Abstract
South Busoga Central Forest Reserve, an isolated reserve in Uganda has been degraded for over forty years. To restore the reserve, the National Forestry Authority established plantations in some parts of the reserve and plans to reserve 40% under native vegetation. Until now, no study exists to inform management of the availability of native species in the degraded area to support natural forest regeneration. The objectives of this study were to: (i) establish if there are seeds of native forest species in the soil seed bank of plantations and unplanted areas (ii) assess the potential of the understory of plantations to serve as ‘seed sources’. A seed bank assay of soils from plantations; Eucalyptus grandis, Maesopsis eminii and Pinus caribaea and unplanted areas was performed. Understory vegetation was sampled in 125 subplots of 5 × 5 m, randomly distributed in the plantations. In the seed bank, we recorded a total of 56 species in plantations and 22 in unplanted. There were more species in soils under E. grandis (12) > M. eminii (10) > P. caribaea (9) > unplanted area (3). Apart from Ficus sp. and Drypetes sp. that are woody, the rest of the species were non-woody. In the understory, we encountered a total of 56 species but only fifteen were native tree species with occurrence of less than five individuals each. These results show that sustained forest degradation depletes soil seed banks of native forest species and that plantations of exotic species can also nurse native species in their understory.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
South Busoga Central Forest Reserve (SBCFR) is a unique ‘forest’ in terms of history, structure and management. From 1920 to 1941, logging of valuable timber species; Celtis durandii Engl., Antiaris toxicaria Lesch. and Milicia excelsa (Welw.) C.C. Berg increased when saw mills were established in the area to the extent that by 1926, the stock of these tree species had dwindled. As such, the Forest Department initiated plantation scheme to regenerate the stock. In 1938, 62,160 hectares of the forest were gazetted as a crown forest and in 1948, the title of the forest changed from ‘crown’ forest to South Busoga Central Forest reserve (National Forestry Authority (NFA), 2012). However, managing the gazetted area became a challenge hence some areas of the reserve were degazetted and by 1968 the reserve area reduced from 62.382 hectares to 16.382 hectares, the present total area of the reserve to date (NFA 2012).
In the 1970s, South Busoga Central Forest reserve was used by the Forest Department for production of timber by establishment of plantations of Pinus caribaea Morelet, M. excelsa, Senna sp. and Tectona grandis L. Unfortunately, during insurgency of the 1970s under Iddi Amin these plantations were cleared. The neighboring communities then encroached and settled in the reserve. This encroachment resulted into enclaves within the forest reserve where people have settled permanently and are cultivating crops such as maize, sweet potatoes, cassava and bananas. Currently, the entire forest reserve has been transformed into a mosaic of farmland and forest plantation.
In an effort to improve the status of South Busoga forest reserve as an important biodiversity area, NFA has undertaken reforestation efforts and established 10,104 ha under plantations of E. grandis, M. eminii and P. caribaea. In addition, NFA plans to maintain 40% of the unplanted area under natural vegetation cover to conserve biodiversity (NFA 2012).
Establishing plantations in degraded forests is a common reforestation practice (Kremer and Bauhus 2020). Plantations are established for economic (Cuong et al. 2020) as well as ecological functions (Ulya et al. 2019; Kumar et al. 2020) such as biodiversity conservation (Brockerhoff et al. 2008; Bremer and Farley 2010; Onyekwelu and Olabiwonnu 2016). Plantations are also established to enhance natural regeneration by facilitating understory floristic diversity (Duan et al. 2010; Dagan and Izhaki 2019; De Stefan et al. 2019) and soil seed bank diversity (Leitao et al. 2010; Wang et al. 2017) by attracting seed dispersers (Wunderle 1997; Barlow et al. 2007; Zamora and Montagnini 2007; Guo et al. 2015). However, factors such as type of species planted (exotic vs native), age of the plantation, proximity of ‘seed sources’ and disturbance regime influence floristic understory and seed bank diversity of forest plantations (Quine and Humprey 2009; Ritter et al. 2018; Chetan et al. 2019). For example, some plantations of native tree species have high understory floristic diversity than exotic tree plantations (Lamb et al. 2005; Bremer and Farley 2010; Medina et al. 2020). On the other hand, some authors have reported low understory floristic diversity in plantations of native tree species (Wang et al. 2009; Longworth and Williamson 2018).
In the African content, Senbeta et al. (2001) reported high understory diversity in four monoculture exotic species plantations established in degraded Afromontane forest in Ethiopia. On the other hand, the soil seed bank had only 10% of the understory floristic diversity represented. However, few studies have compared understory floristic diversity and soil seed bank status in plantations of different species (e.g. Senbeta and Teketay 2002) yet this is important for planning for forest regeneration in degraded forest areas in many countries in Africa. Many studies on understorey floristic diversity and soil seed banks in plantations (Parrotta 1995; Zhang et al. 2014, 2019; Kremer and Bauhus 2020; but see Senbeta et al. 2001) are from other regions. In addition, most existing studies have focused on either floristic understory diversity e.g. Bone et al. (1997); Geldenhuys (1997); Senbeta et al. (2001) and/or soil seed banks (Senbeta and Teketay 2002; Kassa et al. 2020). There are relatively fewer studies on understorey floristic diversity and soil seed banks in exotic and native tree species plantations (e.g. Onyekwelu and Olabiwonnu 2016) despite existence of efforts to rehabilitate degraded forests by establishing plantations. Hence knowledge of soil seed bank status in native and exotic plantations and the potential of the understory vegetation to serve as ‘seed sources’ for regeneration of unplanted areas is limited and yet crucial for management decisions. To understand regeneration in plantations, it is important to analyze understory floristic diversity and seed bank status. In this study, we investigated floristic diversity and soil seed bank status in E. grandis, M. eminii and P. caribaea plantations and unplanted areas of South Busoga Central Forest Reserve (SBCFR). Knowledge of understorey floristic diversity and soil seed bank status would inform management decisions and realization of NFA plans to maintain 40% of the SBCFR under native vegetation for biodiversity conservation. The research questions of this study are (i) How does enrichment planting affect soil seed bank richness, diversity and density? (ii) What is the potential of the understory vegetation of plantations to serve as ‘seed sources? We hypothesize that there is no difference in species richness, diversity and density of seeds of native forest species in soil seed bank in plantations and unplanted areas.
Methods
Study site
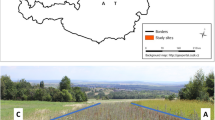
This study was conducted in South Busoga Central Forest Reserve, an isolated reserve located in the Northern shores of Lake Victoria within geographical coordinates of 0° 09′ and 0° 20′ N and 33° 27′ and 33° 39′ E in Mayuge district (Fig. 1), covering an area of 164 km2. It lies at an altitudinal range of 1140 to 1300 m above sea level. The site has a tropical climate with two rainfall peaks (April–May and October–November). Generally, the area receives an average rainfall ranging between 1270 and 1481 mm annually. It experiences a mean monthly minimum temperatures ranging between 17 and 19 °C and the mean maximum temperatures ranging between 27 and 30 °C. In the North and East is surrounded by heavily populated agricultural land, and in the west it grades into Lake Victoria shores. It is a low-lying forest with extensive shores fringe of Cyperus papyrus and scattered rock outcrops. The original tropical semi-deciduous forest has been degraded and only scattered natural trees e.g. Albizia sp., Spathodea campunulata P.Beauv. exist in originally designated strict nature reserve zone. The remaining part of the forest (90%) is consisting of grassland and abandoned agricultural land. About 2950 ha of these grassland and abandoned agricultural land has been developed into plantation. Three species (E. grandis, P. caribaea and M. eminii) in single stands have been planted in 10,104 ha in parts of the reserve. The National Forest Authority plans to maintain 40% of the area under natural vegetation cover to conserve biodiversity (NFA 2012).
Determination of understorey plant species abundance
Fieldwork was conducted in February 2017. To standardize for age effects, twenty-five plots of 10 × 30 m were randomly laid in 13-year-old E. grandis (7), M. eminii (9) and P. caribaea (9) plantation forests, respectively. Understory vegetation to 2 m hight was sampled by dividing the 10 × 30 m plot into twelve 5 × 5 m subplots and sampling 6 alternating subplots along the middle of the 10 × 30 m. The common plant species were identified in situ, recorded and number of individuals counted. Species, which could not be identified in the field, were collected, pressed and delivered to Makerere University Herbarium for proper identification.
Sampling of soil for seed bank determination
In each of the replanted forest areas, we collected soil samples from same plots where above ground vegetation was sampled. Within the 10 × 30 m plot, a line across the longest distance was drawn. Along this line, five soil samples were taken at 5 m intervals using a soil core (12 cm depth and 4.5 cm diameter). The soil was systematically sampled so that all the five soil samples would be situated within the 10 × 30 m plot where the vegetation had been sampled. The surface area sampled by the core was 15.9 cm2 (~ 79.5 cm2/plot) and volume of soil was 190.8 cm3. The soil was removed from the core and divided as follows: two cm top soil was removed and placed in a separate envelope, while the 10 cm soil bottom soil was placed in another envelop. This was repeated for the whole length of the 10 × 30 m, every time keeping the 2 cm layers soil together in one envelop and the 10 cm layer in another envelop. On the overall, every plot of 10 × 30 m plot had two soil samples (2 cm soil envelope and 10 cm soil envelope). In total, we had 25 samples from the three plantations distributed as E. grandis (7), M. eminii (9) and P. caribaea (9) depending on the size of the plantation. In addition, we randomly collected 29 composite soil samples from unplanted areas. Soil samples were collected randomly from unplanted because the soil had been homogenized through cultivation activity. Similarly, we did not divide the soil sample into top 2 cm and bottom 10 cm because the area was cultivated and hence soil layering disturbed and we did not sample vegetation as the farmers maintain the area through weeding. All soil samples were kept in sealed paper bags and stored at 25 °C and ambient humidity.
Soil seed bank determination
In November 2017, seedling assay experiment was set up. Soil samples from each plot were sieved through 2.0 mm mesh sieve to remove debris. The soil samples were spread in a 30 × 27 cm seed trays on sand excavated beneath the floor of a lake and hence did not contain viable seeds. There were additional 24 control trays containing only lake sand to track lake sand contamination and airborne seeds. The trays were kept in a white polythene shelter about 100 km west of the South Busoga Central forest reserve. The trays were kept moist by watering and seedling emergency monitored. Seedlings were identified and removed. Those that could not be identified were replanted and identified later. After a period of 9 months, no more seedlings emerged and the experiment was terminated.
Understory and seed bank species richness and diversity
To determine species richness and diversity for understory and seed bank, the total number of species counted was taken to be the species richness while for species diversity the Shannon–Wiener Diversity Index (H) was calculated in Microsoft Excel using the following expression. Where Pi is the proportion of total sample represented by species i; obtained by dividing no. of individuals of species i by total number of individuals in sample. S is the number of species, = species richness. ln is the natural logarithm.
Seed density was computed by dividing seed abundance per plot by the volume of soil expressed in litres.
Data analysis
Seven seedlings which died before identification were removed from the data set. Although Phyllanthus amarus Schumach. & Thonn., Acalypha sp. and Oxalis corniculata L. occurred in the control trays, their densities were higher in the soil samples, hence were left in the data set. Data was analyzed in SPSS (version 20). Homogeneity of variance test showed that all variables measured deviated from normal. Therefore, Kruskal–Wallis was used to test for differences in soil seed banks from plantations and the unplanted area. Where the medians were significantly different, post-hoc tests with Bonferroni adjustment were performed. Pairwise comparisons were selected when the main test was significant and adjusted significance values used. Analysis of Similarity (ANOSIM) in species composition between soil seed banks and aboveground vegetation was done with Community Analysis Package (CAP 4.1.3) Software.
Results
Native species richness, diversity and density of soil seed banks in plantations and unplanted areas
Fifty-six plant species were recorded in seed bank in plantations of E. grandis, P. caribaea and M. eminii. Out of the 56 species, 27 were native woody species. Of the 27 native species, 10 were shrubs dominated by the family Euphorbiaceae (Acalypha lanceolata Willd., A. brachystachya Hornem., A. racemosa Wall. ex Baill., A. acrogyna Pax) and Asteraceae (Abutilon mauritianum (Jacq.) Medik.), two trees (Ficus sp. and Drypetes sp.) and 15 were non-woody. Out of the 56 species recorded, seven species (~ 13%) (A. mauritianum, A. acrogyna, A. brachystachya, A. lanceolata, A. ornata, Drypetes sp. and Ficus sp.) occurred only in the soil seed bank (Table 1).
Twenty-two species were recorded in unplanted area. Nine were native species while 13 were non-native. Eight of the native species were non-woody and one species (A. ornata) was woody.
There was no statistically significant difference in native species seed density between top and lower layers of the same soil samples in plantations. Median density of non-native species was higher in lower 10 cm layer in Pinus caribaea plantation (Table 2). The median species richness of native species was similar in E. grandis and P. caribaea and lower in M. eminii though not significantly different due high variability, except between P. caribaea and unplanted (K-W Χ22 = 7.75, p ≤ 0.036). The median species richness of non-native species was not significantly different in plantations and unplanted area. The median seed density of native species in plantations was significantly different from the unplanted area (Kruskal–Wallis (K–W) in P. caribaea (K–W Χ22 = 7.58, p = 0.036) and non-significant in E. grandis and M. eminii plantations and unplanted. The native seed densities medians were 34.60, 46.13, 24.11 and 4.19 seeds per liter for E. grandi, M. eminii, P. caribaea and unplanted areas, respectively. The median seed density of non-native species was not significantly different in plantations and unplanted area (K–W Χ22 = 6.11, p = 0.106). Median Shannon diversity of native species was highest in E. grandis (0.679), followed by M. eminii (0.421) and P. caribaea (0.260) and were not significantly different from unplanted. However, Shannon diversity of non-native species was lower in unplanted than in P. caribaea plantation (K–W Χ22 = 12.52, p = 0.002) (Fig. 2).
Box plot of native and non-native species richness (A, B), Seed density (C, D) and Species richness (E, F) of soil samples from plantations; E. grandis (Eucal), M. eminii (Maes), P. caribaea (Pine) and Unplanted (Unpl). The thick line represents the median; the out limits of the box represent first and third interquartile range
Fifty-six species in total were encountered in the understory vegetation. Thirty-eight of these were native while the rest were non-native. Of the native tree species encountered, only fifteen were tree species. These were however represented by a few individuals each: Blighia unijugata Baker, Ficus exasperata Vahl and A. toxicaria with 1 individual each, Milicia excelsa (Welw.) C. C Berg and Psydrax parviflora (Afzel.)Bridson with two individuals each, Albizia coriaria Oliv. three individuals (Table 1).
Eighteen out of 56 species recorded were present only in understorey and only four species were present in both understorey and soil seed bank (Table 1). In terms of conservation status, all the understorey floristic and soil seed bank species were of least concern conservation status. There were 13 non-woody native forest species dominated by (Cassia hirsuta L. and Cissus petiolata Hook.f.). The median species richness of native forest species was similar in E. grandis and M. eminii but high in P. caribaea but not significant. Similarly, Shannon diversity of the native forest species was not significantly different among the plantations. Likewise, understory non-native forest species richness and diversity did not differ significantly in the plantations. Proportion of native trees and shrubs was lower in both seed bank and understory vegetation of the plantations (Fig. 3).
Results from two-dimensional NMDS ordination (iterations = 200; stress = 0.088) show that species composition of seed bank and understory was dominated by non-native species (Fig. 4). However, native and non-native species in soil seed bank and understory were different as indicated by findings from Similarity Percentage analysis (SIMPER), which showed 98.03 percent dissimilarity between native and non-native species composition in seed bank and understorey.
Discussion
Low diversity of native woody species in the soil seedbank
Results from this study show that the soil seed bank in South Busoga Central Forest Reserve is devoid of a substantial amount of viable seeds of native tree species as only two tree species (Ficus sp. and Drypetes sp.) occurring in very low densities germinated in the entire study. For instance, the unplanted area, earmarked for forest regeneration representative of native species formerly present in South Busoga forest reserve, had only one native woody species. Even in the adjacent areas with plantations (Fig. 3a) where colonization would be expected, floristic understorey diversity of native woody species was very low with only four species common to seed bank and understorey. This contrasts sharply with the high diversity of native species that the reserve had before it was encroached. Previously, the reserve was known for a diversity of native tree species dominated by M. excelsa, Ficus sur Forssk., A. toxicaria, M. lutea (Benth.) K. Schum, Ficus natalensis Hochst., A. coriaria and M. eminii (Davenport et al. 1996). However, in the soil seedbank, only a small proportion of the once dominant species were encountered.
Although soil seed banks are a potential source of regeneration after forest degradation, restoration of forests converted into other land uses such as crop cultivation presents a unique challenge to managers.
Our findings of poor representation of native woody species in soil seed banks of plantations in South Busoga Central Forest Reserve are comparable to findings of Chen et al. (2013) who reported low representation of woody species in soil seed banks from plantations in formerly cultivated secondary forest sites in China. Limited representation of woody species was attributed to continuous weeding that killed tree and shrub seedlings before maturity and hence curtailing reproduction and seed input into the soil seed bank from such species. Similarly, Wang et al. (2009) found no indigenous tree seeds in soil seed banks of plantations established in degraded lands in south China. The plausible explanation for poor representation of woody species in the soil seed bank could be due to prolonged cultivation of sites before establishment of plantations. This view is supported by findings that plantations established in sites degraded through other means rather than cultivation had rich woody species in the soil seed bank (Cohen et al. 2004; Sakai et al. 2005; Zeng-Hui et al. 2013). However, our findings contradict Weerasinghe et al. (2019) who found that seed banks of P. caribaea plantations on former tea plantations contained pioneer tree and shrubs. This suggests that other factors other than prolonged cultivation, such as proximity to natural vegetation might contribute to native species diversity and abundance in soil seed banks. For South Busoga Forest Reserve, seed inputs from nearby natural forest may be difficult since the nearest natural forest, Mabira Central Forest reserve, is located about 90 km away and the landscape in between is a sugar cane dominated matrix which is a hostile environment for seed dispersers like birds (Uwimbabazi et al. 2017).
Understorey floristic diversity and soil seed bank
The understorey floristic species and soil seed bank shared only four species of native shrubs and hence the dissimilarity in species composition between understorey and soil seed bank. Many authors have found dissimilarity between understorey floristic and soil seed bank species (Senbeta and Teketay 2002; Wang et al. 2009; Overdyck and Clarkson 2012). Presence of shrubs in the understorey is important as leaves of shrubs form understorey cover that provisions for forage niches for birds (Castaño-Villa et al. 2019; Goded et al. 2019), although a study by Castaño-Villa et al. (2019) found a contrary.
Strengths and weakness of the study
Although only about 7% of the understorey floristic woody species were present in the soil seed bank, the unplanted area had only one species. This finding is supported by earlier studies which have shown that plantations play a role in biodiversity conservation especially when established in degraded landscapes (Bremer and Farley 2010) as in this case SBCFR. The limited woody species in our study may also be explained by germination conditions. Some tropical forest species germinate under certain environmental conditions (Kyereh et al. 1999; Mng’omba et al. 2007) hence seeds requiring special conditions to germinate might have been left out from our seedling assay. However, this cannot adequately explain low return on germination of woody species observed in this study as the conditions used in this study have been previously used to germinate tropical forest species with great success (Dainou et al. 2011; Chen et al. 2013; Esaete et al. 2014; Luo et al. 2017). For South Busoga forest reserve, prolonged cultivation and absence of nearby natural forest to input seeds are therefore the most plausible reasons to account for limited number of viable seeds in the soil seed bank.
In addition, one of the weaknesses of soil seed bank studies is bias in sampling were a comparatively smaller area is sampled for soil seed banks compared to the area sampled for the aboveground vegetation species (Vandvik et al. 2015). In this study, although the soil samples were taken from the same plots were the understorey floristic vegetation was sampled, each soil core sampled an area of 0.00795 m2/plot compared to 150 m2/plot sampled for understorey floristics. Hence the area for soil seed bank was much smaller and might have resulted in low representation of understorey species in the soil bank.
Implications of poor soil seed bank for forest restoration and conservation
National Forestry Authority management plans (2012–2022) show that 40% of the South Busoga Central forest reserve was earmarked for conservation of biodiversity (NFA 2012). The good news from this study show that the soil seed bank (under plantation and unplanted area) is rich in non-woody species. This is feasible because, in addition to herbaceous perennials, ephemeral species like annuals produce large numbers of seeds with hard, impermeable seed coats that enable the embryos to survive for a long time in the soil (Baskin and Baskin 1998). In addition, such species benefit by spreading seed germination overtime, a strategy known as biological bet-hedging; an adaptation to escaping unfavorable conditions and spreading risk of seedling failure (Gao et al. 2014; Gremer et al. 2016; Fan et al. 2018).
Non-woody species are an important food reservoir for wildlife and help in maintaining biological diversity of wildlife such as birds and insects (Barlow et al. 2007; Edwards et al. 2011; Godoi et al. 2016). Therefore, a diverse non-woody soil seed bank in the unplanted area and the adjacent plantations is also important because of the potential of attracting seed dispersers and hence chances of receiving seeds from other sources and ultimately aiding in regeneration and conservation of biodiversity. However, the depleted soil seed bank in terms of native woody species observed in this study should be the main concern for restoration and conservation. The lack of woody species erodes hope of natural regeneration of Mayuge Central forest reserve even if encroachers were completely evicted since ecological restoration of communities is facilitated by the availability of target species in the seed bank (Bossuyt and Honnay 2008).
Conclusion and recommendation
Species richness, diversity and density of non-woody species was high in both areas (degraded and later put under plantations and areas that were unplanted) despite the over four decades of cultivation. However, the presence of very few viable seeds of native woody species in South Busoga Forest Reserve shows that prolonged cultivation depletes soil seed banks of native woody species and that plantations of exotic species can also nurse native species in their understory.
Since there are no nearby natural forests that can facilitate seed rain, we recommend enrichment planting of native species if the goal of conserving at least 40% of the SBCFR is to be realized.
Availability of data and materials
The data used for in this article is available from the authors and database of the project.
Code availability
Not applicable.
References
Akinyemi DS, Oseni SR, Oke SO (2018) Effect of heat on soil seedbank of three contrasting physiognomies in Shasha forest reserve; Southwestern Nigeria. Acta Oecologia 94:22–30. https://doi.org/10.1016/j.actao.2018.03.009
Barlow J, Mestre LAM, Gardner TA, Peres CA (2007) The value of primary, secondary and plantation forests for Amazonian birds. Biol Cons 136:212–231. https://doi.org/10.1016/j.biocon.2006.11.021
Baskin CC, Baskin JM (1998) Seeds. Ecology, biogeography, and evolution of dormancy and germination. Academic Press, San Diego
Bone R, Lawrence M, Magombo Z (1997) The effect of a Eucalyptus camaldulensis (Dehn) plantation on native woodland recovery on Ulumba Mountain, southern Malawi. For Ecol Manag 99:83–99
Bossuyt B, Honnay O (2008) Can the seed bank be used for ecological restoration? An overview of seed bank characteristics in European communities. J Veg Sci 19:875–884
Bremer LL, Farley KA (2010) Does plantation forestry restore biodiversity or create green deserts? A synthesis of the effects of land-use transitions on plant species richness. Biodivers Conserv 19:3893–3915
Brockerhoff EG, Jactel H, Parrotta JA, Quine C, Sayer J (2008) Forests and biodiversity: oxymoron or opportunity. Biodivers Conserv 17:925–951
Castaño-Villa GJ, Santisteban-Arenas R, Hoyos-Jaramillo A, Estévez-Varón JV, Fontúrbel FE (2019) Foraging behavioural traits of tropical insectivorous birds lead to dissimilar communities in contrasting forest habitats. Wildl Biol 1:1–6. https://doi.org/10.2981/wlb.00483
Chazdon RL, Guariguata MR (2016) Natural regeneration as a tool for large-scale forest restoration in the tropics: Prospects and challenges. Biotropica 48:716–730
Chen H, Cao M, Tang Y (2013) Soil seed banks in plantations and tropical seasonal forests of Xishuangbanna, West China. J Trop For Sci 24:375–386
Chetan HC, Ganesan R (2019) Ganesh T (2019) Seed and seedling establishment in abandoned tea plantations role of ecological and edaphic factors, southern Western Ghats, India. Trop Ecol 60:566–580
Cohen S, Braham R, Sanchez’ F (2004) Seed bank viability in disturbed longleaf Pinus caribaea sites. Restor Ecol 12:503–515
Cuong T, Chinh TTQ, Zhang Y, Xie Y (2020) Economic performance of forest plantations in Vietnam. Eucalyptus, Acacia mangium and Manglietia conifera. Forests 11:284. https://doi.org/10.3390/f11030284
Dagan U, Izhaki I (2019) Understory vegetation in planted and pine forests governs bird community composition and diversity in the eastern Mediterranean region. For Ecol 6:29. https://doi.org/10.1186/s40663-019-0186-y
Daïnou K, Bouduin A, Bourland N, Gillet JF, Fétéké F, Doucet JL (2011) Soil seed bank characteristics in Cameroonian rainforests and implications for post-logging forest recovery. Ecol Eng 37:1499–1506
Davenport T, Howard P, Mathews R (1996) South Busoga Forest Reserve. Biodiversity report. Forest Department, Kampala
De Stefano A, Blazier MA, Comer CE, Dean TJ, Wigley TB (2019) Understory vegetation richness and diversity of Eucalyptus benthamii and Pinus elliottii plantations in the Midsouth US. For Sci 66:66–81. https://doi.org/10.1093/forsci/fxz051
Duan W, Ren H, Fu S, Wang J, Zhang J, Yang L, Huang C (2010) Community comparison and determinant analysis of understory vegetation in six plantations in South China. Restor Ecol 8:206–214. https://doi.org/10.1111/j.1526-100X.2008.00444.x
Edwards DP, Larsen TH, Docherly TD, Ansell FA, Hsu WW, Derhe MA, Hamer KC, Wilcore DS (2011) Degraded lands worth protecting the biological importance of South Asia’s repeatedly logged forests. Proc R Soc B 278:82–90
Esaete J, Eycott AE, Reinio J, Telford RJ, Vandvik V (2014) The seed and fern spore bank of a recovering African tropical forest. Biotropica 46:677–686
Fan B, Zhou Y, Ma Q, Yu Q, Zhao C, Sun K (2018) The bet-hedging strategies for seedling emergence of Calligonum mongolicum to adapt to the extreme desert environments in Northwestern China. Front Plant Sci 9:1167. https://doi.org/10.3389/fpls.2018.01167
Galloway AD, Holmes PM, Gaertner M, Esler KJ (2017) The impact of Pinus caribaea plantations on fynbos above-ground vegetation and soil seed banks composition. S Afr J Bot 113:300–307
Gao R, Young X, Young F, Wei L, Huang Z, Walck JL (2014) Aerial and soil seed banks enable populations of an annual species to cope with unpredictable dune ecosystem. Ann Bot 114:279–287
Geldenhuys CJ (1997) Native forest regeneration in pine and eucalypt plantations in Northern Province, South Africa. For Ecol Manag 99:101–115
Goded S, Ekroos J, Domínguez J, Azcarate JG, Guiti JA, Smith HG (2019) Effects of eucalyptus plantations on avian and herb species richness and composition in North-West Spain. Global Ecol Conserv 19:e00690. http://creativecommons.org/licenses/by-nc-nd/4.0/
Godoi MN, Souza FL, Laps RR, Ribeiro DB (2016) Composition and structure of bird communities in vegetational gradients of Bodoquena Mountains, western Brazil. Ann Brazil Acad Sci 88:211–225
Gremer JR, Kimball S, Venable DL (2016) within-and among-year germination in Sonoran Desert winter annuals: bet hedging and predictive germination in a variable environment. Ecol Lett 19:1209–1218. https://doi.org/10.1111/ele.12655
Guevara S, Merono-Casasola P, Ŝanchez-Rìos G (2005) Soil seed banks in the tropical agricultural fields of Lo Tuxtlas, Mexico. Trop Ecol 46:219–227
Guo H, Zhao H, Wang S, Wang X, Zhu Z (2015) Determining the recruitment limitation of three native woody species in the Chinese Pinus caribaea (Pinus caribaea tabuliformis Carr.) plantations on the Loess Plateau, China. Scand J For Res 30:538–546
Kassa G, Molla E, Abiyu A (2020) Effects of eucalyptus tree plantations on soil seed bank and soil physicochemical properties of Qimbaba forest. Cogent Food Agric 5:1711297. https://doi.org/10.1080/23311932.2019.1711297
Kremer KN, Bauhus J (2020) Drivers of native species regeneration in the process of restoring natural forests from mono-specific, even-aged tree plantations: a quantitative review. Restor Ecol 28:1074–1086
Kumar R, Bhatnagar PR, Kakade V, Dobhal S (2020) Tree plantation and soil water conservation enhances climate resilience and carbon sequestration of agroecosystem in semi-arid degraded ravine lands. Agric For Meteorol 282–283:107857. https://doi.org/10.1016/j.agrformet.2019.107857
Kyereh B, Swaine MD, Thompson J (1999) Effect of light on the germination of forest trees in Ghana. J Ecol 87:772–783
Lamb D, Erskine PD, Parrota JA (2005) Restoration of degraded tropical forest landscapes. Rev Sci 310:1628–1632
Leitao FHM, Margues MCM, Ceccon E (2010) Young restored forests increase seedling recruitment in abandoned pastures in the Southern Atlantic forest. Rev Biol Trop 58:1271–1282
Longworth B, Williamson GB (2018) Composition and diversity of woody plants in tree plantations versus secondary forests in costa Rican Lowlands. Trop Conserv Sci 11:1–13
Luo X, Cao M, Zhang M, Song X, Li J, Nakmura A, Kitching R (2017) Soil seed banks along elevational gradients in tropical, subtropical and subal Pinus caribaea forests in Yunna province, South west China. Plant Divers 39:273–286
Medina M, Flores MP, Goya JF, Campanello PI, Pinazo MA, Ritter LJ, Arturi MF (2020) Native tree regeneration in native tree plantations: understanding the contribution of Araucaria angustifolia to biodiversity conservation in the threatened Atlantic Forest in Argentina
Mng’omba SA, du Toit ES, Akinnifesi FK (2007) Germination characteristics of tree seeds: Spotlight on Southern Africa tree species. Tree and forestry science biotechnology Global Science books
National Forestry Authority (NFA) (2012) Forest management plan for South Busoga Central Forest Reserve management plan area. For the period 1st July 2012─30th June 2022. Kampala, Uganda
Onyekwelu JC, Olabiwonnu AA (2016) Can forest plantations harbour biodiversity similar to natural forest ecosystems over time? Int J Biodivers Sci Ecosyst Serv Manag 12:108–115
Overdyck E, Clarkson BD (2012) Seed rain and soil seed banks limit native regeneration within urban forest restoration plantings in Hamilton City, New Zealand. N Z J Ecol 36:177–190
Parrota JA (1995) Influence of overstory composition on understory colonization by native species in plantations on a degraded tropical site. J Veg Sci 6:627–636
Pirard R, Secco LD, Warman R (2016) Do timber plantations contribute to forest conservation? Environ Sci Policy 57:122–130
Quine CP, Humphrey JW (2009) Plantations of exotic tree species in Britain: irrelevant for biodiversity or novel habitat for naïve species? Biodivers Conserv 19:1503–1512. https://doi.org/10.1007/s10531-009-9771-7
Ritter LJ, Campanello PI, Goya JF, Pinazo MA, Arturi MF (2018) Plant size dependent response of native tree regeneration to landscape and stand variables in loblolly pine plantations in the Atlantic Forest, Argentina. For Ecol Manage 429:457–466
Sakai A, Sato S, Sakai T, Kuramoto S, Tabuchi R (2005) A soil seed bank in a mature conifer plantation and establishment of seedlings after clear-cutting in southwest Japan. J For Res 10:295–304
Senbeta F, Teketay D (2002) Soil seed banks in plantations and adjacent natural dry Afromontane forests of central and Southern Ethiopia. Trop Ecol 43:229–242
Senbeta F, Teketay D, Naslund B (2001) Native woody species regeneration in exotic tree plantations at Munessa-Shashemene Forest, southern Ethiopia. New For 24:131–145
Ulya NA, Nulia A, Kunarso A, Martin E, Waluyo EA (2019) Valuation of goods and services derived from plantation forest in peat swamp forest area: the case of South Sumatra Province. IOP Conf Ser Earth Environ Sci 308:012047. https://doi.org/10.1088/1755-1315/308/1/012047
Uwimbabazi M, Eycott AE, Babweteera F, Sande E, Telford T, Vandvik V (2017) Avian guild assemblages in forest fragments around Budongo Forest Reserve, Western Uganda. Ostrich 88:267–276. https://doi.org/10.2989/00306525.2017.1318186
Vandvik V, Klanderud K, Meineri E, Måren IE, Töpper J (2015) Seed banks are biodiversity reservoirs: species—area relationships above versus below ground. Oikos 125:218–228
Wang J, Ren H, Yang L, Li D, Guo Q (2009) Soil seed banks in four 22-year-old plantations in South China: implications for restoration. For Ecol Manage 258:2000–2006
Wang N, Jiao J, Jia Y, Wang D (2017) Influence of afforestation on the species diversity of the soil seed bank and understory vegetation in the hill-gullied loess Plateau, China. Int J Environ Res Public Health 14:1285. https://doi.org/10.3390/ijerph14101285
Weerasinghe M, Ashton MS, Hooper ER, Singhakumara BMP (2019) Floristics of soil seed banks on agricultural and disturbed land cleared of tropical forests. Restor Ecol 27:138–147
Wunderle JM Jr (1997) The role of animal seed dispersal in accelerating native forest regeneration on degraded tropical lands. For Ecol Manag 99:223–235
Zamora CO, Montagnini F (2007) Seed rain and seed dispersal agents in pure and mixed plantations of native trees and abandoned pastures at La Selva biological Station, Costa Rica. Restor Ecol 15:453–461
Zeng-hui H, Yang Y, Ping-sheng L, De-quan D, Bo Z, Bing-fei H (2013) Characteristics of soil seed bank in plantation forest in the rocky mountain region of Beijing, China. J For Res 24:91–97
Zhang D, Zhang J, Yang W, Wu F, Huang Y (2014) Plant and soil seed bank diversity across a range of ages of Eucalyptus grandis plantations afforested on arable lands. Plant Soil 376:307–325
Acknowledgements
We are grateful to the Uganda National Council for Science and Technology (NS 511), National Forestry Authority, particularly the management of Busoga Central Forest Reserve and Tree seed Center Banda for their time and support.
Funding
NORAD funded this research through NORHED Grant number UGA-13/0019.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Esaete, J., Muwanika, V.B., Nampanzira, D.K. et al. Understory floristic diversity and soil seed bank status of planted and unplanted portions of South Busoga Forest Reserve, Eastern Uganda. Trop Ecol 64, 558–570 (2023). https://doi.org/10.1007/s42965-022-00282-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42965-022-00282-6