Abstract
Storage of wheat in conventional packaging materials is not safe as seeds gain moisture from surrounding air of high relative humidity which promotes growth of fungal and insect pests and loss of quality during storage. Implementing the dry chain, initial drying to low moisture content followed by storage in hermetic bags to maintain low moisture may prevent these losses without using fumigants or chemicals. Different levels of initial moisture contents (SMC), i.e., 8, 10, 12, and 14% and packaging materials, including hermetic super bags along with paper, woven polypropylene (PP), jute, and cloth bags were used as two factors for this experiment. After 4 months of storage, small variation in SMC of seed was observed in super bags while SMC increased significantly in conventional packaging materials. Higher storage losses (≈9%), grain quality losses and aflatoxin B1, B2, G1, and G2 contamination (1–2 ppb) in conventional packaging materials were linked to high seed moisture contents. Storage in hermetic bags at 8 and 10% SMC ideally preserved seed quality. In conclusion, hermetic storage of wheat at low seed moisture maintains a dry chain and prevents aflatoxin contamination and grain quality losses and offers an organic approach to avoid contamination of food grains.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Globally, 50–60% losses have been recorded in cereal grains due to poor storage practices and lack of technical efficiency (Kumar and Kalita 2017). Direct and indirect losses of stored grain vary according to regions with 10% losses in temperate regions and almost 50% losses in humid tropical regions (Wijayaratne et al. 2018). Pakistan alone is facing economic losses worth 76 to 90 million USD every year due to improper storage facilities of wheat grains (FAO 2013). Increased production without minimizing the postharvest losses is not profitable as one third of total production is lost at this stage (Bradford et al. 2018). Therefore, food security in developing countries can be ensured by reducing postharvest storage losses.
Farmers in developing countries mostly store their wheat produce in porous jute and polypropylene bags. Most of the farmers used these grains as seed for the next sowing season. Even if properly dried, wheat seeds stored in porous bags gained moisture, especially during monsoon season when relative humidity (RH) is higher due to excessive rains (Afzal et al. 2019). Studies have suggested that high moisture content during storage is responsible for seed aging, storage insect pests, and aflatoxin contamination (Afzal et al. 2017). Use of pesticides for controlling pests of stored grains is a successful approach but leaves behind toxic residues (Mobolade et al. 2019). Use of chemical fumigants is now globally discouraged due to environmental pollution, contamination of food grains with toxic residues, and development of resistance in stored grain pests (Kumar et al. 2017). Moreover, high RH in storehouses leads to growth of aflatoxin-producing molds such as Aspergillus flavus and A. parasiticus (Abdel-Hadi et al. 2012; Wu et al. 2014). In developing countries around 4.5 billion people are prone to chronic aflatoxin exposure (Williams et al. 2004). Wheat being the staple food in many countries contributes significantly for human exposure to aflatoxin (WHO 2017).
There is a need to find organic storage solution which has potential to protect food grains from both storage insect pests and aflatoxin-producing molds. Storage of food grains in hermetic bags has proved an ideal approach to reduce stored grain pest infestation and aflatoxin contamination (Afzal et al. 2017; Martin et al. 2015; Williams et al. 2014). The basic idea of hermetic bag technology is to cut off oxygen supply inside the bags, which leads to reduce insect pest infestation and works well for grain storage. Seed storage at low moisture is vital for both grain and seed during the supply chain. Drying of seeds to low moisture content is safe enough to retain seed viability and then packaging in hermetic containers to keep seeds continuously dry is the basic principle of the dry chain (Bradford et al. 2018; Afzal et al. 2019). Super bags are hermetic in nature and their oxygen and water-resistant properties make them suitable to implement the dry chain. In this study, prospects of the dry chain have been explored using hermetic super bags to control aflatoxin contamination, storage, and grain quality losses.
Materials and methods
Experiment detail
Study was conducted in Seed Physiology Laboratory, Department of Agronomy, University of Agriculture, Faisalabad, Pakistan. Freshly harvested wheat seed free from insect pests was obtained from Wheat Research Institute Faisalabad, Pakistan. Initial seed moisture contents and germination were 10% and 99.5% respectively. Seed was dried to four (8, 10, 12, and 14%) seed moisture levels. Conventional packaging materials, i.e., paper, polypropylene, jute, and cloth bags, were purchased from a local grain market while super bags of 10 kg capacity were provided by GrainPro Inc., USA. Hermetic super bags (SuperGrainbag® Premium RT) are from high-strength PE with excellent water and gas barrier properties. Packed seed was stored for 4 months and RH and temperature of store room was recorded.
Seed drying
Seed was dried to various moisture levels in an airtight plastic container by mixing it with zeolite seed drying beads. The drying bead calculator developed by Bradford’s lab was used to calculate the quantity of beads required for drying the seeds to various moisture levels (Bradford et al. 2016). Wheat seeds were dried up to 8% seed moisture contents by using seed drying beads (Rhino Research Group) made of zeolite clay material. To dry 4 kg of wheat seed, a total of 190 g of drying beads having 23% drying capacity were mixed in it. As initial seed moisture content was 10%, so seeds were packed without applying any drying treatment.
Equilibrating and increasing seed moisture contents
Saturated salt solution of K2S2O3 at 25 °C was placed in an airtight container along with wheat seed to maintain 63% RH (Greenspan 1977) which gave 12% equilibrium moisture contents. Similarly, in order to maintain 14% equilibrium SMC, seed was placed in an airtight plastic container containing saturated solution of NaCl for 14 days at 25 °C temperature that maintains 75% RH. Values of equilibrium relative humidity were converted to equilibrium moisture contents using moisture calculator as described by Bradford et al. (2016).
Determination of seed moisture contents
Sampling was done after 4 months to determine seed moisture contents. Seed sample (5 g) was dried at 103 °C in an oven for the period of 17 h (ISTA 2015). Dry weight of sample was recorded and seed moisture contents were calculated using following equation of International Seed Testing Association.
Biochemical analysis of stored seed
Omeg Analyzer (Omeg Analyzer G™, Bruins Instruments, USA) was used for photometric determination of starch and protein contents. For determination of malondialdehyde contents, seed sample (1 g) was homogenized in 10% solution of trichloroacetic acid (TCA). Absorbance of supernatant collected after centrifugation of reaction mixture was recorded at 450, 532, and 600 nm to determine malondialdehyde contents (Zheng and Tian 2006).
Assessment of storage losses
Randomly drawn 100 g samples of stored wheat were sieved with a standard sieve set (Fisherbrand™ stainless steel sieves 8 in. dia., Fisher Scientific, USA) to count the population of stored grain pests. The number of larvae and adults of Khapra Beetle was counted in a 100-g seed sample. Percentage of weight loss was calculated with the help of equation given by Adams and Schulten (1978).
Here “U” represent the weight of undamaged grains whereas weight of damaged grains is denoted by “D”. The number of damaged grains is represented by “Nd” while “Nu” is used for the number of undamaged grains.
Determination of aflatoxin contamination
Thoroughly grounded wheat flour (50 g) of randomly drawn seed samples from each replicate was used for quantification of aflatoxins using VicamAfla HPLC column. HPLC-grade methanol (1.5 mL) was used to elute the affinity column. 1.5 mL purified water was added to make the final volume up to 3 mL. Sample derivatization was done before injection (Saleemi et al. 2017). For detection of peak, 1 mL sample from the bottom layer was injected to the LC column. The sample was analyzed on HPLC (20A Prominence HPLC System with Fluorescent detector, Shimadzu Scientific Instruments, Japan). For quantification of aflatoxin, obtained peaks were compared with the standard peaks prepared from Sigma Chemical Corporation St. Louis, MO, over a range of 1–50 ppb. Certified Reference Material (CRM) of SUPELCO Bellefonte, PA, was used to assure the analytical quality of the applied method. The limit of quantification (LOQ) was 0.05 ppb whereas limit of detection was 0.02 ppb.
Temperature and RH of store room
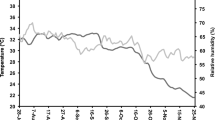
Data of ambient RH and temperature of store room were recorded on an hourly basis, and daily average of RH and temperature is presented (Fig. 1). Minimum temperature (20.7 °C) was observed on the 29th of November, 2016, and maximum (33.2 °C) was observed on September 16, 2016. Mean temperature was 32.1, 32.2, 32.1, 28.9, and 23 °C during July, August, September, October, and November 2016 respectively. Average RH was 64.2, 66.2, 62, 60.7, and 58.3% recorded during July, August, September, October, and November 2016 respectively. Minimum RH (53.7%) was recorded on October 22, 2016, and maximum (73.9%) on August 06, 2016.
Statistical analysis of data
For statistical analysis of data, different packaging materials and seed moisture levels were taken as factors. Completely randomized design (CRD) with factorial arrangement was used for keeping three replications. Data were analyzed using statistical software Statistix 8.1. Treatment means were compared with Tukey’s test at a 5% probability level.
Results
Seed moisture contents
Wheat seed had maximum moisture contents when stored in super bags at 14% initial seed moisture contents (SMC) after 4 months of storage. Minimum SMC were recorded for the seed having 8% initial SMC and were stored in super bags (Fig. 2). An increase in seed moisture contents was observed (from initial 8% SMC to ≈12% SMC) in all packaging materials except super bags. Super bags showed resistance to significant change in initial moisture levels. Moisture contents of the seed slightly decreased in super bags having 14% initial SMC.
Grain quality attributes
Maximum starch contents were measured in wheat stored in super bags at 8–12% SMC while minimum starch contents were found in wheat stored in cloth, jute, and paper bags having 12 and 14% initial SMC (Fig. 3). Highest protein contents were found in seeds stored in hermetic super bags at 8 and 10% initial SMC under Trogoderma granarium infestation. Minimum protein contents were measured from the seeds stored at 14% SMC in polypropylene (PP) bags (Fig. 4). Maximum malondialdehyde contents were present in wheat stored in cloth and jute bags at 8–14% SMC and in PP bags at 8–12% SMC (Fig. 5).
Insect pest population and weight losses during storage
Larvae and adults of Khapra Beetle (Trogoderma granarium) were found during storage. Population estimates of larvae and adults of Khapra Beetle were made by manual counting. No larvae and adults were found in hermetic super bags at all levels of moisture contents. On contrary, severe attack of Khapra beetle was observed in traditional packaging materials. The highest number of larvae was counted from cloth bags at both 12 and 14% SMC (Fig. 6). Adult population was maximum in cloth and polypropylene bags at 12 and 14% initial SMC (Fig. 7). Significantly lower (<1%) quantitative weight losses were observed for the grains stored in super bags while higher weight losses (≈9%) were observed in the rest of all packaging materials (Fig. 8). Losses in weight were higher in conventional packaging materials at 12 and 14% initial SMC whereas lowest weight losses were observed in grains stored at 8 and 10% initial SMC.
Aflatoxin contamination
Aflatoxins B1, B2, G1, and G2 were not detected in wheat seed stored in super bags at 8, 10, and 12% initial SMC. Similarly, no traces of aflatoxin B1 were found in seeds stored at 8% SMC in paper, PP, and jute bags and in cloth bags at 10% initial SMC (Table 1). Seed stored in paper and jute bags at 8 and 10% initial SMC and seed stored in PP bags at 8% initial SMC had no aflatoxin B2 contamination. Wheat seeds stored at 8 and 10% initial SMC in paper and PP bags and in cloth bags at 8% initial SMC had no aflatoxin G1. Seeds stored in paper, PP, and jute bags at 8% initial SMC had also no detectable concentration of aflatoxin G2. Maximum concentrations of aflatoxin B1, B2, G1, and G2 were measured in seeds stored in super bags at 14% initial SMC (Table 1).
Discussion
Water vapor transmission rate of super bag is very low (≤5 g−2 day−1) as there is a gas coating done in between multiple layers of polyethylene, which helps to restrict the entry and exit of moisture into the grains (GrainPro 2020). Except super bags, seed moisture contents varied in all conventional packaging materials due to their permeable structure (Fig. 2). In traditional packaging materials, seed moisture contents increased with the increased ambient RH of the storage environment and vice versa. Moisture contents of stored maize increased up to 3.14% when stored in woven polypropylene bags as compared to hermetic PICS bags having only 0.25% increase in moisture contents (Afzal et al. 2017). Data of seed store indicate that mean RH was around 60–65% and maximum RH was 75% in August 2016 (Fig. 1). High ambient RH elevated the grain moisture in traditional permeable bags as seeds gain and lose moisture depending upon ambient RH (McDonald 2007).
Contamination of food grains with aflatoxin-producing molds is a serious threat for developing countries where around 4.5 billion people are at the risk of chronic exposure to aflatoxins (Williams et al. 2004). High seed moisture contents (14%) in super bags and in traditional porous bags resulted in higher aflatoxin contamination. Aflatoxin-producing molds grow in the conventionally stored grains under high temperature and RH (Hell et al. 2010). For cereal seeds like wheat, 13% seed moisture contents or higher are the baseline for fungal growth and aflatoxin contamination (Bewley et al. 2013). Porous nature of the traditional packaging material allowed the moisture ingress into the seeds under conditions of high RH (Bakhtavar et al. 2019) that hasten the mold growth and aflatoxin production in maize. However, in our study, individual concentration of aflatoxin B1, B2, G1, and G2 in conventional packaging materials and aflatoxin B2, G1, and G2 in super bags at 14% SMC is below compared to permissible limit of individual aflatoxin, but total aflatoxin concentration is higher than the permissible limit (4 ppb) in food adopted by majority of the countries in the world. Total aflatoxins are less than the permissible limit (20 ppb) for animal feed. Concentration of aflatoxin B1 found in seeds stored in super bags at 14% SMC is also higher than the permissible limit (2 ppb) adopted by majority of countries for food grains but less than the permissible limit (5 ppb) for animal feed.
Decline in protein contents was due to stored grain pests feeding on embryo of wheat grains. Embryo portion of grains mainly consisted protein that is why most of stored grain pests preferably attack on it (Emery and Nayak 2007). High seed moisture contents resulted in higher activities of reactive oxygen species (ROS) that are responsible for production of MDA through lipid peroxidation (Bailly 2004) and poor quality of grains. Hermetically sealed super bags hindered the free access of oxygen to the seeds stored inside super bags, hence reducing the chances of ROS productions and seed deterioration.
Wheat storage in hermetic super bags at lower seed moisture contents (the dry chain) resulted in better storage under conditions of high RH without using any fumigant or chemical to control insect pests. Storage losses and aflatoxin contamination were much lower in super bags at 8 and 10% SMC while wheat storage in conventional packaging materials resulted in higher storage and quality losses as well as aflatoxin contamination. Higher aflatoxin contamination and grain quality losses were observed in wheat stored in super bags at 14% SMC. This fact highlights the importance of drying before adoption of hermetic storage for grains.
References
Abdel-Hadi A, Schmidt-Heydt M, Parra R, Geisen R, Magan N (2012) A systems approach to model the relationship between aflatoxin gene cluster expression, environmental factors, growth and toxin production by Aspergillus flavus. J R Soc Interface 9:757–767. https://doi.org/10.1098/rsif.2011.0482
Adams J, Schulten G (1978) Losses caused by insects, mites and microorganisms. In: Harris K, Lindblad C (eds) Postharvest grain loss assessment methods. American Association of Cereal Chemists, New York, pp 83–95
Afzal I, Bakhtavar MA, Ishfaq M, Sagheer M, Baributsa D (2017) Maintaining dryness during storage contributes to higher maize seed quality. J Stored Prod Res 72:49–53. https://doi.org/10.1016/j.jspr.2017.04.001
Afzal I, Khalid E, Basra SMA et al (2019) Maintaining seed quality of maize and wheat through dry chain technology in Pakistan. Int J Agric Biol 22:1363–1368
Bailly C (2004) Active oxygen species and antioxidants in seed biology. Seed Sci Res 14:93–107
Bakhtavar MA, Afzal I, Basra SMA (2019) Moisture adsorption isotherms and quality of seeds stored in conventional packaging materials and hermetic super bag. PLoS One 14:e0207569. https://doi.org/10.1371/journal.pone.0207569
Bewley J, Bradford K, Hilhorst H, Nonogaki H (2013) Seeds : physiology of development, germination and dormancy, 3rd edn. Springer, New York
Bradford KJ, Dahal P, Bello P (2016) Using relative humidity indicator paper to measure seed and commodity moisture contents. Agric Environ Lett 1:1–4. https://doi.org/10.2134/ael2016.04.0018
Bradford KJ, Dahal P, Van Asbrouck J et al (2018) The dry chain: reducing postharvest losses and improving food safety in humid climates. Trends Food Sci Technol 71:84–93. https://doi.org/10.1016/j.tifs.2017.11.002
Emery R, Nayak M (2007) Insect pests of stored grain. In: Bailey P (ed) Pests of field crops and pastures identification and control. CSIRO Publishing, Collingwood, pp 40–61
FAO (2013) Pakistan: Review of wheat sector and grain storage issues
GrainPro (2020) Product specification of GrainPro super grain bag. https://grainpro.com/grainpro-twist-tie/. Accessed 16 May 2019
Greenspan L (1977) Humidity fixed points of binary saturated aqueous solutions (Lewis Greenspan 1976). J Res Natl Inst Stand Technol 81A:89–96. https://doi.org/10.6028/jres.081A.011
Hell K, Mutegi C, Fandohan P (2010) Aflatoxin control and prevention strategies in maize for sub-Saharan Africa. In: Carvalho MO, Fields PG, Adler CS et al (eds) Proceedings of the 10th International Working Conference on Stored Product Protection. Estoril, Portugal, pp 534–541
ISTA (2015) International rules for seed testing. International Seed Testing Association. Basserdorf
Kumar D, Kalita P (2017) Reducing postharvest losses during storage of grain crops to strengthen food security in developing countries. Foods 6:1–22. https://doi.org/10.3390/foods6010008
Kumar S, Mohapatra D, Kotwaliwale N, Singh KK (2017) Vacuum hermetic fumigation: a review. J Stored Prod Res 71:47–56. https://doi.org/10.1016/j.jspr.2017.01.002
Martin DT, Baributsa D, Huesing JE, Williams SB, Murdock LL (2015) PICS bags protect wheat grain, Triticum aestivum (L.), against rice weevil, Sitophilus oryzae (L.) (Coleoptera: Curculionidae). J Stored Prod Res 63:22–30. https://doi.org/10.1016/j.jspr.2015.05.001
McDonald MB (2007) Seed moisture and the equilibrium seed moisture content curve. Seed Technol 29:7–18
Mobolade AJ, Bunindro N, Sahoo D, Rajashekar Y (2019) Traditional methods of food grains preservation and storage in Nigeria and India. Ann Agric Sci 64:196–205. https://doi.org/10.1016/j.aoas.2019.12.003
Saleemi MK, Khan MZ, Khan A, Hameed MR, Khatoon A, Abadin Z, Hassan ZU (2017) Study of fungi and their toxigenic potential isolated from wheat and wheat bran. Toxin Rev 36:80–88. https://doi.org/10.1080/15569543.2016.1233890
WHO (2017) Evaluation of certain contaminants in food (Eighty-third report of the Joint FAO/WHO Expert Committee on Food Additives). WHO Technical Report Series WHO Technical Report Series, No.1002
Wijayaratne LKW, Arthur FH, Whyard S (2018) Methoprene and control of stored-product insects. J Stored Prod Res 76:161–169. https://doi.org/10.1016/j.jspr.2016.09.001
Williams JH, Phillips TD, Jolly PE, Stiles JK, Jolly CM, Aggarwal D (2004) Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. Am J Clin Nutr 80:1106–1122
Williams SB, Baributsa D, Woloshuk C (2014) Assessing Purdue Improved Crop Storage (PICS) bags to mitigate fungal growth and aflatoxin contamination. J Stored Prod Res 59:190–196. https://doi.org/10.1016/j.jspr.2014.08.003
Wu F, Groopman JD, Pestka JJ (2014) Public health impacts of foodborne mycotoxins. Annu Rev Food Sci Technol 5:351–372. https://doi.org/10.1146/annurev-food-030713-092431
Zheng X, Tian S (2006) Effect of oxalic acid on control of postharvest browning of litchi fruit. Food Chem 96:519–523. https://doi.org/10.1016/j.foodchem.2005.02.049
Acknowledgements
The authors acknowledge Higher Education Commission Pakistan for provision support in research materials.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bakhtavar, M.A., Afzal, I. Preserving wheat grain quality and preventing aflatoxin accumulation during storage without pesticides using dry chain technology. Environ Sci Pollut Res 27, 42064–42071 (2020). https://doi.org/10.1007/s11356-020-10212-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10212-5