Abstract
Bacillus sp. WD22, previously isolated from refinery effluent, degraded 71% of C8 hydrocarbons present in 1.0% v/v PCO in seawater (control medium), which reduced to 16.3%, on addition of yeast extract. The bacteria produced a biosurfactant in both media, whose surface was observed to be amorphous in nature under FESEM-EDAX analysis. The biosurfactant was characterized as a linear surfactin by LCMS and FT-IR analysis. The critical micelle concentration was observed as 50 mg/L and 60 mg/L at which the surface tension of water was reduced to 30 mN/m. Purified biosurfactant could emulsify petroleum-based oils and vegetable oils effectively and was stable at all tested conditions of pH, salinity and temperature up to 80 °C. The biosurfactant production was found to be mixed growth associated in control medium, while it was strictly growth associated in medium with yeast extract as studied by the Leudeking-Piret model.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Petroleum crude oil (PCO) is the world’s most used energy source because it is relatively abundant, highly energy dense and can be easily transported across the world [1]. Most of the PCO transportation is done via marine vessels, barges, rail cars, underground and underwater pipelines, which results in widespread contamination [2]. The contamination of PCO in the marine environment often occurs due to shell and barge washing, exploration activities and natural oil seepage into sea beds with oil spills, contributing to more than 60% of oil release. The repercussions of transfer of PCO into marine environment are daunting as PCO consists of substantial quantities of polyaromatic hydrocarbons (PAH) in addition to asphaltenes, resins and aliphatic hydrocarbons [3]. They are proven to exhibit damaging toxicity in marine birds and animals in addition to severely harming marine ecosystems [4]. Hence, the complete removal of PCO from the polluted marine environment is very essential. Among various methods of PCO removal, bioremediation, by the use of indigenous microorganisms, is the most sustainable technique, as it completely mineralizes the pollutants, thereby facilitating their removal from the environment. The successful use of bioremediation in cleaning up of oil spill by Exxon Valdez oil tanker in the Gulf of Alaska in 1989 opened up prospects of this technology in treatment of oil spills [5]. Since PCO is highly hydrophobic, its solubility in the oil is limited, and in order to overcome this, chemical surfactants are applied to enhance the PCO solubility, which often results in secondary pollution [6]. Another advantage of using microbes for remediation is that, on exposure to oils, most of them secrete biosurfactants extracellularly or attached onto their cell wall with potential advantages such as low toxicity, biodegradability and stability to wide range of salinity, pH and temperature [7].
Biosurfactant production and PCO degradation depends on factors like carbon and nitrogen sources and culture conditions such as pH, temperature and agitation speed [8, 9]. When crude oil spills occur in the marine environment, there is a sudden spike in the carbon content; however, the nitrogen becomes a rate-limiting factor as seas and oceans have very low amounts of nitrogen [10]. Addition of organic nitrogen could possibly increase the biosurfactant production and subsequent PCO degradation by microorganisms. Degradation of PCO in seawater by microbes is currently gaining significance as recent literature shows substantial studies on the same. Strains of Pseudomonas, Rhodococcus, Bacillus and indigenous bacterial communities have been employed enhanced degradation of PCO in seawater with an aim to employ them for oil spills [11,12,13]. Our research group has previously reported biosurfactant-mediated degradation of PCO in seawater by strains of Acinetobacter and Pseudomonas, on supplementation with glucose and yeast extract [14, 15].
Hence, the present study reports the biosurfactant-mediated degradation of PCO in seawater by Bacillus sp. WD22, previously isolated from refinery effluent by our research group [16]. The aim of the study is to analyse the composition, properties and production kinetics of biosurfactant secreted on exposure to PCO in seawater supplemented with glucose and yeast extract, which has been reported for the first time.
Materials and methods
Substrates, chemicals and bacteria
Sea water used in this study was obtained from eastern shore of Arabian Sea in Surathkal situated in Karnataka, India. PCO was obtained from a refinery industry situated in Mangalore, Karnataka, India. Bacillus sp. WD22 (GenBank Accession ID: MK355614) was previously isolated from refinery effluent [16]. All the other substrates and chemicals used were of analytical grade.
Growth of Bacillus sp. WD22, PCO degradation and biosurfactant production in seawater under nutrient supplementation
Control medium was prepared by supplementing sea water (100 mL) with 1.0 g/L of glucose. To study the effect of nitrogen supplementation on degradation of PCO by Bacillus sp. WD22, another medium was prepared by supplementing yeast extract (0.05 g/L) to the control. The media were sterilized at 121 °C, 15 lbs pressure for 20 min, and inoculated with 5.0% v/v of 16-h-old Bacillus sp. WD22 (108 CFU/mL). Inoculated media were then incubated at an agitation speed of 100 rpm, 27 °C at pH 8.1 (measured pH of sea water). The growth of Bacillus sp. WD22 was determined by measuring dry weight of biomass after 15 days of incubation.
Estimation of degradation of PCO by gas chromatography (GC)
Media were centrifuged at 10,000 rpm for 15 min, and cell-free supernatant (CFS, 80 mL) was separated carefully devoid of any PCO. The left over CFS with residual PCO was mixed with hexane (1:4), and the oil was extracted using an extraction funnel. The moisture content was removed by passing it over anhydrous sodium sulphate. PCO was also extracted from the control sample (media without addition of bacteria).
Extracted PCO samples (1.0 µL) were injected to a GC (Perkin Elmer Clarus 680) with flame ionization detector for a run time of 32 min with helium as the carrier gas. The inlet initial and final temperature was maintained at 60 °C and 250 °C, respectively. The following formula was used for the estimation of % degradation of PCO.
where Ac and As are the total area under the curve for control and bacteria-treated PCO.
Screening tests for detection of biosurfactant production
The production of biosurfactant by Bacillus sp. WD22 during degradation of PCO in sea water under nutrient supplementation was tested by emulsification index measurement, bacterial adhesion to hydrocarbons (BATH test) and oil clearance assay [17].
Equal volumes of CFS and PCO were vortexed vigorously for 15 min and allowed to stand for 24 h. The height of the emulsified layer and total height was measured, and emulsification index, as a measure of emulsification activity, was calculated as follows:
where He and Ht are the heights of emulsified layer and the total solution, respectively [18].
The ability of bacteria to adhere to PCO was determined by performing the BATH test. Bacillus sp. WD22 cells separated by centrifugation were taken and suspended in phosphate buffer pH 7.0, and optical density at 600 nm (OD600) was measured. The suspension (2.0 mL) was vortexed at high speed with PCO (100 μL) and allowed to stand for 1 h post which OD600 was measured again. The adherence of Bacillus sp. WD22 to PCO was calculated as follows:
where ODo and ODi are the optical densities after and before mixing of cells with PCO, respectively [19].
CFS (10 μL) was dropped to PCO (0.5 mL) spread over 20 mL of distilled water on a Petri plate. The clearance of PCO on addition of CFS depicts the presence of biosurfactant [14].
Extraction of biosurfactant produced by Bacillus sp. WD22
Biosurfactant produced by Bacillus sp. WD22 was extracted by the acid precipitation method. CFS was acidified to pH 2.0 and refrigerated for 16 h for precipitation of biosurfactant. CFS was then centrifuged at 8000 rpm for 20 min, and the pellet (precipitated biosurfactant) was dissolved in phosphate buffer pH. It was dialysed using a dialysis membrane with distilled water overnight to separate the salts of sea water [20]. Extraction was performed with chloroform/methanol mixture (2:1) as the solvent post which the organic phase was separated. On complete evaporation at 50 °C, a white powdery mass was left behind which was the dried biosurfactant. The weight was measured, and yield was calculated in both cases. Biosurfactants were then stored at − 20 °C until analysis.
Compositional and structural analysis of purified biosurfactants
The qualitative analysis of biosurfactants was performed by Anthrone test, Molisch test and ethanol emulsion test to detect the presence of proteins, carbohydrates and lipids. The quantification of proteins and lipids was then carried out by Lowry’s method [21] and modified phenol/sulphuric acid method [22], respectively.
The structure of biosurfactant produced in control medium and medium with yeast extract was analysed and compared by field emission scanning electron microscopy and energy dispersive X-ray (FESEM/EDAX) and liquid chromatography mass spectroscopy (LCMS). Dried biosurfactant were subjected to gold sputtering and fixed on to aluminium stubs. They were then visualized under different magnifications by FESEM (JEOLJSM-7800F). EDAX was performed to determine the elemental composition. In order to study the composition of biosurfactant by LCMS, 1 μg/ml of dried biosurfactant dissolved in acetonitrile was injected to LCMS (1260 Infinity LC fitted with 6410 Triple Quadruple MS, Agilent Technologies, USA). The analysis was performed in full scan positive mode from m/z 200 to 2000 with conditions.
Estimation of critical micelle concentration (CMC)
The measurement of surface tension of solutions of varying concentrations of purified biosurfactant was carried out by using a Sigma 70 Tensiometer (KSV Instruments Ltd., Finland) using the DuNouy method. The CMC of each of the biosurfactant grown in both media was determined by plotting a graph of surface tension and biosurfactant concentration.
Estimation of stability of purified biosurfactant produced by Bacillus sp. WD22
Aqueous solution (2.0 mL) of purified biosurfactant (250 mg/L) was maintained at different pH (2.0–12.0) and salinity conditions (2–12%) for 2 h. To analyse the stability under different temperatures, solutions were stored at 4 °C, 25 °C, 37 °C, 40 °C and 50 °C for 24 h, 60–80 °C for 2 h and 90–100 °C for 30 min. After incubation, the solutions were mixed with PCO (2 ml) by vortexing, and E24 index was calculated as explained earlier.
Emulsification activity of purified biosurfactants produced by Bacillus sp. WD22
Emulsification activity of purified biosurfactants produced by Bacillus sp. WD22 was tested against petroleum oils such as PCO, used engine oil (UEO), fresh motor oil (FMO) and vegetable oils such as sunflower, gingelly, palm, coconut and olive oil. Briefly, equal volumes of aqueous solutions of purified biosurfactant (250 mg/L) and oils were vortexed, and E24 index was calculated.
Bacillus sp. WD22 growth and biosurfactant production kinetics
Control medium and medium with yeast extract were prepared and sterilized as described earlier. Same inoculum and culture conditions were also maintained. The bacterial growth, biosurfactant yield, E24 index and glucose concentration were monitored at interval of 24 h up to 15 days. Sea water without the inoculum was maintained as the control sample. Biosurfactant yield, biomass concentration and E24 index were obtained as described earlier. The concentration of glucose was measured by DNSA method.
Logistic model was used to determine the kinetic parameters of growth of Bacillus sp. WD22:
where µmax, Xo and Xmax are the maximum specific growth rate (h−1), initial biomass concentration (g L−1) and maximum biomass concentration (g L−1); A = X/Xmax [14].
In order to study the biosurfactant production kinetics of Bacillus sp. WD22 in sea water supplemented with PCO and nutrients, the linear form of Leudeking and Piret model was employed.
where Pt and Po are the product concentrations at time t and 0, respectively, \(\alpha\) and \(\beta\) are the growth-associated and non-growth-associated constants, respectively, and
On the basis of values of α and β, type of biosurfactant production kinetics can be determined.
Statistical analysis
All experiments were performed in triplicates. The results obtained are expressed as mean ± standard deviation.
Results and discussion
Degradation of PCO by Bacillus sp. WD22 in seawater supplemented with nutrients
The pH of medium and temperature of incubation was adjusted to 8.1 and 27 °C as the average pH and subsurface temperature of Indian seawater ranges between 7.5–8.4 and 25–28 °C, respectively [3, 23]. The growth of Bacillus sp. WD22 was less in presence of yeast extract (3.73 ± 1.02 g/L) as compared to its absence (3.95 ± 2.38 g/L) after 15 days. The bacteria degraded 71.33% of C8 hydrocarbons in the control medium (Table 1). However, when yeast extract was added, the degradation reduced to 16.25%. In the absence of yeast extract, the bacteria utilized C8 hydrocarbons, whereas supplementation of yeast extract provided nutrients for its growth [24]. PCO degradation reduced by 47% on addition of ammonium chloride as the nitrogen source by a bacterial consortium in soil, while Bacillus subtilis, isolated from petroleum contaminated soil, degraded 65% of 0.3% v/v PCO at pH 7.0, NaCl 1.0% in 5 days [24, 25]
Screening tests for detection of biosurfactant activity
The emulsification of PCO by CFS was more in the control medium (E24 index = 42.67 ± 0.25%) than the medium with yeast extract (32.78 ± 0.16%). Similar patterns were observed for cell surface hydrophobicity (17.07 ± 0.56% and 26.02 ± 0.28%) as well as PCO clearance (Fig. S1). This outcome corroborates with the findings of PCO degradation in control and medium with yeast extract. Bacillus thuringiensis SH24 when grown in minimal salt medium supplemented with PCO showed an E24 index of 35 ± 1.75% against paraffin oil[26], while Bacillus subtilis B30 isolated from oil contaminated soil gave an E24 index of > 50% against crude oil [27].
Yield of biosurfactant
Post extraction of biosurfactant from CFS by acid precipitation technique, salt contamination of sea water was removed by dialysis. Biosurfactant yield was considerably low in case of medium with yeast extract (0.52 ± 0.106 g/L) than the control medium (0.76 ± 0.85 g/L). Nutrient deficiency in control medium could possibly have induced biosurfactant production to enhance PCO bioavailability. Bacillus stratosphericus strain FLU5 isolated from contaminated seawater produced 0.23 g/L of lipopeptide biosurfactant in presence of 1.0% v/v PCO as sole carbon source at 37 °C in 10 days [28].
Structural and compositional analysis of biosurfactant
The qualitative analysis of purified biosurfactants produced by Bacillus sp. WD22 contained proteins and lipids in both media. Biosurfactants was found to contain proteins and lipids in the weight ratio 1.84:98.16 and 1.08:98.92 in the presence and absence of yeast extract, respectively. Biosurfactant produced by Bacillus sp. isolated from green coffee grains contained 2.5%, 53% and 44% of carbohydrates, proteins and lipids, respectively [29].
On FESEM analysis of biosurfactants produced in both media, it was observed that there were no structural variations (Fig. 1). The surface of biosurfactants produced by Bacillus sp. WD22 was amorphous in nature. The structure of a biosurfactant produced by Bacillus aryabhattai was observed to be polymeric in nature [30]. The elemental analysis revealed the presence of carbon, oxygen in high amount, nitrogen and phosphorous in low amount confirming the biosurfactants as lipoprotein [14]. Trace amounts of chlorine, potassium, sodium, magnesium and sulphur in the elemental analysis are the elemental remnants from sea water (Fig. 2).
Biosurfactant produced by Bacillus sp. WD22 was characterized as a linear surfactin with a C14 β hydroxy fatty acid in both control medium and medium with yeast extract. Figures 3 and 4 depict the characteristic fragmentation pattern of [M + H] + ion m/z 1026 eluted out at retention time of 5.58 min and 5.23 min, respectively. The fragment at m/z 618.25 and m/z 611.16 are sodium adducts of protonated molecule attached to the peptide fragment Leu-Leu-Asp-Leu [31]. A biosurfactant secreted by Bacillus tequilensis MK 729,017, on exposure to crude oil, was also identified as a surfactin as per LCMS analysis [32].
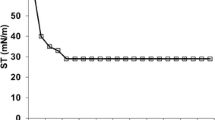
Estimation of CMC
The concentration of surfactant at which lowest surface tension is attained is referred as CMC. The surface tension of water was measured on addition of biosurfactant at various concentrations (5.0–100 mg/L). The ability of biosurfactant to reduce the surface tension of water depends on its purity and also the composition of medium in which it is produced [33]. The CMC for biosurfactant of Bacillus sp. WD22 was 50 mg/L and 60 mg/L for control and yeast extract media, respectively (Fig. 5). Advantage of biosurfactant with low CMC values is that less concentration of biosurfactant is sufficient to reduce the ST to its minimum value.
Stability analysis of purified biosurfactant
The stability of biosurfactant produced by Bacillus sp. WD22 were stable from pH 4.0 to 12.0 (Fig. 6A); however, on increase of NaCl concentration, the stability showed a gradual decrease (Fig. 6B). Biosurfactant produced by B. amyloliquefaciens SH20 was stable up to 15% NaCl after which the emulsification activity reduced [26]. When the temperature was increased beyond 60 °C, the stability of biosurfactant reduced drastically (Fig. 6C). Biosurfactant produced by B. safensis J2 was observed to be stable up to pH 7.0 and temperature 35 °C [34].
Emulsification activity of purified biosurfactant
The ability of biosurfactant to emulsify different hydrocarbon and vegetable oils was depicted in terms of E24 Index (Fig. S2 and S3). Biosurfactant could solubilize most of the oils with maximum emulsification of PCO and gingelly oil. Biosurfactant produced in control medium showed slightly better activity than that in medium with yeast extract. A lipopeptide biosurfactant produced by Bacillus licheniformis L20 emulsified various hydrocarbons and PCO [35].
Kinetics of bacterial growth and biosurfactant production
Bacillus sp. WD22 was grown in the presence of 1.0% PCO and 1.0 g/L glucose for 15 days in control medium and medium with yeast extract (Figs. 7 and 8). There was increased emulsification activity from the beginning of incubation period which gradually slowed down after 8 days in the control medium, whereas the reverse pattern was observed in the medium with yeast extract. Similar pattern was observed with biosurfactant production wherein increased production was observed from first day of incubation in control medium than the medium with yeast extract. This could be possibly due to nutrient limitations in control medium (no yeast extract) resulting in increased solubilization and subsequent uptake of PCO an energy source. In case of medium with yeast extract, the sudden increase in emulsification activity and biosurfactant production was observed after the 8th day. This could be due to depletion of glucose and yeast extract, and hence the bacteria start to produce biosurfactant to utilize PCO for maintenance. This can be corroborated from the observation that the bacteria degraded 71% and 16.25% of C8 hydrocarbons present in PCO in control medium and medium with yeast extract, respectively. Considerable variation was visualized in biosurfactant production, whereas no variation in the glucose utilization and biomass production patterns were noted.
The logistic model and Leudeking-Piret model were used to describe the kinetics of bacterial growth and biosurfactant production by Bacillus sp. WD22 (Figs. 9 and 10). The models successfully described the growth and biosurfactant production on analysing correlation of experimental concentration with model predicted concentration and R2 values (Fig. S4 and S5). The maximum specific growth rate was found to be more in medium with yeast extract than the control medium (Table 2), but the maximum biomass concentration was observed in medium with yeast extract. Biosurfactant production was found to be mixed growth associated in the control medium (α = 0.116 Ug/X and β = 0.0157 Ug/Xh), whereas in the presence of yeast extract, the production was found to be strictly growth associated (negligible β value = 0.0033 Ug/Xh).
Conclusions
Bacillus sp. WD22, previously isolated from refinery effluent, degraded C8 hydrocarbons of PCO in seawater with glucose supplementation, which was repressed on addition of yeast extract. The degradation was aided by a biosurfactant which was confirmed to be surfactin by FT-IR and LCMS analysis. This biosurfactant is capable of emulsifying petroleum-based and vegetable oils with good stability against tested conditions of pH, temperature and salinity. This biosurfactant could be extracted, purified and used for a wide range of environmental applications.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Akbari S, Abdurahman NH, Yunus RM et al (2018) Biosurfactants—a new frontier for social and environmental safety: a mini review. Biotechnol Res Innov 2:81–90. https://doi.org/10.1016/j.biori.2018.09.001
Al-Hawash AB, Dragh MA, Li S et al (2018) Principles of microbial degradation of petroleum hydrocarbons in the environment. Egypt J Aquat Res 44:71–76
Asitok AG, Antai SP, Ekpenyong MG (2017) Water soluble fraction of crude oil uncouples protease biosynthesis and activity in hydrocarbonoclastic bacteria; implications for natural attenuation. Int J Sci 3:1–17. https://doi.org/10.18483/ijsci.1344
Atmayudha A, Syauqi A, Purwanto WW (2021) Green logistics of crude oil transportation: a multi-objective optimization approach. Clean Logist Supply Chain 1:100002. https://doi.org/10.1016/J.CLSCN.2021.100002
Barakat KM, Hassan SWM, Darwesh OM (2017) Biosurfactant production by haloalkaliphilic Bacillus strains isolated from Red Sea Egypt. Egypt J Aquat Res 43:205–211. https://doi.org/10.1016/j.ejar.2017.09.001
Chettri B, Singh AK (2019) Kinetics of hydrocarbon degradation by a newly isolated heavy metal tolerant bacterium Novosphingobium panipatense P5:ABC. Bioresour Technol 294:122190. https://doi.org/10.1016/j.biortech.2019.122190
Cruz JM, Hughes C, Quilty B et al (2018) Agricultural feedstock supplemented with manganese for biosurfactant production by Bacillus subtilis. Waste Biomass Valoriz 9:613–618. https://doi.org/10.1007/s12649-017-0019-6
da Silva RCSF, de Almeida DG, Brasileiro PPF et al (2019) Production, formulation and cost estimation of a commercial biosurfactant. Biodegradation 30:191–201. https://doi.org/10.1007/s10532-018-9830-4
Das AJ, Kumar R (2019) Production of biosurfactant from agro-industrial waste by Bacillus safensis J2 and exploring its oil recovery efficiency and role in restoration of diesel contaminated soil. Environ Technol Innov 16:100450. https://doi.org/10.1016/J.ETI.2019.100450
Datta P, Tiwari P, Pandey LM (2018) Isolation and characterization of biosurfactant producing and oil degrading Bacillus subtilis MG495086 from formation water of Assam oil reservoir and its suitability for enhanced oil recovery. Bioresour Technol 270:439–448. https://doi.org/10.1016/j.biortech.2018.09.047
Goveas LC, Krishna A, Salian A et al (2020) Isolation and characterization of bacteria from refinery effluent for degradation of petroleum crude oil in seawater. J Pure Appl Microbiol 14:473–484. https://doi.org/10.22207/JPAM.14.1.49
Goveas LC, Sajankila SP (2020) Effect of yeast extract supplementation on halotolerant biosurfactant production kinetics coupled with degradation of petroleum crude oil by Acinetobacter baumannii OCB1 in marine environment. Bioresour Technol Rep 11:100447. https://doi.org/10.1016/j.biteb.2020.100447
Han Y, Nambi IM, Clement TP (2018) Environmental impacts of the Chennai oil spill accident – a case study. Sci Total Environ 626:795–806. https://doi.org/10.1016/j.scitotenv.2018.01.128
Hentati D, Chebbi A, Hadrich F et al (2019) Production, characterization and biotechnological potential of lipopeptide biosurfactants from a novel marine Bacillus stratosphericus strain FLU5. Ecotoxicol Environ Saf 167:441–449. https://doi.org/10.1016/J.ECOENV.2018.10.036
Heryani H, Putra MD (2017) Kinetic study and modeling of biosurfactant production using Bacillus sp. Electron J Biotechnol 27:49–54. https://doi.org/10.1016/j.ejbt.2017.03.005
Karlapudi AP, Venkateswarulu TC, Tammineedi J et al (2018) Role of biosurfactants in bioremediation of oil pollution-a review. Petroleum 4:241–249. https://doi.org/10.1016/j.petlm.2018.03.007
Kezrane I, Harouna BM, Hamadache M et al (2020) Use of hydrocarbons sludge as a substrate for the production of biosurfactants by Pseudomonas aeruginosa ATCC 27853. Environ Monit Assess 192:1–16. https://doi.org/10.1007/s10661-020-08269-3
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lyu Y, Zhang T, Dou B et al (2018) A lipopeptide biosurfactant from Bacillus sp. Lv13 and their combined effects on biodesulfurization of dibenzothiophene. RSC Adv 8:38787–38791. https://doi.org/10.1039/c8ra06693k
Marathe SK, Vashistht MA, Prashanth A et al (2018) Isolation, partial purification, biochemical characterization and detergent compatibility of alkaline protease produced by Bacillus subtilis, Alcaligenes faecalis and Pseudomonas aeruginosa obtained from sea water samples. J Genet Eng Biotechnol 16:39–46. https://doi.org/10.1016/j.jgeb.2017.10.001
Medić A, Lješević M, Inui H et al (2020) Efficient biodegradation of petroleum: N-alkanes and polycyclic aromatic hydrocarbons by polyextremophilic Pseudomonas aeruginosa san ai with multidegradative capacity. RSC Adv 10:14060–14070. https://doi.org/10.1039/c9ra10371f
Nouni MR, Jha P, Sarkhel R et al (2021) Alternative fuels for decarbonisation of road transport sector in India: options, present status, opportunities, and challenges. Fuel 305:121583. https://doi.org/10.1016/J.FUEL.2021.121583
Rao P, Pattabiraman TN (1989) Reevaluation of the phenol-sulfuric acid reaction for the estimation of hexoses and pentoses. Anal Biochem 181:18–22. https://doi.org/10.1016/0003-2697(89)90387-4
Sana S, Mazumder A, Datta S, Biswas D (2017) Towards the development of an effective in vivo wound healing agent from Bacillus sp. derived biosurfactant using Catla catla fish fat. RSC Adv 7:13668–13677. https://doi.org/10.1039/c6ra26904d
Satpute SK, Banpurkar AG, Dhakephalkar PK et al (2010) Methods for investigating biosurfactants and bioemulsifiers: a review. Crit Rev Biotechnol 30:127–144. https://doi.org/10.3109/07388550903427280
Singh AK, Sharma P (2020) Disinfectant-like activity of lipopeptide biosurfactant produced by Bacillus tequilensis strain SDS21. Colloids Surf B Biointerfaces 185:110514. https://doi.org/10.1016/J.COLSURFB.2019.110514
Ukhurebor KE, Athar H, Adetunji CO et al (2021) Environmental implications of petroleum spillages in the Niger Delta region of Nigeria: a review. J Environ Manage 293:112872. https://doi.org/10.1016/J.JENVMAN.2021.112872
Vandana, Priyadarshanee M, Mahto U, Das S (2022) Mechanism of toxicity and adverse health effects of environmental pollutants. In: Microb Biodegrad Bioremediation. pp 33–53. https://doi.org/10.1016/B978-0-323-85455-9.00024-2
Velázquez-Aradillas JC, Toribio-Jiménez J, del Carmen Ángeles González-Chávez M et al (2011) Characterisation of a biosurfactant produced by a Bacillus cereus strain tolerant to cadmium and isolated from green coffee grain. World J Microbiol Biotechnol 27:907–913.https://doi.org/10.1007/S11274-010-0533-1/FIGURES/6
Wu HC, Dissard D, Douville E et al (2018) (2018) Surface ocean pH variations since 1689 CE and recent ocean acidification in the tropical South Pacific. Nat Commun 91(9):1–13. https://doi.org/10.1038/s41467-018-04922-1
Yaraguppi DA, Bagewadi ZK, Muddapur UM, Mulla SI (2020) Response surface methodology-based optimization of biosurfactant production from isolated Bacillus aryabhattai strain ZDY2. J Pet Explor Prod Technol 10:2483–2498. https://doi.org/10.1007/s13202-020-00866-9
Datta P, Tiwari P, Pandey LM (2020) Oil washing proficiency of biosurfactant produced by isolated Bacillus tequilensis MK 729017 from Assam reservoir soil. J Pet Sci Eng 195:107612. https://doi.org/10.1016/j.petrol.2020.107612
Goveas LC, Selvaraj R, Vinayagam R, Alsaiari AA, Alharthi NS, Sajankila SP (2022) Nitrogen dependence of rhamnolipid mediated degradation of petroleum crude oil by indigenous Pseudomonas sp. WD23 in seawater. Chemosphere 304:135235. https://doi.org/10.1016/j.chemosphere.2022.135235
Das AJ, Kumar R (2019) Production of biosurfactant from agro-industrial waste by Bacillus safensis J2 and exploring its oil recovery efficiency and role in restoration of diesel contaminated soil. Environ Technol Innov 16:100450. https://doi.org/10.1016/j.eti.2019.100450
Liu Q, Niu J, Yu Y, Wang C, Lu S, Zhang S, Lv J, Peng B (2021) Production, characterization and application of biosurfactant produced by Bacillus licheniformis L20 for microbial enhanced oil recovery. J Clean Prod 307:127193. https://doi.org/10.1016/j.jclepro.2021.127193
Acknowledgements
LCG thanks the Vision Group of Science and Technology, Govt. of Karnataka (VGST-GoK) for funding this research work under RGS-F grant (GRD No 846/315).
Funding
This work is funded by the Vision Group of Science and Technology, Govt. of Karnataka (VGST-GoK) RGS-F grant (GRD No 846/315).
Author information
Authors and Affiliations
Contributions
Louella Concepta Goveas: Conceptualization, methodology, validation, investigation, writing—original draft, writing—review and editing. Shyama Prasad Sajankila: Methodology, supervision. Raja Selvaraj: Validation, supervision.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Luiz Henrique Rosa
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Goveas, L.C., Selvaraj, R. & Sajankila, S.P. Characterization of biosurfactant produced in response to petroleum crude oil stress by Bacillus sp. WD22 in marine environment. Braz J Microbiol 53, 2015–2025 (2022). https://doi.org/10.1007/s42770-022-00811-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-022-00811-4