Abstract
Hydroxypropyl methylcellulose (HPMC) and polyvinyl alcohol (PVA) were mixed in the varying ratios of 1:1 and 1:3 respectively and used to formulate inhibitor systems for corrosion inhibition studies on carbon steel surface induced in 1.0 M H2SO4 solution. The performance of formulated inhibitor systems was assessed using weight measurement, potentiodynamic polarization technique at 28 − 65 °C and theoretical computation respectively. Weight loss measurement result revealed that corrosion resistance of the formulated inhibitor systems was dependent on time, mixing ratio, inhibitor concentration and temperature. Thus, the optimum inhibition performance obtained was from the combination of HPMC and PVA with ratio of 1:1. Also, the adsorption characteristics recorded was attributed to formation of protective complex thin film on the carbon steel surface via Temkin adsorption isotherm. The temperature and inhibition efficiency relations obtained supported physical adsorption mechanism proposed. In addition, potentiodynamic polarization result revealed that action of inhibitor systems modified both partial electrochemical reaction processes and also retained the mixed mode function in its operation. Density function theory revealed the chemistry reactivities of inhibitor system in aqueous phase whereas molecular dynamic simulation interpreted the real experimental condition of metal surface in extreme electrolytic solution in the presence of inhibitor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The continuous research on the response of metals and alloys in various service environments is due to their relevance in fabrication of many industrial metallic structures, tools and appliances. In fact, some of these materials possess either moderate or near-excellent features which make them top choice during material selection applicable to various service environment. On that note, specified grades of metals and alloys are designated for fabrication of pipes, barrels, drill bits, tanks, sheets, tubes, plates, etc. for various applications in different industrial sectors (Al-Sabagh et al. 2011; Oguzie et al. 2007; Verma et al. 2016; Sasikumar et al. 2015). Despite these characteristics, the long-term reliability responses of these materials are not guaranteed when exposed to harsh work environment without adequate protection. For instance, the use of diluted mineral acid solution in many operational processes (such as acid cleaning and pickling, textile dyeing, water and sewage treatment, chemical etching, etc) is a welcome development due to efficiency enhancement but gradually the metallic tools and equipment parts deteriorate via a process called corrosion (Iroha and Nnanna 2019).
Corrosion is an electrochemical process involving metal dissolution at anodic region and hydrogen evolution at cathodic region. The hydrogen gas released creates cracks or minor holes on the metal surface which could later develop to larger pitting effect if not checked or regulated. The resultant effects of dissolution of metals and alloys in acidic environment are many including; pollution and contamination of environment, undue shutdown of plants and machineries, mechanical failures of structures, loss of economy on routine maintenance and replacement of parts, etc. Also, corrosion contributes immensely in the deterioration of structural materials in marine and offshore environment respectively (Shams et al. 2019). Hence, the developmental strategy in checkmating the dissolution activities of metal and alloys during service in acid solution environment is of paramount importance. In pursuance of this developmental goal, non-toxic chemical substances (corrosion inhibitors) either from organic or inorganic sources are the right option with readily availability, ease of modification and presence of inhibiting potency within their structures.
Reports of many researchers in scientific literatures have revealed the use of many organic substances to regulate the activities of metal dissolution in acid environment (Bentiss et al. 1999; Wahdan et al. 2002; El-Shafei et al. 2001; Ebenso and Oguzie 2005; Chidiebere et al. 2012; Oguzie et al. 2010). These materials were able to perform by adsorbing their molecules on the charged metal atoms through chemical or physical interactions and the stability of the interaction determines the effectiveness of the inhibitor. Also, many of these materials have been used alone or in combination with other additives or materials to achieve optimum performance. Equally, many water soluble polymers have responded positively independently (Rajendran et al. 2005; Bereket et al. 2003; Selvaraji et al. 2004; Jianguo et al. 1995; Dubey and Singh 2007; Umoren et al. 2006; Umoren and Obot 2008; Eid et al. 2018) or in combined mixtures (Morooka et al. 2001; Nwanonenyi et al. 2018a; Nwanonenyi et al. 2018b; Tsoeunyane et al., 2019; Umoren et al. 2014; Umoren et al. 2007; Umoren et al. 2018; Mansri et al. 2012) in the fight against metal corrosion in some harsh service environment. However, more work is needed to be done in the search for more corrosion inhibitors because of the complex nature of some service environment and inadequate reliability and durability responses of some inhibitors engaged in corrosion control and metal protection. This necessitated the formation of corrosion inhibitor using mixture of HPMC and PVA despite that these polymers have been engaged individually in the corrosion control course with near-moderate results or performance elsewhere (Umoren et al. 2007; Arukalam 2014). Also, the idea of combining two different inhibitive materials with varying properties in search for better results is a worthwhile and unprecedented in the field of corrosion control research (Obot and Umoren 2011; Umoren et al. 2010a). In this emerging research, the combination of two different polymers as a single inhibitor was to bring the different functional groups of each polymer together in one system with a view of having a better and functional inhibitor. The functional groups of interest in PVA molecule are vinyl group and hydroxyl group whereas in HPMC molecule the centres of attraction include the following hydroxyl group, methyl group, propyl group and phenyl structure.
Therefore, the research was focused on investigating the corrosion resistance behaviour of inhibitor developed from polymer mixtures on carbon steel in 1.0 M H2SO4 acid solution environment using potentiodynamic polarization, gasometric measurement and simulation technique respectively. Also, the impact of temperature on the corrosion studies and mode of inhibition adsorption were monitored and mode of adsorption mechanism. In addition, the density functional theory was used to assess the chemistry reactivities of inhibitor system whereas the molecular dynamic simulation was used to explain the real experimental condition of metal surface induced in extreme electrolytic solution containing different ions and molecules. Finally, use of combined polymer inhibitor from mixture of HPMC and PVA in the fight against corrosion of metal in acidic medium has not been reported elsewhere to the best of our knowledge.
Experimental section
Preparation of metal and test solutions
Carbon steel sheet with the following percentage composition (Si = 0.03, Cr = 0.06, Mn = 0.04, C = 0.06, Cu = 0.06 and remainder was Fe) was used for the corrosion studies. The metal sheet was cut to 3 × 3 × 0.3 cm dimension using mechanical cutter. The surface was polished #1000 and #1200 emery paper, washed with distilled water, dried with warm air and secured with dessicator for subsequent used. The tests solutions were prepared using concentrated H2SO4 acid, HPMC, PVA and distilled water. 1.0 M H2SO4 solution used for blank solution was obtained using serial dilution principle whereas inhibited solution was prepared with 1 L of diluted acid solution and varying ratios (1:0, 1:1 and 1:3) of HPMC and PVA respectively in grams.
Experimental method
Weight loss measurement technique
This was utilized to study the behaviour of combined polymer inhibitor in resisting of carbon steel corrosion in 1.0 M H2SO4 environment and the experimental standard (ASTM G1–90, 1996) adopted has been reported elsewhere (Eid et al. 2018). Prepared metal samples were immersed in 250 mL capacity beakers containing test solutions (150 mL) with the aid plastic thread and glass rod. The beakers were left at room temperature (28 ± 1 °C), metal samples were retrieved after 12 h interval and this process continued for 108 h progressively. In addition, the same experiment was repeated but the beakers were immersed in digital water bath containing water, regulated at 35–65 °C and the metal samples were withdrawn at 8 h interval. Weight loss results obtained were mean values from the triplicate difference of the weight of metal sample before and immersion in the test solutions. The corrosion parameters determined from the weight loss results include the following;
-
(a)
Corrosion rate, CR (mm/yr): This measure the rate at which surface of metal sample exposed to acid solution deteriorates. It is determined from the expression stated below as follow;
where wb, wa, A, t and D represents mass (g) of metal sample before immersion, mass of metal sample after immersion, total surface area (cm2) of metal sample, time (h) of exposure, density (g/cm−3) of metal sample respectively.
-
(b)
Inhibition efficiency, IE (%) and extent of surface coverage, θ: These parameters assess the anticorrosion resistance or behaviour of a material on the surface of metal sample induced in acid solution. These parameters are determined using the expressions below.
where CR1, CR2 represents corrosion rate of carbon steel in acid solution and inhibited system respectively.
Potentiodynamic polarization (PDP) measurement
This was carried out on advanced corrosion testing machine (PARC-263 model). The electrolytic cell, made of cylindrical glass, houses the conventional electrodes; graphite rod (counter), saturated calomel (reference) and carbon steel (working) and test solution. Part of carbon steel sample (1cm2 surface area) was exposed to test solution while the remaining part was coated with wax. Desktop computer (visual monitor), electrochemical workstation and terminals of electrolytic cell were connected using luggin capillaries. Steady state open potential circuit (OCP) was allowed for 30 min before measurements were taken at potential range of ±250 mV and 0.333 mV/s scan rate of corrosion potential. Unstirred state of test solution and aerated room condition was maintained at 28 ± oC. Experimental measurement was repeated three times and average results were taken. Power suite (program software) was used to extrapolate experimental data (Nwanonenyi et al. 2018b).
Theoretical computations
The two simulation modules utilized in the course performing the theoretical computation includes; Dmol3 and molecular dynamic simulation module
-
(i)
Dmol3 simulation:
In this case, electronic molecular structures of repeat unit of polymers (HMPC and PVA) used for the corrosion inhibition was modeled and used to perform the simulation in gaseous and aqueous phase respectively. The geometrical optimization was done with Dmol3 module tool as contained in Material Studio 7.0 software using following parameters; gradient-exchange correlation functional (GGA//PW91), spin unrestricted, DND Basis set, k-point set: (gamma) 1x1x1. The COSMO control was used to include the solvation effect (aqueous phase) in the simulation and the following structural descriptors were determined; geometrical optimized structure, total electronic density, frontier molecular orbitals and energy gap. The chemical reactivities of inhibitor systems were dependent on structural descriptors of individual polymeric inhibitor used. For instance, optimized structure presents molecular structure of inhibitors that replicate real life situation, total electron density provides information on how electron hovers around the inhibitor molecules, frontier molecular orbitals give information on the highest (EHOMO) and lowest (ELUMO) occupied molecular energy orbitals and energy gap (ΔEgap = ELUMO- EHOMO) between the occupied molecular energy. In addition, Fukui functions present the positions of electrophilic and nucleophilic sites within the inhibitor structures.
-
(ii)
Molecular dynamic simulation:
This was performed using Forcite dynamic and quench simulation tool of Material studio 7.0 software. The (iron) Fe crystal was imported into 3D atomistic document, optimized with Forcite module tool and cleaved along (110) plane. Surface of the Fe crystal was optimized using Forcite module tool and enlarged to (15 × 15) supercell range which offered large surface for the interaction of inhibitor molecules, crystal surface and electrolytic solution. Vaccum slab crystal thickness of 25.0 Ǻ and 1.00 Ǻ position was built to accommodate the Fe crystal surface, inhibitor systems active species in an approximated electrolyte solution containing 516H2O molecules, 7H3O+ and 7SO42− ions respectively. Thus, Amorphous cell tool was used to pack the constituents of electrolyte solution into crystal surface. The geometry optimization was done with Forcite module with maximum iteration number of 1000, COMPASS force field and forcefield charges in a simulation box (15 × 15 × 29.0927 Ǻ). Finally, Forcite Quench simulation module was used to perform the molecular dynamics simulation with COMPASS force field, number steps of 5000, every quench steps of 250, time step of 1 fs, NVT ensemble; 5.0 ps total simulation time and 298 K. The binding energy (EBinding) provides information on the strength of interaction between the Fe (110) surface and inhibitor molecule in electrolytic solution and it was determined according Eq. (4) stated below (Njoku et al. 2017);
where Etotal is the total energy of the entire system, Eelectrolytic solution is the total energy of electrolytic solution (water molecules, H3O+ and SO42− ions respectively) and Einhibitor system is the total energy of inhibitor system.
Results and discussion
Weight loss measurement results
The measurement of level of materials loss with distinct properties of metal on short and long term application during corrosion reaction process is the characteristic features of weight loss technique. In addition, this technique shows the degree of visual effect or physical deterioration on the metal surface by the surrounding environment or corrodent (Nwanonenyi et al. 2018b). Thus, the efficacy and aptness of the technique has been reported elsewhere (Oguzie 2006; Oguzie 2005).
Effect of time response on the level of material loss
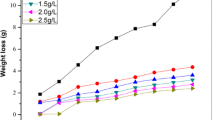
In weight loss measurement, time factor plays significant role (either in a short run or long run applications) in assessing the response of inhibitor on the corrosion behaviour of metal surface in aggressive environment. Hence, the performance or long-term reliability response (or durability) of corrosion inhibitor is dependent on time and other factors (Nwanonenyi et al. 2018a; Nwanonenyi et al. 2018b). The time effect on the level of material reduction for corrosion of carbon steel in blank acid solution and inhibited systems at 28 ± 1 °C is presented in Table 1. Data on Table 1 evidently illustrate that material dissolution varies linearly with time and no evidence of passivation manifestation for all the cases studied despite variations in ratio of mixing and concentrations of polymeric inhibitors. Furthermore, critical assessment on the level of material deterioration confirmed that metal surface reduced significantly with increase in time of exposure in aggressive solutions. Also, it was observed that ratio of mixing used together with inhibitor concentrations were able to cushion the prevailing corrosion effect on metal surface exposed to 1.0 M H2SO4 solution at varying time. This behaviour revealed that corrosion resistance offered by the inhibitor systems performed better at reduced exposure time, high concentration and mixture HMPC and PVA at ratio of 1: 1.
Effect of inhibitor concentration and mixing ratio on corrosion rate and inhibition efficiency
The results of corrosion rate obtained from weight loss measurements are presented in Table 1. Corrosion rate shows evidence of visual manifestation or physical damage done by the corrosive ions on the metal surface due to evolution of hydrogen gas at the cathodic region. Also, the corrosion rate value is a parameter used to assess how potent corrosive ions are in a system. Thus, the higher the rate of hydrogen gas evolution in the corrosion system the higher the corrosion rate value. Data on Table 1 revealed the effect of ratio of inhibitor mixtures and inhibitor concentration on the corrosion rate of carbon steel in 1.0 M H2SO4 solution. It is clearly seen that rate at which dissolution of metal in 1.0 M H2SO4 solution occurred was also dependent on the inhibitors mixing ratios and inhibitor concentration variations. Thus, the varying increase observed in the concentration of inhibitor recorded gradual reduction in the corrosion rate. This evidenced that used of HPMC and PVA on individual and composite basis respectively as corrosion inhibitor adsorbed hydrogen gas evolved at cathodic regions of carbon steel surface with drastic reduction in the destructive impact caused by the gas release. The corrosion rate response was not completely reduced or eliminated within the conditions of studies and this suggests that corrosion ions potency was conditioned by the amount of inhibitor present within the corrodent system and also there exist negligible corrosion sites within the metal surface. In addition, this is attributed to effect of varying inhibitive species within the inhibitor system.
The reliability and durability response of corrosion inhibitors is accessed based on their ability to sustain the impact of corrosion menace on the metal surface and their contributions in the formation of stable corroded product over a certain period of time (Nwanonenyi et al. 2018b). Hence, it is believed that the following factors such as nature of inhibitor, charge on metals surface, nature corrosive ions, temperature, etc. play active role in determining the effectiveness of corrosion inhibitor. The major challenge in controlling corrosion of metal in solution via inhibitor adsorption is that metal surface protected by film layer still has some free or unprotected sites within the surface where negligible corrosion rates occur. Thus, resulting to difficulty in achieving 100% inhibition efficiency in a corrosion inhibition system. Clearly, it seen that ratios of mixture and inhibitor concentrations were responsible for the efficiency of inhibition exhibited by all the inhibitors on the corrosion of carbon steel surface in 1.0 M H2SO4 solution. Thus, the gradual increment in the concentration of all the inhibitors gave rise to increased inhibition efficiency and it supports the evidence that inhibitor effectiveness observed could due to amount of inhibitor presence within the inhibited system. Hence, it seen in all cases that increment in inhibition efficiency based on concentration of inhibitor is as follows; 5.0 g/L > 3.0 g/L > 1.0 g/L. Equally, it seen that effectiveness of inhibitors obtained from the combination of HMPC and PVA showed better results compared to individual polymers whereas HMPC performed better than PVA. This could be attributed to presence of varying inhibitive functional groups within the inhibited system and effective interaction between the polymers. The order of inhibition efficiency observed according to ratios of mixture is as follows; HPMC: PVA (1:1), HPMC: PVA (3:1) and HPMC: PVA (1:3). Hence, the result of 1:1 ratio of HPMC and PVA mixture behaviour obtained exhibited best performance compared to other inhibited systems although the response was sustained as time of exposure progresses. It is believed that result manifested by combination of HPMC and PVA in the ratio of 1:1 is evidence of better synergistic effect from the combined ratio in controlling the corrosion menace on the metal surface. The result observed is in agreement with the findings of Mansri, et al., (2012) (Mansri et al. 2012).
Potentiodynamic polarization results
The PDP measurement was done to ascertain the impact of each inhibitor (at maximum concentration, 5.0 g/L) in regulating the corrosion process at anodic and cathodic region respectively of carbon steel surface at 28 ± oC. Curves of polarization shown in Fig. 1 revealed effective modification on both regions of corrosion process by the inhibitors of study. Table 2 presents the results of corrosion parameters (corrosion current densities (icorr), corrosion potential (Ecorr), anodic Tafel slope (ba) and cathodic Tafel slope (bc) extrapolated from the polarization curves. Also, data on Table 1 illustrates significant reduction in both current densities (icorr) and corrosion potential (Ecorr) together with displacement in anodic and cathodic region respectively. Evidently, this remarkable response obtained indicates that displacement in cathodic region by inhibitor portrays retardation of evolution of hydrogen gas more effectively compared to anodic dissolution. Also, displacement in anodic region shows dominance control of anodic dissolution over hydrogen gas evolution by the inhibitor. In addition, variations observed in displaced corrosion potential values are attributed to effectiveness of inhibitor caused by the amount or level of inhibitive species present within the inhibitor system. Furthermore, the reduction observed in the partials reactions of corrosion process suggests that all the inhibitors of study exhibited mixed-type function in resistance corrosion behaviour of carbon steel in 1.0 M H2SO4 solution. This is because the value of corrosion potential displacement obtained in this research did not exceed 85 mV (Ferreira et al. 2004). Hence, in classifying inhibitor based on performance (cathodic, anodic or mixed-type inhibitor) 85 mV is a threshold value. The slight changes in icorr values observed could be attributable to competition between free inhibitor molecules and adsorbed molecules. The values of icorr1 in absence of inhibitor and presence of inhibitor (icorr2) were used to compute the efficiency of inhibition (IE %) using the equation below;
where icorr1 and icorr2 represent current density in the absence of inhibitor and presence of inhibitor respectively.
Adsorption studies
Adsorption interaction between the molecules of inhibitor and charge on metal surface is one of the factors responsible for inhibition of corrosion on the metal surface exposed to aggressive work environment. Also, this force of attraction occurs by physical attraction, chemical attraction or resultant combination of both depending on the nature of chemical structure possessed by the available inhibitor species. Thus, the inhibitor molecules are able to engage themselves in the inhibitor-metal interaction (or adsorption process) through their inherent functional groups. The control of corrosion on metal surface by adsorption inhibition is a surface phenomenon which could occur by mono, double or multi layer adsorption. Any of these modes of adsorption may lead to stable adsorption though factors such nature of metal temperature, chemical structure of inhibitor, pH of corrosion medium, etc. (Obot and Obi-Egbedi 2010) are determining parameters. Furthermore, regression model (adsorption isotherm) is usually employed to have better insight on the mode of inhibition adsorption and also the nature adsorption surface (homogeneous and heterogeneous). This is done by fitting the results of degree of surface coverage obtained from weight loss measurement (as shown in Eq. (3)) above into the regression model to have proper and accurate information on the interaction existing between the inhibitor molecules and metal surface.
In this case, the regression model that gave proper insight on the mode of adsorption for the corrosion inhibitor system with optimum performance (that is, HPMC: PVA (1:1)) was the Temkin adsorption isotherm and it is given in Eq. (6) as;
where θ = degree of surface coverage, C = inhibitor concentration, Kd = equilibrium constant of adsorption process, α = inhibitor molecules interaction parameter (negative value signifies repulsion whereas positive values represent attraction). The plot of (θ) against InC gave linear graphs as shown in Fig. 2 and the adsorption parameter α and Kd were obtained from the slope and intercept respectively. Also, the correlation co-efficient (R2) and other adsorption parameters determined from the linear plot are presented in Table 3. The results of correlation co-efficient obtained are closeness to unity, thus indicating the best fit and that adsorption occurred via multilayer adsorption on a heterogeneous surface. The positive value of inhibitor molecules interaction parameter (α) represents the attraction or interaction ability of the inhibitor molecules on the metal surface. It is observed that α decreases with increase in time (as shown in Table 3), thus indicating weakening or desorption of the interaction existing between the adsorbed inhibitor molecules and metal surface (Obot and Obi-Egbedi 2010). Also, this result reflects the reduction in the performance of the combined polymer inhibitor caused by the rise in time. Hence, result of α is used to assess the adsorption potential of the inhibitor, inhibitive performance and degree of surface coverage.
The relationship between the free energy of adsorption (ΔGads) and equilibrium adsorption constant (Kads) of the inhibitor system on the surface of carbon steel is expressed according to Eq. (7) stated below:
where R = molar gas constant, T = absolute temperature and 55.5 = concentration of water in solution. The computed result of free energy of adsorption (ΔGads) obtained are shown in Table 3. Values of ΔGads obtained are negative and this suggests that adsorption of inhibitor system onto carbon steel surface is spontaneous. In addition, the values of ΔGads obtained are less than −20 KJ/mol, thus indicating that adsorption process for the inhibitor system occurred via electrostatic interaction (physical adsorption mechanism) between the molecules of inhibitor and charge onto the carbon steel surface (Ferreira et al. 2004).
Temperature response considerations
In metal corrosion and inhibition studies temperature effect is an important factor to be given due consideration since it provides information on the amount heat present, its effect within corroding system and controls the performance of inhibitor system. Thus, the parameters such as corrosion rate, efficiency of inhibition and long-term reliability and stability response of the inhibitor system are dependent on temperature. In this study, the effect of varying temperatures within the range of 35 – 65 °C was used to monitor the corrosion behaviour of carbon steel in 1.0 M H2SO4 solution and the potentials of different concentrations of inhibitor systems in inhibiting or regulating the corrosion process on the carbon steel surface induced in the same corrodent system. It is clearly seen in Table 4 that increment in temperature gave a resultant increment in the corrosion rates of carbon steel induced in 1.0 M H2SO4 solution even in the presence of different concentrations of inhibitor systems. The response observed is attributed to active agitations caused by corrosion ions within the corrodent system due to acquisition of more kinetic energy from change in heat effect and gradual desorption of adsorbed protective film on the metal surface. Hence, this behaviour supports evidence of physical interactions between inhibitor species and charge on the metal surface (Nwanonenyi et al. 2018a; Nwanonenyi et al. 2018b). In addition, the inhibitor system that exhibited best result is the case of mixture of HPMC and PVA in the ratio of 1:1.
Furthermore, the heat interaction parameters (such as apparent activation energies (Ea), enthalpy and entropy of adsorption of the corrosion inhibition process) for the inhibited system (HPMC: PVA at the ratio of 1:1) that exhibited better inhibition efficiency response or performance were determined in this study. Activation energy (Ea) represents the minimum amount of energy that must be exceeded for a chemical reaction to start or proceed and it is expressed using Arrhenius Eq. (8) stated below (Oguzie 2005);
where A, R, T represents pre-exponential factor, universal gas constant, absolute temperature respectively. Logarithm of corrosion rate is plotted against reciprocal of absolute temperature as shown in Fig. 3 and activation energy is obtained from slope (that is, slope = (−Ea/R) of the plot. Table 5 presents the results obtained and it reveals values of activation energy for the inhibited system is higher compared to corrodent system. Thus, the response obtained supports the physical adsorption proposed for the inhibition mechanism (Obot and Obi-Egbedi 2010). Also, it is observed that gradual increase in the amount of inhibitor within the corrodent system gives rise to increase in the values of activation energy. This is clear evidence that as much inhibitor was introduced into the corrodent system corrosion ions needed an additional energy to break the barrier holding the reaction to proceed.
The enthalpy and entropy of adsorption for corrosion and inhibition process was evaluated Transition-State Equation stated below (Nwanonenyi et al. 2017; Nwanonenyi et al. 2020) to determine the independency of the parameters with temperature for the case of mixture of HPMC and PVA at ratio of 1: 1;
where ∆Hads, ∆Sads, CR, R, N, h, T represents enthalpy of adsorption, entropy of adsorption, corrosion rate, universal gas constant, Avogadro’s constant, Planck’s constant and absolute temperature respectively. Plot of logarithm of RCH/T versus 1/T is presented in Fig. 4 for the corrosion and inhibition process at different inhibitor concentrations. The values of enthalpy (∆Hads) and entropy (∆Sads) were determine from slope (−∆Hads/2.303R) and intercept [(log(R/Nh) + ∆Sads/2.303R)] respectively and results obtained are shown in Table 4. Enthalpy values obtained are positive suggesting evidence of endothermic process whereas negative values of entropy suggests free movement of molecules of inhibitor within the solution. High values of enthalpy observed in the inhibited system compared to blank solution suggest physical adsorption mechanism predominance over the inhibition process (Anyiam et al. 2020a).
Synergistic effect
The inhibition efficiency manifested by the inhibitor systems in this study showed better results in their combined forms compared to their individual basis even at low and elevated temperatures. Thus, synergistic parameter stated below was used to evaluate the effect of synergism on the inhibitive performance of the inhibitor system on carbon steel in 1.0 M H2SO4 solution considering the case of polymer mixture that showed better inhibition performance (that is, HPMC and PVA mixture at the ratio of 1:1).
where I1, I2, I11 + 2 represents inhibition efficiency of HPMC, inhibition efficiency of PVA, inhibition efficiency of mixed HPMC and PVA respectively. Also, 11 + 2 = (I1+ I2). There is synergistic interaction effect between the inhibitor systems when the value of S1 > 1 whereas no interaction exists and antagonistic interaction effect occurs when S1 approaches unit and SI < 1 respectively (Anyiam et al. 2020b). The computed values for the synergistic parameters obtained are presented in Table 5 and it illustrated that the values of synergistic parameters are greater unity thus indicating the inhibition characteristics manifested by the inhibitor system was due to synergistic effect. The synergistic effect result obtained is in agreement with findings of Umoren, Li, Wang (2010) (Umoren et al. 2010b).
Theoretical computation consideration
Dmol3 simulation results
Figure 5 presents the results of theoretical simulation performed and it is seen that HPMC and PVA repeat unit respectively are saturated with electron and flat-lying molecular orientations characteristics from the total electron density result (Table 6). Regions of HOMO and LUMO orbital respectively in PVA repeat unit are seen around the vinyl and hydroxyl group respectively and this reflects the positions of electrophilic and nucleophilic site, respectively. In HPMC repeat unit, both regions of HOMO and LUMO are seen around the phenyl and methyl group respectively, and this also reflects the position for electrophilic and nucleophilic attack respectively. The donation of electrons to d-orbital of metal atom surface occurs at HOMO regions whereas the oxidized electrons released from Fe to Fe2+ are accepted at regions of LUMO. Thus, it is level of response from the donation and acceptance of electrons at HOMO and LUMO region respectively that determines the nature of stable interaction and hydrophobicity formed by the inhibitor system. Computed values of ELUMO, EHOMO and ∆Egap obtained for HPMC and PVA respectively in both gas and aqueous phase are presented in Table 7. The result of theoretical simulation obtained reflects the position of experimental findings and is a clear indication that HPMC molecule exhibited enhanced inhibition efficiency compared to PVA molecule. It observed that energy gap value (or the effectiveness inhibitor) of HPMC and PVA repeat unit respectively at gas phase is higher compared to aqueous phase and this revealed that water or solvation effect weakens the strength of inhibitor. In addition, it is revealed that function of PVA molecule in the inhibition performance of the inhibitor systems was supportive mode.
Molecular dynamic simulation (MDS) results
The molecular quench dynamic simulation technique was employed to probe the nature of adsorption interaction that exists between the metal surface and inhibitor molecule in the approximated electrolyte solution containing important ions; 516H2O, 7H3O+ and 7SO42− and in gas phase. Also, our motive here is to interpret the real experimental process that involves corrosion and adsorption inhibition using theoretical basis. The structures of results of adsorption interactions between Fe (110) surface and inhibitor system in extreme electrolytic solution obtained and gas phase are presented Fig. 6. It is observed from the structures that strong and reasonable adsorption interaction occurred between the metal surface and inhibitor systems whereas the presence of multi-component active ingredients (such as water molecules, ions) within the electrolytic solution caused drastic reduction in the interaction energy (as seen the values of binding energy of interaction in case of gas phase and aqueous phase). Hence, the observed behaviour could be caused due to followings reasons (Obot and Umoren 2011); (i) synergistic interaction between inhibitor molecules and charges on metal surface (ii) dispersive interaction caused by pi-electron delocalization and polarization of atoms within inhibitor molecular structure. (iii) possible protonation of inhibitor molecule by the electrolytic solution (iv) nature of adsorption interaction orientation exhibited by inhibitor molecule on metal surface. It is believed that these reasons stated above are the factors responsible for the outstanding inhibition performance observed in the experimental results. The result of binding energy adsorption interaction between metal surface and inhibitor molecules in aqueous (electrolytic solution constituents) and gas phase obtained are −157.65 kJ/mol and − 596.68 kJ/mol. Also, the binding energy of adsorption between the metal surface and electrolytic solution constituents is −153.32 kJ/mol.
Mechanism for corrosion resistance
It is believed that some reactive centres (adsorption centres) or functional groups within the structural arrangment of inhibitor molecules play significant role in assessing whether the molecules possess the abitily to resistance corrosion or not. Probably, the inhibitive molecules that are able to resistance or regulated the corosion on metals surface in solution act through adsorption. Hence, the adsorption capacity of corrosion inhibitors depends on the form of adsorption centres presence within the molecular structure of the inhibitor. For instant, reactive centres containing heteroatoms, cyclic rings, π-bond, aromatic structure, etc. exhibit good inhibitive effectiveness. It is believed that presence of multiple centres of adsorption will offer better resistance to corrosion than single centre of adsorption. This is because many inhibiting species within the inhibited system will interact more (by either physical or chemical molecular attractive) with the charges on metal surface, promote more surface coverage and offer improved protection from stable adsorbed and corroded product. Hence, the interaction formed between the inhibiting species within the molecules of HPMC and PVA, and the charge on carbon steel surface provided the protective complex film that modified the dissolution of carbon steel induced in 1.0 M H2SO4 solution in this research. Also, the inhibitor systems exhibited a remarkable improvement in the inhibition efficiency of carbon steel in 1.0 M H2SO4 solution recorded as the against the results of individual performance of HPMC and PVA. Possibly, this implies that presence of multiple inhibitive species or adsorption centres within the corrodent system containing metal promotes the degree at which the surface metal will be sheltered.
Conclusion
The result of this work is another welcome development in the field of corrosion science and metal protection. It is another experimental proof that use of composite materials as corrosion inhibitor are in position to offer better results compared to single material. It is clearly seen that combined polymer inhibitor from mixture of HPMC and PVA resisted the corrosion of carbon steel in 1.0 M H2SO4 solution to a certain degree dependent on concentration at all temperatures. The inhibition mode was mixed type evidenced by PDP studies. The possible positions or functional groups through which transfer electrons occurred in HPMC molecule include hydroxyl, methyl and phenyl group respectively whereas in PVA molecule it is through vinyl group and hydroxyl group. This is believed to responsible for the varying of inhibition characteristics observed in the performance of each polymer as corrosion inhibitor. Evidently, it is clear that time and temperature response respectively plays significant role during metal corrosion process because it was observed that effective inhibition was better favoured at lower time and temperature respectively at different conditions of inhibition. Again, the combination of the groups and structures synergistically increase the potentials of combined polymer inhibitor to form protective film over the carbon steel surface. The interpretation of the real experimental conditions using theoretical basis through molecular dynamic simulation module revealed the role multi-components ions and molecules in the corrosion and inhibition process. The results recorded in this research revealed that carbon steel corrosion and inhibition in 1.0 M H2SO4 solution in the presence of HPMC, PVA and HPMC/PVA was dependent of time, inhibitor concentration, inhibitor mixing ratio and temperature. Also, the inhibitor developed provides an alternative means of regulating corrosion on carbon steel surface in 1.0 M H2SO4 solution using eco-friendly and non-toxic materials from polymer background. The experimental methods used were efficient and the developed inhibitor can be introduced in surface coatings where carbon steel is used in construction of metallic tube, plates, pipes sheets, rods and bars respectively. Finally, through the of advanced surface probe techniques the experimental variables could be used to expand further research.
References
Al-Sabagh AM, Abd-El-Bary HM, El-Ghazawy RA, Mishrif MR, Hussein BM (2011) Corrosion inhibition efficiency of linear alkyl benzene derivatives for carbon steel pipelines in 1M HCl. Egypt J Pet 20:33–45
Anyiam CK, Ogbobe O, Oguzie EE, Madufor IC, Nwanonenyi SC, Onuegbu GC, Obasi HC, Chidiebere MA (2020a) Corrosion inhibition of galvanized steel in hydrochloric acid medium by a physically modified starch. SN Appl Sci 2. https://doi.org/10.1007/s42452-020-2322-2
Anyiam CK, Ogbobe O, EE EEO, Madufor IC (2020b) Synergistic study of modified sweet potato starch and KI for corrosion protection of mild steel in acidic media. J Bio Tribo Corrosion 6(70):70
Arukalam IO (2014) Durability and synergistic effects of KI on the acid corrosion inhibition of carbon steel by hydroxypropyl methylcellulose. Carbohydr Polym 112:291–299
Bentiss F, Lagrenee M, Traisnel M, Hornez JC (1999) The corrosion inhibition of carbon steel in acidic media by a new triazole derivative. Corros Sci 41:789–803
Bereket GA, Yurt A, Turk H (2003) Inhibition of corrosion of low carbon steel in acidic solution by selected polyelectrolytes and polymers. Anti-Corrossion Methods Mater 50(6):422–535
Chidiebere MA, Ogukwe CE, Oguzie KL, Eneh CN, Oguzie EE (2012) Corrosion inhibition and adsorption behavior of Punica granatum extract on carbon steel in acidic environments: experimental and theoretical studies. Ind Eng Chem Res 51:668–677
Dubey AK, Singh G (2007) Corrosion inhibition of carbon steel in sulphuric acid solution by using polyethylene glycol methyl ether (PEGME). Port Electrochim Acta 25:221–235
Ebenso EE, Oguzie EE (2005) Corrosion inhibition of carbon steel in acidic media by some organic dyes. Mater Lett 59:2163–2165
Eid MSA, Habafy MA, Reham AM, Samy MS, Shaban Asmaa S (2018) Control the corrosion of mild steel using synthesized polymers based on polyacrylamide. Egypt J Pet 27(4):897–910
El-Shafei AA, Moussa MH, El-Far AA (2001) The corrosion inhibition character of thiosemicarbazide and its derivatives for C-steel in hydrochloric acid solution. Mater Chem Phys 70:175–180
Ferreira ES, Giancomelli C, Giancomelli FC, Spinelli A (2004) Evaluation of the inhibitor effect of L-ascorbic acid on the corrosion of carbon steel. Mater. Chem Phys 83:129–134
Iroha NB, Nnanna LA (2019) Electrochemical and adsorption study of the anticorrosion behavior of Cefepime on pipeline steel surface in acidic solution J. mater. Environ. Sci 10:898–908
Jianguo J, Lin W, Otieno-Alego V, Schweinsberg DP (1995) Polyvinylpyrrolidone and polyethylenimine as corrosion inhibitors for the corrosion of low carbon steel in phosphoric acid. Corrosion Sci 37(6):975–985
Mansri A, Bouras B, Tennouga L, Medjahed K (2012) Effect of iodide ion on corrosion inhibition of mild steel in 1M H2SO4 by polyacrylamide with different macromolecular weight and polyacrylamide poly4-vinylpyridine mixture. Der Pharma Chemica 4(5):1803–1811
Morooka M, Sekine I, Tanaki T, Hirosett N, Yuasa M (2001) Effects of polymer-polymer complexes on the corrosion of carbon steel in cooling water system (part 2): corrosion investigation in polymethacrylicacid/polyacrylamide system. Corrossion Eng 50(3):106–114
Njoku DI, Li Y, Lgaz H, Oguzie EE (2017) Dispersive adsorption of Xylopia aethiopica constituents on carbon steel in acid-chloride medium: a combined experimental and theoretical approach. J Mol Liq 249:371–388. https://doi.org/10.1016/j.molliq.2017.11.051
Nwanonenyi SC, Arukalam IO, Obasi HC, Ezeamaku UL, Eze IO, Chukwujike IC, Chidiebere MA (2017) Corrosion inhibitive behavior and adsorption of millet (Panicum miliaceum) starch on carbon steel in hydrochloric acid environment. J Bio Tribo Corros 3:54
Nwanonenyi SC, Obasi HC, Chidiebere AM (2018a) Inhibitive performance of carboxymethyl cellulose and additives on corrosion of carbon steel in acidic and alkaline environments. J Bio Tribo Corrosion 4. https://doi.org/10.1007/s40735-018-0148-x
Nwanonenyi SC, Obasi HC, Chukwujike IC, Chidiebere MA, Oguzie EE (2018b) Inhibition of carbon steel corrosion in 1M H2SO4 using soy polymer and polyvinylpyrrolidone, Chemistry Africa, DOI https://doi.org/10.1007/s42250-018-00035-w
Nwanonenyi SC, Obasi HC, Obidiegwu MU, Chukwujike IC (2020) Anticorrosion response of polymer mixture on mild steel in hydrochloric acid environment. Emergent Materials 3:663–673. https://doi.org/10.1007/s42247-020-00120-2
Obot IB, Obi-Egbedi NO (2010) Adsorption properties and inhibition of carbon steel corrosion in sulphuric acid solution by ketoconazole: experimental and theoretical investigation. Corros Sci 52:198–204
Obot IB, Umoren SA, Obi-Egbedi (2011) Corrosion inhibition and adsorption behaviour for aluminium by extract of Aningeria robusta in HCl solution: synergistic effect iodide ions, J Mater Environ Sci 2(1), 60–71
Oguzie EE (2005) Corrosion inhibition of carbon steel in hydrochloric acid solution by methylene blue dye. Mater Lett 59(8–9):1076–1079
Oguzie EE (2006) Studies on the inhibitive effect of Occimum viridis extract on the acid corrosion of carbon steel. Mater Chem Phys 99(2–3):441–446
Oguzie EE, Li Y, Wang FH (2007) Corrosion inhibition and adsorption behavior of methionine on carbon steel in sulfuric acid and synergistic effect of iodide ion. J Colloid Interface Sci 310(2007):90–98
Oguzie EE, Enenebeaku CK, Akalezi CO, Okoro SC, Ayuk AA, Ejike EN (2010) Adsorption and corrosion-inhibiting effect of Dacryodis edulis extract on low-carbon-steel corrosion in acidic media. J Colloid Interface Sci 349:283–292
Rajendran S, Sridevi SP, Anthony N, John Amalraj A, Sundearavadivelu M (2005) Corrosion behaviour of carbon steel in polyvinyl alcohol. Anti-Corrossion Methods Mater 52(2):102–107
Sasikumar Y, Adekunle AS, Olasunkanmi LO, Bahadur Baskar IR, Kabanda MM, Obot IB, Ebenso EE (2015) Experimental, quantum chemical and Monte Carlo simulation studies on the corrosion inhibition of some alkyl imidazolium ionic liquids containing tetrafluoroborate anion on MS in acidic medium. J Mol Liq 21:105–118
Selvaraji SK, Kennedy AJ, Amalraj AJ, Rajendran S, Palaniswamy N (2004) Corrosion behaviour of carbon steel in the presence of polyvinylpyrrolidone. Corrossion Rev 22(3):219–232
Shams A, Faisal K, Yahui Z, Susan C (2019) Optimization of zinc-nickel film electrodeposition for better corrosion resistant characteristics. Can J Chem Eng 97:2426–2439. https://doi.org/10.1002/cjce.23521
Tsoeunyane MG, Makhatha ME, Arotiba OA (2019) Corrosion inhibition of carbon steel by poly(butylene succinate)-L-histidine extended with 1,6-diisocynatohexane polymer composite in 1M HCl. Corrosion, International Journal of. https://doi.org/10.1155/2019/7406409
Umoren SA, Obot IB (2008) Polyvinyl pyrrolidone and polyacrylamide as corrosion inhibitors for carbon steel in acidic medium. Surf Rev Lett 25(3):277–284
Umoren SA, Ebenso EE, Okafor PC, Ogbobe O (2006) Water soluble polymers as corrosion inhibitors of carbon steel in acidic medium. Pigment Resin Technol 35(6):346–352
Umoren SA, Ogbobe O, Okafor PC, Ebenso EE (2007) Polyethylene glycol and polyvinyl alcohol as corrosion inhibitors for aluminium in acidic medium. J Appl Polym Sci 105:3363–3370
Umoren SA, Solomon MM, Udosoro II, Udoh AP (2010a) Synergistic and antagonistic effects between halide ions and carboxymethyl cellulose for the corrosion inhibition of carbon steel in sulphuric acid solution. Cellulose 17:635–648
Umoren SA, Li Y, Wang FH (2010b) Electrochemical study of corrosion inhibition and adsorption behaviour for pure iron by polyacrylamide in H2SO4: synergistic effect of iodide ions. Corros Sci 52(5):1777–1786
Umoren SA, Eduok UM, Solomon MM (2014) Effect of polyvinylpyrrolidone – polyethylene glycol blends on the corrosion inhibition of aluminium in HCl solution, 43(5), 299–313, https://doi.org/10.1108/PRT-09-2013-0079
Umoren SA, AlAhmary AA, Gasem ZM, Solomon MM (2018) Evaluation of chitosan and carboxymethyl cellulose as ecofriendly corrosion inhibitors for steel. Int J Biol Macromol 117:1017–1028
Verma C, Olasunkanmi LO, Obot IB, Ebenso EE, Quraishi MA (2016) 2, 4-Diamino-5- (phenylthio)-5 H-chromeno [2, 3-b] pyridine-3-carbonitriles as green and effective corrosion inhibitors: gravimetric, electrochemical, surface morphology and theoretical studies. RSC Adv 6:53933–53948
Wahdan MH, Hermas AA, Morad MS (2002) Corrosion inhibition of carbon-steels by propargyltriphenylphosphonium bromide in H2SO4 solution. Mater Chem Phys 76:111–118
Acknowledgments
The support from the World Bank Africa Centres of Excellence for Impact (ACE Impact) project (NUC/EC/507/1/304) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
I, the corresponding attest on behalf of other authors that there is no case of competing interest in this work.
Rights and permissions
About this article
Cite this article
Nwanonenyi, S.C., Ezeani, E.O., Obele, C.M. et al. Protection of carbon steel surface in extreme environment using polymer mixture: effects of time, inhibitor concentration, mixing ratio and synergy. Saf. Extreme Environ. 2, 245–258 (2020). https://doi.org/10.1007/s42797-021-00029-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42797-021-00029-x