Abstract
To elucidate the contribution of non-structural carbohydrates (NSCs) stored in leaf sheaths to developing grains and the corresponding molecular regulatory mechanisms of rice plant when source leaves suffered the early aging during the grain-filling stage, the carbohydrate changes and transcriptional expression of several gene families related to carbohydrate metabolism were comprehensively investigated in leaf sheaths of two rice cultivars: early senescence leaf (esl) mutant and its wild type. Results showed that the translocation rate of NSCs in leaf sheaths and the contribution of NSCs to grain development were significantly enhanced in source leaves with early senescence at the grain-filling stage. The decrease of starch in leaf sheaths of esl mutant rice between the heading and harvest stages was lower than that in the wild type, whereas the translocation of sucrose and fructose in leaf sheaths of esl mutant rice was significantly higher than those in the wild type. The expression of SUT4 in leaf sheaths of esl mutant rice at the early grain-filling stage was probably responsible for the rapid sucrose loading into the phloem of leaf sheaths. The remarkably low expression of CIN1, SuSy1, and SuSy2 in leaf sheaths of esl mutant rice reflected the low sucrose metabolism. Meanwhile, the transcriptional levels of genes for starch metabolism-related enzymes in leaf sheaths of esl mutant rice were significantly lower than those of the wild type. Therefore, starch metabolism in leaf sheaths of esl mutant rice was depressed when source leaves underwent the early senescence during the grain-filling stage. The possible relationships among NSC translocation, carbohydrate metabolism, and transcription regulation in leaf sheaths of esl mutant rice were also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In rice plants, non-structural carbohydrates (NSCs), which temporarily stored in leaf sheaths before the heading stage, are translocated to the growing panicles and would contribute greatly to rice yield after the heading stage (Song et al. 1990; Ishikawa et al. 1993; He et al. 2005). The NSCs of leaf sheaths are effective in improving the relative growth rate of grains and increasing the percentage of ripened grains during the grain-filling stage (Weng et al. 1982; Tsukaguchi et al. 1996). Thus, the NSCs of leaf sheaths in rice have drawn the interest of agronomists as important factors for higher yield.
Depending on the cultivars and environmental conditions, the carbohydrates in leaf sheaths serve as alternative carbon buffer compounds for grain filling to compensate for the deficiencies of photosynthetic products under stress conditions (Liang et al. 1994; He et al. 2005; Li et al. 2008). The previous studies have revealed that the NSCs are temporarily stored in leaf sheaths before heading; these carbohydrates contributed approximately to 30% of final grain yield (Cock and Yoshida 1972; Samonte et al. 2001). Yang et al. (2001a, 2004) stated that the remobilization of NSCs from leaf sheaths to grains was enhanced by moderate water stress during the grain-filling stage in rice and wheat, thereby accelerating the rate of grain filling. Abou-khalifa et al. (2008) found that the reserved carbohydrates in leaf sheaths would contribute up to 50% of grain yield when source leaves were cut off during the grain-filling stage. However, various stresses also accelerate rice plant early senescence during the grain-filing stage, shorten grain filling period, and reduce the grain weight and setting percentage of grains, thereby displaying certain adverse effects to grain yield and quality (Weng et al. 1982; Song et al. 1990; Yang et al. 2001a, 2003). Therefore, the rational remobilization of NSCs pre-stored in leaf sheaths before heading seems to be crucial in cases where quickened leaf senescence occurs during the grain-filling stage because of adverse environmental conditions. The contribution of carbohydrates from leaf sheaths to grain yield needs to be further investigated when the source leaves suffered the early aging during the grain-filling stage.
Starch is transiently stored in leaf sheaths as one of the major NSCs. This carbohydrate is considered to mitigate the yield loss caused by unfavorable climate during the grain-filling stage (Ishimaru et al. 2004). ADP-Glucose pyrophosphorylase (AGPase; EC 2.7.7.27), starch synthase (SS; EC 2.4.1.21), and starch branching enzyme (SBE; EC 2.4.1.18) are generally known to play major roles in the starch synthesis of leaf sheaths and cereal endosperm (James et al. 2003; Hirose et al. 2006). AGPase catalyzes the first reaction in starch synthesis and produces the activated glucosyl donor: ADP-glucose. SS is composed of soluble (SSS) and granule-bound isoforms (GBSS); this enzyme elongates the amylose and amylopectin chains (Hirose and Terao 2004). SBE cleaves α-1,4 bonds on amylose and amylopectin molecules, and reattaches the released glucan segments via α-1,6 linkages to form branches on the starch polymers (Hurkman et al. 2003). Perez et al. (1971) observed that a change in starch content of leaf sheaths during the heading period was associated with the activity of granule-bound starch synthase (GBSS). Watanabe et al. (1997) reported that GBSS probably functioned in collaboration with AGPase, SSS, and SBE in leaf sheaths during the grain-filling stage. In addition, the transcriptions of APDL2, GBSSII, SSSI, SBEI, SBEIII, and SBEIV genes were also highly correlated with the rapid starch accumulation in leaf sheaths of rice (Hirose et al. 2006; Chen and Wang 2008). By contrast, α-amylase and β-amylase are responsible for starch digestion in plant (Liao et al. 2010). After the heading period, starch in leaf sheaths is digested by the reactions catalyzed by α-amylase and β-amylase; the products of starch cleavage are essential for growing grains via remobilization to the panicles (Smith et al. 2005). In addition to starch, sucrose, and its components, glucose and fructose play major roles in the transport and metabolism of carbon in leaf sheaths of rice (Scofield et al. 2007). After the heading period, carbohydrates in leaf sheaths are converted back to sucrose and returned to the long-distance transport pathway to the filling grains on the panicle catalyzed by sucrose transporters (SUTs), which are mainly dominated by the abundant expression of SUT1 and SUT4 in leaf sheaths during the grain-filling stage (Aoki et al. 2003; Scofield et al. 2007; Chen and Wang 2008). Besides sucrose transport, the level of sucrose in leaf sheaths also participates in the sucrose metabolism catalyzed by cytosolic fructose-1,6-bisphosphatase (cyFBPase), sucrose phosphate synthase (SPS, EC 2.1.4.14), sucrose synthase (SuSy, EC 2.4.1.13), and cell wall invertase (CIN) (Winter 2000). Among these enzymes, SPS and cyFBPase are responsible for irreversible sucrose synthesis from UDP-glucose and fructose-6-phosphate (Serrato et al. 2009). SuSy catalyzes the reversible conversion of sucrose and UDP into UDP-glucose and fructose, whereas CIN participates in the irreversible hydrolysis of sucrose to glucose and fructose (Sturm 1999). In rice, SPS, SuSy, and CIN exist in different isoforms and are encoded by a few families of isogenes, which exhibit distinct expression profiles in a wide range of tissues at different developmental stages (Ji et al. 2005; Hirose et al. 2008; Okamura et al. 2011), thereby maintaining the continuous cycle of sucrose synthesis and degradation. The rapid cycling of sucrose would allow cells to respond promptly to variations in sucrose supply and the cellular demand for carbon sources under biosynthesis processes (i.e., starch and cell wall synthesis) (Fernie et al. 2002). Thus, the metabolism of starch and sucrose in leaf sheaths reflects the functional transition of the sink–source of leaf sheaths in rice (Hirose et al. 1999; Chen and Wang 2008). However, the previous studies mainly focused on the sucrose transport and starch metabolism in leaf sheaths during the grain-filling stage. To date, little is known regarding the involvement of sucrose and starch in rice leaf sheaths when source leaves suffer from the early aging. In addition, the molecular mechanism for the conversion between sucrose and starch in leaf sheaths has yet to be elucidated when the source leaves undergo the early senescence during the grain-filling stage.
Therefore, this study aimed to advance the current understanding of the contribution rate of carbohydrates in leaf sheaths to growing grains when the source leaves suffered from the early aging. An additional aim was to elucidate the molecular regulatory mechanism of carbohydrate metabolism in leaf sheaths during the grain-filling stage. The rice cultivars: early senescence leaf (esl) mutant and its wild type, were employed to investigate the translocation rate of NSCs from leaf sheaths to growing grains and to determine the changes of starch, sucrose, glucose, and fructose in leaf sheaths between the heading and harvest stages. Meanwhile, the genotype-dependent differences in the transcriptional expression and temporal patterns of key genes involved in the sucrose and starch metabolism in leaf sheaths were analyzed by quantitative real-time reverse transcription PCR (qRT-PCR).
Materials and methods
Plant materials
The esl mutant was obtained by gamma-irradiated indica restore line Fu142. A stable esl inherited mutant was obtained by successive self-pollination; plant phenotype selection was performed from the M2 to M8 generations. The M8 generation seeds of esl and its corresponding control Fu142 were used in this study. Compared with a wild-type cultivar, the esl mutant did not exhibit noticeable phenotypic abnormalities, including leaf appearance in seedling and tillering stage. However, the leaves of esl mutant displayed exacerbated lesions and accelerated senescence at the late tillering stage, initially from the tips of the lower leaves, followed by exacerbated brown lesions spreading to cover the whole leaf blade, but the topmost 1–2 fully expanded leaves and heart leaf still retained normal green appearance. After anthesis, the flag leaf of esl mutant appeared senescence symptoms, and the exacerbated lesions gradually spreaded from the leaf tip downward to the whole leaf blade during the grain-filling stage, until completely withered approximately 25 days post anthesis. By contrast, the wild type still remained green during the same period (Li et al. 2014).
Rice seeds were sown in the seedling nursery at the experimental farm of the Zijingang campus (120°04′ E, 30°18′ N) of Zhejiang University in Hangzhou, China. A completely randomized field plot design was arranged with three replications for each cultivar. Each replication was planted in 10 × 12 rows, which were spaced at 18 cm × 18 cm, with one rice seedling for each hill. The field management was implemented according to local cultivation mode, and the soil type was periodically waterlogged paddy soil, with 1.69 g/kg total N, 24.5 mg/kg available P, and 103.7 mg/kg exchangeable K. At the full heading stage, 80–100 rice plants with uniform anthesis day were randomly selected and tagged. After the flowering stage, pollen fertilization was conducted prior to the grain-filling stage. From the beginning of the grain-filling stage, the leaf sheaths of the upper flag leaf with tagged plants were sampled at a 7-day interval, with three independent biological replicates at 9:00 a.m. Leaf sheaths were immediately frozen in liquid nitrogen and kept at −80 °C for gene expression analyses.
To calculate the translocation rate of NSCs in leaf sheaths, fresh leaf sheaths were sampled at the heading and harvest stages for NSC measurement. At maturity, ten tagged panicles were harvested from each replicate. The total grain number per panicle, filling grain number per panicle, seed-setting rate, weight of filling grains per panicle, and the harvest index were determined.
Determination of NSCs in leaf sheath of flag leaf
Fresh leaf sheaths were baked at 105 °C for about 30 min for deactivation of biological enzymes, and then dried at 60 °C until the constant weight. Dried sample was ground to powder by a pulverizer. 0.5 g sample powder was extracted with 80% (v/v) ethanol at 80 °C for 30 min, with three repeats. After centrifugation at 5000g for 15 min, the supernatant was collected for the determination of sucrose, glucose, and fructose, and the pellets were used to determine starch content.
The contents of sucrose, glucose, and fructose were determined according to the method described by Luo and Huang (2011). For sucrose determination, 0.4 mL supernatant was mixed together with 200 µL 2 mol L−1 NaOH in a 10 mL tube, and then the solution was incubated in boiling water for 5 min. After the solution cooled down to room temperature, 2.8 mL 30% HCl and 0.8 mL 0.1% dioxybenzene were added in the tube. Subsequently, the mixed solution was incubated at 80 °C for 10 min. The absorbance at 480 nm wavelength was measured, and sucrose content was calculated as the sucrose standard curve. For fructose determination, 1 mL supernatant was mixed with 2 mL 0.1% dioxybenzene and 1 mL water, and then was incubated at 80 °C for 10 min. After the sample cooled down, the absorbance was measured at 480 nm, and fructose content was calculated as the fructose standard curve. The determination of glucose content was based on the oxidation of glucose catalyzed by glucose oxidase. 2 mL supernatant and 4 mL of glucose oxidase reagent (0.1 mg mL−1 o-dianisidine, 0.1 mg mL−1 horseradish peroxidase, and 1 U mL−1 glucose oxidase) were incubated at 30 °C for 10 min in a 15 mL tube, followed by adding 8 mL of 10 mol L−1 H2SO4 solution for preventing the reaction. Within 1 h, the mixture was measured at 460 nm. The glucose content was calculated as glucose standard curve.
The determination of starch content was conducted according to the method of Nakamura and Miyachi (1982). The starch in residual pellets was first converted into glucose by perchloric acid, and the glucose could form into furfural derivatives and react with anthrone reagent. The residual pellets were rinsed with 20 mL distilled water in a 50 mL flask, and then were gelatinized in boiling water for 15 min. After the sample cooled down, 2 mL 9.2 mol L−1 perchloric acid was added, and the mixture was gelatinized again in boiling water for 15 min. After centrifugation at 5000g for 5 min, the supernatant was transferred into a new flask, and distilled water was added to a final volume of 50 mL. 2 mL of supernatant was mixed with 5 mL of anthrone reagent, and the mixed solution was boiled for 10 min. The absorbance at 620 nm was measured, and starch content was calculated as the standard curve.
RNA isolation and cDNA preparation
Frozen leaf sheath tissues (100 mg) were crushed to fine powder by a mortar and pestle, using liquid N2. Total RNA was extracted with the Trizol reagent (Invitrogen), according to the manufacturer’s protocol. The RNA quality was evaluated with a spectrophotometer (NanoDropTM 1000, Thermo Fisher Scientific, and USA), and genomic DNA pollution was removed by RNase-free DNase I at 37 °C. About 1 µg RNA was reverse transcribed for the cDNA synthesis using an oligo (dT) primer in 50 µL reaction buffer. The reaction condition was incubated at 37 °C for 15 min and then stopped after heating at 95 °C for 5 min.
Quantitative real-time PCR (qRT-PCR)
Aliquots of the first-strand cDNA mixture corresponding to 20 ng of total RNA were used as templates for qRT-PCR analysis, using the SYBR Green Real-time PCR Master Mix reagent Kit (TOYOBO, Osaka, Japan). The reaction was performed on the Bio-Rad CFX96 System (Bio-Rad, USA) according to the manufacturers’ protocols. The reaction procedures were 30 s at 95 °C, followed by 40 cycles of 95 °C for 5 s, 58 °C for 10 s, and 72 °C for 15 s, then a melting curve protocol from 58 to 95 °C following the final cycle of PCR to detect a single gene-specific peak for all primers tested. The gene-specific primer pairs were designed by Primer Premier 5.0 (Premier, Canada), as listed in Table S1. Actin was used as the internal control gene. The samples were normalized using ACTIN expression, whereas the relative transcript levels were analyzed using the \( 2^{{ - {\Delta \Delta }C_{\text{T}} }} \) method (Schmittgen and Livak 2008). The average value and standard error were calculated from three independent biological replicates.
Statistical analysis
All determinations were performed in at least three independent experiments. Statistical significances were estimated by the analysis of variance (ANOVA), using SPSS statistical software package (Chicago, USA). Differences were considered significant at a probability level of P < 0.05. Standard deviation (SD) was calculated and shown in the figures.
Results
Genotype-dependent differences in the NSC translocation rate of leaf sheaths and its contribution to grain yield
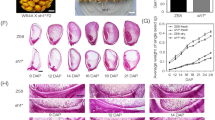
As shown in Table 1, the NSCs in leaf sheaths of esl mutant rice at the heading and harvest stages were 239.70 and 104.51 mg g−1, respectively, which were significantly lower than those in the wild type, from 294.93 to 196.81 mg g−1. However, the NSCs translocation rate in leaf sheaths of esl mutant rice reached up to 56.4%, but it was only 33.3% in wild-type cultivar. On the other hand, the agronomic traits of esl mutant rice were significantly lower than that of the wild type, such as the total grain number, filling grain number per panicle, seed-setting rate, weight of filling grains, and the harvest index. However, the contribution of NSCs in leaf sheaths to grain filling was up to 25.60% for esl mutant rice, which was significantly higher than that in wild type at only 3.52%. These results suggested that the early leaf senescence enhanced the translocation rate of NSCs in leaf sheaths during the grain-filling stage and promoted the contribution of NSCs in leaf sheaths to fill grains in esl mutant rice.
In addition, Table 2 depicts the differences in the sugar composition of NSCs in leaf sheaths of two rice cultivars between the heading and harvest stages. Starch, sucrose, and fructose were the dominant sugars in both rice cultivars. By contrast, the sucrose and starch content in leaf sheaths of esl mutant rice was significantly lower than in the wild type, whereas fructose and glucose showed similar amounts in both cultivars, which accounted for 81.77–85.6 and 19.53–25.70 mg g−1 at the heading stage, respectively. However, the decreased amount of sucrose, fructose, and starch varied in two rice cultivars. Sucrose and fructose in leaf sheaths of esl mutant exhibited a clear decline between the heading and harvest stages; the amounts of decrease were significantly higher than those in the wild type. By contrast, the decrease of starch in leaf sheaths of esl mutant rice was significantly lower than that in the wild type, thereby suggesting that the translocation of sucrose and fructose was markedly strengthened in the leaf sheaths of esl mutant rice, whereas starch remobilization was hardly executed. Therefore, the NSC translocation in leaf sheaths of esl mutant rice basically depended on the transduction of sucrose and fructose. By contrast, this process primarily relied on the starch remobilization in the wild type.
Transcriptional regulation of key genes involved in the sucrose transduction in leaf sheaths of two rice cultivars during the grain-filling stage
To explore the molecular mechanism of genotype-dependent differences in sucrose transduction, the transcriptional profiles of SUT, cyFBP, SPS, CIN, and SuSy isoforms and gene families were analyzed in the leaf sheaths of two rice cultivars. Among the five SUT isoforms, SUT1 and SUT4 were highly expressed in leaf sheaths, whereas SUT2 was detected with extremely low expression, but no transcripts were detected for SUT3 and SUT5 (Fig. 1a). Compared with the wild-type cultivar, SUT1 in leaf sheaths of esl mutant rice showed low and relatively constant expression patterns during the grain filling period, except for the similar expression level of the wild type during the initial stage of grain filling. In the wild-type cultivar, SUT1 transcription remained relatively constant at the early and middle stages of grain filling before reaching its maximum expression level at the final stage of grain filling (Fig. 1b). These results indicated that genotype-dependent variations existed in the temporal transcriptional pattern of SUT1 during the grain-filling stage, which might cause differences in the sucrose transport of leaf sheaths of two rice cultivars. Besides, SUT4 in leaf sheaths of esl mutant rice displayed higher expression than the wild type during the initial stage of grain filling before dropping to a similar transcript pattern as the wild type, which gradually decreased and maintained its low expression from 14 days to the final stage of grain filling (Fig. 1c). Therefore, SUT4 may play an important role in sucrose transport of leaf sheaths of esl mutant rice at the early stage of grain filling.
Genotypic difference in the SUT isoform gene expression and their temporal patterns in leaf sheaths of two rice cultivars; a comparison of five SUT isoform expression at anthesis; b, c time course of SUT1 and SUT4 expression, respectively, during the grain-filling stage. Vertical bars represent standard errors (n = 3). Asterisks represent significant differences (P < 0.05)
The cyFBP and SPS isoform genes in leaf sheaths of esl mutant rice showed significantly lower expression than the wild type during the grain-filling stage (Fig. 2). By contrast, cyFBP in leaf sheaths of esl mutant rice showed similar expression as that in the wild type during the initial stage of grain filling before slowly decreasing. Meanwhile, cyFBP in the wild type remained relatively consistent during the grain-filling stage (Fig. 2a). Among the five SPS isoforms, SPS2, SPS6, and SPS8 exhibited high expression in the leaf sheaths, whereas SPS1 displayed extremely low expression. However, the SPS11 transcript was not detectable in leaf sheaths (Fig. 2b). The temporal transcriptional patterns of SPS1, SPS2, SPS6, and SPS8 in leaf sheaths of esl mutant rice gradually decreased during the grain-filling stage (Fig. 2c–f). Specifically, SPS2, SPS6, and SPS8 hardly had any transcripts starting from the 14th day of grain filling (Fig. 2d–f). Compared with esl mutant rice, the wild type presented decreasing expression patterns for SPS2, SPS6, and SPS11 at the early stage of grain filling and the increasing amount of transcripts at the mid-late stage of grain filling (Fig. 2d–f). These results suggested that the sucrose synthesis in leaf sheaths of esl mutant rice manipulated by SPS2, SPS6, SPS8, and cyFBP was weakened by the early leaf senescence.
Genotypic differences in SPS isoform gene expression and temporal patterns of cyFBP and SPS isoforms in leaf sheaths of two rice cultivars; b comparison of five SPS isoform expression at anthesis; a, c–f the time course of cyFBP, SPS1, SPS2, SPS6, and SPS8 expression during the grain-filling stage, respectively. Vertical bars represent standard errors (n = 3). Asterisks represent significant differences (P < 0.05)
To characterize the conversion of sucrose and other monosaccharide in leaf sheaths at the molecular level, the transcription analysis of CIN and SuSy isoform genes was conducted. As shown in Fig. 3a, CIN1 and CIN4 were preferentially expressed in leaf sheaths, but the CIN2 transcript was not detected. Among the six SuSy isoform genes, the transcript of SuSy2 was the most abundant in leaf sheaths, but the least abundant level of SuSy1 transcript was detected, whereas the transcripts of SuSy3 and SuSy4 were seldom observed. However, no transcripts of SuSy5 and SuSy6 were detected in leaf sheaths (Fig. 3d). By contrast, the temporal transcripts of CIN1 and CIN4 in leaf sheaths of esl mutant rice were retained at a lower level than those in wild-type cultivars during the grain-filling stage with the declining expression pattern for CIN1 and the relatively constant transcript amount of CIN4 (Fig. 3b, c). However, SuSy1 and SuSy2 were observed to be expressed more highly in leaf sheaths of esl mutant rice as compared with those in wild-type cultivars during the initial stages of grain filling before gradually declined to less than those in the wild type in accordance with the grain filling (Fig. 3e, f). These results suggested that the conversion between sucrose and other monosaccharides in leaf sheaths of esl mutant rice chiefly occurred in the initial stage of grain filling, which were modulated by the transcription of CIN1, SuSy1, and SuSy2.
Genotypic differences in the expression of CIN and SuSy isoforms and their temporal patterns of CIN1, CIN4, SuSy1, and SuSy2 in leaf sheaths of two rice cultivars; a, d comparison of CIN and SuSy isoform genes at anthesis; b, c, e, f time course of CIN1, CIN4, SuSy1, and SuSy4 expression during the grain-filling stage, respectively. Vertical bars represent standard errors (n = 3). Asterisks represent significant differences (P < 0.05)
Transcriptional regulation of starch metabolism-related genes in leaf sheaths during the grain-filling stage
Among the seven genes coding for AGPase, transcripts of AGPS2a for the small subunit of the enzyme and AGPL3 for the large subunit were largely expressed in leaf sheaths. AGPS1, AGPL1, and AGPL2 were held at low expression amounts, whereas the transcripts for AGPS2b and AGPL4 were not detected (Fig. 4a). The esl mutant rice presented obviously higher transcripts for AGPS2a than the wild type in the initial stage of grain filling, and then declined to moderate level until 7 days of grain filling (Fig. 4c). AGPL2 and AGPL3 in leaf sheaths of the esl mutant rice showed nearly the similar expression patterns as those in the wild type (Fig. 4e, f). Surprisingly, AGPS1 and AGPL1 in leaf sheaths of esl mutant rice exhibited low expression during the grain-filling stage, whereas those in the wild type began to elevate their transcription from 14 days of grain filling and reached the peak levels during the final stage of grain filling (Fig. 4b, d), thereby implying that starch was deposited in leaf sheaths of the wild type in the final stage of grain filling.
Genotypic differences in AGP isoform gene expression and the temporal patterns in leaf sheaths of two rice cultivars; a comparison of seven AGP isoform gene expression at anthesis; b–f time course of AGPS1, AGPS2a, AGPL1, AGPL2, and AGPL3 expression during the grain-filling stage, respectively. Vertical bars represent standard errors (n = 3). Asterisks represent significant differences (P < 0.05)
In addition to AGPase, the transcript of GBSSII, one of the two GBSS genes, expressed largely in leaf sheaths, whereas the transcript of the other GBSS gene, GBSSI, was not detectable (Fig. 5a). The transcript of GBSSII in leaf sheaths of esl mutant rice was lower than that in the wild type during the grain-filling stage, on the contrary, GBSSII transcript in the wild type peaked at the final stage of grain filling (Fig. 5b), coinciding with the increases in the transcripts of AGPS1 and AGPL1. Apart from GBSSII, five SS genes, SSSI, SSSIIc, SSSIIIb, SSSIVa, and SSSIVb were detected to express in leaf sheaths, including high expression amount for SSSI and SSSIIIb, and low expression abundance for SSSIIc, SSSIVa, and SSSIVb (Fig. 5a). By contrast, the transcripts of SSSI and SSSIIIb in leaf sheaths of esl mutant rice were significantly lower than those in the wild type during the grain-filling stage (Fig. 5c, e). Compared with the wild-type cultivar, the esl mutant showed an obviously ascending transcription pattern for SSSIVa during the grain-filling stage (Fig. 5f), suggesting that SSSIVa maybe have special contribution to starch synthesis in leaf sheaths of esl mutant rice. However, SSSIIc and SSSIVb displayed low and irregular transcriptional patterns in leaf sheaths of two rice cultivars during the grain-filling stage (Fig. 5d, g), implying that SSSIIc and SSSIVb probably functioned in constitutive synthesis for starch in leaf sheaths.
Genotypic differences in the starch synthase isoform gene expression and their temporal patterns in leaf sheaths of two rice cultivars; a comparisons of the gene expression of ten starch synthase isoforms at anthesis; b–g time course of GBSSII, SSSI, SSSIIc, SSSIIIb, SSSIVa, and SSSIVb expression during the grain-filling stage, respectively. Vertical bars represent standard errors (n = 3). Asterisks represent significant differences (P < 0.05)
The expression of α-amylase gene family and β-amylase were identified in leaf sheaths of two rice cultivars during the grain-filling stage. As shown in Fig. 6a, Amy2A and Amy4A exhibited high transcription in leaf sheaths. The transcription level of Amy3C was relatively low, whereas no transcript was detected for other amylase isoforms (Fig. 6a). By contrast, the temporal transcriptional pattern of Amy2A in leaf sheaths of esl mutant rice was significantly lower than that in the wild type during the grain-filling stage. In particular, almost no transcripts were present for Amy2A in leaf sheaths of esl mutant rice after 14 days of grain filling. By contrast, Amy2A in wild-type cultivars showed significantly high expression in leaf sheaths during the whole grain-filling stage (Fig. 6b). These results implied that the transcription of Amy2A in leaf sheaths was genotype-dependent, which probably caused the differential remobilization of starch in leaf sheaths of two rice cultivars. On the other hand, Amy4A in leaf sheaths of esl mutants presented a significantly higher transcript level than that in the wild type during the initial stage of grain filling. Subsequently, these levels rapidly dropped to the minimum level, thereby causing a decrease in the wild type on 14th day of the grain-filling stage. However, Amy4A in the wild-type cultivar displayed relatively constant at the early stage of grain filling (0–7 days) before slightly declining at 7 days of grain-filling stage (Fig. 6c), thereby suggesting that Amy4A merely functioned during the early stage of grain filling in starch remobilization of leaf sheaths of esl mutant rice, however, the total remobilization of starch in leaf sheaths of esl mutant rice was weaker than that in the wild type during the entire grain-filling stage.
Genotypic differences in the amylase isoform genes and their temporal patterns in leaf sheaths of two rice cultivars; a comparison of ten α-amylase isoform genes and β-amylase expression at anthesis; b, c time course of Amy2A and Amy4A expression during the grain-filling stage, respectively. Vertical bars represent standard errors (n = 3). Asterisks represent significant differences (P < 0.05)
Hierarchical cluster analysis of expression profiles for genes involved in the carbohydrate metabolism in leaf sheaths
According to the temporal expression profiles, 36 genes associated with carbohydrate metabolism in leaf sheaths of two rice cultivars were classified by hierarchical cluster analysis during the grain-filling stage. The data showed that these genes were divided into five general groups (Fig. 7). As shown in Fig. 7, these genes related to sucrose synthesis (cyFBP, SPS isoform genes), starch synthesis (AGPase and SS isoform genes), and starch degradation (Amy4A and Amy2A) belonged to Groups IV and V, which showed extremely low expression abundance in the leaf sheath of esl mutant. On the other hand, genes encoding Frk, CIN, and SUT isoforms were classified as Group I, which displayed slightly lower expression in the esl mutant rice than in the wild type during the grain-filling stage. In addition, these genes corresponding to SuSy isoforms, Hxk isoforms, and SUT4 were classified as Groups II and III, which exhibited higher expression in the wild type than in the esl mutant rice at the early stage of grain filling.
Discussion
In rice plants, NSCs are accumulated in leaf sheaths before heading then translocated to the panicle for grain filling, so it can greatly affect the yield formation (He et al. 2005). The contribution of accumulated NSCs in leaf sheaths to the grain weight ranged from 9.1 to 42.2%, depending on the cultivars and environmental conditions (Ramasamy et al. 1997; Ntanos and Koutroubas 2002). Arai-Sanoh et al. (2013) reported that the translocation rate of NSCs in leaf sheaths of six rice cultivars ranged from 4.3 to 66.8%, which resulted in differential contribution to the final grain yield. Yang et al. (2001a) found that NSC remobilization in leaf sheaths was increased by 23.8–27.1% by water-deficit stress, and the final contribution of remobilized NSCs to grains was significantly enhanced by water stress. Notably, the contribution of NSCs in leaf sheaths to grain yield could reach up to 50% when the source leaves were collected during the grain-filling stage (Abou-khalifa et al. 2008). By contrast, the NSC translocation from leaf sheaths to grains was restrained once the top halves of the panicles were removed from the rice plants during the grain-filling stage (Arai-Sanoh et al. 2013). In this study, the NSC translocation rate from leaf sheaths to grains and its contribution to the developing grains reached as much as 56.4 and 25.60% in esl mutant rice, whereas those of the wild type were merely 33.3 and 3.52%, respectively (Table 1). Therefore, the NSC translocation from leaf sheaths to grains and its contribution to the final grain yield were significantly promoted by the source leaves of esl mutant rice with early senescence during the grain-filling stage. These results indicated that esl mutant rice had increased reliance on NSCs temporarily stored in leaf sheaths before heading to complete the grain filling, and NSCs in leaf sheaths of esl mutant rice played an important compensating role in grain filling for the deficiencies of photo-assimilates from source leaves, given that the source leaves suffered early senescence during the grain-filling stage. This result is consistent with previously research and reinforces the current opinion that NSCs in leaf sheaths serve as alternative carbon buffer compounds for grain filling to compensate for the deficiencies of photosynthesis (Yang et al. 2001a, 2003). Yang et al. (2001a) found that the NSC remobilization enhanced by water deficit led to a high harvest index. However, the seed-setting rate and harvest index of esl mutant rice were significantly lower than those of wild cultivars in this study (Table 1), which may be attributed to the deficiency of carbohydrates supplied from photosynthesis and the shortened grain filling period. In general, grain filling of rice is mainly affected by carbohydrates supplied from two sources, photosynthesis after heading and reserved NSCs in leaf sheaths before heading. In the esl mutant rice employed in the current study, the flag leaf began to exhibit senescence symptoms after anthesis and became nearly withered at approximately the 20th day of the grain-filling stage (Li et al. 2014), which severely reduced the photosynthetic capacity and shortened the grain-filling period. The deficiency of carbohydrates supplied from the flag leaf and the shortened grain-filling period did not cater to the carbohydrate demand of developing grains despite enhancing the NSC remobilization in leaf sheaths and caused the poor grain filling and a low harvest index in esl mutant rice.
In rice, NSCs are mainly composed of starch, sucrose, glucose, and fructose. Starch is the dominant carbohydrate in leaf sheaths; starch accumulation in the leaf sheaths reaches a maximum value at approximately the date of heading before its remobilization begins (Watanabe et al. 1997; Hirose et al. 2006). This process of starch changes in leaf sheaths is defined as the sink–source transition (Hirose et al. 1999; Ishimaru et al. 2004). He et al. (2005) reported that the maximum starch content of leaf sheaths ranged from 88 to 132 mg g−1 in the IR72 (indica), Takanari (indica), IR65598-112-2 (tropical japonica), and Nipponbare (temperate japonica) rice cultivars. In this study, the starch content in leaf sheaths of esl mutant rice was 68.44 mg g−1 at the heading stage. The decrease of starch in esl mutant cultivars between the heading and harvest stages was only 4.48 mg g−1, which was significantly lower than those in wild cultivar at 105.92 and 33.08 mg g−1, respectively (Table 2). These results suggested that the starch of leaf sheaths of esl mutant rice only reached to the constitutive status and retained the basal level in leaf sheaths. The starch level of the leaf sheaths was too low to be remobilized in esl mutant rice. By contrast, the level of starch accumulation in the wild type before heading was enough to be remobilized. Furthermore, the remobilization of starch in leaf sheaths was first converted into sucrose before its translocation to the sink tissues via the long-distance vascular pathway (Scofield et al. 2007). In addition, sucrose is also considered to be one of the NSCs stored in leaf sheaths before heading (He et al. 2005). In this study, the decrease of sucrose and fructose in leaf sheaths of esl mutant rice was 52.03 and 67.67 mg g−1, respectively, which was significantly higher than those in the wild type (Table 2). Therefore, sucrose and fructose were preferentially remobilized out of the leaf sheaths of esl mutant rice because of the deficiency of starch accumulation. By contrast, the sucrose level in the wild type remained relatively constant because of the compensating effect of starch transduction. Therefore, the starch in leaf sheaths was the primary carbohydrate remobilized in normal rice during the grain-filling stage, except when the starch accumulation in leaf sheaths before heading was not enough to be remobilized in rice under various stresses. In addition, sucrose and fructose would be preferentially remobilized to the developing grains.
Sucrose translocation from leaf sheaths to developing grains was accompanied by increased activities of SUTs (Berthier et al. 2009). The sucrose loading into phloem by SUTs was a key step of the long-distance vascular pathway (Scofield et al. 2007). The SUT1, SUT2, and SUT4 isoforms were expressed in leaf sheaths of rice (Aoki et al. 2003). Among the three isoforms, SUT1 primarily functioned in the phloem loading of sucrose retrieved from the apoplasm along the transport pathway (Scofield et al. 2007). SUT1 and SUT4 had important roles in the sucrose translocation of leaf sheaths (Chen and Wang 2008). In this study, the SUT1 transcript levels in leaf sheaths of esl mutant rice were lower than that in the wild type; SUT4 was preferentially expressed in the leaf sheaths of the esl mutant at the beginning of grain-filling stage (Fig. 1), thereby suggesting that the genotype-dependent temporal patterns of SUT1 probably caused the difference in the sucrose transport of two rice cultivars. The remarkably high expression of SUT4 in esl mutant rice was possibly closely associated with the swift sucrose translocation out of leaf sheaths in the initial stage of grain filling. In addition, cyFBPase and SPS participated in the sucrose biosynthesis in leaf sheaths of rice during the grain-filling stage (Hirose et al. 1999; Yang et al. 2001b). The transcripts of their encoding genes in this study remained relatively lower in the leaf sheaths of esl mutant rice than in the wild type, especially the transcripts of SPS isoforms during the initial and final stages of grain filling (Fig. 2). Therefore, the leaf sheaths of esl mutant rice had poor sucrose synthesis compared with the wild-type cultivar. Meanwhile, the sucrose synthesis in leaf sheaths of the wild-type cultivar was enhanced in the initial and ending stages of grain filling, which probably caused the sucrose re-accumulation in leaf sheaths of the wild-type cultivar. The utilization of sucrose as a source of carbon and energy depended on its cleavage by SuSy or CIN in leaf sheaths of rice plant. Hirose et al. (1999) proposed that a portion of the imported sucrose in leaf sheaths would be utilized for sheath growth by the expression of SuSy isoform genes in rice. SuSy catalyzed the cleavage of sucrose to form UDP-Glc and Fru, which were important intermediate materials for various physiological metabolisms (Yang et al. 2004). In this study, the SuSy1 and SuSy2 transcripts in leaf sheaths of esl mutant rice were significantly higher than those in the wild type at the beginning of grain filling, and eventually reached a lower level than those of the wild type (Fig. 3g–i). Therefore, a small portion of the sucrose was split into available intermediate monosaccharides in leaf sheaths, which probably participated in the sheath growth of esl mutant rice at the beginning of grain-filling stage. On the other hand, the intermediate monosaccharides were also responsible for the conversion between sucrose and starch in leaf sheaths and were crucial for development, growth, and carbon partitioning in rice (Sturm and Tang 1999; Hirose et al. 2002). In this study, the transcripts of CIN1 and CIN4 in leaf sheaths of esl mutant rice were lower than those in the wild type during the whole grain-filling stage (Fig. 3d–f), which probably caused the low hexose level in leaf sheaths of esl mutant rice, thereby indicating its weaker intermediate metabolism because of various factors.
During the sink–source transition of leaf sheaths, the changes in starch were accompanied by a coordinated change in the transcripts of specific members of the AGP, SS, and amylase gene families (Takahashi et al. 2005; Hirose et al. 2006). Specifically, the differential expression of AGPL2, GBSSII, and SSSI was strongly associated with the starch content in leaf sheaths (Chen and Wang 2008). In addition, a growing number of evidence have revealed that the expression of a few genes for starch synthesis could be regulated by the sugar level in rice plants (Dian et al. 2003, 2005). Takashi et al. (2005) reported that the expression of AGPS1, AGPL1, and AGPL4 in rice was stimulated by either sucrose or glucose, and the transcripts of SSSI and SSSIIIb in rice leaves were enhanced in response to sucrose (Dian et al. 2005). Our results confirmed that the transcripts of AGPS1, AGPS2a, AGPL1, AGPL3, GBSSII, SSSI, SSSIIIb, and Amy4A in leaf sheaths of esl mutant rice were significantly lower than those in the wild type between the heading and harvest stages (Figs. 4, 5, 6). These transcription levels simultaneously changed with the hexose level (glucose and fructose; Table 2). Meanwhile, the starch content in leaf sheaths of esl mutant rice was significantly lower than that in wild-type cultivars between the heading and harvest stages (Table 2). Therefore, the esl mutant rice had decreased starch metabolism (synthesis and degradation) in leaf sheaths during the grain-filling stage, instead of the wild type. However, the high expression of AGPS1 and AGPL1 in leaf sheaths of the wild type at the late grain-filling stage possibly favored the re-accumulation of starch in leaf sheaths of the wild type.
In conclusion, the remobilization of NSCs in leaf sheaths and the contribution to developing grains were obviously enhanced, given the deficiencies of photo-assimilates from source leaves. NSCs in leaf sheaths played an important compensating role in grain filling when the source leaves experienced the early aging during the grain-filling stage. Starch accumulation in leaf sheaths of esl mutant rice before heading was not enough for remobilization; sucrose and fructose were preferentially remobilized to the developing grains. The remarkably high expression of SUT4 in leaf sheaths of esl mutant rice in the initial stage of grain filling was possibly closely associated with the swift sucrose translocation out of leaf sheaths. By contrast, the decreasing expression of SPS, SuSy, and CIN isoforms in leaf sheaths of esl mutant rice possibly resulted in the poor sucrose metabolism during the grain-filling stage. In addition, the presence of fewer hexose substrates may have restricted the transcripts of genes for starch synthesis-related enzymes in leaf sheaths of esl mutant rice, thereby leading to the decreased starch synthesis and remobilization during the grain-filling stage.
Author contribution statement
FC and ZL designed the research and wrote the paper. ZL, FW, and WL conducted the research. ZL, QZ, and JL analyzed the data and performed statistical analysis.
References
Abou-khalifa AAB, Misra AN, Salem AEA (2008) Effect of leaf cutting on physiological traits and yield of two rice cultivars. Afr J Plant Sci 2:147–150
Aoki N, Hirose T, Scofield GN, Whitfeld PR, Furbank RT (2003) The sucrose transporter gene family in rice. Plant Cell Physiol 44:223–232
Arai-Sanoh Y, Ida M, Zhao R, Nishitani K, Yoshinaga S, Takai T, Nakano H, Iwasawa N, Kondo M (2013) Varietal differences in cell wall β-(1 → 3), (1 → 4)-glucan and nonstructural carbohydrate in rice stems during the grain filling stage. Plant Prod Sci 16:1–7
Berthier A, Desclos M, Amiard V, Morvan-Bertrand A, Demmig-Adams B, Adams WW, Turgeon R, Prud’homme MP, Noiraud-Romy N (2009) Activation of sucrose transport in defoliated Lolium perenne L.: an example of apoplastic phloem loading plasticity. Plant Cell Physiol 50:1329–1344
Chen HJ, Wang SJ (2008) Molecular regulation of sink-source transition in rice leaf sheaths during the heading period. Acta Physiol Plant 30:639–649
Cock JH, Yoshida S (1972) Accumulation of 14C-labelled carbohydrate before flowering and its subsequent redistribution and respiration in rice plant. Jpn J Crop Sci 41:226–234
Dian W, Jiang H, Chen Q, Liu F, Wu P (2003) Cloning and characterization of the granule-bound starch synthase II gene in rice: gene expression is regulated by nitrogen level, sugar and circadian rhythm. Planta 218:261–268
Dian W, Jiang H, Wu P (2005) Evolution and expression analysis of starch synthase III and IV in rice. J Exp Bot 56:623–632
Fernie AR, Willmitzer L, Trethewey RN (2002) Sucrose to starch: a transition in molecular plant physiology. Trends Plant Sci 7:35–41
He HY, Koike M, Ishimaru T, Ohsugi R, Yamagishi T (2005) Temporal and spatial vatiations of carbohydrate content in rice leaf sheath and their varietal differences. Plant Prod Sci 8:546–552
Hirose T, Terao T (2004) A comprehensive expression analysis of the starch synthase gene family in rice (Oryza sativa L.). Planta 220:9–16
Hirose T, Endler A, Ohsugi R (1999) Gene expresseion of enzymes for starch and sucrose metabolism and transport in leaf sheaths of rice (Oryza sativa L.) during the heading period in relation to the sink to source transition. Plant Prod Sci 2:178–183
Hirose T, Takano M, Terao T (2002) Cell wall invertase in developing rice caryopsis: molecular cloning of OsCIN1 and analysis of its expression in relation to its role in grain filling. Plant Cell Physiol 43:452–459
Hirose T, Ohdan T, Nakamura Y, Terao T (2006) Expression profiling of genes related to starch synthesis in rice leaf sheaths during the heading period. Physiol Plant 128:425–435
Hirose T, Scofield GN, Terao T (2008) An expression analysis profile for the entire sucrose synthase gene family in rice. Plant Sci 174:534–543
Hurkman WJ, McCue KF, Altenbach SB, Korn A, Tanaka CK, Kothari KM, Johnson EL, Bechtel DB, Wilson JD, Anderson OD, DuPont FM (2003) Effect of temperature on expression of genes encoding enzymes for starch biosynthesis in developing wheat endosperm. Plant Sci 164:873–881
Ishikawa T, Akita S, Li Q (1993) Relationship between contents of non-structural carbohydrates before panicle initiation stage and grain yield in rice (Oryza sativa L.). Jpn J Crop Sci 62:130–131
Ishimaru K, Kosone M, Sasaki H, Kashiwagi T (2004) Leaf contents differ depending on the position in a rice leaf sheath during sink-source transition. Plant Physiol Biochem 42:855–860
James MG, Denyer K, Myers AM (2003) Starch synthesis in the cereal endosperm. Curr Opin Plant Biol 6:215–222
Ji XM, Van den Ende W, Van Laere A, Cheng SH, Bennett J (2005) Structure, evolution, and expression of the two invertase gene families of rice. J Mol Evol 60:615–634
Li ZW, Xiong J, Li ZF, Qi XH, Chen HF, Shao CH, Wang JY, Liang YY, Lin WX (2008) Analysis of differential expression of proteins in rice leaf sheath during grain filling. Acta Agron Sin 34:619–626
Li Z, Wang F, Lei B, Cao Z, Pan G, Cheng F (2014) Genotypic-dependent alteration in transcriptional expression of various CAT isoenzyme genes in esl mutant rice and its relation to H2O2-induced leaf senescence. Plant Growth Regul 73:237–248
Liang JS, Cao XZ, Zhang HY, Song P, Zhu QS (1994) The changes and affecting factors of stem-sheath reserve contents of rice during grain filling. Chinese J Rice Sci 8:151–156
Liao DQ, Zhang HL, Li ZC, John B (2010) Characterization of evolution and tissue-expression of rice (Oryza sativa L.) α-amylase genes. Acta Agron Sin 36:17–27
Luo XL, Huang QF (2011) Relationships between leaf and stem soluble sugar content and tuberous root starch accumulation in Cassava. J Agric Sci 3:64–72
Nakamura Y, Miyachi S (1982) Effect of temperature on starch degradation in Chlorella vulgaris 11 h cells. Plant Cell Physiol 23:333–341
Ntanos DA, Koutroubas SD (2002) Dry matter and N accumulation and translocation for Indica and Japonica rice under Mediterranean conditions. Field Crop Res 74:93–101
Okamura M, Aoki N, Hirose T, Yonekura M, Ohto C, Ohsugi R (2011) Tissue specificity and diurnal change in gene expression of the sucrose phosphate synthase gene family in rice. Plant Sci 181:159–166
Perez CM, Palmiano EP, Baun LC, Juliano BO (1971) Starch metabolism in the leaf sheaths and culm of rice. Plant Physiol 47:404–408
Ramasamy S, Ten Berge HFM, Purushothaman S (1997) Yield formation in rice in response to drainage and nitrogen application. Field Crop Res 51:65–82
Samonte SOPB, Wilson LT, McClung AM, Tarpley L (2001) Seasonal dynamics of nonstructural carbohydrate partitioning in fifteen diverse rice (Oryza sativa L.) genotypes. Crop Sci 41:902–909
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3:1101–1108
Scofield GN, Hirose T, Aoki N, Furbank RT (2007) Involvement of the sucrose transporter, OsSUT1, in the long-distance pathway for assimilate transport in rice. J Exp Bot 58:3155–3169
Serrato AJ, Barajas-López JD, Chueca A, Sahrawy M (2009) Changing sugar partitioning in FBPase-manipulated plants. J Exp Bot 60:2923–2931
Smith AM, Zeeman SC, Smith SM (2005) Starch degradation. Annu Rev Plant Biol 56:73–98
Song X, Agata W, Kawamitsu Y (1990) Studies on dry matter and grain production of F1 hybrid rice in China. III Grain-production character from the viewpoint of time changes in non-structural carbohydrate and nitrogen contents during the yield production. Jpn J Crop Sci 59:107–112
Sturm A (1999) Invertase, primary structures, functions, and roles in plant development and sucrose partitioning. Plant Physiol 121:1–7
Sturm A, Tang GQ (1999) The sucrose-cleaving enzymes of plants are crucial for development, growth and carbon partitioning. Trends Plant Sci 4:401–407
Takahashi S, Ishimaru K, Yazaki J, Fujii F, Shimbo K, Yamamoto K, Sakata K, Sasaki T, Kishimoto N, Kikuchi S (2005) Microarray analysis of sink-source transition in rice leaf sheaths. Breed Sci 55:153–162
Takashi A, Mizuno K, Fujimura T (2005) Gene expression of ADP-glucose pyrophosphorylase and starch contents in rice cultured cells are cooperatively regulated by sucrose and ABA. Plant Cell Physiol 46:937–946
Tsukaguchi T, Horie T, Ohnishi M (1996) Filling percentage of rice spikelet as affected by availability of non-structural carbohydrates at the initial phase of grain filling. Jpn J Crop Sci 65:445–452
Watanabe Y, Nakamura Y, Ishii R (1997) Relationship between starch accumulation and activities of the related enzymes in the leaf sheath as a temporary sink organ in rice (Oryza sativa). Aust J Plant Physiol 24:563–569
Weng JH, Agata W, Takeda T, Hakoyama S (1982) Studies on dry matter and grain production of rice plants. Influence of the reserved carbohydrate until heading stage and the assimilation products during the ripening period on grain production. Jpn J Crop Sci 51:500–509
Winter H (2000) Regulation of sucrose metabolism in higher plants: localization and regulation of activity of key enzymes. Crit Rev Biochem Mol 35:253–289
Yang J, Zhang J, Wang Z, Zhu Q (2001a) Activities of starch hydrolytic enzymes and sucrose-phosphate synthase in the stems of rice subjected to water stress during grain filling. J Exp Bot 52:2169–2179
Yang J, Zhang J, Wang Z, Zhu Q, Wang W (2001b) Remobilization of carbon reserves in response to water deficit during grain filling of rice. Field Crop Res 71:47–55
Yang J, Zhang J, Wang Z, Zhu Q, Liu L (2003) Involvement of abscisic acid and cytokinins in the senescence and remobilization of carbon reserves in wheat subjected to water stress during grain filling. Plant Cell Environ 26:1621–1631
Yang J, Zhang J, Wang Z, Xu G, Zhu Q (2004) Activities of key enzymes in sucrose-to starch conversion in wheat grains subjected to water deficit during grain filling. Plant Physiol 135:1621–1629
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (No. 31271655), the Project Funded by China Postdoctoral Science Foundation (No. 2015M580560), and the Natural Science Foundation of Fujian Province (No. 2016J01100).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Srivastava.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, Z., Wang, F., Lin, W. et al. Carbon reserve and remobilization in leaf sheaths during the grain-filling stage in response to leaf early senescence. Acta Physiol Plant 39, 10 (2017). https://doi.org/10.1007/s11738-016-2304-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-016-2304-6