Abstract
Purpose
The purpose of this study was to review the different hermetic storage (HS) systems used in Sub-Saharan Africa (SSA) and their effectiveness against the agents of storage quality deterioration.
Method
Relevant studies on grain HS in SSA conducted in the past two decades for effectiveness against the agents of storage losses are reviewed. Specifically, the study comprehensively reviewed the effectiveness of HS technologies against insect-induced damage and weight loss, seed germination, insect infestation, and mold and mycotoxin contamination. Traditional grain storage methods and HS technologies used in SSA are reviewed, including those suitable for smallholder farmers and traders. Future developments and modifications to HS are also discussed.
Results
Most grain HS studies are carried out in SSA where post-harvest storage losses are considered one of the world’s largest. Scholarly studies compared the performance of HS against traditional technologies for storage periods of up to 7 months and a few extending to 1 year or more. Commonly studied HS technologies include hermetic layered bags and grain silos. In general, HS offers superior protection against the agents of grain deterioration for long-term storage compared to conventional storage technologies.
Conclusion
HS technologies are highly effective in protecting stored grains from quantitative and qualitative storage losses and thus guarantee that stored grains can attract better prices and are safe and nutritious to the consumer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The world population will be over 9 billion people by the middle of the twenty-first century (UN, 2019), and food production must grow by up to 70% to meet the new food demand (Affognon et al., 2015; Binns et al., 2021; Hodges et al., 2011). Developing nations in Sub-Saharan Africa (SSA) and South Asia will contribute the largest to this projected population rise and hence food demand (Hodges et al., 2011; Kumar and Kalita, 2017). Even with current populations, these nations are already struggling with many challenges such as low agricultural production and productivity. The challenge to feed the world will need redress amidst worsening climate crises, natural resource depletion, increasing urbanization, environmental degradation, and land demand for alternative uses. World leaders, scientists, and philanthropists are progressively engaged in rethinking possible strategies to achieve this goal amidst all challenges.
Among the options to meet the food demand are to (1) increase food production on existing land, (2) sustainably bring more land into agricultural production, and (3) make more food available through the global reduction of post-harvest food losses and food wastes. All these interventions demand the full awareness that any food security effort need not only be suitable but environmentally, socially, and economically sound, acceptable, and sustainable (Guenha et al., 2014; Movilla-Pateiro et al., 2020). Increasing food production on existing land can play an important role but will be constrained by the finite resources of land, water, and the biosphere (Elferink and Schierhorn, 2016; Godfray et al., 2010). Besides, bringing more land acreage into agricultural production may be unsustainable in the long term due to shrinking agricultural land and rapid urbanization (Bob, 2010) and can inevitably lead to biodiversity loss. An integrated approach should, thus, not only aim at increasing food production and productivity but should, together with other strategies, focus on reducing post-harvest losses (PHLs) through proper and sustainable post-harvest handling practices (Guenha et al., 2014; Silva et al., 2018).
While different crops are grown globally, grain crops are considered important in food security and nutrition as sources of vital dietary calories and proteins to consumers (Alonso-Amelot and Avila-Núñez, 2011; Mesterházy et al., 2020). Globally, cereal grains are the key sources of calories with rice, wheat, and maize being considered the most popular staple food crops in most developing countries (Kumar and Kalita, 2017). Due to its importance, cereal grains supply the calorie needs of over 60% of the population in developing countries (Awika, 2011). Like cereals, legume grains contribute about 33% of the global dietary protein needs (Vance et al., 2000). This is significant in developing economies where the majority of the population lacks adequate physical and financial access to protein sources of other origins. In SSA, maize, rice, wheat, millet, and sorghum are the most commonly cultivated cereal grains, with common beans, chickpea, cowpea, pigeon pea, and groundnuts being the most cultivated legumes (Raheem et al., 2021; Vanlauwe et al., 2019).
Despite the contribution of grains to food security and nutrition in developing countries, PHLs in the sector remain high. PHLs are measurable quantitative and qualitative food losses that occur at any point between crop harvesting and consumption due to several causes linked to physical, physiological, and environmental factors. These factors may include grain mechanical damage, heightened temperature and relative humidity, mold and mycotoxin contamination, invasion by pests (insects, rodents, birds), inappropriate handling, storage, and processing (Abass et al., 2014; Hengsdijk and De Boer, 2017). In SSA, quantitative grain PHLs are estimated at between 10 and 30% (APHLIS, 2020; Brown et al., 2013) and could reach up to 50% or more when considered together with quality losses (Rickman, 2002; Shee et al., 2019). On a global scale, about one-third of the food produced for human consumption, estimated at USD 1.0 trillion, is lost or wasted annually in PHLs (World Bank, 2011). In SSA, grains worth about USD 4 billion out of estimated annual grain production of USD 27 billion are reportedly lost annually as PHLs (World Bank, 2011). Among the causes of PHLs, grain storage losses are considered the main loss factor in developing countries (Kumar and Kalita, 2017), estimated at between 10 and 20% of stored commodities mainly resulting from insect pests (Philip and Throne, 2010). The causes of grain PHLs in storage include poor storage conditions, lack of proper storage infrastructure, high temperature, and relative humidity. These lead to mold development, high respiration rate, and increased insect activity, with negative consequences on the quality of stored grains.
Due to high incidences of storage insect pest infestations, many smallholder farmers in developing countries sell their grains shortly after harvest for fear of losses to storage pests (Mutungi et al., 2015). These farmers soon buy the grains at often relatively higher prices than sold during the lean seasons. When grains are not sold immediately after harvest, smallholder farmers manage insect pests using various forms of cultural practices and application of botanicals (Kamanula et al., 2010; Said and Pashte, 2015) or through the use of synthetic pesticides (Upadhyay and Ahmad, 2011). The use of botanicals such as wood ash, animal dung, and admixtures are often not very protective for long-term grain storage and become unsuitable when grain quantity becomes large (Mutungi et al., 2014). Despite its effectiveness, there is available scientific evidence linking synthetic pesticide residues in foods to health complications and environmental contamination (Dubey et al., 2008; Loganathan et al., 2011). Besides, the development of insect resistance (Benhalima et al., 2004; Chulze, 2010; Collins, 2006), lack of knowledge on proper applications and use (De Groote et al., 2013; Mutungi et al., 2014), high cost and unavailability (Tefera et al., 2010), and reduced grain quality in some cases have disadvantaged the use of synthetic pesticides for the preservation of grains during storage.
Alternative safe, effective, and eco-sustainable storage technologies that eliminate synthetic pesticide use without compromising grain quality have attracted the attention of scientists, medical practitioners, consumers, and policymakers to minimize storage losses due to insects. One such technology is the use of hermetic or airtight storage. HS use has attracted global interest and the attention of all stakeholders involved in the recent few decades (Baributsa and Ignacio, 2020). Due to their ability to store grains safely and for sufficiently long periods without the need to use synthetic pesticides (Baributsa et al., 2010; Carvalho et al., 2012; Kumar and Kalita, 2017), HS technologies have become popular for a wide range of commodities in Africa, Asia, and in South and Central America (Sudini et al., 2015). The success of the technology is due to the drastic elimination of oxygen (O2) with a parallel build-up of carbon dioxide (CO2) in the internal atmosphere due to the aerobic respiration of insects, grains, and fungi (Moreno-Martinez et al., 2000; Odjo et al., 2020). In such a modified grain atmosphere, insect death occurs either through hypoxia or desiccation (Moreno-Martinez et al., 2000; Murdock et al., 2012).
The success of any grain storage technology relies on its efficacy to protect the stored commodity from the agents of deterioration. These deteriorations include, among others, grain damage and weight loss, nutritional changes, grain moisture content changes, insect infestations, and mold and mycotoxin contamination. The degree of grain storage deterioration depends on the storage method used and storage conditions. HS technologies prevent or minimize stored grain quantity and quality deterioration, hence reducing storage losses. While HS has been in use since ancient times, its scientific research in SSA caught momentum in the past two decades or so. Numerous studies have demonstrated the effectiveness of different HS technologies for protecting stored grains under various storage conditions. However, there is a need to document evidence-based information on how HS technologies affect grain quality parameters in storage compared to traditional techniques as undertaken by research scientists in the past 20 years. The objective of this review paper was thus three-fold: first, discuss the factors responsible for grain storage losses in the context of SSA; secondly, describe the HS technologies commonly used for grain storage in SSA; and lastly, comprehensively review research studies conducted in the past two decades to demonstrate the effectiveness of HS technologies on grain quality.
Major Factors of Post-Harvest Storage Concern in Sub-Saharan Africa
Grain deterioration factors in storage consist of biotic and abiotic factors (Moses et al., 2015). The biotic factors are related to the living organisms in the grain ecosystem and include insect pests, rodents, birds, fungi, and the grain itself (Befikadu, 2014). In SSA, insect pests are considered the most significant biotic factor of storage concern (Midega et al., 2016; Njoroge et al., 2014). Abiotic factors include the non-living component of the grain ecosystem that affects the functioning of biotic components. These include temperature, relative humidity, intergranular gaseous levels, and grain moisture content (Moses et al., 2015). Moisture content and temperature are the two most important abiotic factors that significantly affect grain storage quality and shelf-life(Gonzales et al., 2009; Kumar and Kalita, 2017). Continuous and unfavorable interactions that occur over time between the biotic and abiotic variables cause grain deterioration (Moses et al., 2015), with the extent of interactions determining the degree of deterioration in storage.
Biotic Factors
Insect Pests
Stored grain insect pests are the most devastating biotic factor in storage grain deterioration (Moses et al., 2015) and can cause severe grain losses amounting to up to 40% of stored grains (Abass et al., 2014; Boxall, 2002; Bradford et al., 2018; Ognakossan et al., 2018; Tapondjou et al., 2002; Tefera et al., 2010). Most storage insect pest species are either beetle (Coleoptera) or moths (Lepidoptera)(Table 1), although there are some other types (Hodges and Maritime, 2012; Upadhyay and Ahmad, 2011). Both grubs and adult insects attack stored grains for beetles, while caterpillars are the destructive life stage among the moths (Upadhyay and Ahmad, 2011).
The severity of insect infestation in stored grains is a factor of the geography and climatic conditions and is a concern in the tropical and sub-tropical regions where the climatic conditions favor their rapid multiplication (Moses et al., 2015). In SSA, the maize weevil (Sitophilus zeamais) and the larger grain borer (Prostephanus truncatus Horn) are the most notorious and damaging storage insect pests known (Mutambuki et al., 2019; Vowotor et al., 2005), although several others also exist. P. truncatus is more destructive and can cause extensive grain damage amounting to over 30% dry weight loss in endemic situations (Cugala et al., 2007). P. truncatus first originated in Mexico and Central America and was accidentally introduced to Africa in the late 1970s where it has prevailed and thrived to date (Arthur et al., 2019).
The effect of insect pests on stored grains is both quantitative and qualitative. Insects cause considerable quantitative weight loss by penetrating the grain kernels, feeding on the endosperm and selectively removing the grain nutritious portion (Befikadu, 2014; Hodges and Maritime, 2012). In Niger, several grain crops lost between 7.4 and 83.9% of their original weight due to insect infestation after 6 months in storage (Baoua et al., 2015). In Zimbabwe, weight losses of maize due to insect pests were between 2 and 13% after 8 months of storage in traditional granaries (Giga et al., 1991). In Ethiopia, wheat grains suffered substantial insect-induced damage of between 3.6 and 13.6% with corresponding weight loss of between 0.7 and 2.5% after 6 weeks of storage (Kalsa et al., 2019). As insects feed, grain moisture content increases due to the aerobic respiration process that causes qualitative grain loss through the development of molds (Befikadu, 2014). The heat generated due to the metabolic process causes an increase in grain temperature and hence increased reproduction rate of storage insects. As the population of insects increases, more moisture is produced in the grain, and the cyclic process continues, sometimes at even faster rates. Qualitative grain deterioration by insects is also through their excreta, dead insects, partial consumption of grains which reduce the quality and consumer appeal of the grain, and reduced nutritional content (Johnson, 2020; Stathers et al., 2020; Taddese et al., 2020).
Rodents and Birds
Rodents are notorious for invading stored grains irrespective of the grain storage period and can, like insect pests, affect both grain quality and quantity (Brown et al., 2013). In Kenya, rodents are the second most important cause of storage losses after insect pests, responsible for between 30 and 43% of the storage weight losses of maize (Ognakossan et al., 2016). When present in grain stores, rodents can consume daily food equivalent to 10% of their body weight (Mills, 1996). In East Africa, the main rodent species of post-harvest storage concern include the black rat (Rattus rattus), the house mouse (Mus musculus), and the Natal multimammate mouse (Mastomys natalensis) (Makundi et al., 1999). These have become highly successful commensal invaders of grain stores with extreme adaptability and a high procreation rate. R. rattus and M. musculus inhabit houses and storage structures, while M. natalensis moves from the field to storage structures in search of food whenever there is scarcity in the field (Ognakossan et al., 2016).
There are remarkably few studies in SSA quantifying grain post-harvest storage losses due to rodents (Brown et al., 2013). A study in Kenya reported cumulative weight losses of 2.2–6.9% and 5.2–18.3% in shelled maize and dehusked cobs respectively after 3 months of storage due to R. rattus(Ognakossan et al., 2016). Apart from causing direct physical weight losses due to feeding, rodents in grain stores can lead to several other storage challenges such as damage to storage structures like sacks and buildings and contamination of grains with urine, hair, and fecal droppings (Brown et al., 2013; Ognakossan et al., 2018). Grains contaminated by rodent droppings may harbor pathogens rendering them unsafe and unfit for human consumption (Hodges et al., 2014). Another problem imposed by rodents is that their urine may participate in the proliferation of molds due to increased water activity of the grains, and rodent feeding itself could facilitate the dissemination of fungal spores (Ognakossan et al., 2018).
Bird infestation of grain stores in SSA is rarely a problem but can be a concern when doors, windows, and ventilators are left open, providing easy access to the interior of stores. The bird species of concern in grain storage facilities are predominantly the same species that cause problems in domestic and commercial centers of many rural and urban areas (McCarthy, 2003). In SSA, bird species commonly known to invade grain stores are sparrows (Passer domesticus), pigeons (Columbia livia domestica), and chicken (Gallus gallus L.). Birds cause deterioration of stored grains through grain consumption, contamination with excreta, and the introduction of mites (Mills, 1996).
Mold and Mycotoxin Contamination
Molds (fungi) responsible for grain deterioration consist of two main groups based on their predominance at different crop growth stages and harvest, affected by environmental conditions. The first group is the field fungi which colonize ripening grains on field standing crops during the crop maturation stage. Examples of field fungi include Alternaria and Fusarium(Mannaa and Kim, 2017). The second group is the storage fungi which may be present in minute numbers before harvesting but increase substantially during storage due to favorable environmental conditions (Mannaa and Kim, 2017). Storage fungi mainly consist of Aspergillus and Penicillium(Bradford et al., 2018).
Mold contamination of stored grains can cause undesirable qualitative and quantitative changes. These changes include grain discoloration, heating, dry matter loss, increased fatty acid content, mycotoxin production, loss of germination, and degradation of lipids and proteins (Magan and Aldred, 2007). Grain contamination by certain molds is also considered a serious food safety concern in the tropical and sub-tropical regions where the ambient relative humidity is high. This is due to the carcinogenic nature of the mycotoxins produced by these molds (Kaaya and Kyamuhangire, 2006). Mycotoxins are secondary metabolites produced by certain fungal species, mostly Aspergillus, Fusarium, and Penicillium(Mannaa and Kim, 2017), and are toxic to humans and animals (Bradford et al., 2018). Among the mycotoxins, aflatoxin, ochratoxin, deoxynivalenol, zearalenone, fumonisin, trichothecenes, and patulin significantly contaminate stored grains (Wagacha and Muthomi, 2008). Mycotoxin contamination by aflatoxin and fumonisin renders grains unsafe for human consumption (Tefera, 2012). Consumption of aflatoxin-contaminated foods results in aflatoxicosis that may cause death in extreme cases through liver inflammation (Tefera, 2012).
Mold and mycotoxin contaminations are influenced mainly by two environmental factors: temperature and relative humidity (Moses et al., 2015). Relative humidity of at least 70% promotes mold growth in grains (Ng'ang'a et al., 2016a). Relative humidity at the grain surface layer is a factor of moisture content of the grains in equilibrium with a relative humidity of 70%, which for most grains is about 14% (Hodges and Maritime, 2012). When moisture content increases to 15–19%, molds of Aspergillus spp. and Penicillium spp. grow. This causes an increase in the grain respiratory activity (Magan and Aldred, 2007), which leads to an increase in temperature due to the aerobic respiration process resulting in grain heating, and hence spoilage. It is thus imperative that grains are stored at a moisture content of at most 14% to avoid mold growth and mycotoxins.
Abiotic (Physical) Factors
The principal physical factors which interact to influence stored grain microenvironment include temperature, relative humidity, moisture content of the stored grain, and gaseous concentrations (especially oxygen and carbon dioxide in the grain interstitial environment). The first three factors are discussed in this section while gaseous concentrations are discussed under the principle of grain hermetic storage.
Temperature and Relative Humidity
Proper temperature and relative humidity management are considered the two most important management strategies that can be used to protect stored grains from the devastating effects of insect pests and molds. Most storage insect pests thrive in an optimal temperature range of between 25 and 34 °C for most species and between 15 and 30 °C for mold development (Moses et al., 2015; Taruvinga et al., 2014). Most SSA countries fall within this temperature range and suffer huge grain storage losses to insect infestations exacerbated by poor storage practices and infrastructure in these regions. Outside these temperature ranges (colder or hotter), the development of insects and molds is constrained despite the tolerance exhibited by certain species and life stages of certain pests (Loganathan et al., 2011).
Relative humidity, the percentage of water vapor in the air between the grains at a given temperature, represents the equilibrium between the air humidity and the grain moisture content. The relative humidity of the intergranular air is responsible for determining which living organisms can develop in stored grains (Mills, 1996). Relative humidity above 65% facilitates the development of mold and insect pests which may cause grain deterioration (Taruvinga et al., 2014). There is a direct linear relationship between grain temperature and relative humidity: an increase in grain temperature causes an increase in grain relative humidity (Devereau et al., 2002). High temperature and relative humidity regimes that characterize the tropical regions of SSA are favorable for insect growth and development, shortening their life cycle. This results in increased insect pest populations (Phophi et al., 2020; Van Dyck et al., 2015). Besides insect infestation, the high temperature and relative humidity in SSA increase the susceptibility of stored grains to mycotoxin contamination (Magan et al., 2011).
Moisture Content
Moisture content is the amount of moisture per unit weight of grain sample. The amount of moisture present in grains determines the extent of physical, biological, and biochemical activities in stored grains (Suleiman et al., 2013). As dry grains are hygroscopic, they may gain or lose moisture in traditional storage systems due to fluctuations in the external relative humidity environment (Diarra and Amoah, 2019). Grains at harvest have high moisture content in the range of 16–20% or more and must be quickly dried to at most 14% before storage to prevent deterioration (Magan et al., 2010). When inadequately dried before storage, grain temperature will rise due to the respiration process of the grains resulting in the development of spoilage molds (Magan et al., 2010).
The moisture content of stored grains has a key role in deterioration, and each grain has recommended storage moisture content without significant quality deterioration. The moisture content of dry grain varies between 6 and 15% depending on the type of grain and duration of storage (Taruvinga et al., 2014). Also, at a given relative humidity, insect pests prefer grains with higher moisture content, probably because of the soft texture nature of these grains (Pixton and Warburton, 1971). As a rule of thumb, the higher the moisture content of stored grains, the greater the degree of susceptibility to deterioration to insect pests and mold deterioration.
Conventional Grain Storage Technologies in Sub-Saharan Africa
There are several traditional methods used for grain storage in SSA. These mainly include jute bags, cloth bags, woven polypropylene (PP) bags, and woven baskets. There are also several others. These are extensively described in the work of Mobolade et al. (2019). The woven PP bag is the most commonly used traditional grain storage method globally (Nduku et al., 2013). These bags, which mostly come in sizes ranging from 25 to 100 kg, are widely used by smallholder farmers due to their availability, affordability, and convenience in handling and use (Mobolade et al., 2019). The limitation of the traditional storage methods such as the woven PP bags is that they permit the exchange of air and moisture between grains and the ambient environment. This allows the biotic organisms enclosed in the grain to continue their metabolic life unrestricted unless a control measure such as the use of synthetic insecticides is in place. HS has come in place to fulfill this gap by restricting interactions between the ambient environment and internal grain atmosphere.
Principle of Grain Hermetic Storage
HS is credited for long-term and intermediate storage of grains without synthetic insecticides due to their ability to create a hostile internal environment detrimental to the growth, development, reproduction, and survival of insect pests within the stored grains. This is because HS provides a barrier that limits O2 and moisture exchange between the grain’s internal atmosphere and external environment (Odjo et al., 2020). The effectiveness of HS depends on the integrity of the hermetic seal, the nature of the stored commodity, the type and prevalence of insect pests, and the mechanical strength of the barrier material used (Njoroge et al., 2014).
HS that relies on the grain ecology to create low O2 with a parallel high CO2 atmosphere is a form of a biogenerated modified atmosphere, also referred to as Organic HS (Cheng et al., 2013; Villers et al., 2006). The low O2 and high CO2 environment are due to the physiological process of aerobic respiration of insects, grains, and molds in the sealed structures of HS (Calderon and Navarro, 1980; Jonfia-Essien et al., 2010; Quezada et al., 2006; Sanon et al., 2011; Tubbs et al., 2016; Williams et al., 2014). In order of ranking, insects are the largest consumer of enclosed O2 followed by molds and lastly grains (Moreno-Martinez et al., 2000). The grain respiration process accounts for a minimal drop in O2 and a rise in CO2(Baoua et al., 2012). When O2 level is reduced to 3% v/v or below, insects stop feeding and eventually die due to hypoxia (Moreno-Martinez et al., 2000). Besides the death of insects, fungal development also ceases at such low O2 levels (Moreno-Martinez et al., 2000).
Organic HS is the most common form of HS in developing countries (Villers et al., 2006) since it relies on the grain ecology alone to control insect pests. In specialized applications requiring rapid disinfestation, O2 levels can be quickly decreased through (1) the application of a high vacuum sufficient enough to eliminate all stages of insect pests (termed vacuum HS); and (2) through purging with an external CO2 gas (termed gas-hermetic fumigation). In either case, the O2 levels can be quickly reduced to at most 2% in a short time (Navarro et al., 2003; Villers et al., 2006).
The role of reduced O2 levels in protecting stored grains from insect pests is two-fold. The first role is that O2 provides a basis upon which oxidative metabolism occurs to provide energy without which growth, reproduction, and survival become difficult for the living organisms responsible for grain deterioration. The O2 enclosed in the biogenerated interstitial grain atmosphere in HS is consumed according to the general respiration equation as shown in Eq. (1). The depletion in O2 levels rather than the CO2 accumulation is responsible for the constrained development and survival of insect pests in the stored grains (Bailey, 1965; Murdock et al., 2012). The CO2 levels attained in a biogenerated HS, usually at most 20%, may not cause insect mortality but may impose behavioral changes in certain insects (Willis and Roth, 1954). Despite the limited effects of CO2 levels in Organic HS on insect mortality, the synergistic effects of low O2 levels and elevated CO2 levels are more effective in controlling insects compared to the effect of the individual gases (Calderon and Navarro, 1980; Cheng et al., 2013).
The second role of reduced O2 in insect pest control in hermetic grain storage is that it serves as a supply of metabolic water for the insects as shown in the respiration process in Eq. (1). This metabolic water is essential to sustain the vital life processes of insects. At limiting O2 levels of HS, this source of metabolic water is interrupted (Murdock and Baoua, 2014). Due to this, enclosed insects or their life stages gradually desiccate and die since they continue to respire, losing water from their tissues. The early instars of the insect pest life stage are particularly more vulnerable to asphyxiation and desiccation as they are metabolically more active (Murdock et al., 2012).
The Contribution of Grain Hermetic Storage to Food Security
Improved storage such as the use of HS can affect all the four internationally recognized pillars of food security of availability, access, utilization, and stability (Napoli et al., 2011). Storage of grains in HS containers prevents grain insect infestations and moisture loss due to environmental relative humidity changes, all responsible for physical grain losses if unchecked. Storage in HS guarantees food security by causing improvement in food availability through reduced physical food losses (Adeola, 2020; Tefera, 2012). This effort can lessen the burden to improve local food availability through food imports and food aid donations at the national and regional levels. Reduced post-harvest storage losses also help improve grain supply in the markets relative to the demand, resulting in increased food access by the consumers. This may result in food price reductions on the one hand and improved farmers’ incomes due to superior grain quality sale on the other, empowering farmers to gain financial access to other foods which they do not physically produce (Delgado et al., 2021).
Besides improved food availability and access, HS of grains helps guarantee food utilization by consumers through preservation of the stored grain nutritional values such as calories, proteins, and vitamins (Sheahan and Barrett, 2017), in addition to micronutrient quality preservation. Grain storage loss reduction is also critical in improved food safety due to the management of fungal or pest infestations that would otherwise result in consumer health implications through the consumption of otherwise contaminated foods. In so doing, there is an improvement in food utilization by the consumer. HS of grains also contributes to improved food price stability due to an increase in the amount of stored food, even during the crop off-seasons(Bendinelli et al., 2020).
Forms of Hermetic Storage Commonly used in SSA
HS of grains is used in several forms to meet the different user scales and needs. Despite the diversity, they work under the same principle as discussed in the previous section. As discussed in the following section, these include the hermetic layered bags, metal and plastic silos, plastic drums, Cocoons, and other containers.
Hermetic Layered Bags
Hermetic layered bags are one of the many methods of airtight storage based on a biogenerated modified atmosphere. Most hermetic bags used for grain storage consist of two main components: (i) an inner (single or double) liner of high-density polyethylene (HDPE) bag, and (ii) an outer bag. The inner HDPE liner consists of a thin, transparent, and low permeability multi-layer plastic that limits the permeability of gases so that a low O2 environment is created and maintained at levels that hinder the development of all stages of insect pests. When the HDPE liner is pierced or damaged, the bag loses its protective integrity. In the case of bags with double inner HDPE liners, the second liner provides extra safety should one of the liners become damaged. The outer bag, usually a woven PP or jute bag, offers extra strength and protection during handling.
There are two main forms of hermetic layered bags used for grain storage in SSA. These are the Purdue Improved Crop Storage (PICS™) bag and the SuperGrain™ (SG) bag. There has been widespread adoption of both PICS bags and SG bags in West and Central Africa due to their efficacy against storage pests, but also due to their low cost, little space requirement, durability, and ease of manufacture and use (Baributsa et al., 2010; Ndegwa et al., 2016). Despite their effectiveness, hermetic bags have some limitations which include susceptibility of HDPE liners to physical damage and perforations from certain insect species and rodents (Chigoverah and Mvumi, 2016; De Groote et al., 2013; García-Lara et al., 2013; Manandhar et al., 2018).
Purdue Improved Crop Storage Bag
PICS bag is a trademark hermetic layered bag originally developed in the late 1980s by Scientists from Purdue University with financial support from the United States Agency for International Development (USAID) in a Bean/Cowpea Collaborative Research Support Program (Moussa et al., 2014; Murdock and Baoua, 2014). Initially developed to manage insect pests in cowpea grains in West and Central Africa (Murdock et al., 2012), PICS bag successes, utilization, and adoption have extended to several grain commodities spanning territories beyond the Central and West Africa corridors. It consists of three-layered plastic bags. These consist of (1) two 80 μm inner HDPE bags, one surrounded by the second to create a low permeability seal, and (2) an outer bag made of woven PP bag which endows mechanical strength to the two inner HDPE bags and the storage bag as a whole (Baributsa et al., 2010; Murdock and Baoua, 2014). For PICS bags to be effective in controlling insect pests, each of the two inner liners should have a minimum thickness of 80 μm as liners less than this have proven to be less effective (Sanon et al., 2011).
PICS bags are considered the most cost-effective grain storage option for smallholder farmers in Africa (Ibro et al., 2014) and is promoted and marketed in the continent at a retail price of about USD 2–3 per bag (Baoua et al., 2018; Murdock and Baoua, 2014). PICS bags come in three different sizes of 25 kg, 50 kg, and 100 kg bags in SSA (Baributsa and Ignacio, 2020; Jones et al., 2011).
SuperGrain Bag
SG bags were initially developed for rice seed storage in a 5-year research project of the International Rice Research Institute in the Philippines (Villers et al., 2006). It has since been used to store other grain commodities. They are produced commercially by GrainPro Inc. in the Philippines and share a very close similarity to the PICS bag. While the PICS bag consists of two inner HDPE liners, the SG bag consists of a single HDPE liner having a thickness of about 78 μm, surrounded by an outer woven PP bag. SG bags are marketed in capacities ranging from 25 kg to 100 kg with prices ranging between USD 3 to 5 for 90 kg bags (Ndegwa et al., 2016).
Despite the registered success of SG bags in protecting stored grains, its protective action can be compromised in the presence of some species of insects such as the P. truncatus(Chigoverah and Mvumi, 2016; De Groote et al., 2013), due mainly in part to the perforations of the single HDPE liner by certain species of insect pests. Under such a circumstance, single-use for one season is recommended. Due to this, a more recent variant of the SG bag (SGB-IV R™) has been introduced with superior HDPE characteristics of greater toughness and puncture resistance while still retaining the original 78 μm thickness (De Bruin et al., 2012; García-Lara et al., 2013).
Other Hermetic Layered Bags
While the PICS and the SG bags are the two most common forms of hermetic layered bags used in SSA, several other brands have emerged in the recent past by either innovating or imitating pre-existing bags. These include the Elite bags and the AgroZ® bags, all manufactured in East Africa. AgroZ bags are double-layered (one 90 μm co-extruded inner liner combining HDPE and metallocene linear low-density polyethylene surrounded by an outer woven PP bag) bags developed and distributed by A to Z Textile Mills Ltd in Tanzania (Baributsa and Ignacio, 2020). Elite hermetic bags consist of a triple layer similar to PICS bags manufactured by Elite Innovations (K) Ltd based in Kenya.
Metallic Silo
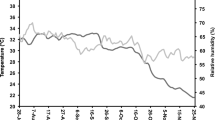
A metallic silo (Fig. 1) is an airtight cylindrical structure made of a galvanized iron sheet to protect grains from post-harvest storage losses (Tefera et al., 2010). The metallic silo technology is proven to be very effective for considerable storage periods, offering protection to stored grains from not only insect pests but also rodents and birds (Chigoverah and Mvumi, 2016; De Groote et al., 2013; Kumar and Kalita, 2017; Tefera et al., 2010). Compared to the hermetic bags, metallic silos are physically stronger and offer superior grain protection for a much longer duration. The metallic silo first originated from Central America where the POSTCOSECHA (the Spanish word for post-harvest) program promoted its use in Central American countries from the early 1990s to 2000s (Hellin et al., 2008). However, one of the concerns regarding the use of metallic silos was the continued treatment of stored grains with phostoxin, a highly toxic grain fumigant, to offer extra protection (Yusuf and He, 2011). In SSA, the use of grain fumigants in many countries is, by law, restricted to licensed fumigation companies. To ensure a rapid depletion in O2 levels, candles are lit and placed in metallic silos before sealing the top lid (Mubayiwa et al., 2021). This practice used in SSA has demonstrated that there is no need to use phostoxin fumigant in metallic silo grain storage.
Metallic silo used for grain storage in developing countries (Manandhar et al., 2018)
Despite their effectiveness in reducing grain storage losses, metallic silos have some limitations that include (1) high initial costs compared to the hermetic bags, (2) limited availability of galvanized iron sheets used for their manufacture, and (3) a limited number of trained artisans for their construction (Chegere et al., 2020; De Groote et al., 2013; Gitonga et al., 2013). Besides, failure to ensure hermetic seal during manufacture or improper usage may significantly jeopardize their protective integrity (Chigoverah and Mvumi, 2016; Manandhar et al., 2018). Due to these constraints, the adoption and use of metallic silos for grain storage in SSA have been relatively low to date.
Plastic Silo and Plastic Barrel
Like metallic silos, the plastic silo is used in many parts of SSA for grain storage. They may have the same design as the metallic silo with an inlet or outlet hole; plastic barrels commonly used for water harvesting are also frequently used for airtight grain storage in some communities (Abass et al., 2018). They can be made airtight like the hermetic bag or the metallic silo. While effective for grain storage, plastic barrels have other competing uses in water storage and are often considered expensive for smallholder farmers’ grain storage (Ibro et al., 2014).
Despite the effectiveness of the silos and barrels for grain storage in smallholder farmer subsistence settings, containers need to be filled with grains to be effective (Covele et al., 2020). Smallholder farmers, however, produce a small quantity of grains that may not fill the larger size containers to ensure hermeticity. This is not necessary in the case of the hermetic bags.
Grain Cocoon
Grain Cocoon™ is a commercially available large-scale hermetic device with flexible liners used for grain storage without synthetic pesticides (Villers et al., 2006). The device is a large plastic bag that consists of two plastic halves joined together using an airtight zipper following loading of the device with bags containing grains. Grain Cocoon consists of a flexible UV-resistant polyvinyl chloride that guards against attack by rodents and prevents the exchange of air and moisture between the stored grains and the external environment. It can be used multiple times for many years under very harsh climatic conditions without getting damaged easily.
Like the SG bags, Cocoons are manufactured and distributed by GrainPro Company in the Philippines. The effectiveness of Cocoons for grain storage protection is reported, but scientific documentation of their performance in comparison with other storage methods is scanty (Chigoverah et al., 2018). A study in Ghana on the storage of cocoa beans (Jonfia-Essien et al., 2008; Jonfia-Essien et al., 2010) and Zimbabwe on maize storage (Chigoverah et al., 2018; Chigoverah et al., 2016) are some of the few documented studies in SSA on the use of Cocoons for grain storage. In all cases, they were highly effective for the protection of stored grain quality. In Sri Lanka, the use of Grain Cocoon for soybean storage was highly effective for 8 months (Gunathilake, 2020).
Alternative Hermetic Containers
While hermetic bags and silos are the common forms of HS commercially marketed for smallholder grain storage in SSA, there exist alternative containers that can equally be suitable (Williams et al., 2017). These include, among others, recycled plastic bottles, jerry cans, and metal drums. Plastic bottles, usually used water and beverage bottles, with most being small in capacity, are mainly suitable for seed storage (Odjo et al., 2020). This is beneficial as seeds are usually kept in small quantities for smallholder farmers compared to the harvest. Due to this, plastic bottles function as alternative storage containers in some areas. They tend to be small in size and are generally available, free of charge, in almost all urban and rural areas of SSA. While plastics remain an environmental nuisance in Africa, their utilization as alternative grain storage units can offer an environmental relief. Used empty oil drums for grain storage among smallholder farmers are also noticeable in some areas in SSA. However, these may provide an incomplete airtightness unless treated with a sealing material (Mann et al., 1999).
Advantages and Disadvantages of Hermetic Storage Systems
With HS, the quality of stored grains can be maintained longer for use as seeds or grains compared to traditional open storage systems such as the woven PP bags. The O2-deficient and CO2-enhanced atmosphere created in HS offers an advantage by providing an unfavorable environment for the development and survival of insect pests without the use of insecticides. Through this, insect infestation is eliminated, maintaining grain integrity of germination, moisture content, damage, weight loss, etc. for several months (Guenha et al., 2014; Villers et al., 2008). By protecting the quality of stored grains for longer, farmers can keep their stored commodities longer and earn higher incomes through sales during lean seasons (Guenha et al., 2014; Navarro et al., 1994). Besides, the use of HS guarantees the food security status of smallholder farming households.
HS use is also a relatively cheap grain storage method since it eliminates the need for synthetic insecticides or fumigation, offering significant economic and environmental benefits. The rapid adoption and use of HS in SSA can be linked to the increasing awareness of the dangers of synthetic pesticides and the demand by consumers for safe, environmentally friendly, and sustainable storage technologies. Some HS units, such as the silos, offer advantages through rodent protection, ease of installation and use, and the ability to be used for several years without damage. In addition to the above, HS is used to store products without the growth of mycotoxins such as aflatoxin in corn and peanut and ochratoxin in coffee (Nyarko et al., 2021; Villers et al., 2008).
Despite their effectiveness, some HS systems are highly susceptible to physical damage. This damage may occur due to the puncture of the inner HDPE liners by sharp objects or careless handling. Other sources of damage include abrasions and perforations due to certain insect pests and rodents (De Groote et al., 2013; García-Lara et al., 2013). These punctures and other physical damage breach the protective integrity of the hermetic seal and hence useful life of HS systems. The problem of physical damage to HS systems is more common in HDPE liners of HS layered bags. Some HS systems, such as the silos, have high initial costs and require knowledge and skills to construct and operate for them to be effective (Manandhar et al., 2018).
Effects of Hermetic Storage on Different Grain Quality Parameters
Effect on Grain Damage and Weight Loss
Grain damage refers to the visual physical evidence of deterioration in grain characterized by a hole, crack, or discoloration (De Groote et al., 2013). It is considered a more qualitative than quantitative grain assessment criterion usually reported as a percentage of damaged grains (Boxall, 2002). Grain damage is an important quality parameter as it influences the consumers’ willingness to buy grain and the price payable (Compton et al., 1998; Mishili et al., 2011). Other qualitative factors that affect consumer grain buying choice include live insect pests, dead pest remains, and insect excreta on produce. Grain weight loss, meanwhile, is the disappearance of otherwise edible food, usually expressed as a percentage weight loss (Boxall, 2002; De Groote et al., 2013). Weight loss of grains during storage occurs due to several factors related to loss of mass and moisture. Moisture weight loss may result from relative humidity differences between grains and the ambient environment, evaporation occurring as the grain tries to equilibrate with the ambient relative humidity (Walker et al., 2018). Mass weight loss is attributed mainly to pests (insects, rodents, and birds) feeding on the grains, fungal infestation, and grain metabolic activity (Covele et al., 2020). However, weight loss due to grain metabolic activities is negligible (Baoua et al., 2014b).
HS has the potential to keep stored grain damage and weight loss at levels comparable to that of insect-free grains (De Groote et al., 2013; Martin et al., 2015), partly due to its ability to eliminate or minimize the devastating actions of insect pests. This effectiveness is attributed mainly to reduced O2 levels inside HS devices which bring about behavioral changes in insects besides reduced growth, development, and reproduction. These limitations help prevent insect multiplication in hermetically stored grains (Chigoverah and Mvumi, 2016; Murdock et al., 2012). Besides, the ability of HS to limit relative humidity changes of grains against external changes helps prevent weight loss of stored grains. Several scholars have studied the effectiveness of HS in preventing grain damage and weight loss with successful results, retaining the quality and quantity of grains following long periods in storage.
In the study of the effectiveness of two different HS technologies on grain damage and weight loss, García-Lara et al. (2020) stored artificially infested maize grains in specialized hermetic containers: plastic hermetic silo (Bioxilo™) and plastic hermetic bag (sBag™) for 12 months. These were compared with storage in woven PP bags. Grains stored in woven PP bags recorded the highest degree of damage, with 51% and 60% total grain damage at 8 and 12 months respectively. In contrast, a minimal degree of grain damage of less than 10% occurred in grains stored in plastic hermetic bags and hermetic silos after 8 months. The 1000-kernel weight was unchanged after 8 months in hermetically stored grains while it decreased substantially in the traditional woven PP bag (12.8%).
Several other scholars have demonstrated the protective action of HS against the damaging effects of insect-induced grain damage and weight loss in stored grains (Atta et al., 2020; Baoua et al., 2014a; Baoua et al., 2012; Somavat et al., 2017; Yakubu et al., 2011). Table 2 shows the comparison of HS against traditional storage technologies on grain damage and weight loss. While treatment with synthetic insecticides may protect stored grains against insect-induced damage and weight loss, insecticidal potency is known to wear out with time (Mubayiwa et al., 2021; Mutungi et al., 2014). Due to this, insect re-infestation occurs later in storage which leads to profuse grain damage and weight loss.
Effect on Seed Germination
Access to good quality seeds is the foundation of smallholder farmer crop production in developing countries where at least 90% of cultivated crops depend on farmer-saved seeds (Mutungi et al., 2015; Neate and Guei, 2010). The private seed companies in developing countries tend to concentrate on hybrid seed production targeting affluent farmers who can afford to pay for new seeds every planting season. Smallholder farmers, however, are constrained by resources and may not be able to pay for these seeds and should therefore be able to store a portion of their harvested open-pollinated seed varieties to guarantee next season planting.
The time difference between crop harvesting and the next planting season is usually long, sometimes lasting several months; this may necessitate grains destined for use as seed to be stored well to guarantee high germinability in the next planting season. Several factors may compromise the ability of stored seeds to germinate such as natural aging, seed exposure temperature, insect infestation and seed damage (Martin et al., 2015), and relative humidity and seed moisture changes during storage (Guberac et al., 2003; Martin et al., 2015; Mutungi et al., 2015; Njoroge et al., 2014; Silva et al., 2018). All these factors are negatively correlated with seed germination. Insect pests are known to target the nutrient-dense portion of stored grains and thus destroy the embryo while feeding, reducing the seed germination potential and vigor of such planted seeds (Baoua et al., 2014b; Baributsa et al., 2017; Vales et al., 2014). High relative humidity of at least 80% causes fungal invasion of stored grain; proliferation of fungal spores in storage results in loss of seed viability and seedling vigor (Mutungi et al., 2014). Besides, seed germination may be severely affected by the complex changes resulting from increased heat and moisture due to the grain metabolic processes.
The type of storage is another factor that affects the germination capacity and vigor of stored seeds. Grains stored in different forms of HS containers have been shown to maintain their germination potential higher and longer than those in conventional non-hermetic counterparts (Anankware et al., 2012; Bakhtavar et al., 2019; Ellis and Hong, 2006; Freitas et al., 2016; Sudini et al., 2015). Table 2 shows the comparisons of hermetic and non-hermetic storage of grains on seed germination. From this, it is easily noticeable that hermetically stored grains preserve seed germination better than traditional storage practices. HS retains or minimizes decline in the germination of stored seeds by controlling insect multiplication and protecting against insect-induced damage during storage, thus promoting the ability of stored seeds to maintain their viability (Martin et al., 2015; Vales et al., 2014). Besides, HS protects stored seeds against fluctuating external relative humidity that would otherwise affect seed germinability.
Effect on Insect Infestation
The success of HS, perhaps, is its ability to minimize insect growth and multiplication in stored grains without synthetic insecticides. The technology is thus not only environmentally friendly but also cost-effective for smallholder farmers in developing countries as they do not have to buy grain insecticides. The biogenerated O2-depleted and CO2-enriched modified atmosphere causes insect mortality due to hypoxia and desiccation. In so doing, the insect population cannot grow in hermetically stored grains. Not only do O2 and CO2 changes cause insect mortality in hermetically stored grains, but also cause a cessation in egg production as well as larval and pupal development responsible for sustaining future pest populations (Amadou et al., 2016). Scholarly studies have shown HS technologies to be superior to conventional storage in protecting against stored grain insect infestations.
Freitas et al. (2016) studied the effectiveness of storing common beans in hermetic silo bags and used plastic bottles on common bean weevil infestation during 120 days of storage. There was no increase in the level of insect infestation by Acanthoscelides obtectus pests in grains stored in HS (silo bags and plastic bottles) during 120 days of storage. In contrast, infestation in non-hermetic glass containers increased by 54% after 120 days.
Baributsa et al. (2020) evaluated the performance of five post-harvest storage methods for maize grain storage during seven months in Benin. Naturally infested grains were stored in different hermetic bag brands (PICS bag, AgroZ bag, SG bag) and non-hermetic bags (woven PP bag and ZeroFly bag which is an insecticide-treated bag). Grains used in the experiment had a mean initial infestation of 52.5 insects per 500 g of grains. After 7 months of storage, there were no live insect pests in any of the HS bags investigated. In the woven PP and ZeroFly bags, however, live insect populations ranged between 1.8 and 5.3 insects per 500 g for S. zeamais, R. dominica, T. castaneum, and Cryptolestesferrugineus insect pests. Even though the ZeroFly storage is pre-treated with insecticides, it was unable to control insect infestations during storage.
Table 2 shows the effectiveness of HS devices in protecting stored grains from insect infestations. From this table, HS controls insect infestation in grains better than traditional storage methods. Despite the protective ability of HS for stored grains, some insect pests such as R. dominica, C. maculatus, and P. truncatus are shown to perforate the HDPE liners of hermetic bags (Chigoverah and Mvumi, 2016; De Groote et al., 2013; García-Lara et al., 2013). This interferes with the airtight seal and compromises the integrity of the hermetic protection.
Effect on Grain Moisture Content, Mold, and Mycotoxin Contamination
The moisture content of stored grains is the most important physical factor in grain deterioration since it favors the growth and proliferation of toxigenic molds (Sawant et al., 2012). When stored under non-hermetic conditions, grains absorb and lose moisture in humid conditions and dryer environments, respectively, until the attainment of equilibrium moisture content (Baoua et al., 2014a; Williams et al., 2014). Apart from exposure to varying external relative humidity levels, grains stored in non-hermetic devices may also gain moisture due to high insect activity and heavy fungal growth resulting from the breakdown of organic matter to yield CO2, moisture, and heat (Murdock et al., 2012; Njoroge et al., 2014).
HS devices possess an excellent ability to minimize grain moisture changes by (1) functioning as a barrier to moisture migration between the ambient environment and stored grains (Covele et al., 2020; Lane and Woloshuk, 2017; Williams et al., 2017) and (2) limiting moisture production resulting from the aerobic respiration process due to depleted O2 levels. Due to these, HS storage maintains the moisture content of stored grains at relatively constant levels compared to traditional methods (Aboagye et al., 2017; Baoua et al., 2014b; Chigoverah and Mvumi, 2016; Lane and Woloshuk, 2017; Likhayo et al., 2018; Suleiman et al., 2018; Tubbs et al., 2016; Williams et al., 2014). This is beneficial provided the grain is stored at a safe moisture level (Ng'ang'a et al., 2016b). This characteristic is highly desirable in the tropical and sub-tropical regions where the ambient relative humidity is always high and grains would gain moisture under no protective action of non-hermetic containers. By maintaining a constant grain moisture content, HS helps prevent the deleterious effects that result from moisture content changes (Ognakossan et al., 2013; Williams et al., 2017).
Yewle et al. (2022) investigated the performance of four different hermetic bag brands (PICS bag, SG bag, and two other brands marketed in India) on stored green gram moisture content during 6 months of storage. The woven PP and jute bags were used as non-hermetic containers for comparison. The average moisture content of green gram increased by about 2% in woven PP and jute bags following 6 months of storage. In all the hermetically stored grains, there was no substantial difference in the moisture content of grains between the onset and after six months of storage.
Mold and mycotoxin contamination in storage is a problem mostly in improperly dried grains (Suleiman et al., 2018), but may also occur due to moisture gain resulting from other factors such as insect activity aside from ambient relative humidity changes. In non-hermetic storage, the high insect population favors mold infestation and hence mycotoxin contamination due to the insect feeding activity as well as the ability to generate localized heat and moisture within the grains (Ng'ang'a et al., 2016b). The growth and accumulation of molds and mycotoxins are low if grains are stored and kept at a moisture content of at most 14% before storage (Magan and Aldred, 2007). Besides proper drying, the O2 depleted atmosphere characteristic of HS is a constraint to the development of molds and hence mycotoxin accumulation (Tubbs et al., 2016). The low mold growth and mycotoxin contamination in airtight storage are due to the limited biological activity under such conditions. Several studies have demonstrated the effectiveness of HS technologies on mold and mycotoxin contaminations with positive outcomes.
Williams et al. (2014) studied the effectiveness of PICS bags for maize storage on moisture content, mold, and mycotoxin contamination. Maize grains of moisture levels of 12, 15, 18, and 21% and inoculated with Aspergillus flavus-infested grains were stored in either PICS bags or woven PP bags at 26 °C for 1 month and 2 months. In woven PP bags, grain moisture content decreased in both storage periods, leveling around 6% after 2 months irrespective of the initial moisture content. In contrast, the moisture content of grains stored in PICS bags remained unchanged at the end of storage. A. flavus growth and aflatoxin accumulation were not recorded in any maize stored in PICS bags irrespective of the initial moisture content after 1 or 2 months. In the woven PP bags, low moisture maize (12 and 15%) did not register detectable levels of aflatoxin B1 but levels of 56.2 ppb and 47.7 ppb occurred in high moisture maize of 18 and 21% moisture content respectively after 2 months of storage.
Ng'ang'a et al. (2016b) compared the performance of PICS bags over the woven PP bags and jute bags for storage of shelled maize under on-farm conditions on aflatoxin contamination during 35 weeks of storage. After 35 weeks, there was a marginal change in mold infestation and aflatoxin contamination in grains stored in PICS bags of at most 14% moisture content. In contrast, mold infestations increased up to 6-fold while total aflatoxin content increased 5–8-fold in woven PP bags and jute bags in the same storage period. While marginal changes in mold and mycotoxin contamination are reported in hermetically stored grains, mold and aflatoxin contamination increased in all the bags with pre-storage moisture content greater than 14%.
In another study, Diarra and Amoah (2019) investigated the effectiveness of storage of maize grains in SG bags on aflatoxin contamination during 3 months of storage. Maize grains pre-infested with A. flavus were stored in either SG bags or woven PP bags for 90 days. Total aflatoxin content in SG bags changed marginally and remained below the recommended safe limit of 20 ppb following 90 days of storage. In contrast, total aflatoxin increased profusely in the woven PP bags, exceeding the recommended safe limit at the end of storage.
Despite the protective action of HS on moisture content changes and mold and mycotoxin contaminations, changes in grain moisture content have been reported in some studies. Fusseini et al. (2016) reported an increase in moisture content as well as aflatoxin content in maize grains in PICS bags following storage at different temperatures under laboratory conditions. This study indicated that the largest increase in aflatoxin content occurred at a cooler temperature of 16 °C. Under on-farm storage conditions in Kenya, the number of Aspergillus spp. also increased during storage in both hermetic and non-hermetic storage (Maina et al., 2016). The study of mold and mycotoxin contamination may thus not be conclusive due to these conflicting results.
Future Developments and Modifications in Hermetic Storage
The use of HS technologies has become an important consideration in the wake of recognition of the environmental and health implications of fumigants and other synthetic pesticides to control insect pests in stored grains. This is because conventional storage technologies commonly used in developing countries do not adequately protect stored grains without synthetic pesticides. Grain fumigants such as methyl bromide are no longer used in most developed countries unlike in developing countries where they are indiscriminately used due to lack of legislation and regulation. HS is an alternative environmentally friendly technology, and increasing awareness, adoption, and use in SSA are still growing. The authors anticipate the following developments and modifications in the use of HS in the future.
HS technologies are likely to become less costly and more available as manufacturers and distributors become more available and widely distributed in SSA. This would increase technology availability and accessibility to all farming households. One of the major challenges faced by grain farmers is the lack of availability and high cost of hermetic technologies in rural villages and markets. Currently, there are 15 plastic companies globally that manufacture PICS bags, 80% of whom are in SSA and the rest in Asia and Latin America (Baributsa and Ignacio, 2020). Previously, the PICS bag, SG bag, and the metallic silo were the popular marketed forms of a biogenerated HS in SSA. However, the last 10 years or so has seen the manufacture of new HS brands imitating or innovating existing ones. Newer manufacturers are likely to enter the market and this will help improve the overall availability and access of HS technologies.
Furthermore, as cheaper technologies to scavenge O2 and exude CO2 become accessible, a shift from a biogenerated modified atmosphere to controlled atmosphere storage is predicted. This would cause rapid depletion of O2 in a short time without reliance on the biotic components to reduce O2 levels. In addition, with the growing interest of large-scale users in HS containers such as the hermetic bags and Grain Cocoons, it is anticipated that the use and monitoring of these devices will become automated with time, including processes such as sealing and monitoring grain quality.
Conclusions
Considerable grain quantity and quality storage losses due to insects, molds, and mycotoxin contaminations are still a threat that negatively impact food security, nutrition, and household income of smallholder farmers in SSA. These effects emanate from the interactions between biotic and abiotic factors whose degree determines the magnitude of storage losses. As an improved storage method, HS plays a significant role in smallholder agriculture by significantly reducing storage losses without the need for synthetic insecticides. The use of a biogenerated modified atmosphere HS is currently being promoted and disseminated in many parts of SSA and other regions of the world because of their effectiveness. As seen by this review, HS technology is superior to traditional methods in protecting the integrity of stored grains regarding insect infestation, seed viability, grain damage and weight loss, mold growth, and mycotoxin contamination. The potential benefits realizable from HS use include improved food security, household income, and enhanced international grain trade where quality is highly emphasized. Despite their success in protecting stored grains, their adoption and use have been low in many parts of SSA due to high acquisition costs, lack of knowledge on use and manufacture, and lack of availability in smallholder farming communities where they are needed.
Abbreviations
- SSA:
-
Sub-Saharan Africa
- PHLs:
-
post-harvest losses
- HS:
-
Hermetic storage
- HDPE:
-
High-density polyethylene
- PICS:
-
Purdue improved crop storage bag
- PP:
-
polypropylene bag
- SG:
-
SuperGrain bag
References
Abass, A. B., Fischler, M., Schneider, K., Daudi, S., Gaspar, A., Rüst, J., Kabula, E., Ndunguru, G., Madulu, D., & Msola, D. (2018). On-farm comparison of different postharvest storage technologies in a maize farming system of Tanzania Central Corridor. Journal of Stored Products Research, 77, 55–65. https://doi.org/10.1016/j.jspr.2018.03.002
Abass, A. B., Ndunguru, G., Mamiro, P., Alenkhe, B., Mlingi, N., & Bekunda, M. (2014). Post-harvest food losses in a maize-based farming system of semi-arid savannah area of Tanzania. Journal of Stored Products Research, 57, 49–57. https://doi.org/10.1016/j.jspr.2013.12.004
Aboagye, D., Darko, J. O., & Banadda, N. (2017). Comparative study of hermetic and non-hermetic storage on quality of cowpea in Ghana. Chemical and Biological Technologies in Agriculture, 4(1), 1–6. https://doi.org/10.1186/s40538-017-0091-y
Adeola, E. H. (2020). A post-harvest management practices among rice farmers in Imo State Nigeria. European Journal of Biology and Biotechnology, 1(4). https://doi.org/10.24018/ejbio.2020.1.4.32
Affognon, H., Mutungi, C., Sanginga, P., & Borgemeister, C. (2015). Unpacking postharvest losses in sub-saharan Africa: A meta-analysis. World Development, 66, 49–68. https://doi.org/10.1016/j.worlddev.2014.08.002
Alemayehu, S., Abay, F., Ayimut, K. M., Assefa, D., Chala, A., Mahroof, R., Harvey, J., & Subramanyam, B. (2020). Evaluating different hermetic storage technologies to arrest mold growth, prevent mycotoxin accumulation and preserve germination quality of stored chickpea in Ethiopia. Journal of Stored Products Research, 85, 101526. https://doi.org/10.1016/j.jspr.2019.101526
Alonso-Amelot, M. E., & Avila-Núñez, J. L. (2011). Comparison of seven methods for stored cereal losses to insects for their application in rural conditions. Journal of Stored Products Research, 47(2), 82–87. https://doi.org/10.1016/j.jspr.2011.01.001
Amadou, L., Baoua, I., Baributsa, D., Williams, S., & Murdock, L. (2016). Triple bag hermetic technology for controlling a bruchid (Spermophagus sp.)(Coleoptera, Chrysomelidae) in stored Hibiscus sabdariffa grain. Journal of Stored Products Research, 69, 22–25. https://doi.org/10.1016/j.jspr.2016.05.004
Anankware, P., Fatunbi, A., Afreh-Nuamah, K., Obeng-Ofori, D., & Ansah, A. (2012). Efficacy of the multiple-layer hermetic storage bag for biorational management of primary beetle pests of stored maize. Academic Journal of Entomology, 5(1), 47–53. https://doi.org/10.5829/idosi.aje.2012.5.1.61332
APHLIS. (2020). Dry weight loss: All countries—All crops - 2020. Retrieved from https://www.aphlis.net/en/page/20#/datatables?year=21&tab=dry_weight_losses&metric=prc
Arthur, F. H., Morrison, W. R., & Morey, A. C. (2019). Modeling the potential range expansion of larger grain borer, Prostephanus truncatus (Coleoptera: Bostrichidae). Scientific Reports, 9(1), 1–10. https://doi.org/10.1038/s41598-019-42974-5
Atta, B., Rizwan, M., Sabir, A. M., Gogi, M. D., & Ali, K. (2020). Damage potential of Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) on wheat grains stored in hermetic and non-hermetic storage bags. International Journal of Tropical Insect Science, 40(1), 27–37. https://doi.org/10.1007/s42690-019-00047-0
Awika, J. M. (2011). Major cereal grains production and use around the world. In Advances in Cereal Science: Implications to Food Processing and Health Promotion (pp. 1-13): American Chemical Society. https://doi.org/10.1021/bk-2011-1089.ch001
Bailey, S. (1965). Air-tight storage of grain; its effect on insect pests—IV Rhyzopertha dominica (F.) and some other Coleoptera that infest stored grain. Journal of Stored Products Research, 1(1), 25–33. https://doi.org/10.1016/0022-474X(65)90005-6
Bakhtavar, M. A., Afzal, I., & Basra, S. M. A. (2019). Moisture adsorption isotherms and quality of seeds stored in conventional packaging materials and hermetic Super Bag. PLoS One, 14(2), e0207569. https://doi.org/10.1371/journal.pone.0207569
Baoua, I., Amadou, L., Abdourahmane, M., Bakoye, O., Baributsa, D., & Murdock, L. (2015). Grain storage and insect pests of stored grain in rural Niger. Journal of Stored Products Research, 64, 8–12. https://doi.org/10.1016/j.jspr.2015.04.007
Baoua, I., Amadou, L., Bakoye, O., Baributsa, D., & Murdock, L. (2016). Triple bagging hermetic technology for post-harvest preservation of paddy rice Oryza sativa L. in the Sahel of West Africa. Journal of Stored Products Research, 68, 73–79. https://doi.org/10.1016/j.jspr.2016.04.006
Baoua, I., Amadou, L., Baributsa, D., & Murdock, L. (2014a). Triple bag hermetic technology for post-harvest preservation of Bambara groundnut (Vigna subterranea (L.) Verdc.). Journal of Stored Products Research, 58, 48–52. https://doi.org/10.1016/j.jspr.2014.03.001
Baoua, I., Amadou, L., Ousmane, B., Baributsa, D., & Murdock, L. (2014b). PICS bags for post-harvest storage of maize grain in West Africa. Journal of Stored Products Research, 58, 20–28. https://doi.org/10.1016/j.jspr.2014.03.001
Baoua, I. B., Bakoye, O., Amadou, L., Murdock, L. L., & Baributsa, D. (2018). Performance of PICS bags under extreme conditions in the sahel zone of Niger. Journal of Stored Products Research, 76, 96–101. https://doi.org/10.1016/j.jspr.2018.01.007
Baoua, I. B., Margam, V., Amadou, L., & Murdock, L. L. (2012). Performance of triple bagging hermetic technology for postharvest storage of cowpea grain in Niger. Journal of Stored Products Research, 51, 81–85. https://doi.org/10.1016/j.jspr.2012.07.003
Baributsa, D., Bakoye, O. N., Ibrahim, B., & Murdock, L. L. (2020). Performance of five postharvest storage methods for maize preservation in Northern Benin. Insects, 11(8), 541. https://doi.org/10.3390/insects11080541
Baributsa, D., Baoua, I., Bakoye, O., Amadou, L., & Murdock, L. (2017). PICS bags safely store unshelled and shelled groundnuts in Niger. Journal of Stored Products Research, 72, 54–58. https://doi.org/10.1016/j.jspr.2017.03.007
Baributsa, D., & Ignacio, C. M. C. (2020). Developments in the use of hermetic bags for grain storage. In Advances in Postharvest Management of Cereals and Grains (pp. 171-198). https://doi.org/10.19103/AS.2020.0072.06
Baributsa, D., Lowenberg-DeBoer, J., Murdock, L., & Moussa, B. (2010). Profitable chemical-free cowpea storage technology for smallholder farmers in Africa: opportunities and challenges. Julius-Kühn-Archiv, 425, 1046–1052. https://doi.org/10.5073/jka.2010.425.340
Befikadu, D. (2014). Factors affecting quality of grain stored in ethiopian traditional storage structures and opportunities for improvement. International Journal of Sciences: Basic and Applied Research, 18(1), 235–257.
Bendinelli, W. E., Su, C. T., Péra, T. G., & Caixeta Filho, J. V. (2020). What are the main factors that determine post-harvest losses of grains? Sustainable Production and Consumption, 21, 228–238. https://doi.org/10.1016/j.spc.2019.09.002
Benhalima, H., Chaudhry, M., Mills, K., & Price, N. (2004). Phosphine resistance in stored-product insects collected from various grain storage facilities in Morocco. Journal of Stored Products Research, 40(3), 241–249. https://doi.org/10.1016/S0022-474X(03)00012-2
Binns, C. W., Lee, M. K., Maycock, B., Torheim, L. E., Nanishi, K., & Duong, D. T. T. (2021). Climate change, food supply, and dietary guidelines. Annual Review of Public Health, 42, 233–255. https://doi.org/10.1146/annurev-publhealth-012420-105044
Bob, U. (2010). Land-related conflicts in sub-Saharan Africa. African Journal on Conflict Resolution, 10(2). https://doi.org/10.4314/ajcr.v10i2.63310
Boxall, R. (2002). Damage and loss caused by the larger grain borer Prostephanus truncatus. Integrated Pest Management Reviews, 7(2), 105–121. https://doi.org/10.1023/A:1026397115946
Bradford, K. J., Dahal, P., Van Asbrouck, J., Kunusoth, K., Bello, P., Thompson, J., & Wu, F. (2018). The dry chain: Reducing postharvest losses and improving food safety in humid climates. Trends in Food Science and Technology, 71, 84–93. https://doi.org/10.1016/j.tifs.2017.11.002
Brown, P. R., McWilliam, A., & Khamphoukeo, K. (2013). Post-harvest damage to stored grain by rodents in village environments in Laos. International Biodeterioration & Biodegradation, 82, 104–109. https://doi.org/10.1016/j.ibiod.2012.12.018
CABI. (2019). Acanthoscelides obtectus (bean bruchid). In invasive species compendium. : CAB International. Retrieved from https://www.cabi.org/isc/datasheet/2503.
Calderon, M., & Navarro, S. (1980). Synergistic effect of CO2 and O2 mixtures on two stored grain insect pests. In Developments in Agricultural Engineering (Vol. 1, pp. 79-84): Elsevier. https://doi.org/10.1016/B978-0-444-41939-2.50013-9
Carvalho, M. O., Pires, I., Barbosa, A., Barros, G., Riudavets, J., Garcia, A. C., Brites, C., & Navarro, S. (2012). The use of modified atmospheres to control Sitophilus zeamais and Sitophilus oryzae on stored rice in Portugal. Journal of Stored Products Research, 50, 49–56. https://doi.org/10.1016/j.jspr.2012.05.001
Chegere, M. J., Lokina, R., & Mwakaje, A. G. (2020). The impact of hermetic storage bag supply and training on food security in Tanzania. Food Security, 12(6), 1299–1316. https://doi.org/10.1007/s12571-020-01052-9
Cheng, W., Lei, J., Ahn, J.-E., Wang, Y., Lei, C., & Zhu-Salzman, K. (2013). CO2 enhances effects of hypoxia on mortality, development, and gene expression in cowpea bruchid, Callosobruchus maculatus. Journal of Insect Physiology, 59(11), 1160–1168. https://doi.org/10.1016/j.jinsphys.2013.08.009
Chigoverah, A., Mvumi, B., Muchechemera, C., & Dator, J. (2018). Are GrainPro Cocoons™ an effective alternative to conventional phosphine fumigation in large-scale control of stored-maize insect pests? Journal of Pest Science, 91(4), 1393–1406. https://doi.org/10.1007/s10340-018-0996-7
Chigoverah, A., Mvumi, B., Muchechemera, C., & Dator, J. V. (2016). Grainpro Cocoons™ as an alternative to phosphine fumigation for large scale grain storage in Zimbabwe. Paper presented at the Proceedings of the 10th International Conference on Controlled Atmosphere and Fumigation in Stored Products (CAF2016), CAF Permanent Committee Secretariat, Winnipeg, Canada.
Chigoverah, A. A., & Mvumi, B. M. (2016). Efficacy of metal silos and hermetic bags against stored-maize insect pests under simulated smallholder farmer conditions. Journal of Stored Products Research, 69, 179–189. https://doi.org/10.1016/j.jspr.2016.08.004
Chulze, S. (2010). Strategies to reduce mycotoxin levels in maize during storage: a review. Food Additives and Contaminants, 27(5), 651–657. https://doi.org/10.1080/19440040903573032
Collins, P. (2006). Resistance to chemical treatments in insect pests of stored grain and its management. Paper presented at the Proceedings of the 9th International Working Conference on Stored Product Protection.
Compton, J., Floyd, S., Magrath, P., Addo, S., Gbedevi, S., Agbo, B., Bokor, G., Amekupe, S., Motey, Z., & Penni, H. (1998). Involving grain traders in determining the effect of post-harvest insect damage on the price of maize in African markets. Crop Protection, 17(6), 483–489. https://doi.org/10.1016/S0261-2194(98)00041-6
Covele, G., Gulube, A., Tivana, L., Ribeiro-Barros, A. I., Carvalho, M. O., Ndayiragije, A., & Nguenha, R. (2020). Effectiveness of hermetic containers in controlling paddy rice (Oryza sativa L.) storage insect pests. Journal of Stored Products Research, 89, 101710. https://doi.org/10.1016/j.jspr.2020.101710
Cugala, D., Sidumo, A., Santos, L., Mariquele, B., Cumba, V., & Bulha, M. (2007). Assessment of status, distribution and weight lost due to Prostephanus truncatus (Horn)(Coleoptera: Bostrichidae) in Mozambique. Paper presented at the 8th African Crop Science Society Conference, El-Minia, Egypt, 27-31 October 2007.
De Bruin, T., Villers, P., Wagh, A., & Navarro, S. (2012). Worldwide use of hermetic storage for the preservation of agricultural products. Paper presented at the Proceedings of 9th International Controlled Atmosphere and Fumigation Conference (CAF).
De Groote, H., Kimenju, S. C., Likhayo, P., Kanampiu, F., Tefera, T., & Hellin, J. (2013). Effectiveness of hermetic systems in controlling maize storage pests in Kenya. Journal of Stored Products Research, 53, 27–36. https://doi.org/10.1016/j.jspr.2013.01.001
Delgado, L., Schuster, M., & Torero, M. (2021). Quantity and quality food losses across the value chain: a comparative analysis. Food Policy, 98, 101958. https://doi.org/10.1016/j.foodpol.2020.101958
Devereau, A., Myhara, R., & Anderson, C. (2002). Physical factors in post-harvest quality. In P. Golob, G. Farrell, & J. E. Orchard (Eds.), Crop post-harvest: Science and technology (Vol. 1, pp. 69-92): Blackwell Science Ltd
Diarra, M., & Amoah, R. S. (2019). Physical factors in the hermetic SuperGrainBag® and effect on the larger grain borer [Prostephanus truncatus (Horn)(Coleoptera: Bostrichidae)] and aflatoxin production by Aspergillus flavus during the storage of ‘Obatanpa’maize (Zea mays L.) variety. Journal of Stored Products Research, 83, 84–91. https://doi.org/10.1016/j.jspr.2019.05.016
Dubey, N., Srivastava, B., & Kumar, A. (2008). Current status of plant products as botanical pesticides in storage pest management. Journal of Biopesticides, 1(2), 182–186.
Elferink, M., & Schierhorn, F. (2016). Global Demand for Food is Rising. Can we meet it Harvard Business Review, 7(4).
Ellis, R., & Hong, T. (2006). Temperature sensitivity of the low-moisture-content limit to negative seed longevity–moisture content relationships in hermetic storage. Annals of Botany, 97(5), 785–791. https://doi.org/10.1093/aob/mcl035
Freitas, R. S., Faroni, L. R., & Sousa, A. (2016). Hermetic storage for control of common bean weevil, Acanthoscelides obtectus (Say). Journal of Stored Products Research, 66, 1–5. https://doi.org/10.1016/j.jspr.2015.12.004
Fusseini, I., Torkpo, K., & S., & Afreh-Nuamah, K. (2016). Assessment of levels of temperature conditions on the effectiveness of multiple-layer hermetic bag for bio-rational management of aflatoxin in stored maize. AIMS Agriculture and Food, 1(3), 342–353. https://doi.org/10.3934/agrfood.2016.3.342
García-Lara, S., García-Jaimes, E., & Ortíz-Islas, S. (2020). Field effectiveness of improved hermetic storage technologies on maize grain quality in Central Mexico. Journal of Stored Products Research, 87, 101585. https://doi.org/10.1016/j.jspr.2020.101585
García-Lara, S., Ortiz-Islas, S., & Villers, P. (2013). Portable hermetic storage bag resistant to Prostephanus truncatus, Rhyzopertha dominica, and Callosobruchus maculatus. Journal of Stored Products Research, 54, 23–25. https://doi.org/10.1016/j.jspr.2013.04.001
Giga, D., Mutemerewa, S., Moyo, G., & Neeley, D. (1991). Assessment and control of losses caused by insect pests in small farmers' stores in Zimbabwe. Crop Protection, 10(4), 287–292. https://doi.org/10.1016/0261-2194(91)90007-E
Gitonga, Z. M., De Groote, H., Kassie, M., & Tefera, T. (2013). Impact of metal silos on households’ maize storage, storage losses and food security: An application of a propensity score matching. Food Policy, 43, 44–55. https://doi.org/10.1016/j.foodpol.2013.08.005
Godfray, H. C. J., Beddington, J. R., Crute, I. R., Haddad, L., Lawrence, D., Muir, J. F., Pretty, J., Robinson, S., Thomas, S. M., & Toulmin, C. (2010). Food security: The challenge of feeding 9 billion people. Science, 327(5967), 812–818. https://doi.org/10.1126/science.1185383
Gonzales, H. B., Armstrong, P. R., & Maghirang, R. G. (2009). Simultaneous monitoring of stored grain with relative humidity, temperature, and carbon dioxide sensors. Applied Engineering in Agriculture, 25(4), 595–604. https://doi.org/10.13031/2013.27466
Guberac, V., Maric, S., Lalic, A., Drezner, G., & Zdunic, Z. (2003). Hermetically sealed storage of cereal seeds and its influence on vigour and germination. Journal of Agronomy and Crop Science, 189(1), 54–56. https://doi.org/10.1046/j.1439-037X.2003.00596.x
Guenha, R., Salvador, B. d. V., Rickman, J., Goulao, L. F., Muocha, I. M., & Carvalho, M. O. (2014). Hermetic storage with plastic sealing to reduce insect infestation and secure paddy seed quality: a powerful strategy for rice farmers in Mozambique. Journal of Stored Products Research, 59, 275–281. https://doi.org/10.1016/j.jspr.2014.06.007
Gueye, M., Goergen, G., Badiane, D., Hell, K., & Lamboni, L. (2008). First report on occurrence of the larger grain borer Prostephanus truncatus (Horn)(Coleoptera: Bostrichidae) in Senegal. African Entomology: Journal of the Entomological Society of Southern Africa, 16(2), 309–311https://hdl.handle.net/10520/EJC32771
Gunathilake, C. C. (2020). Effect of modified atmospheric conditions to soybean seeds under hermetically sealed large capacity storage. Agricultural Engineering International: CIGR Journal, 22(3), 213–218.
Hellin, J., Kanampiu, F., Erenstein, O., Thorpe, W., Singh, J., Varma, A., & King, A. (2008). Metal silos bring food security to El Salvador. Appropriate Technology, 35, 69–70.
Hengsdijk, H., & De Boer, W. (2017). Post-harvest management and post-harvest losses of cereals in Ethiopia. Food Security, 9(5), 945–958. https://doi.org/10.1007/s12571-017-0714-y
Hodges, R., Bernard, M., & Rembold, F. (2014). APHLIS-Postharvest cereal losses in Sub-Saharan Africa, their estimation, assessment and reduction. In. Luxembourg: .
Hodges, R. J., Buzby, J. C., & Bennett, B. (2011). Postharvest losses and waste in developed and less developed countries: opportunities to improve resource use. The Journal of Agricultural Science, 149(S1), 37–45. https://doi.org/10.1017/s0021859610000936
Hodges, R. J., & Maritime, C. (2012). Postharvest quality losses of cereal grains in Sub-Saharan Africa. African Postharvest Losses Information System, 22.
Ibro, G., Sorgho, M. C., Idris, A. A., Moussa, B., Baributsa, D., & Lowenberg-DeBoer, J. (2014). Adoption of cowpea hermetic storage by women in Nigeria, Niger and Burkina Faso. Journal of Stored Products Research, 58, 87–96. https://doi.org/10.1016/j.jspr.2014.02.007
Jayakumar, M., Arivoli, S., Raveen, R., & Tennyson, S. (2017). Repellent activity and fumigant toxicity of a few plant oils against the adult rice weevil Sitophilus oryzae Linnaeus 1763 (Coleoptera: Curculionidae). Journal of Entomology and Zoology Studies, 5(2), 324–335.
Johnson, J. B. (2020). An overview of near-infrared spectroscopy (NIRS) for the detection of insect pests in stored grains. Journal of Stored Products Research, 86, 101558. https://doi.org/10.1016/j.jspr.2019.101558
Jones, M., Alexander, C. E., & Lowenberg-DeBoer, J. (2011). Profitability of hermetic purdue improved crop storage (PICS) bags for African common bean producers. Retrieved from https://ageconsearch.umn.edu/record/117708/files/11-6.pdf
Jonfia-Essien, W., Navarro, S., & Dator, J. (2008). Effectiveness of hermetic storage in insect control and quality preservation of cocoa beans in Ghana. Paper presented at the 8th .Int. Conf. Controlled Atmosphere and Fumigation in Stored Products.
Jonfia-Essien, W., Varro, S., & Villers, P. (2010). Hermetic storage: a novel approach to the protection of cocoa beans. African Crop Science Journal, 18(2). https://doi.org/10.4314/acsj.v18i2.65797
Kaaya, A. N., & Kyamuhangire, W. (2006). The effect of storage time and agroecological zone on mould incidence and aflatoxin contamination of maize from traders in Uganda. International Journal of Food Microbiology, 110(3), 217–223. https://doi.org/10.1016/j.ijfoodmicro.2006.04.004
Kalsa, K. K., Subramanyam, B., Demissie, G., Worku, A. F., & Habtu, N. G. (2019). Major insect pests and their associated losses in quantity and quality of farm-stored wheat seed. Ethiopian Journal of Agricultural Sciences, 29(2), 71–82.
Kamanula, J., Sileshi, G. W., Belmain, S. R., Sola, P., Mvumi, B. M., Nyirenda, G. K. C., Nyirenda, S. P., & Stevenson, P. C. (2010). Farmers' insect pest management practices and pesticidal plant use in the protection of stored maize and beans in Southern Africa. International Journal of Pest Management, 57(1), 41–49. https://doi.org/10.1080/09670874.2010.522264
Kumar, D., & Kalita, P. (2017). Reducing postharvest losses during storage of grain crops to strengthen food security in developing countries. Foods, 6(1). https://doi.org/10.3390/foods6010008
Lane, B., & Woloshuk, C. (2017). Impact of storage environment on the efficacy of hermetic storage bags. Journal of Stored Products Research, 72, 83–89. https://doi.org/10.1016/j.jspr.2017.03.008
Likhayo, P., Bruce, A. Y., Tefera, T., & Mueke, J. (2018). Maize grain stored in hermetic bags: Effect of moisture and pest infestation on grain quality. Journal of Food Quality, 2018. https://doi.org/10.1155/2018/2515698
Loganathan, M., Jayas, D., Fields, P., & White, N. (2011). Low and high temperatures for the control of cowpea beetle, Callosobruchus maculatus (F.)(Coleoptera: Bruchidae) in chickpeas. Journal of Stored Products Research, 47(3), 244–248. https://doi.org/10.1016/j.jspr.2011.03.005
Magan, N., & Aldred, D. (2007). Post-harvest control strategies: Minimizing mycotoxins in the food chain. International Journal of Food Microbiology, 119(1-2), 131–139. https://doi.org/10.1016/j.ijfoodmicro.2007.07.034
Magan, N., Aldred, D., Mylona, K., & Lambert, R. J. (2010). Limiting mycotoxins in stored wheat. Food Additives and Contaminants, 27(5), 644–650. https://doi.org/10.1080/19440040903514523
Magan, N., Medina, A., & Aldred, D. (2011). Possible climate-change effects on mycotoxin contamination of food crops pre-and postharvest. Plant Pathology, 60(1), 150–163. https://doi.org/10.1111/j.1365-3059.2010.02412.x
Maina, A. W., Wagacha, J. M., Mwaura, F. B., Muthomi, J. W., & Woloshuk, C. P. (2016). Postharvest practices of maize farmers in Kaiti District, Kenya and the Impact of hermetic storage on populations of Aspergillus Spp. and Aflatoxin Contamination. Journal of Food Research, 5(6), 53. https://doi.org/10.5539/jfr.v5n6p53
Makundi, R. H., Oguge, N. O., & Mwanjabe, P. S. (1999). Rodent pest management in East Africa—an ecological approach.
Manandhar, A., Milindi, P., & Shah, A. (2018). An overview of the post-harvest grain storage practices of smallholder farmers in developing countries. Agriculture, 8(4), 57. https://doi.org/10.3390/agriculture8040057
Mann, D., Jayas, D., Muir, W., & White, N. (1999). Predicting the gas-tightness of grain storage structures. Canadian Agricultural Engineering, 41(4), 259–265.
Mannaa, M., & Kim, K. D. (2017). Influence of temperature and water activity on deleterious fungi and mycotoxin production during grain storage. Mycobiolology, 45(4), 240–254. https://doi.org/10.5941/myco.2017.45.4.240
Martin, D., Baributsa, D., Huesing, J., Williams, S., & Murdock, L. (2015). PICS bags protect wheat grain, Triticum aestivum (L.), against rice weevil, Sitophilus oryzae (L.)(Coleoptera: Curculionidae). Journal of Stored Products Research, 63, 22–30. https://doi.org/10.1016/j.jspr.2015.05.001
McCarthy, P. (2003). Bird management in grain storage facilities. Paper presented at the Australian Postharvest Technical Conference (CSIRO and GRDC, Canberra, 2003).
Mesterházy, Á., Oláh, J., & Popp, J. (2020). Losses in the grain supply chain: Causes and solutions. Sustainability, 12(6), 2342. https://doi.org/10.3390/su12062342
Midega, C. A., Murage, A. W., Pittchar, J. O., & Khan, Z. R. (2016). Managing storage pests of maize: Farmers' knowledge, perceptions and practices in western Kenya. Crop Protection, 90, 142–149. https://doi.org/10.1016/j.cropro.2016.08.033
Mills, J. (1996). Quality of stored cereals. In R. J. Henry & P. S. Kettlewell (Eds.), Cereal grain quality (pp. 441-478). Dordrecht: Springer. https://doi.org/10.1007/978-94-009-1513-8_14
Mishili, F. J., Temu, A., Fulton, J., & Lowenberg-DeBoer, J. (2011). Consumer preferences as drivers of the common bean trade in Tanzania: A marketing perspective. Journal of International Food & Agribusiness Marketing, 23(2), 110–127. https://doi.org/10.1080/08974438.2011.558761
Mobolade, A. J., Bunindro, N., Sahoo, D., & Rajashekar, Y. (2019). Traditional methods of food grains preservation and storage in Nigeria and India. Annals of Agricultural Science, 64(2), 196–205. https://doi.org/10.1016/j.aoas.2019.12.003
Moreno-Martinez, E., Jiménez, S., & Vazquez, M. E. (2000). Effect of Sitophilus zeamais and Aspergillus chevalieri on the oxygen level in maize stored hermetically. Journal of Stored Products Research, 36(1), 25–36. https://doi.org/10.1016/S0022-474X(99)00023-5
Moses, J., Jayas, D., & Alagusundaram, K. (2015). Climate change and its implications on stored food grains. Agricultural Research, 4(1), 21–30. https://doi.org/10.1007/s40003-015-0152-z
Moussa, B., Abdoulaye, T., Coulibaly, O., Baributsa, D., & Lowenberg-DeBoer, J. (2014). Adoption of on-farm hermetic storage for cowpea in West and Central Africa in 2012. Journal of Stored Products Research, 58, 77–86. https://doi.org/10.1016/j.jspr.2014.02.008
Movilla-Pateiro, L., Mahou-Lago, X., Doval, M., & Simal-Gandara, J. (2020). Toward a sustainable metric and indicators for the goal of sustainability in agricultural and food production. Critical Reviews in Food Science and Nutrition, 61, 1–22. https://doi.org/10.1080/10408398.2020.1754161
Mubayiwa, M., Mvumi, B. M., Stathers, T., Mlambo, S., & Nyabako, T. (2021). Field evaluation of hermetic and synthetic pesticide-based technologies in smallholder sorghum grain storage in hot and arid climates. Scientific Reports, 11, 3692. https://doi.org/10.1038/s41598-021-83086-3
Murdock, L., & Baoua, I. (2014). On purdue improved cowpea storage (PICS) technology: background, mode of action, future prospects. Journal of Stored Products Research, 58, 3–11. https://doi.org/10.1016/j.jspr.2014.02.006
Murdock, L. L., Margam, V., Baoua, I., Balfe, S., & Shade, R. E. (2012). Death by desiccation: Effects of hermetic storage on cowpea bruchids. Journal of Stored Products Research, 49, 166–170. https://doi.org/10.1016/j.jspr.2012.01.002
Mutambuki, K., Affognon, H., Likhayo, P., & Baributsa, D. (2019). Evaluation of purdue Improved crop storage triple layer hermetic storage bag against Prostephanus truncatus (Horn)(Coleoptera: Bostrichidae) and Sitophilus zeamais (Motsch.)(Coleoptera: Curculionidae). Insects, 10(7), 204. https://doi.org/10.3390/insects10070204
Mutungi, C., Affognon, H. D., Njoroge, A. W., Manono, J., Baributsa, D., & Murdock, L. L. (2015). Triple-layer plastic bags protect dry common beans (Phaseolus vulgaris) against damage by Acanthoscelides obtectus (Coleoptera: Chrysomelidae) during storage. Journal of Economic Entomology, 108(5), 2479–2488. https://doi.org/10.1093/jee/tov197
Mutungi, C. M., Affognon, H., Njoroge, A. W., Baributsa, D., & Murdock, L. L. (2014). Storage of mung bean (Vigna radiata [L.] Wilczek) and pigeonpea grains (Cajanus cajan [L.] Millsp) in hermetic triple-layer bags stops losses caused by Callosobruchus maculatus (F.) (Coleoptera: Bruchidae). Journal of Stored Products Research, 58, 39–47. https://doi.org/10.1016/j.jspr.2014.03.004
Napoli, M., De Muro, P., & Mazziotta, M. (2011). Towards a food insecurity Multidimensional Index (FIMI). (Master in Human Development and Food Security), Università degli studi Roma Tre,
Navarro, S., Donahaye, E., & Fishman, S. (1994). The future of hermetic storage of dry grains in tropical and subtropical climates, Paper presented at the Proceedings of 6th International Working Conference on Stored Product Protection. CAB International.
Navarro, S., Finkelman, S., Sabio, G., Isikber, A., Dias, R., Rindner, M., & Azrieli, A. (2003, 22-26 July 2002). Enhanced effectiveness of vacuum or CO2 in combination with increased temperatures for control of storage insects. Paper presented at the Proceedings of the 8th International Working Conference on Stored Product Protection, York, UK.
Ndegwa, M. K., De Groote, H., Gitonga, Z. M., & Bruce, A. Y. (2016). Effectiveness and economics of hermetic bags for maize storage: Results of a randomized controlled trial in Kenya. Crop Protection, 90, 17–26. https://doi.org/10.1016/j.cropro.2016.08.007
Nduku, T. M., De Groote, H., & Nzuma, J. M. (2013). Comparative analysis of maize storage structures in Kenya. Paper presented at the 2013 Fourth International Conference, , Hammamet,.
Neate, P. J., & Guei, R. G. (2010). Promoting the growth and development of smallholder seed enterprises for food security crops: best practices and options for decision making: Food and Agriculture Organization of the United Nations (FAO).
Ng'ang'a, J., Mutungi, C., Imathiu, S., & Affognon, H. (2016a). Effect of triple-layer hermetic bagging on mould infection and aflatoxin contamination of maize during multi-month on-farm storage in Kenya. Journal of Stored Products Research, 69, 119–128. https://doi.org/10.1016/j.jspr.2016.07.005
Ng'ang'a, J., Mutungi, C., Imathiu, S. M., & Affognon, H. (2016b). Low permeability triple-layer plastic bags prevent losses of maize caused by insects in rural on-farm stores. Food Security, 8(3), 621–633. https://doi.org/10.1007/s12571-016-0567-9
Njoroge, A., Affognon, H., Mutungi, C., Manono, J., Lamuka, P., & Murdock, L. (2014). Triple bag hermetic storage delivers a lethal punch to Prostephanus truncatus (Horn)(Coleoptera: Bostrichidae) in stored maize. Journal of Stored Products Research, 58, 12–19. https://doi.org/10.1016/j.jspr.2014.02.005
Nyarko, S. K., Akyereko, Y. G., Akowuah, J. O., & Wireko-Manu, F. D. (2021). Comparative studies on grain quality and pesticide residues in maize stored in hermetic and polypropylene storage bags. Agriculture, 11(8), 772. https://doi.org/10.3390/agriculture11080772
Odjo, S., Burgueno, J., Rivers, A., & Verhulst, N. (2020). Hermetic storage technologies reduce maize pest damage in smallholder farming systems in Mexico. Journal of Stored Products Research, 88, 101664. https://doi.org/10.1016/j.jspr.2020.101664
Ognakossan, K. E., Affognon, H. D., Mutungi, C. M., Sila, D. N., Midingoyi, S.-K. G., & Owino, W. O. (2016). On-farm maize storage systems and rodent postharvest losses in six maize growing agro-ecological zones of Kenya. Food Security, 8(6), 1169–1189. https://doi.org/10.1007/s12571-016-0618-2
Ognakossan, K. E., Mutungi, C. M., Otieno, T. O., Affognon, H. D., Sila, D. N., & Owino, W. O. (2018). Quantitative and quality losses caused by rodents in on-farm stored maize: a case study in the low land tropical zone of Kenya. Food Security, 10(6), 1525–1537. https://doi.org/10.1007/s12571-018-0861-9
Ognakossan, K. E., Tounou, A. K., Lamboni, Y., & Hell, K. (2013). Post-harvest insect infestation in maize grain stored in woven polypropylene and in hermetic bags. International Journal of Tropical Insect Science, 33(1), 71–81. https://doi.org/10.1017/S1742758412000458
Philip, T., & Throne, J. (2010). Biorational approaches for managing stored-product insect. Annual Review of Entomology, 55, 375–339. https://doi.org/10.1146/annurev.ento.54.110807.090451
Phophi, M. M., Mafongoya, P., & Lottering, S. (2020). Perceptions of climate change and drivers of insect pest outbreaks in vegetable crops in Limpopo Province of South Africa. Climate, 8(2), 27. https://doi.org/10.3390/cli8020027
Pixton, S., & Warburton, S. (1971). Moisture content/relative humidity equilibrium of some cereal grains at different temperatures. Journal of Stored Products Research, 6(4), 283–293. https://doi.org/10.1016/0022-474X(71)90041-5
Quezada, M. Y., Moreno, J., Vázquez, M. E., Mendoza, M., Méndez-Albores, A., & Moreno-Martínez, E. (2006). Hermetic storage system preventing the proliferation of Prostephanus truncatus Horn and storage fungi in maize with different moisture contents. Postharvest Biology and Technology, 39(3), 321–326. https://doi.org/10.1016/j.postharvbio.2005.10.004
Raheem, D., Dayoub, M., Birech, R., & Nakiyemba, A. (2021). The contribution of cereal grains to food security and sustainability in Africa: Potential application of UAV in Ghana, Nigeria, Uganda, and Namibia. Urban Science, 5(1), 8. https://doi.org/10.3390/urbansci5010008
Rickman, J. F. (2002). Grain quality from harvest to market. Paper presented at the 9th JIRCAS International Symposium 2002 - 'Value-Addition to Agricultural Products', , Japan.
Said, P., & Pashte, V. (2015). Botanicals: The protectants of stored grains pests. Trends in Biosciences, 8(15), 3750–3755.
Sanon, A., Dabiré-Binso, L., & Ba, N. (2011). Triple-bagging of cowpeas within high density polyethylene bags to control the cowpea beetle Callosobruchus maculatus F.(Coleoptera: Bruchidae). Journal of Stored Products Research, 47(3), 210–215. https://doi.org/10.1016/j.jspr.2011.02.003
Sawant, A. A., Patil, S., Kalse, S., & Thakor, N. (2012). Effect of temperature, relative humidity and moisture content on germination percentage of wheat stored in different storage structures. Agricultural Engineering International: CIGR Journal, 14(2), 110–118.
Sheahan, M., & Barrett, C. B. (2017). Food loss and waste in Sub-Saharan Africa. Food Policy, 70, 1–12. https://doi.org/10.1016/j.foodpol.2017.03.012
Shee, A., Mayanja, S., Simba, E., Stathers, T., Bechoff, A., & Bennett, B. (2019). Determinants of postharvest losses along smallholder producers maize and Sweetpotato value chains: an ordered Probit analysis. Food Security, 11(5), 1101–1120. https://doi.org/10.1007/s12571-019-00949-4
Shehu, A., Obeng-Ofori, D., & Eziah, V. Y. (2010). Biological efficacy of Calneem™ oil against the tropical warehouse moth Ephestia cautella (Lepidoptera: Pyralidae) in stored maize. International Journal of Tropical Insect Science, 30(4), 207–213. https://doi.org/10.1017/S1742758410000378
Silva, M. G., Silva, G. N., Sousa, A. H., Freitas, R. S., Silva, M. S., & Abreu, A. O. (2018). Hermetic storage as an alternative for controlling Callosobruchus maculatus (Coleoptera: Chrysomelidae) and preserving the quality of cowpeas. Journal of Stored Products Research, 78, 27–31. https://doi.org/10.1016/j.jspr.2018.05.010
Somavat, P., Huang, H., Kumar, S., Garg, M. K., Danao, M.-G. C., Singh, V., Paulsen, M. R., & Rausch, K. D. (2017). Comparison of hermetic storage of wheat with traditional storage methods in India. Applied Engineering in Agriculture, 33(1), 121. 10.13031/aea.11792
Stathers, T. E., Arnold, S. E., Rumney, C. J., & Hopson, C. (2020). Measuring the nutritional cost of insect infestation of stored maize and cowpea. Food Security, 1-24. https://doi.org/10.1007/s12571-019-00997-w
Sudini, H., Ranga Rao, G. V., Gowda, C. L. L., Chandrika, R., Margam, V., Rathore, A., & Murdock, L. L. (2015). Purdue improved crop storage (PICS) bags for safe storage of groundnuts. Journal of Stored Products Research, 64, 133–138. https://doi.org/10.1016/j.jspr.2014.09.002
Suleiman, R., Bern, C., Brumm, T., & Rosentrater, K. (2018). Impact of moisture content and maize weevils on maize quality during hermetic and non-hermetic storage. Journal of Stored Products Research, 78, 1–10. https://doi.org/10.1016/j.jspr.2018.05.007
Suleiman, R., Rosentrater, K. A., & Bern, C. (2013). Effects of deterioration parameters on storage of maize: A review. Journal of Natural Science Research, 3(9), 147–165.
Taddese, M., Dibaba, K., Bayissa, W., Hunde, D., Mendesil, E., Kassie, M., Mutungi, C., & Tefera, T. (2020). Assessment of quantitative and qualitative losses of stored grains due to insect infestation in Ethiopia. Journal of Stored Products Research, 89, 101689. https://doi.org/10.1016/j.jspr.2020.101689
Tapondjou, L., Adler, C., Bouda, H., & Fontem, D. (2002). Efficacy of powder and essential oil from Chenopodium ambrosioides leaves as post-harvest grain protectants against six-stored product beetles. Journal of Stored Products Research, 38(4), 395–402. https://doi.org/10.1016/S0022-474X(01)00044-3
Taruvinga, C., Mejia, D., & Sanz Alvarez, J. (2014). Appropriate seed and grain storage systems for small-scale farmers: key practices for DRR implementers (9251083347). Retrieved from http://www.fao.org/3/i3769e/i3769e.pdf
Tefera, T. (2012). Post-harvest losses in African maize in the face of increasing food shortage. Food Security, 4(2), 267–277. https://doi.org/10.1007/s12571-012-0182-3
Tefera, T., Kanampiu, F., De Groote, H., Hellin, J., Mugo, S., Kimenju, S., Beyene, Y., Boddupalli, P. M., Shiferaw, B., & Banziger, M. (2010). The metal silo: An effective grain storage technology for reducing post-harvest insect and pathogen losses in maize while improving smallholder farmers’ food security in developing countries. Crop Protection, 30(3), 240–245. https://doi.org/10.1016/j.cropro.2010.11.015
Tubbs, T., Baributsa, D., & Woloshuk, C. (2016). Impact of opening hermetic storage bags on grain quality, fungal growth and aflatoxin accumulation. Journal of Stored Products Research, 69, 276–281. https://doi.org/10.1016/j.jspr.2016.10.003
UN (2019, June 17). Growing at a slower pace, world population is expected to reach 9.7 billion in 2050 and could peak at nearly 11 billion around 2100. Retrieved from https://www.un.org/development/desa/en/news/population/world-population-prospects-2019.html
Upadhyay, R. K., & Ahmad, S. (2011). Management strategies for control of stored grain insect pests in farmer stores and public ware houses. World Journal of Agricultural Sciences, 7(5), 527–549.
Vales, M., Rao, G. R., Sudini, H., Patil, S., & Murdock, L. (2014). Effective and economic storage of pigeonpea seed in triple layer plastic bags. Journal of Stored Products Research, 58, 29–38. https://doi.org/10.1016/j.jspr.2014.01.004
Van Dyck, H., Bonte, D., Puls, R., Gotthard, K., & Maes, D. (2015). The lost generation hypothesis: could climate change drive ectotherms into a developmental trap? Oikos, 124(1), 54–61. https://doi.org/10.1111/oik.02066
Vance, C. P., Graham, P. H., & Allan, D. L. (2000). Biological nitrogen fixation: Phosphorus—A critical future need? In F. O. Pedrosa, M. Hungria, G. Yates, & W. E. Newton (Eds.), Nitrogen Fixation: From Molecules to Crop Productivity. Current Plant Science and Biotechnology in Agriculture (Vol. 38, pp. 509-514): Springer, Dordrecht. https://doi.org/10.1007/0-306-47615-0_291
Vanlauwe, B., Hungria, M., Kanampiu, F., & Giller, K. E. (2019). The role of legumes in the sustainable intensification of African smallholder agriculture: Lessons learnt and challenges for the future. Agriculture, Ecosystems and Environment, 284, 106583. https://doi.org/10.1016/j.agee.2019.106583
Villers, P., De Bruin, T., & Navarro, S. (2006). Development and applications of the hermetic storage technology. Paper presented at the Proceedings of the 9th Internafional Working Conference on Stored Products Protecfion.
Villers, P., Navarro, S., & De Bruin, T. (2008). Development of hermetic storage technology in sealed flexible storage structures. Paper presented at the Proceedings of the 8th International Conference on Controlled Atmosphere and Fumigation in Stored Products, CAF.
Vowotor, K., Meikle, W., Ayertey, J., & Markham, R. (2005). Distribution of and association between the larger grain borer Prostephanus truncatus (Horn)(Coleoptera: Bostrichidae) and the maize weevil Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae) in maize stores. Journal of Stored Products Research, 41(5), 498–512. https://doi.org/10.1016/j.jspr.2004.08.002
Wagacha, J., & Muthomi, J. (2008). Mycotoxin problem in Africa: current status, implications to food safety and health and possible management strategies. International Journal of Food Microbiology, 124(1), 1–12. https://doi.org/10.1016/j.ijfoodmicro.2008.01.008
Walker, S., Jaime, R., Kagot, V., & Probst, C. (2018). Comparative effects of hermetic and traditional storage devices on maize grain: Mycotoxin development, insect infestation and grain quality. Journal of Stored Products Research, 77, 34–44. https://doi.org/10.1016/j.jspr.2018.02.002
Waongo, A., Traore, F., Ba, M. N., Dabire-Binso, C., Murdock, L. L., Baributsa, D., & Sanon, A. (2019). Effects of PICS bags on insect pests of sorghum during long-term storage in Burkina Faso. Journal of Stored Products Research, 83, 261–266. https://doi.org/10.1016/j.jspr.2019.07.010
Williams, S. B., Baributsa, D., & Woloshuk, C. (2014). Assessing purdue improved crop storage (PICS) bags to mitigate fungal growth and aflatoxin contamination. Journal of Stored Products Research, 59, 190–196. https://doi.org/10.1016/j.jspr.2014.08.003
Williams, S. B., Murdock, L. L., & Baributsa, D. (2017). Safe storage of maize in alternative hermetic containers. Journal of Stored Products Research, 71, 125–129. https://doi.org/10.1016/j.jspr.2016.12.008
Willis, E. R., & Roth, L. M. (1954). Reactions of flour beetles of the genus Tribolium to carbon dioxide and dry air. The Journal of Experimental Zoology, 127(1), 117–152. https://doi.org/10.1002/jez.1401270107
World Bank. (2011). Missing food: The case of postharvest grain losses in sub-saharan Africa (60371-AFR). Retrieved from http://www.fao.org/3/at454e/at454e.pdf
Yakubu, A., Bern, C. J., Coats, J. R., & Bailey, T. B. (2011). Hermetic on-farm storage for maize weevil control in East Africa. African Journal of Agricultural Research, 6(14), 3311–3319. https://doi.org/10.5897/AJAR10.829
Yewle, N., Swain, K. C., Mann, S., & Guru, P. (2022). Performance of hermetic bags in green gram [Vigna radiata (L.) R. Wilczek] storage for managing pulse beetle (Callosobruchus chinensis). Journal of Stored Products Research, 95, 101896. https://doi.org/10.1016/j.jspr.2021.101896
Yusuf, B. L., & He, Y. (2011). Design, development and techniques for controlling grains post-harvest losses with metal silo for small and medium scale farmers. African Journal of Biotechnology, 10(65), 14552–14561. https://doi.org/10.5897/AJB11.1845
Acknowledgements
This work was funded by the Government of Uganda through the Makerere University Research Innovations Fund (Mak-RIF) [Grant No. RIF1/CAES/022].
Disclaimer
The opinions expressed in this manuscript are those of the authors and do not necessarily reflect the views of the funding organization.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Okori, F., Cherotich, S., Baidhe, E. et al. Grain Hermetic Storage and Post-Harvest Loss Reduction in Sub-Saharan Africa: Effects on Grain Damage, Weight Loss, Germination, Insect Infestation, and Mold and Mycotoxin Contamination. J. Biosyst. Eng. 47, 48–68 (2022). https://doi.org/10.1007/s42853-022-00130-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42853-022-00130-4