Abstract
Our previous studies showed that foliar applied magnesium sulfate during the booting stage can effectively increase wheat grain weight. To explore the effects of foliar application of magnesium sulfate during wheat grain filling on photosynthetic characteristics of flag leaf and grain filling, an experiment was conducted using winter wheat cultivars to assess the effects of foliar application of magnesium sulphate on photosynthetic characteristics of flag leaves, carbohydrate metabolism in grains, and dry matter translocation in different organs in the Zhoumai 27 and Aikang 58 cultivars at different growth stages. The results indicated throughout the different stages of growth, the flag leaves exhibited a high net photosynthetic rate (Pn), stomatal conductance (Gs) and transpiration (Tr), and a decrease in the concentration of intercellular CO2 (Ci). Therefore, foliar application of magnesium sulfate during the booting stage maintained high canopy photosynthesis after anthesis. Simultaneously, exogenous supply of magnesium sulphate enhanced the sucrose synthase (SUS) and invertase (INV) enzyme activities in detached wheat grains, meanwhile reinforced the activities of most starch synthesis enzyme such as ADP-glucose pyrophosphorylase and soluble starch synthase, and consequently lead to a higher content of grain starch. Furthermore, field experiment also confirmed foliar application of magnesium sulphate can improve superior dry matter accumulation and translocation in grain.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Starch synthesis in wheat (Triticum aestivum L.) comes from two types of photosynthate, current photosynthate transferres directly to the grain and photosynthate redistributes from reserve pools in vegetative tissues (Schnyder 2010). The contribution of stored carbohydrates to grain yield has been assessed as varying from 4 to 75% of the total amount of starch in grains, which depends upon the genotype, environment, and field practices (Pollock and Cairns 1991). For example, large kernel types have advantages over the small kernel types in terms of the amount of starch accumulation at mid and late stage (Dai et al. 2009). Pre-anthesis high temperature acclimation alleviates the negative effects of post-anthesis heat stress on stem stored carbohydrates remobilization and grain starch accumulation in wheat (Wang et al. 2012). Each crop plant can fully express its yield potential provided all external conditions are at optimum (Evans and Fischer 1999; Gardner 2015). Under field conditions, mineral nutrition supply is considered one of the major factors affecting carbohydrate partitioning (Ding and Xu 2011). Magnesium is the basic nutrient controlling processes responsible for photosynthesis and assimilate production and partitioning among plant parts. Mineral deficiencies were supposed to affect carbohydrate partitioning. When the plant lacks magnesium, the starch transport in the chloroplast is blocked, resulting in the accumulation of starch in chloroplasts (Cakmak and Marschner 1992; Shearman et al. 2005). Furthermore, a shortage of magnesium leads to decreased rate of root growth, preceding any response of above ground parts (Cakmak et al. 1994; Farhat et al. 2016). However, little is known about effects of foliar application of magnesium sulfate on photosynthetic characteristics, dry matter accumulation and its translocation, and carbohydrate metabolism in grains during wheat grain-filling. Additionally, the agronomic and physiological changes in response to apply foliar magnesium sulfate have not been characterized. The aim of this study was to examine the effects of applied foliar magnesium sulfate at the booting stage, on the agronomic and physiological traits of wheat varieties Zhoumai 27 and Aikang 58 to find out the causes of higher dry matter production of applied foliar magnesium sulfate.
Materials and methods
Field experiments were conducted in Lieshan District, Anhui Province, China (33° 16′ N 117° 23′ E, 40 m above sea level) over two growing seasons (2014/2015 and 2015/2016). Two popular wheat varieties were used, Zhoumai 27 and Aikang 58. Zhoumai 27 was bred by Zhoukou academy of agricultural sciences of Henan province and released in 2011. It was widely used in the Huanghuai winter wheat area of China. Aikang 58, a high yielding variety, was bred by Henan institute of science and technology and released in 2005.
Two popular wheat varieties were sown on 31 October 2014 and 2 November 2015 in plot areas of 6.6 m−2 (3.3 m × 2 m) with 8 rows, with row spacing of 20 cm. The density of plant was 200 plant m−2. The previous studies showed that foliar applied 25 mg L−1 magnesium sulfate during the booting stage can effectively increase wheat grain weight (unpublished). Two levels of magnesium sulphate (0 and 25 mg L−1) were applied during the booting stage as treatments MS0 (foliar applied distilled water, 0.1 L m−2) and MS1 (25 mg L−1, 0.1 L m−2). Each treatment was replicated three times in a randomized trial layout. The treatment of magnesium sulphate was applied by a back-pack electric sprayer with single nozzle within the canopy at a rate of 0.1 L m−2 at a concentration of 25 mg L−1 in the afternoon between 16:00 and 19:00, and the two treatments were sprayed for three consecutive days to strengthen their effects. The field soil was a clay loam with 45.6 g kg−1 organic matter, 175 mg kg−1 alkali-hydrolyzable N, 9.8 mg kg−1 Olsen-P, and 116 mg kg−1 exchangeable K in the 0–20 cm soil profile. 13.68 m2 (3.6 m wide × 3.8 m long in 18 rows, 20 cm apart) with line socket spacing of 20 cm × 10 cm. Nitrogen basal fertilizer was applied at 145 kg ha−1. Other cultivation and management measures were consistent with the field production.
Only tagged tillers were used for measurements of photosynthesis at 7, 14, 21, and 28 days after anthesis (DAA). Ears were tagged at florescence and sampled at 7, 14, 21, 28, and 35 DAA. Two kernels from between 3 and 12 spikelets at the base of each ear were sampled, and 200 kernels were taken at each time point. Of these, 50 kernels were frozen in liquid nitrogen for 30 min and placed in a freezer at − 80 °C.
The greenness of the topmost, fully expanded lamina, was measured in both cultivars after anthesis, using a non-destructive, hand-held chlorophyll meter (SPAD-502; Konica Minolta Sensing Inc., Osaka, Japan). The chlorophyll meter (soil–plant analysis development, SPAD) is a quick, rapid tool for estimating chlorophyll concentration in leaves nondestructively. The SPAD readings were obtained from the one-third and two-third positions of each lamina. Ten laminas were measured in each subplot, and these values were averaged.
Due to unavoidable variation in field measurements of photosynthesis parameters, up to ten tillers per variety were used for the measurements of photosynthesis. Measurements were started on the day after the onset of flowering (day one) and repeated at intervals of 2 days. On each tiller, one measurement of photosynthesis was made (in the morning). The net photosynthetic rate (Pn), stomatal conductance (Gs), transpiration (Tr) and intercellular CO2 (Ci) were measured using a LI-COR 6400 portable photosynthesis system (LI-COR, Inc., Lincoln Nebraska, USA). Measurements from flag leaves were taken on sunny days between 9:00 and 10:30 a.m, to avoid potential stomatal closure during the middle of the day. The CO2 concentration in the leaf chamber was set at 400 mol mol−1 and the photosynthetic active radiation was set at 1100 micromol m−2s−1. On each measurement date, 10 leaves from ten individual plants in each of the three replicates of each treatment were analyzed.
The preparation of enzyme extracts was similar to that described (Nakamura et al. 1989). SS was assayed in the cleavage direction and analysed as previously described (Sung et al. 1989). AGPase was assayed by the method (Nakamura et al. 1989). SSS were determined according to the previous method (Schaffer and Petreikov 1997). Invertase activity were estimated according to the protocol described (Koonjul et al. 2005).
Measurements of sucrose and hexose determinations were performed as described (Oliver et al. 2005). Starch content was measured according to the method described (Buysse and Merckx 1993).
Samples were collected from a 0.25 m2 area in each plot at maturity to estimate the content of dry matter and its components. The samples were separated into leaf, culm and stalk, chaff, and grains, and were dried at 60 °C until they reached a constant weight.
The data were processed in Microsoft Excel 2007 and then graphed with Sigma Plot 10.0 software. Analysis of variance (ANOVA) was performed for grain yield, dry matter weight, ear trait, and plant characteristics using SPSS17.0 (SPSS Institute Inc.).
Results
SPAD values is a parameter that measures the relative amount of chlorophyll in a plant leaves. SPAD readings may represent a useful screening criterion in breeding programs aimed at increasing the rate and duration of leaf photosynthesis. Changes in SPAD readings after anthesis are shown in Table S1, which exhibited a sharp reduction at 21 DAA after anthesis in both varieties in periods from 2014 to 2015 and 2015 to 2016. Responses to MS1 had no significant effect on the SPAD readings at early grain filling stage. However, SPAD readings with the treatment MS1 significantly elevated in both wheat varieties after 21 DDA, suggesting that MS1 treatment improved flag leaf SPAD reading attenuation in the late stage of filling, and delayed leaf senescence during reproductive growth.
The Pn, Gs, Tr and Ci of flag leaves after anthesis in both varieties in both growing seasons are shown in Fig. S1. The Pn of flag leaves under the MS1 treatment was greater than that of flag leaves under MS0 treatment from 7 to 28 DAA in both growing seasons, although only statistically different at the late filling stage (Fig. S1a), suggesting that magnesium sulphate application improved the photosynthetic capacity, and maintained higher photosynthetic rate during later grain filling, was of benefit to carbohydrate supply in kernel. The Gs and Tr under MS1 treatment were greater than those under MS0 treatment from 7 to 28 DAA in both growing seasons. Gs is an important parameter that reflects the gas exchange capacity of leaves and is closely related to the Pn. High Gs facilitates the entry of external CO2 into the leaves, which maintains a high Pn, so the trends in Gs were similar to those of Pn (Fig. S1b). The stomatal conductance of flag leaf initially increased, but slowly declined from anthesis to maturity, and that of MS1 treatment was significantly higher than MS0 treatment, and it increased gradually from 14 to 28 DAA, although only statistically different at the late filling stage (Fig. S1c). MS1 treatment had greater effects on the Ci values of the two wheat varieties in the late grain filling stage in both growing seasons. From 7 to 28 DAA, the Ci of flag leaves of the two varieties were higher in MS1 treatments than MS0 (Fig. S1d), while foliar application of magnesium sulphate failed to significantly increase the Tr of flag leaves for both cultivars from 0 to 7 DAA.
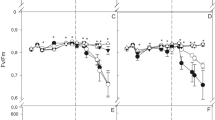
To understand the relationship between duration of photosynthesis in the flag leaf and grain filling, we compared the levels of sugars in MS1 and MS0 plants. The contents of sucrose, glucose, and fructose were measured. In general, panicle grains of the two genotypes had similar sugar levels during most of development in both growing seasons (Fig. 1). Sucrose was the major sugar, whose levels changed relatively little during development. Sucrose levels in MS1 were not significant lower (p < 0.05) than those in MS0 for both varieties (except before 14 DAA) at all stages (Fig. 1a). Hexose levels per grain fell during development. Glucose levels in MS1 were not significant higher (p < 0.05) than that in MS0 for both varieties (except 35 DAA) at all stages, when the level was 53.8–62.5% higher in MS1 grains than in MS0 grains (Fig. 1b). Fructose levels were not significant higher except at 7 DAA, when MS1 grains had 22.6–42.3% more than MS0 grains for both varieties in both growing seasons (Fig. 1c).
Changes in the contents of sucrose, glucose, fructose and starch in two winter wheat cultivars in both growing seasons in MS0 and MS1 plants. Where MS0 is the control and MS1 denotes the MgSO4 treatment at the booting stage. Bars indicate standard errors of the means of three measurements. Asterisks denote significant differences between treatments at p < 0.05 (*) by the LSD test
Starch levels were similar rising tendency through development in MS1 and MS0 plants, starch content in MS1 were higher (p < 0.05) than that in MS0, but not significant before 35 DDA (Fig. 1d). At 35 DAA, starch content in MS1 grains was markedly higher than MS0 grains, which probably contributed to the grain weight difference (Table S2).
Sucrose, the major translocatory form of carbon in cereals, enters per se into the wheat endosperm without its prior inversion into hexoses. Cell wall-bound invertase (Cw-Inv) is an extracellular enzyme catalyzing the cleavage of the transport sugar sucrose into glucose and fructose. Hence, Cw-Inv activity increases the local hexose availability and is therefore thought to be a key enzyme for supplying sink tissues with carbohydrates. Cw-Inv activity in the kernel of MS1 was higher than in MS0 during grain filling. However, Cw-Inv activities in MS1 were not significant higher (P < 0.05) than those in MS0 for all varieties (except 14 DAA) at all stages (Fig. 2a).
Changes in activities of cell wall-bound invertase (Cw-Inv) and sucrose synthase (SS) in two winter wheat cultivars in both growing seasons in MS0 and MS1 plants. Where MS0 is the control and MS1 denotes the MgSO4 treatment at the booting stage. Bars indicate standard errors of the means of three measurements. Asterisks denote significant differences between treatments at p < 0.05 (*) by the LSD test
In wheat kernels, the function of SS is to catalyze the conversion of sucrose into UDPG and fructose and then into starch. Changes in SS activity correspond to the rate of conversion of sucrose. SS activity in the kernel of MS1 was higher than in MS0 during grain filling (Fig. 2b). These results demonstrated that suggesting that magnesium sulphate application may be favorable to increase sucrose content and promote the accumulation of starch in grain.
ADP-glucose pyrophosphorylase (AGPase) catalyse the reaction between Glucose-1-phospate and ATP to produce ADP-glucose. AGPase activity peaked at 21 DDA after anthesis in both cultivars and in both growing seasons. However, AGPase activity in the kernel of MS1 was not significant higher than in MS0 during grain filling except at 21 DAA (Fig. 3a).
Changes in activities of ADP-glucose pyrophosphorylase (AGPase) and soluble starch synthase (SSS) in two winter wheat cultivars in both growing seasons in MS0 and MS1 plants. Where MS0 is the control and MS1 denotes the MgSO4 treatment at the booting stage. Bars indicate standard errors of the means of three measurements. Asterisks denote significant differences between treatments at p < 0.05 (*) by the LSD test
The maximum catalytic activity of soluble starch synthase (SSS) qualifies as the single most important factor controlling the rate of synthesis of starch in wheat endosperm. SSS activity in the kernel of MS1 was higher than in MS0 during grain filling. However, SSS activity was affected markedly even under a short period of MS1 at 21 d after anthesis when other period did not show any significant response (Fig. 3b). The enzyme activity of MS1 grains measured at day 21 was 73% of the control on a grain basis and 68% of the control on a dry weight basis.
The dry matte distribution in various plant parts at maturity in MS1 and MS0 plants is presented in Table S2. In general, comparing the results for there MS1 and MS0, dry matter partitioning was enhanced in the grain of both wheat varieties when magnesium sulphate was applied at the booting stage. Treatment with MS1 resulted in significant and substantial increases in dry matter weight of plant compared to those of the MS0 treatments. In addition, dry matter weight of leaf, culm and stalk, chaff and grain increased significantly with respect to the control in both wheat varieties. Treatment with MS1 resulted in significant and substantial (in the range of 8.49–21.9%) increases in dry matter weight of the same organs compared to those of the MS0 treatments. In addition, treatment with MS1 resulted in significant (in the range of 6–12%) decreases in harvest index compared to those of the MS0 treatment (Table S2). These findings indicated that there were significant differences between the different winter wheat genotypes in the effects of magnesium sulphate and the greater redistribution of dry matter in different organs.
Discussion
Although some photosynthate produced before anthesis is used for grain filling, most of the photosynthate produced during the post anthesis period is the major source for starch synthesis in grain (Gebbing and Schnyder 1999; Maydup et al. 2010). In Mg-deficient plants, Mg deficiency decreased photosynthetic activity (Hermans and Verbruggen 2005). Our study showed that magnesium sulphate application increased photosynthetic rate during grain filling stage (Fig. S1a), suggested photosynthesis is regulated by foliar applied magnesium.
Photosynthesis in the flag leaves could thus account directly for up to 47% of the grain mass. All of this contribution for grain filling presumably comes increasingly from remobilisation of materials in the senescing leaves (Ruuska et al. 2006). The photosynthetic capacity of leaves greatly increased when Mg concentration was enhanced (Dimassi-Theriou and Bosabalidis 1997). The higher photosynthetic rate of flag leaf in MS1 produced more glucose equivalents per plant than was available in control plants (Fig. S1, Fig. 1). In MS1 plants, photosynthesis in the flag leaf could potentially make a greater contribution to grain filling than in control plants, over a longer period, represents a potential increase in grain mass.
Sucrose is one of temporary storage form of the photosynthate in wheat leaf, as well as the main form to transport in wheat plant (Schnyder 2010). And sucrose content in flag leaf was affected not only by sucrose synthesis but by sucrose degradation and sucrose transport. After anthesis, current photosynthate was transported to grain through phloem in the form of sucrose, thereafter degraded for starch synthesis. Magnesium is critically involved in the phloem loading of sucrose and thus carbohydrate partitioning between source and grain (Hermans et al. 2005).
Several studies have confirmed that the remobilization of water-soluble carbohydrates (WSC) in stem generally starts during the period of near constant rate of dry matter accumulation in grains (Bell and Incoll 1990; Li et al. 2017a). And, application of magnesium sulphate, also decreased a significant accumulation of WSC in grain (Fig. 1). Furthermore, the total amount of WSC reserves used for grain filling is much greater resulting in high yields (Bonnett and Incoll 1992; Li et al. 2017b).
Duration of grain filling may be limited by starch synthase activity (Borrill et al. 2015). It is generally observed that this activity is comparable with the rate of starch synthesis, and declines sharply during the later stages of grain development (Zhang et al. 2010). The rate of starch synthesis is controlled predominantly by starch synthase activity (Keeling et al. 1993). Application of magnesium sulphate increased starch synthesis in developing grain in middle and later stages in both genotypes (Fig. 3b). However, grain filling was limited at later stages by the capacity for starch synthesis (Borrill et al. 2015). Previous studies have suggested that the most destructive effect of magnesium deficiency is sugar accumulation in source leaves. Thus, an accumulation of carbohydrates in source leaves and a reduction of root growth are considered as an earlier response to Mg deficiency, since it is involved in biomass formation and carbohydrate partitioning (Hermans and Verbruggen 2005). We observed a significant reduction of soluble sugars (glucose, fructose, and sucrose) and a significant accumulation of starch in grain of wheat grown under Mg applied (Fig. 1), which correspond to the higher invertase activity and SS activity (Figs. 2, 3). Moreover, treatment with MS1 resulted in significant and substantial increases in dry matter weight of the same organs compared to those of the MS0 treatments (Table S2). These findings indicated that there were significant differences between the different winter wheat genotypes in the effects of magnesium sulphate and the greater redistribution of dry matter in different organs.
References
Bell CJ, Incoll LD (1990) The redistribution of assimilate in field-grown winter wheat. J Exp Bot 41:949–960
Bonnett GD, Incoll LD (1992) The potential pre-anthesis and post-anthesis contributions of stem internodes to grain yield in crops of winter barley. Ann Bot 69:219–225
Borrill P, Fahy B, Smith AM, Uauy C (2015) Wheat grain filling is limited by grain filling capacity rather than the duration of flag leaf photosynthesis: a case study using NAM RNAi plants. PLoS ONE 10:1–14
Buysse J, Merckx R (1993) An improved colorimetric method to quantify sugar content of plant tissue. J Exp Bot 44:1627–1629
Cakmak I, Marschner H (1992) Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol 98:1222–1229
Cakmak I, Hengeler C, Marschner H (1994) Partitioning of shoot and root dry matter and carbohydrates in bean plants suffering from phosphorus, potassium and magnesium deficiency. J Exp Bot 45:1245–1250
Dai Z, Yin Y, Wang Z (2009) Comparison of starch accumulation and enzyme activity in grains of wheat cultivars differing in kernel type. Plant Growth Regul 57:153–162
Dimassi-Theriou K, Bosabalidis AM (1997) Effects of light, magnesium and sucrose on leaf anatomy, photosynthesis, starch and total sugar accumulation, in kiwifruit cultured in vitro. Plant Cell Tissue Organ Culture 47:127–134
Ding Y, Xu G (2011) Low magnesium with high potassium supply changes sugar partitioning and root growth pattern prior to visible magnesium deficiency in leaves of rice. Am J Plant Sci 2:601–608
Evans LT, Fischer RA (1999) Yield potential: its definition, measurement, and significance. Crop Sci 39:1544–1551
Farhat N, Elkhouni A, Zorrig W, Smaoui A, Abdelly C, Rabhi M (2016) Effects of magnesium deficiency on photosynthesis and carbohydrate partitioning. Acta Physiol Plant 38:145–156
Gardner WK (2015) Sodium, calcium and magnesium ratios in soils of NW victoria, australia may restrict root growth and crop production. J Plant Nutr 9:1205–1215
Gebbing T, Schnyder H (1999) Pre-anthesis reserve utilization for protein and carbohydrate synthesis in grains of wheat. Plant Physiol 121:871–878
Hermans C, Verbruggen N (2005) Physiological characterization of Mg deficiency in Arabidopsis thaliana. J Exp Bot 56:2153–2161
Hermans C, Bourgis F, Faucher M, Strasser RJ, Delrot S, Verbruggen N (2005) Magnesium deficiency in sugar beets alters sugar partitioning and phloem loading in young mature leaves. Planta 220:541–549
Keeling PL, Bacon PJ, Holt DC (1993) Elevated temperature reduces starch deposition in wheat endosperm by reducing the activity of soluble starch synthase. Planta 191:342–348
Koonjul PK, Minhas JS, Nunes C, Sheoran IS, Saini HS (2005) Selective transcriptional down-regulation of anther invertases precedes the failure of pollen development in water-stressed wheat. J Exp Bot 56:179–186
Li G, Pan J, Cui K, Yuan M, Hu Q, Wang W, Mohapatra PK, Nie L, Huang J, Peng S (2017a) Limitation of unloading in the developing grains is a possible cause responsible for low stem non-structural carbohydrate translocation and poor grain yield formation in rice through verification of recombinant inbred lines. Front Plant Sci 8:1–15
Li Z, Wang F, Lin W, Zhao Q, Liu J, Cheng F (2017b) Carbon reserve and remobilization in leaf sheaths during the grain-filling stage in response to leaf early senescence. Acta Physiol Plant 39:10–24
Maydup ML, Antonietta M, Guiamet JJ, Graciano C, López JR, Tambussi EA (2010) The contribution of ear photosynthesis to grain filling in bread wheat (Triticum aestivum L.). Field Crops Res 119:48–58
Nakamura Y, Yuki K, Park SY, Ohya T (1989) Carbohydrate metabolism in the developing endosperm of rice grains. Plant Cell Physiol 30:833–839
Oliver SN, Van Dongen JT, Alfred SC, Mamun EA, Zhao X, Saini HS, Fernandes SF, Blanchard CL, Sutton BG, Geigenberger P (2005) Cold-induced repression of the rice anther-specific cell wall invertase gene OSINV4 is correlated with sucrose accumulation and pollen sterility. Plant, Cell Environ 28:1534–1551
Pollock CJ, Cairns AJ (1991) Fructan metabolism in grasses and cereals. Annu Rev Plant Biol 42:77–101
Ruuska SA, Rebetzke GJ, van Herwaarden AF, Richards RA, Fettell NA, Tabe L, Cld J (2006) Genotypic variation in water-soluble carbohydrate accumulation in wheat. Funct Plant Biol 33:799–809
Schaffer AA, Petreikov M (1997) Sucrose-to-Starch metabolism in tomato fruit undergoing transient starch accumulation. Plant Physiol 113:739–746
Schnyder H (2010) The role of carbohydrate storage and redistribution in the source-sink relations of wheat and barley during grain filling. New Phytol 123:233–245
Shearman VJ, Sylvesterbradley R, Scott RK, Foulkes MJ (2005) Physiological processes associated with wheat yield progress in the UK. Crop Sci 45:175–185
Sung SJ, Xu DP, Black CC (1989) Identification of actively filling sucrose sinks. Plant Physiol 89:1117–1121
Wang X, Cai J, Liu F, Jin M, Yu H, Jiang D, Wollenweber B, Dai T, Cao W (2012) Pre-anthesis high temperature acclimation alleviates the negative effects of post-anthesis heat stress on stem stored carbohydrates remobilization and grain starch accumulation in wheat. J Cereal Sci 55:331–336
Zhang C, Jiang D, Liu F, Cai J, Dai T, Cao W (2010) Starch granules size distribution in superior and inferior grains of wheat is related to enzyme activities and their gene expressions during grain filling. J Cereal Sci 51:226–233
Acknowledgements
The research was supported by Anhui Provincial Natural Science Foundation (1708085QC62), Scientific Research Foundation of the Higher Education Institutions of Anhui Province, China (KJ2019A0585), National Undergraduate Training Programs for Innovation and Entrepreneurship (201910373033), Anhui University Collaborative Innovation Project (GXXT-2019-033) and the Doctoral Scientific Research Foundation of Huaibei Normal University (15601047).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Changes in the net photosynthetic rate (Pn), the transpiration rate (Tr), the stomatal conductance (Gs) and the intercellular CO2 (Ci) in two winter wheat cultivars in both growing seasons in MS0 and MS1 plants. Where MS0 is the control and MS1 denotes the MgSO4 treatment at the booting stage. Bars indicate standard errors of the means of three measurements. Asterisks denote significant differences between treatments at p < 0.05 (*) by the LSD test (PDF 333 kb)
Rights and permissions
About this article
Cite this article
Ba, Q., Zhang, L., Chen, S. et al. Effects of foliar application of magnesium sulfate on photosynthetic characteristics, dry matter accumulation and its translocation, and carbohydrate metabolism in grain during wheat grain filling. CEREAL RESEARCH COMMUNICATIONS 48, 157–163 (2020). https://doi.org/10.1007/s42976-020-00026-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42976-020-00026-z