using Bradford’s method [29] using BSA as the standard.
Malondialdehyde Content
The level of lipid peroxidation in the leaf tissue was measured in terms of malondialdehyde (MDA) (a product of lipid peroxidation) content determined by thiobarbituric acid (TBA) reaction using the method of Heath and Packer [30], with minor modifications as described by Dhindsa et al. [31] and Zhang and Kirham [23]. A 0.25 g leaf sample was homogenized in 5.0 ml 0.1 % TCA. The homogenate was centrifuged at 10,000×g for 5 min. To a 1 ml aliquot of the supernatant, 4.0 ml of 20 % TCA containing 0.5 % TBA was added. The mixture was heated at 95 °C for 30 min and then quickly cooled in an ice bath. After centrifugation at 10,000×g for 10 min, the absorbance of the supernatant at 532 nm was read and the value for non-specific absorption at 600 nm was subtracted. The MDA content was calculated by using an extinction coefficient of 155 mM−1 cm−1.
Quantitative Real-Time PCR Analyses
Total RNA from the leaves of two genotypes C306 and HD2687 at the anthesis stage was isolated using TRIZOL reagent (Invitrogen). The complementary DNA (cDNA) was synthesized using a ProtoScript first strand cDNA synthesis kit (BioLabs) from 1 μg of total RNA according to the manufacturer’s protocol. The reverse transcription reaction was carried out at 44 °C for 60 min followed by 92 °C for 10 min. Gene-specific primers (Table 2) were designed using IDT Primer Quest, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) RNA primer was used as internal control. Primer efficiencies were calculated on a standard curve prepared by twofold dilution of control cDNA. Real-time quantitative PCR was performed on iCycler (BioRad 3600) with the QuantiTect SYBER I Green in a 20 μl reaction volume that contained 10 μl of SYBER Bio Pars (GUASNR), 6 μl of each forward and reverse primers (10 pmol), 5 μl of diluted cDNA template and the appropriate amount of sterile, double-distilled water.
Statistical Analysis of the Data
The experiment was laid out in a completely randomized block design with four replications. All physiological experiments were repeated three times independently. Significance of data was tested by analysis of variance (ANOVA) and Duncan’s multiple range test at p < 0.05 and where applicable at p < 0.01 using SAS 9.3 and JMP 9.0 software. Values are presented in the graph as the mean ± standard error (SE).
Results and Discussion
Relative Water Content
Gradual reduction in RWC was observed in all the four genotypes at tillering, anthesis and 15DAA under medium (MS) and severe (SS) water stress treatment (Fig. 1). Significant difference for genotype, treatment and genotype × treatment interaction was observed (Table 3). Results showed that all cultivars maintained relatively higher RWC at the tillering stage compared to anthesis and 15DAA under both the treatments (MS and SS). The maximum percent reduction in RWC was observed in HD2687 at all the three growth stages under both medium and severe stress. Genotype C306 maintained higher RWC at anthesis and 15DAA while AKAW3717 and PBW 343 exhibited medium behaviour and HD2687 showed the lowest value under water stress. This indicated that sensitive cultivars respond to drought with a faster decrease in RWC than tolerant cultivars. This variation in RWC between cultivars may be attributed to differences in the ability of the cultivars to absorb more water from the soil and/or the ability to control water loss through the stomata [24]. RWC indicate the water stress of the cells and have significant association with yield and stress tolerance [2, 19].
Chlorophyll Index
Under normal conditions, the chlorophyll content was nearly same in all the four genotypes and decreased more in severe stress compared to medium stress at all the three growth stages. Chlorophyll content decreased significantly in all the genotypes at tillering, anthesis and 15DAA under both the treatments (Fig. 2). Chlorophyll loss is associated with environmental stress, and the variation in the total chlorophyll/carotenoids ratio may be a good indicator of stress in plants [32]. However, maximum reduction in chlorophyll content was recorded in genotype PBW343, i.e. 41, 42 and 45 % at tillering, anthesis and 15 DAA respectively in severe stress. Drought tolerant genotypes AKAW3717 and C306 showed less reduction in chlorophyll index under medium and severe stress compared to HD2687 and PBW343, depicting one of the contributing traits for drought tolerance as chlorophyll maintenance is essential for photosynthesis under drought stress. Many previous studies have reported that tolerant wheat genotypes have higher chlorophyll content and lower percent reduction under stress [33, 34]. Percent decrease was observed more in drought susceptible genotypes as compared to tolerant genotypes at all three stages of growth. This trait has been successfully used by many workers for screening and selection of drought tolerant wheat cultivars [2].
Phenotypic and Yield Traits
Stress factors, especially drought, negatively affect plant growth and development and cause a sharp decrease of plant productivity [35].
Plant Height
When stress was imposed at the tillering stage except PBW343, no significant difference was found for plant height in both medium and severe stress with respect to control (Fig. 3). Similarly, no significant effect of water stress on plant height at anthesis and 15DAA was observed in any of the genotypes.
Thousand Grain Weight
Water stress that occurred at different growth stages showed a significant effect on the thousand grain weight (TGW) of genotypes. When the stress was imposed at the tillering stage, a significant reduction in TGW was observed in all the genotypes under severe stress except AKAW3717 (Fig. 4). The disturbed plant physiological conditions caused by drought treatment at the tillering stage might reduce the TGW. At the anthesis stage, maximum reduction in TGW was observed in genotype HD2687 (25 %) whereas at 15DAA, genotype AKAW3717 showed the highest reduction in TGW under severe stress. Under medium stress at 15DAA, no significant difference for TGW was observed in C306. In this study, two stages, i.e. 15DAA and anthesis, were found sensitive to TGW reduction under drought. The stem water soluble carbohydrates and the current photosynthesis are very important for grain development in wheat. This material is translocated from leaves, culm and head to the grain at the grain filling stage [36]. The decreased TGW due to drought at anthesis and 15DAA would have been due to decreased water supply and soluble carbohydrates and a reduction in the number of endoplast cells and amyloplasts in the grain [37].
Grain Yield
Grain yield/plant was significantly reduced in all the genotypes except AKAW3717 at the tillering stage under severe stress with respect to the control (Fig. 5). No effect between the treatments on the genotypes for grain yield was observed at the tillering stage except PBW343. When water stress was imposed at anthesis, genotypes AKAW3717, PBW343 and HD2687 showed a significant reduction in grain yield under medium and severe stress as compared to the control (Fig. 5) while in C306, reduction was observed under severe stress only. At 15DAA, all the genotypes showed reduction under severe stress. Although reduction was observed at all the stages, the tillering and anthesis stages were found to be sensitive to drought stress as maximum reduction in grain yield was observed in the genotypes under severe stress. Root growth continues in the early 60 days of plant growth [38], and drought at early stages would have damaged the root system and impaired water and nutrient uptake. The most sensitive stages for grain set are associated with the processes of microsporogenesis, anther dehiscence and fertilization [39], and just after fertilization for grain size [40]. The negative effect of drought stress on yield and yield performance has been well documented as a major problem in many developing countries of the world [41].
ANOVA was used to find interactions showing significant differences between the genotypes, treatment and genotype × treatment on yield traits. Despite highly significant differences between genotypes and traits investigated, all the treatments were found significant (Table 4). Thus, the stress treatments highlighted the optimum differential between genotypes, though an interaction between genotype and treatment was found significant for grain yield at the tillering stage and for TGW at the anthesis stage.
Assessment of the Drought Susceptibility Index of Genotypes
Relative drought tolerance, i.e. drought susceptibility index (DSI) of wheat genotypes, was calculated on the basis of grain yield/plant (g) as given in Fig. 6. Genotypes having a DSI less than 1.0 are considered as drought tolerant and more than 1.0 treated as drought susceptible. All the genotypes under medium stress treatment showed a relatively low DSI compared to those under severe stress treatment. In this study, DSI values ranged from 0.18 to 1.9. The highest value of DSI was observed in PBW343 (1.5) and the lowest in AKAW3717 (0.46) at the tillering stage under severe water stress. At the anthesis stage, AKAW3717 and HD2687 showed high values of DSI, i.e. 1.69 and 1.13 respectively, while C306 had the lowest DSI (0.26) under severe water stress which showed its drought tolerant nature. Genotype PBW343 at the anthesis stage showed a relatively low DSI (0.9). Under medium and severe stress, over the three stages, C306 showed consistently low DSI depicting its drought tolerant nature while genotype HD2687 showed comparatively high DSI value.
Biochemical Analysis
MDA, SOD, CAT and POX are important biochemical indices for evaluating the redox status of a genotype under drought stress. A differential response was observed in the level of MDA content and antioxidant enzymes between the genotypes at the anthesis stage at 1d and 10d in four genotypes.
Antioxidant Enzyme Activity
In the present study, the enzyme activities were recorded on 1d and 10d under severe water stress at the anthesis stage. The antioxidant enzymes analysed were SOD, CAT and POX. There was not much change in the level of SOD under controlled conditions in all the four genotypes, but the level increased with the severity of the stress and time in C306 and AKAW3717 (Fig. 7a). In HD2687 and PBW343, initially, there was a slight increase in the enzyme activity on 1d but, at 10d, a significant decrease was observed in PBW343. Variable responses of SOD to dehydration stress have been reported in literature including increased activity [42], lack of effect [43] and decreased activity [44] depending on the tissue and stage of development.
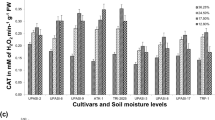
The initial level of catalase was found low and decreased significantly with severe water stress in C306 and AKAW3717. The activity of catalase increased significantly at 10d in both genotypes HD2687 and PBW343 (Fig. 7b). Kaur et al. [45] reported an initial increase in catalase in both C306 and PBW343, but later, either remained unchanged or decreased. In barley, however, lower initial levels of catalase were reported in 21-day-old seedlings subjected to water stress. However, the level increased with the increase in severity of stress [46]. In general, increased SOD and CAT activities were reported for mild water deficit [47], whereas severe or prolonged drought stress caused a decline in the activities of these enzymes [48]. Hameed et al. [49] also reported that with increasing severity magnitude, CAT activity decreased. The level of peroxidase in control plants was higher for HD2687 and PBW343. But in C306 and AKAW3717, although the initial control level of peroxidase was not very high, the level increased with an increase in the stress, while in HD2687, a significant decrease was observed up to 10d. Peroxidase activity increased significantly in PBW343 up to 10d as compared to the control (Fig. 7c). The increase was much more in C306 as compared to AKAW3717. There are several reports that the activity of peroxidase greatly increased in response to water stress in wheat [23]. Further, higher peroxidase activity has been shown to be associated with higher water retention in Nicotiana tabacum [50] and wheat leaves [51]. This means genotypes maintaining higher peroxidase activity in leaves under water stress may also have higher water retention and subsequent stress tolerance. Since both the catalase and peroxidase perform the same function of detoxifying the H2O2, catalase activity was compensated by the significant increase in the peroxidase activity in the tolerant cultivars. Under drought stress, an increase in peroxidase activity has earlier been reported in wheat [52, 53]. Previously, a decreased catalase activity with a simultaneous increase in peroxidase activity under heat stress has been reported in leaves and roots of creeping bentgrass [54].
The wheat genotypes responded differently to water stress in terms of activities of SOD, CAT and POX. SOD and POX activities were expressed more in the tolerant genotype (C306) than the susceptible genotype (HD2687). This further suggests that different wheat genotypes have discrete water stress thresholds and therefore they have different physiological adaptive mechanisms to regulate their redox status [55].
Lipid Peroxidation (MDA)
Lipid peroxidation estimated as thiobarbituric acid reactive substances (TBARS) increased with water stress in all the genotypes at the anthesis stage. Lower levels of MDA at 1d in genotypes C306 and AKAW3717 showed a stable performance under drought stress at the anthesis stage (Fig. 7d). However, on 10d in AKAW3717, the MDA content increased. The highest MDA content was observed in HD2687 and PBW343 in severe stress and minimum in C306 while AKAW3717 showed an intermediate response. In both the susceptible cultivars, more lipid peroxidation occurred on 10d, indicating less membrane stability under drought stress. Kaur et al. [45] also reported higher MDA levels in PBW343 than C306 under water stress. Marcinska et al. [56] also observed a differential response in drought-susceptible and drought-tolerant genotypes at the seedling stage. The relative tolerance of a genotype to water stress as reflected by its lower lipid peroxidation and higher membrane stability has been reported in wheat [57].
Gene Expression in Response to Water Stress
Water stress can trigger a wide variety of plant responses, including regulation of gene expression. The expression of antioxidant enzyme-related genes was analysed by qRT-PCR in the drought tolerant (DT) genotype C306 and drought susceptible (DS) genotype HD2687 at the anthesis stage on 1d and 10d under severe stress. The genes included Cu/Zn-SOD which encodes chloroplastic copper/zinc superoxide dismutase; Fe-SOD, which encodes iron-superoxide dismutase; Mn-SOD, which encodes mitochondrial manganese superoxide dismutase, CAT—catalase and POX—peroxidase. Studying the relation of gene expression and stress tolerance at the level of RNA abundance can give reliable information on antioxidant enzyme-related gene activation than any other enzyme activity [58].
A slight upregulation in the transcript level of Cu/Zn-SOD was observed at 10d in C306 (DT), while its expression was significantly downregulated under severe water stress in HD2687 (DS) at 10d as compared to the control (Fig. 8a). Xu et al. [59] have shown that transcript levels for Cyt Cu/Zn-SOD and Chl Cu/Zn-SOD were enhanced significantly during drought, whereas SOD activity was pronouncedly decreased in leaves of Kentucky bluegrass. The expression of Mn-SOD was found significantly upregulated at both 1d and 10d in C306 (DT) as compared to the control (Fig. 8b). The highest increase in transcript level of Mn-SOD was observed at 10d in C306 (42.8 %) as compared with the well-watered (WW) condition and significantly decreased in HD2687 at 1d and 10d. The transcript level of Mn-SOD goes along with the changes in the profile of SOD activity. Our data showed the higher expression of Mn-SOD in the drought tolerant genotype at the anthesis stage under severe water stress which suggests that Mn-SOD might play an important role in drought tolerance. Similarly, Baek et al. [60] reported the significant upregulation and role of the Mn-SOD gene under water stress in wheat. Wang et al. [61] also showed that in the presence of Mn-SOD, drought tolerance increased in transgenic rice. The transcript level of Fe-SOD under severe water stress was downregulated in both the genotypes C306 (DT) and HD2687 (DS). Compared with the control, the highest decrease in the transcript level of Fe-SOD was found at 10d in C306 (38.7 %) (Fig. 8c). The susceptible genotype has shown limited expression of Mn-SOD and Fe-SOD, which showed their sensitivity to water stress. The overall relative fold expression of Fe-SOD was comparatively less than that of Cu/Zn-SOD in both the genotypes. The results revealed that genes Mn-SOD and Cu/Zn-SOD contributed mainly to the overall change in SOD activity and scavenging of O2 − in wheat genotypes.
The transcript level of CAT was significantly inhibited by water stress in both the genotypes. In relation to the well watered condition and increased time of stress severity, expression of this gene was significantly downregulated in both C306 (DT) and HD2687 (DS) genotypes (Fig. 9a). The gene expression of CAT did not go along with the catalase activity change in HD2687. The discrepancy between gene expression and enzyme activity indicates that enzyme activity changes were not caused by the mRNA level but were regulated at the post-transcriptional level, which in part might be enzyme inactivation/activation or degradation/synthetic processes induced by drought.
The trend of variation in the expression level of POX (Fig. 9b) was similar to the expression of Mn-SOD. The transcript level of POX increased from 1d to 10d in genotype C306 while it decreased significantly in HD2687 from 1d to 10d. It is evident that the expression of genes related to antioxidant enzymes appears to be intimately associated with a cascade of physiological, cellular and molecular events developed in parallel and dependent not only on the degree of genotype tolerance [62] but also on the severity and exposure time to water stress.
Conclusion
Oxidative damage is an important factor that could decrease plant yield. Drought tolerant genotypes showed membrane protection compared to the control, however, the fact that the activity of any antioxidant enzyme cycle was superior to that of the control may be indicative of cultivar stability. Our results indicated that drought resistant wheat cultivars had higher SOD and peroxidase activities and lower MDA content than drought sensitive wheat cultivars at the anthesis stage, suggesting that drought resistant wheat cultivars protected themselves more efficiently than the drought sensitive wheat cultivars under drought stress. Genotypic differences in drought tolerance could be attributed to the ability of wheat plants to acclimate and induce antioxidant defence under stress. As revealed in the study, water stress imposed major disturbances in the antioxidant gene expression, causing alterations in wheat transcript profiles. Noticeably, the higher transcript level of Mn-SOD in the drought tolerant versus drought susceptible genotype suggests that activation of this stress responsive gene could be involved in the antioxidant protection of wheat during drought. This system provides an excellent opportunity to study the mechanism involved in gene regulation in response to stressful conditions.
Abbreviations
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- POX:
-
Peroxidase
- CAT:
-
Catalase
- MDA:
-
Malondialdehyde
References
Kirigwi, F. M., van Ginkel, M., Trethowan, R., Sears, R. G., Rajaram, S., & Paulsen, G. M. (2004). Evaluation of selection strategies for wheat adaptation across water regimes. Euphytica, 135, 361–371.
Almeselmani, M., Abdullah, F., Hareri, F., Naaesan, M., Ammar, M. A., Kanbar, O. Z., & Saud, A. A. (2011). Effect of drought on different physiological characters and yield component in different varieties of Syrian durum wheat. Journal of Agricultural Science, 3, 127–133.
Lorenz, G. F., Bennett, J. M., & Loggale, L. B. (1987). Difference in drought resistance between two corn hybrids in water relations and root length density. Agronomy Journal, 79, 802–807.
Winter, S. R., Musick, J. T., & Porter, K. B. (1998). Evaluation of screening techniques for breeding drought resistant winter wheat. Crop Science, 28, 512–516.
Grzesiak, S., Grzesiak, M. T., & Filek, W. (2003). Evaluation of physiological screening tests for breeding drought resistant Triticale (x Triticosecale Wittmack). Acta Physiologiae Plantarum, 25, 29–37.
Tan, Y., Liang, Z., & Shao, H. (2006). Effect of water deficits on the activity of anti-oxidative enzymes and osmoregulation among three different genotypes of radix astragali at seeding stage. Colloid Surface Biointerfaces, 49, 60–65.
Singh, T., & Malik, D. S. (1983). Effect of water stress at three growth stages on the yield and water-use efficiency of dwarf wheat. Irrigation Science, 4, 239–245.
Zhang, X., Wang, Y., Sun, H., Chen, S., & Shao, L. (2013). Optimizing the yield of winter wheat by regulating water consumption during vegetative and reproductive stages under limited water supply. Irrigation Science, 31, 1103–1112.
Mogensen, V. O., Jensen, H. E., & Rab Md, A. (1985). Grain yield, yield components, drought sensitivity and water use efficiency of spring wheat subjected to water stress at various growth stages. Irrigation Science, 6, 131–140.
Loggini, B., Scartazza, A., Brugnoli, E., & Navari-Izzo, F. (1999). Antioxidative defense system, pigment composition, and photosynthetic efficiency in two wheat cultivars subjected to drought. Plant Physiology, 119, 1091–1099.
Lascano, H. R., Antonicelli, G. E., Luna, C. M., Melchiorre, M. N., Gomez, L. D., Racca, R. W., Trippi, V. S., & Casano, L. M. (2001). Antioxidant system response of different wheat cultivars under drought: field and in vitro studies. Australian Journal of Plant Physiology, 28(11), 1095–1102.
Shinozaki, K., & Yamaguchi-Shinozaki, K. (2007). Gene networks involved in drought stress response and tolerance. Journal of Experimental Botany, 58, 221–227.
Sheoran, S., Pandey, B., Sharma, P., Narwal, S., Singh, R., Sharma, I., & Chatrath, R. (2013). In silico comparative analysis and expression profile of antioxidant proteins in plants. Genetics and Molecular Research, 12(1), 537–551.
Cornic, G., & Fresneau, C. (2002). Photosynthetic carbon reduction and oxidation cycles are the main electron sinks for photosystem II activity during a mild drought. Annals of Botany, 89, 887–894.
Harb, A., Krishnan, A., Ambavaram, M. M. R., & Pereira, A. (2010). Molecular and physiological analysis of drought stress in Arabidopsis reveals early responses leading to acclimation in plant growth. Plant Physiology, 154, 1254–1271.
Bota, J., Medrano, H., & Flexas, J. (2004). Is photosynthesis limited by decreased Rubisco activity and RuBP content under progressive water stress? New Phytologist, 162, 671–681.
De Carvalho, M. H. C. (2008). Drought stress and reactive oxygen species: production, scavenging and signaling. Plant Signaling & Behavior, 3, 156–165.
Bartosz, G. (1997). Oxidative stress in plants. Acta Physiologia Plantrum, 19, 47–64.
Almeselmani, M., Deshmukh, P. S., Sairam, R. K., Kushwaha, S. R., & Singh, T. P. (2006). Protective role of antioxidant enzymes under high temperature stress. Plant Science, 171, 382–388.
Sairam, R. K., Deshmukh, P. S., & Shukla, D. S. (1997). Tolerance of drought and temperature stress in relation to increased antioxidant enzyme activity in wheat. Journal of Agronomy and Crop Science, 178, 171–178.
DaCosta, M., & Huang, B. (2007). Changes in antioxidant enzyme activities and lipid peroxidation for bentgrass species in response to drought stress. Journal of the American Society for Horticultural Science, 132, 319–326.
Munns, R. (2005). Genes and salt tolerance: bringing them together. New Phytologist, 167, 645–663.
Zhang, J., & Kirkham, M. B. (1994). Drought stress-induced changes in activities of superoxide dismutase, catalase and peroxidase in wheat species. Plant and Cell Physiology, 35, 785–791.
Keyvan, S. (2010). The effects of drought stress on yield, relative water content, proline, soluble carbohydrates and chlorophyll of bread wheat cultivars. Journal of Animal and Plant Sciences, 8(3), 1051–1060.
Barrs, H. D., & Weatherley, P. E. (1962). A re-examination of the relative turgidity technique for estimating water deficits in leaves. Australian Journal of Biological Sciences, 15, 413–428.
McCord, J. M., & Fridovich, I. (1969). The utility of superoxide dismutase in studying free radical reactions. I: Radicals generated by the interaction of sulfite, dimethyl sulfoxide, and oxygen. The Journal of Biological Chemistry, 244, 6056–6063.
Jebara, S., Jebara, M., Limani, F., & Aouani, M. E. (2005). Changes in ascorbate peroxidase, catalase, guaiacol peroxidase and superoxide dismutase activities in common bean (Phaseolus vulgaris) nodules under salt stress. Journal of Plant Physiology, 102, 929–936.
Rao, L., Perez, D., & White, E. (1996). Lamin proteolysis facilitates nuclear events during apoptosis. Journal of Cell Biology, 135, 1441–1455.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Annals of Biochemistry, 72, 248–254.
Heath, R. L., & Packer, L. (1968). Photoperoxidation in isolated chloroplasts. Kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics, 125, 189–198.
Dhindsa, R. S., Dhindsa, P., & Thorpe, T. A. (1981). Leaf senescence correlated with increased levels of membrane permeability and lipid peroxidation and decreased levels of superoxide dismutase and catalase. Journal of Experimental Botany, 32, 93–101.
Hendry, G. A. F., & Price, A. H. (1993). Stress indicators: chlorophylls and carotenoids. In G. A. F. Hendry & J. P. Grime (Eds.), Methods in comparative plant ecology (pp. 148–152). London: Chapman & Hall.
Sairam, R. K., Deshmukh, P. S., Shukla, D. S., & Ram, S. (1990). Metabolic activity and grain yield under moisture stress in wheat genotypes. Indian Journal of Plant Physiology, 33, 226–231.
Nyachiro, J. M., Briggs, K. G., Hoddinott, J., & Johnson-Flanagan, A. M. (2001). Chlorophyll content, chlorophyll fluorescence and water deficit in spring wheat. Cereal Research Communication, 29, 135–142.
Pan, X. Y., Wang, Y. F., Wang, G. X., Cao, Q. D., & Wang, J. (2002). Relationship between growth redundancy and size inequality in spring wheat population mulched with clear plastic film. Acta Phytoecology Sinica, 26, 177–184.
Waldren, P. R., & Flowerday, A. D. (1979). Growth stages and distribution of dry matter N, P and K in winter wheat. Agronomy Journal, 71, 391–397.
Saini, H. S., & Westgate, M. E. (2000). Reproductive development in grain crops during drought. Advances in Agronomy, 68, 59–95.
Fageria, N. K., Baligar, V. C., & Charles, A. J. (1991). Growth and mineral nutrition of field crops. (Books in soils, plants, and the environment series). New York: Marcel Dekker Inc.
Craudfurd, P. Q., Vadz, V., Jagadish, S. V. K., Prasad, P. V. V., & Zaman-Allah, M. (2013). Crop science experiments designed to inform crop modelling. Agricultural and Forest Meteorology, 170, 8–18.
Gooding, M. J., Ellis, R. H., Shewry, P. R., & Schofield, J. D. (2003). Effects of restricted water availability and increased temperature on the grain filling, drying and quality of winter wheat. Journal of Cereal Science, 37, 295–309.
Passioura, J. (2007). The drought environment: physical, biological and agricultural perspectives. Journal of Experimental Botany, 58, 113–117.
Quatracci, M. F., Sgherri, C. M., Pinzino, C., & Navari-Izzo, F. (1994). Superoxide production in wheat plants differently sensitive to drought. Proceedings of The Royal Society of Edinburgh, 102B, 287–290.
Bartoli, C. G., Simonthacchi, M., Tambussi, E. A., Beltrano, J., Montaldi, E. R., & Puntarulo, S. (1999). Drought and watering-dependent oxidative stress: effect on antioxidant content in Triticum aestivum L. leaves. Journal of Experimental Botany, 50, 375–383.
Srivalli, B., Sharma, G., & Khanna-Chopra, R. (2003). Antioxidative defence system in an upland rice cultivar subjected to increasing intensity of water stress followed by recovery. Physiologia Plantarum, 119, 503–512.
Kaur, M., Gupta, A. K., & Zhawar, V. K. (2014). Antioxidant response and lea gene expression under exogenous ABA and water deficit stress in wheat cultivars contrasting in drought tolerance. Journal of Plant Biochemistry and Biotechnology, 23(1), 18–30.
Harb, A., Awad, D., & Samarah, N. (2015). Gene expression and activity of antioxidant enzymes in barley (Hordeum vulgare L.) under controlled severe drought. Journal of Plant Interactions, 12(1), 109–116.
Feng, Z., Jin-Kui, G., Ying-Li, Y., Wen-Liang, H., & Li-Xin, Z. (2004). Changes in the pattern of antioxidant enzymes in wheat exposed to water deficit and rewatering. Acta Physiologiae Plantarum, 26, 345–352.
Simova-Stoilova, L., Demirevska, K., Petrova, T., Tsenov, N., & Feller, U. (2008). Antioxidative protection in wheat varieties under severe recoverable drought at seedling stage. Plant, Soil and Environment, 54, 529–536.
Hameed, A., Bibi, N., Akhter, J., & Iqbal, N. (2011). Differential changes in antioxidants, proteases, and lipid peroxidation in flag leaves of wheat genotypes under different levels of water deficit conditions. Plant Physiology and Biochemistry, 49, 178–185.
Mercado, J. A., Matas, A. J., Heredia, A., Valpuesta, V., & Quesada, M. A. (2004). Changes in the water binding characteristics of the cell walls from transgenic Nicotiana tabacum leaves with enhanced levels of peroxidase activity. Physiologia Plantarum, 122, 504–512.
Khanna-Chopra, R., & Selote, D. S. (2007). Acclimation to drought stress generates oxidative stress tolerance in drought-resistant than-susceptible wheat cultivar under field conditions. Environmental and Experimental Botany, 60, 276–283.
Devi, R., Kaur, N., & Gupta, A. K. (2012). Potential of antioxidant enzymes in depicting drought tolerance of wheat (Triticum aestivum L.). Indian Journal of Biochemistry & Biophysics, 49, 257–265.
Valifard, M., Moradshahi, A., & Kholdebarin, B. (2012). Biochemical and physiological responses of two wheat (Triticum aestivum L.) cultivars to drought stress applied at seedling stage. Journal of Agriculture Science and Technology, 14, 1567–1578.
Liu, X., & Huang, B. (2000). Heat stress injury in relation to membrane lipid peroxidation in creeping bentgrass. Crop Science, 40, 503–510.
Shao, H. B., Guo, Q. J., Chu, L. Y., Zhao, X. N., Su, Z. L., Hu, Y. C., & Cheng, J. F. (2007). Understanding molecular mechanism of higher plant plasticity under abiotic stress. Colloids Surfaces B., 54(1), 37–45.
Marcinska, I., Czyczyło-Mysza, I., Skrzypek, E., Filek, M., et al. (2013). Impact of osmotic stress on physiological and biochemical characteristics in drought-susceptible and drought-resistant wheat genotypes. Acta Physiologia Plantarum, 35, 451–461.
Khan, N., & Naqvi, F. N. (2010). Effect of water stress on lipid peroxidation and antioxidant enzymes in local bread wheat hexaploids. Journal of Food, Agriculture and Environment, 8(2), 521–526.
Lin, K., Huang, H., & Lin, C. (2010). Cloning, expression and physiological analysis of broccoli catalase gene and Chinese cabbage ascorbate peroxidase gene under heat stress. Plant Cell Reports, 29, 575–593.
Xu, L., Han, L., & Huang, B. (2011). Antioxidant enzyme activities and gene expression patterns in leaves of Kentucky bluegrass in response to drought and post-drought recovery. Journal of the American Society for Horticultural Science, 136(4), 247–255.
Baek, K.-H., & Skinner, D. Z. (2003). Alteration of antioxidant enzyme gene expression during cold acclimation of near-isogenic wheat lines. Plant Science, 165, 1221–1227.
Wang, X., Cai, J., Jiang, D., Liu, E., Dai, T., & Cao, W. (2005). Enhanced drought tolerance of transgenic rice plants expressing a pea manganese superoxide dismutase. Journal of Plant Physiology, 162, 465–472.
Hu, L., Li, H., Pang, H., & Fu, J. (2012). Responses of antioxidant gene, protein and enzymes to salinity stress in two genotypes of perennial ryegrass (Lolium perenne) differing in salt tolerance. Journal of Plant Physiology, 169(2), 146–156.
Acknowledgments
This work is an outcome of the project DWR/RPP/10-5.4. The authors are thankful to the Indian Institute of Wheat & Barley Research (IIWBR) and Indian Council of Agricultural Research (ICAR, New Delhi) for providing the research facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sheoran, S., Thakur, V., Narwal, S. et al. Differential Activity and Expression Profile of Antioxidant Enzymes and Physiological Changes in Wheat (Triticum aestivum L.) Under Drought. Appl Biochem Biotechnol 177, 1282–1298 (2015). https://doi.org/10.1007/s12010-015-1813-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1813-x