Abstract
Previous studies carried out in our laboratory described the antimicrobial activity of the whole hexanic extract (HE) of Achyrocline satureioides (Lam.) DC against Paenibacillus larvae, the causal agent of American Foulbrood (AFB) a disease of the honey bee larvae. In this study, the HE was partitioned into five main fractions by chromatographic techniques leading to the isolation of four known compounds: two prenylated phloroglucinol α-pyrones (1 and 3), 5,7-dihydroxy-3,8-dimethoxyflavone (gnaphaliin A) (2), and 23-methyl-6-O-demethylauricepyrone (4). Isolated compounds were further analyzed towards structural elucidation using 1H RMN and 13C RMN spectroscopic techniques. For the first time, the antimicrobial activity of the isolated compounds was evaluated against P. larvae strains by broth microdilution method and compared with that of the whole HE. Compounds 1–4 displayed minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) values ranging between 0.07 and 62.5 μg/mL and 0.26 and 12.5 μg/mL, respectively. The lowest MIC and MBC values were obtained with compounds 3 and 4, respectively. The antimicrobial activity of each single compound and the combination of them showed that the presence of all compounds is needed for the antimicrobial efficacy of whole HE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Honeybees play a vital role in the pollination of crops, fruit, and wild flowers. Thus, factors affecting bee health also affect a sustainable and profitable agriculture as well as many non-agricultural ecosystems.
Honeybees are affected by numerous pathogens like viruses, bacteria, fungi, and parasites, which represent a significant threat to bee health. The Gram-positive, spore-forming bacterium, Paenibacillus larvae (P. larvae), is the causative agent of American foulbrood (AFB), the most severe bacterial disease that affects larvae of the honeybee Apis mellifera. AFB presents worldwide distribution, causing a significant decrease in honeybee populations and production of honey, pollen, propolis, royal jelly, and beeswax [1, 2].
AFB is a notifiable disease in many countries, and its treatment is often regulated by law. AFB control measures include the burning destruction of infected hives or the use of antibiotics for the treatment of the disease, although it is banned in most European countries, limiting the international marketing. In Argentina, since 2016, the Servicio Nacional de Sanidad y Calidad Agroalimentaria (SENASA) has forbidden veterinary products used in beekeeping made on the base of the active principle oxytetracycline, used for the treatment of AFB [3]. The fact that antibiotics can mitigate, but not eliminate the disease provokes infected hives to be treated constantly to prevent a foulbrood outbreak [4,5,6]. However, antibiotics are only effective on the rod cell but not on the spore, which can persist in the hives for years [7]. In addition, chemical residues can persist in honey affecting its quality and safety for human consumption. All these factors contribute to increase the antimicrobial resistance to antibiotics.
As a consequence, the requirement for a reduction in the use of antibiotics in food prompts a need to develop safer active compounds. In recent years, many researchers have been investigating new strategies that can be alternatives to the use of antibiotics. The use of natural substances (i.e., plant extracts and essential oils or their compounds) in apiculture offers an interesting alternative against synthetic antibiotics. Plant extracts are of particular interest because they are relatively safe, widely accepted by consumers, and can be exploited for multiple uses. Taking into consideration the large potential of plants as sources for antimicrobial compounds, many systematic investigations were carried out and screened in different parts of the world [8,9,10,11,12].
South America has historically been a notable reservoir of plant resources. Many of them have been evaluated for their biological activities, including for the control of diseases in honeybees. In this way, essential oils [13,14,15,16] and propolis [17] have been tested for the control of P. larvae, and several plant extracts showed antibacterial activity against this pathogen also [10, 18, 19].
It has been shown that extracts from Achyrocline satureioides (Lam.) DC have antimicrobial activity against Gram-positive and Gram-negative bacteria [20]. This plant belongs to the Asteraceae family, and it is known as “Marcela” in folk medicine; it is a native plant from the southeastern region of South America and grows in sandy or stony soils, on hilly or plain terrain. It is common in Brazil, Uruguay, Venezuela, Colombia, Bolivia, Paraguay, Peru, and also central and northeastern of Argentina [21,22,23]. In this last country, it mostly grows in humid areas and sandy soils, such as the mountains of Córdoba, San Luis, and Tandil and on coastal dunes of the Province of Buenos Aires [24].
The extracts of A. satureioides are characterized by their high content of polyphenols and flavonoids [25, 26]. Numerous authors found that the yield of the dry extract and the content of flavonoids vary according to the extraction method used. On the other hand, pharmacological activities of A. satureioides have been the subject of numerous studies in the past few years [25]. The large volume of related literature and the positive results reported by most authors reflect the undoubted potential of this plant as a natural medicine. Also, toxicological studies of A. satureioides aqueous extracts, performed in mice and rats, demonstrated low acute toxicity when it was administered intraperitoneally and no toxicity was observed after oral administration [27].
In a previous study, we determined that extracts from A. satureioides presented an antagonistic effect on the growth of P. larvae strains. Four different extracts were obtained by liquid–liquid extraction from an aqueous-ethyl alcohol macerate of the aerial parts of the plant. The crude hexanic extract (HE) showed the highest anti-P. larvae activity [28, 29]. The biological activity of the HE against the growth of P. larvae strains tested in our work was comparable with those informed by other authors for different natural products and for the antibiotic oxytetracycline [30,31,32].
The aim of the present study was to isolate and identify by 1H NMR and 13C RMN spectroscopic techniques the compounds present in the HE of A. satureioides and test, for the first time, their antibacterial activity against P. larvae, comparing them with the inhibitory activity of the whole HE.
Material and methods
All reagents and solvents were of analytical grade and used without further purification. All experiments were carried out with ultrapure water from LABCONCO equipment model 90,901–01 (HPLC grade water).
Cleaning and drying of plant material
The aerial parts of A. satureioides were collected from Santa Mónica, Córdoba, Argentina (32° 5′ 26.5″ S, 64° 37′ 1.9″ W) during the flowering season. A voucher specimen was stored at the Herbarium of the Faculty of Agronomy and Veterinary, Universidad Nacional de Río Cuarto, as file herbarium 4658. Leaves, fine stems, and flowers were collected, cleaned, and dried. Dried specimens were stored until they were used for extraction.
Extract preparation
The air-dried powdered plant material (200 g) was extracted by maceration with an ethanol–water (1:1) mixture at room temperature. The extracting mixture was renewed until the material was exhausted (until it came out colorless). This macerate was filtered with Whatman Nº 2 paper obtaining the hydro-alcoholic extract (HAE). The HAE was concentrated under reduced pressure in a rotary evaporator, and the aqueous solution obtained (approximately 2 L) was submitted to liquid–liquid extraction with hexane. Then, the HE was concentrated to dryness in a rotary evaporator under reduced pressure and was kept in dark flasks at 4 °C until they were used.
Phytochemical screening of HE
Preliminary phytochemical screening tests of the dry HE were carried out to identify useful constituents by standard methods [33], testing for alkaloids, tannins, flavonoids, anthraquinones, steroids, and triterpenoids.
Test for alkaloids
A small amount (0.09 g) of the HE was dissolved in 15 mL of 5% HCl and kept in a water bath at 60 °C for about 2 min. The extract was then filtered off and allowed to cool, and then 1 mL of the extract was separated in two test tubes. Few drops of Dragendorff’s reagent (potassium iodide-bismuth nitrate) were added in the tube and appearance of orange-red precipitate was taken as positive. Few drops of Mayer’s reagent (composed of mercuric chloride and potassium iodide dissolved in distilled water) were added to the second tube and appearance of buff-colored precipitate designates the existence of alkaloids.
Test for steroids and triterpenoids
The HE (0.09 g) was extracted with two 15 mL portions of petroleum ether. The extract was filtered, and evaporated in a water-bath, up to 5 mL. Then, 10 mL of water–methanol (10:90) was added and stirred. The mixture was allowed to completely separate into two layers. The ether layer was separated by two-dimensional TLC on silica gel G eluting first with cyclohexane-ethyl acetate (95:5) and then, with petroleum ether—ethyl ether—acetic acid (80:20:1) and sprayed with Liebermann-Burchard reagent. Then, it was heated at 105 °C for 10–15 min. The residue was extracted with two 10 mL portions of ethanol–water (1:7) at 60 °C, and it was filtered. The filtrate (called solution 1 hereafter) was used for the determination of flavonoids, anthraquinones, and tannins.
Test for flavonoids
One milliliter of solution 1 was put into two clean test tubes. One test tube was reserved as a control. Concentrated HCl (0.5 mL) was added to the other test tube followed by addition of magnesium turnings. The turning to a red color confirmed the presence of flavonoids.
Test for tannins
One milliliter of the solution 1 was added to a 1 mL of solution of gelatin containing 10% NaCl. The mixture was centrifuged at 2000 rpm, the residue was dissolved in 2 mL of urea solution 10 M, and few drops of 5% ferric chloride were added. The occurrence of a blackish blue color showed the presence of gallic tannins and a green-blackish color indicated presence of catechol tannins [34].
Test for anthraquinones
Five milliliters of solution 1 was placed in a dry test tube. One milliliter of hydrogen peroxide (20 volumes) and 1 mL of H2SO4 (5%) were then added. This mixture was heated in a steam bath for 15 min. It was then cooled and extracted with 3 mL of benzene and stirred with 1 mL of 5% NaOH containing 2% NH4OH. A pink-red color in the ammoniacal (lower) layer showed the presence of anthracene derivatives.
Hexanic extract fractioning
Column chromatography (CC)
The HE was subjected to silica gel CC for the isolation of the active principles. An appropriate column of 5-cm diameter and 50-cm length was used. A small plug of fiberglass was placed at the bottom of column helped with a glass rod. The column was packed with 30 gr of Silica gel (60 Å pore size, 35–75-μm diameters, Sigma, USA). It was washed with water, rinsed with acetone, and then completely dried. A mixture of n-hexane–acetone (8:2, v/v) was used as mobile phase. A 3-mm layer of sand was carefully placed on the flat top of the dry silica gel bed, and the column was clamped for pressure packing and elution. Then, the HE was seeded on top of the silica bed, the mobile phase poured carefully in, and N2 gas pressure of 50 psi was applied. The knob at the bottom was slowly opened to release the solvent. One hundred fractions of 2 mL each were separately collected and subjected to thin-layer chromatography (TLC) using a mixture of n-hexane:acetone (8:2 v/v) as mobile phase. The content of those flasks showing a TLC band with the same retardation factor (Rf) value were gathered in a single flask and the solvent evaporated by using a rotary evaporator. The number of flasks was reduced from one hundred to five. These purified fractions were kept in a dark flask at 4 °C until they were used for further analysis on thin layer or radial centrifugal chromatography.
TLC
TLC analysis of the HE of A. satureioides was performed with silica gel on PET foils (20 × 20 cm of 0.25-mm thick) with fluorescent indicator at 254 nm (Fluka, Germany). Different mixtures of n-hexane and ethyl acetate were used as mobile phase (see below). The chromatographic plates were revealed with UV light at 365 nm and 254 nm with anisaldehyde solutions sprayed onto them, heating the plates at 120 °C.
Centrifugal thin-layer chromatography
A Harrison Research Chromatotron (model 7924 T) was used in this experiment. The rotor stationary phase was made of silica gel 60 F256 with calcium sulfate hemihydrate (Merck) with an average particle size of 15 μm, a thickness of 2 mm, and a diameter of 24 cm. The rotation rate was 800 rpm. Solvent was delivered from a reservoir at a rate of 1 mL/min to 10 mL/min.
Identification of compound via spectroscopic techniques
The 1H RMN and 13C RMN spectra of isolated compound were recorded in Bruker Avance 400 at 400 and 100 MHz, respectively, in CDCl3, with TMS as the internal reference. The compounds were identified by comparison with published spectroscopic data.
Microbial strains
Two strains of P. larvae were used in this study: P larvae Tipica (Pl T) and P. larvae strain 9 (Pl 9). The first one belonged to the Centro de Investigación en Abejas Sociales de la Universidad Nacional de Mar del Plata (CIAS-UNMDP), and Pl 9 was isolated from infected colonies from Rio Cuarto region. It was biochemically and molecularly identified using previously described techniques [35]. There were grown on MYPGP agar (Mueller–Hinton broth, yeast extract, potassium phosphate, glucose, and sodium pyruvate), where P. larvae colonies were small, regular, mostly rough, flat or rose, and whitish to beige colored. They were Gram-positive rod, catalase negative; they could hydrolyze gelatin and casein, but not starch. The identification and molecular confirmation of Pl 9 strain were carried out using the rRNA 16S gene. The data was incorporated to GenBank’s database, which confirmed the identity of the microorganism [29]. The P. larvae strains were kept at − 20 °C on MYPGP agar with 20% v/v of glycerol until used.
Minimum inhibitory concentration (MIC) determination
The antimicrobial activity of the HE and the isolated compounds against P. larvae strains was determined by a broth microdilution method [36,37,38]. Colonies grown for 48 h in a MYPGP agar plate were suspended in sterile peptone water until reaching a final optical density at 600 nm of 0.1.
Preparation of HE/compounds dilutions: 6 mg of HE/compounds was dissolved in 15% dimethyl sulfoxide (DMSO), which was previously determined as the non-toxic concentration of solvent for the growth of P. larvae. HE dilutions were prepared at a concentration range of 0.048 to 600 µg/mL. In a microplate, 100 μL of brain heart infusion was placed in each well, then 100 μL of HE was added to well 1, and serial dilutions were performed until well 11. Finally, 50 μL of the previously prepared inoculum were added to each well, except for the negative control. The microplate was incubated in microaerophilia at 37 °C for 48 h, and then 10 μL of resazurin (0.01%) was added to each well. It was incubated again during 1:30 h at the same conditions. MIC was defined as the lowest concentration of compounds that inhibited visible growth indicated by a blue color and bacterial growth was shown by a pink color; DMSO at 15% was used as the negative control (blue color). The assays were repeated three times.
Minimum bactericidal concentration (MBC) determination
For the minimum bactericidal concentration (MBC) determination, 100 µL of the MIC dilution and the previous ones were inoculated in MYPGP agar and incubated in microaerophilia at 37 °C for 48 h. MBC was considered as the last dilution without cell growth [39, 40].
Determination of associations between compounds
Briefly, 100 µl of dilutions of compound “A” was distributed in the rows of a microplate and 100 µl of compound “B” was placed in the columns. Then, different combinations were made between isolated compounds so as to reach different combinations of them [41]. Then, 50 µl of the inoculum was added to each well (optical density at 600 nm: 0.8–0.12); microplates were incubated at 37 °C for 24 h and revealed with rezasurin. The interactions were stablished using the fractional inhibitory concentration index (FICI) with the formula: FICI = (MIC Ab/MIC A) + (MIC Ba/MIC B), where MIC A and MIC B were the minimum inhibitory concentrations of compounds “A” and “B” and MIC Ab and MIC Ba were the minimum inhibitory concentration of A or B at a defined concentration of B or A, respectively. Synergistic effect was observed when FICI value < 1; an additive effect when FICI value = 1; an indifferent effect when 1 < FICI value ≤ 2 and an antagonistic effect when FICI value > 2.
Results and discussion
HE yields and chemical compositions
The yields of HE from A. satureioides were expressed as percentage (w/w) of original dry vegetal and varied between 0.5 and 0.75%. Phytochemical tests on samples of HE for the identification of bioactive constituents were carried out using the standard phytochemical procedures described in experimental section. The results confirmed the presence of tannins and flavonoids in the HE, which was in agreement with preliminary studies carried out in our laboratory [42].The presence of anthraquinones and terpenoids were also observed, while alkaloids were not found. The results are shown in Table 1.
For the testing of the biological activity of natural products and for the structure elucidation of potential bioactive compounds, very small quantities of the substances in extreme pure stage are necessary. Therefore, isolation of pure compounds from their extracts is one of the most important requirements in natural product research.
The compounds present in the HE were investigated in order to establish the chemical profile potentially responsible for the antimicrobial activity. Firstly, the HE was fractionated by CC on silica gel and the collected fractions were analyzed by TLC as previously described in the experimental section. Eluted samples showing the same Rf values were put together, resulting in five main fractions (F1 to F5) (Table 2). The obtained fractions were dried under vacuum using a rotary evaporator preparing them for a subsequent purification. The F1 and F5 fractions were purified by preparative TLC, F2 by radial centrifugal chromatography and F3 and F4 by successive column chromatography (Table 2).
Several mobile phases with varying solvent ratios were tested for F1 and F5 (data not shown here). The TLC plates were developed using a mixture 15% n-hexane:ethyl acetate as the mobile phase for F1. Separation of F5 components was achieved by two successive TLCs using the same solvent system but with different proportions: 25% and 30%, respectively. In the semi-preparative TLC plates, two spots were collected from F1 and F5. Each of the desired bands was scraped off the backing material. The isolated compounds were placed in tubes with acetone, left standing for 1 h, filtered and stored in dry and previously weighed dark glass containers until further characterization by spectroscopic techniques.
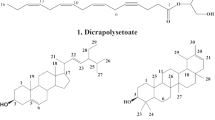
Two main compounds were separated from F1 and F5, hereinafter referred to as compound 1 and 2, respectively (Fig. 1). Comparing the 1H NMR and 13C NMR data (see Table 3) with Refs. [20, 43], the obtained products were identified as (S)-3-[{5,7-dihydroxy-2,2-dimethyl-8-(2-methylbutanoyl)-2H-chromen-6-yl}methyl]-6-ethyl-4-hydroxy-5-methyl-2H-pyran-2-one, (1) and 5,7-dihydroxy-3,8-dimethoxyflavone (gnaphaliin A) (2).
F2 was further purified by radial centrifugal chromatography. The chromatotron was first fitted with a 1-mm silica gel plate, and 50 mL of n-hexane was placed in the solvent reservoir. Once the plate was equilibrated, the F2 was loaded onto the plate via the reservoir inlet. The purification step was carried out using a mixture of n-hexane and ethyl acetate in a gradient as mobile phase. The eluents were collected in series of one hundred forty fractions for each solvent system. After TLC analysis (using a mixture of n-hexane and ethyl acetate (8:2 v/v) as the mobile phase), fractions of each solvent system with similar Rf value were combined as it was assumed that the same compound was present in each collection. Therefore, the number of fractions was reduced to eleven and all were analyzed by spectroscopic techniques. The results showed that the main compound of these fractions was 1, described above.
Reverse phase column chromatography was applied for the isolation of compounds from 100 mg of a mixture of F3 and F4. The purification step was carried out using a gradient mixture of n-hexane and ethyl acetate as the eluent. Collected fractions (two hundred fractions) were monitored by TLC using a mixture of n-hexane and ethyl acetate (4:1, v/v) as mobile phase. Fractions with similar Rf value were combined as it was assumed that the same compound was present in each collection. Therefore, the number of fractions was reduced to ten and all were analyzed by spectroscopic techniques. The results showed that four compounds were isolated. Two of them were the compounds 1 and 2, described above. Comparing the 1H NMR and 13C NMR data (see Table 3) with Refs. [12, 20], the third compound was identified as another prenylated phloroglucinol α-pyrones, 3-[{4,6-dihydroxy-7-(2-(S)-methylbutanoyl)-2-(prop-1-en-2-yl)-2,3-dihydrobenzofuran-5-yl}methyl]-6-ethyl-4-hydroxy-5-methyl-2H-pyran-2-one and the fourth was the compound known as 23-methyl-6-O-desmethylauricepyrone hereinafter referred to as compound 3 and 4, respectively (Fig. 1).
At the end of the purification process, the amount of the isolated compounds was: 1 (4.1% yield based on whole HE), 2 (3.0%), 3 (0.3%) and 4 (2.5%).
It is important to clear out that different composition and concentrations would be obtained if the plants were collected from a different place. Nowadays, it is known that variability in both parameters could be observed due to the recollection place, the weather conditions, the part of the plant, or the presence of a plague. In addition, the harvesting time, how to keep it dry, the age of the plant, and the extraction method also influence the quality and quantity of the compounds. However, in this study, it was observed that the chemical composition of A. satureioides did not show significant differences with the composition of hexane extracts of the same plant collected from other geographical area or collection period reported by other authors. This could be relevant if a different hexane extraction method is applied [20, 29]. These results would indicate that A. satureioides has a relatively stable chemical composition which is essential from the point of view of its application as an antibacterial agent or for any other use.
Evaluation of antibacterial activity of isolated compounds and HE
The antimicrobial activity of extracts obtained from A. satureioides with solvents of different polarities against P. larvae growth was previously studied, HE being the most active [29]. In order to identify the metabolites responsible for the antibacterial activity against P. larvae described for HE, the antibacterial activity of compounds 1–4 was assayed. The results are summarized in Table 4, and the antibacterial activity of HE was included for comparative purposes.
It was observed that all isolated compounds and the whole HE were active against the growth of P. larvae strains except for compound 2 that did not show activity against Pl T, within the range of concentrations studied. The evaluation of the antimicrobial activity of the compounds, 1 to 4, displayed MIC and MBC values ranging from 0.07 to 62.5 μg/mL and 0.26 to 12.5 μg/mL, respectively. The best MIC was obtained with compound 3 followed by 4 and the HE. The lowest anti-P larvae activity was demonstrated for compound 2, chemically recognized as a flavonoid. This result was consistent with other authors who observed that flavonoids isolated from different plant extracts presented lower antimicrobial activity against P. larvae compared to other chemical compounds [19]. Compounds 3 and 4 also showed the strongest bactericidal effect on PI 9, with MBCs of 0.50 and 0.26 μg/mL, respectively. The whole HE had previously showed a MIC of 0.4 μg/mL and 2.34 μg/mL for Pl 9 and Pl T, respectively, which was in the range obtained for the isolated compounds, but it was only observed bactericidal effect of it on Pl T.
The antimicrobial activity of a phytochemical has been defined as significant when MIC is below 10 μg/mL, moderate when 10 μg/mL < MIC < 100 μg/mL or low when MIC > 100 μg/mL [44]. Therefore, the results showed that both the isolated compounds and HE significantly inhibited the growth of P. larvae strains, with MIC values below 10 μg/mL, in most cases. All values were comparable or less than those informed by other authors for different natural products against P. larvae [18, 19].
It is important to emphasize that compounds 1, 3, and 4 isolated in this work match with compounds numbered 1, 2, and 5 previously reported by Casero et al. [20], which also demonstrated antimicrobial activity against clinically significant bacteria, reporting better activity on Gram-positive bacteria. Similar results were obtained in our work considering that P. larvae is Gram-positive bacteria.
The results presented here showed that all the isolated compounds and the whole HE had very effective bioactivity and could be considered to replace the use of traditional antibiotics for the treatment against AFB in bees. This is more promising taking into consideration that more recent studies by our research group have shown that the HE from this plant is not toxic to larvae and bees at the MIC concentrations reported in this work [45].
Associations between compounds
Above was described the antimicrobial activity of the EH and four isolated compounds of A. satureioides against two P. larvae strains. The main interest in the isolation and identification of single compounds of a HE is based on the need to identify the degree of biological activity each one has in the whole HE so that this one can be replaced by an individual agent or a mix. This implies that interactions between isolated compounds should be done in order to asses if these compounds have better or worse activity than the whole extract. Many times, it has been reported that the antimicrobial activity of the whole vegetal fraction was more effective and had a wider range than the individual activity of isolated compounds [46, 47].
Therefore, different combinations between isolated compounds were performed in order to evaluate the antimicrobial interactions: synergisms, additivity, antagonisms, and indifference. In all combinations, between compounds 1, 2, and 3, a synergistic effect was observed for both strains (FICI values < 1), except for the 1/3 mixture for Pl 9, which showed an additive effect (FICI value = 1). No antagonism was seen in any of the experiments. The remaining combinations of the other interactions showed inconclusive results.
The results obtained with the combinations 1/3 were used to build isobolograms. Plotting the MIC value of one compound of a combination on the x-axis and the other on the y-axis resulted in the isobolograms shown in Fig. 2. The line that connects the two points on the graph shows all the combinations that would theoretically result in additivity, those that are below the line would correspond to a synergistic effect and those that are above it to an antagonistic effect [48].
The MIC values of 1/3 combinations on Pl 9 strains were located on the straight line showing the additive effect of both compounds while for Pl T the three combinations were below the lines, indicating a synergistic effect (Fig. 2).
It could be noticed in this research work that the concentrations needed to reach inhibition with each single compound was higher than the effective concentration of the whole HE, supporting the idea of a better activity of a complex mixture of compounds. Similar statements were given by many authors who studied the antimicrobial interactions between different essential oils, or by the terpene constituents of them describing the whole extract as a more effective inhibitor than single compounds [46,47,48,49]
Conclusions
The HE of A. satureioides constitutes an alternative as a new source of antibacterial compounds for the treatment of AFB. This research helped in identifying, for the first time, the active principles of the HE responsible for the antibacterial activity against P. larvae, the causal agent of AFB disease in honey bee colonies. Though (S)-3-[{5,7-dihydroxy-2,2-dimethyl-8-(2-methylbutanoyl)-2H-chromen-6-yl}methyl]-6-ethyl-4-hydroxy-5-methyl-2H-pyran-2-one (1), gnaphaliin A (2), 3-[{4,6-Dihydroxy-7-(2-(S)-methylbutanoyl)-2-(prop-1-en-2-yl)-2,3-dihydrobenzofuran-5-yl}methyl]-6-ethyl-4-hydroxy-5-methyl-2H-pyran-2-one (3) and 23-methyl-6-O-desmethylauricepyrone (4) were identified as the major chemical compounds.
The results showed that both the isolated compounds and whole HE significantly inhibited the growth of P. larvae strains, with MIC values below 10 μg/mL, in most cases.
In addition, different combinations between compounds 1 and 4 were tested, showing, in most cases, a synergistic effect for both P. larvae strains (FICI values < 1). However, the complete HE resulted more effective than the combinations studied and the individual compounds, except for compounds 3 and 4 towards Pl 9.
To conclude, these products represent a potential alternative to replace traditional antibiotics and reduce residues in honey. However, the efficacies of these agents need to be further evaluated under field conditions in beehives.
Data availability
Not applicable.
References
Genersh E (2010) American foulbrood in honeybees and its causative agent. Paenibacillus larvae J Invertebr Pathol 103:S10–S19
Lindström A, Fries I (2015) Sampling of adult bees for detection of American foulbrood (Paenibacillus larvae subsp. larvae) spores in honey bee (Apis mellifera) colonies. J Apic Res 44(2):82–86
Se recuerda que no está permitido el uso de antibióticos en las colmenas. Available from: www.senasa.gob.ar. Accessed July 3, 2021.
Evans JD (2003) Diverse origins of tetracycline resistance in the honey bee bacterial pathogen. Paenibacillus larvae J Invertebr Pathol 83:46–50
Alippi AM, López AC, Reynaldi FJ, Grasso DH, Aguilar OM (2007) Evidence for plasmidmediated tetracycline resistance in Paenibacillus larvae, the causal agent of American Foulbrood (AFB) disease in honeybees. Vet Microbiol 125:290–303
Reybroeck W, Daeseleire E, De Brabander HF, Herman L (2012) Antimicrobials in beekeeping. Vet Microbiol 158:1–11
López Uribe, M, Underwood, R (2019) Honey bee diseases: American Foulbrood. College of Agricultural Sciences. The Pennsylvania State University 323 Agricultural Administration Building University Park, PA 16802.
Nascimento GGF, Locatelli J, Freitas PC, Silva GL (2000) Antibacterial activity of plant extracts and phytochemicals on antibiotic resistant bacteria. Braz J Microbiol 31:247–256
Ghosh A, Kanti Das B, Roy A, Mandal B, Chandra G (2008) Antibacterial activity of some medicinal plant extracts. J Nat Med 62:259–262
Flesar J, Havlik J, Kloucek P, Rada V, Titera D, Bednar M, Stropnicky M, Kokoska L (2010) In vitro growth-inhibitory effect of plant-derived extracts and compounds against Paenibacillus larvae and their acute oral toxicity to adult honey bees. Vet Microbiol 145:129–133
Damiani N, Gende LB, Maggi MD, Palacios S, Marcangeli JA, Eguaras MJ (2011) Repellent and acaricidal effects of botanical extracts on Varroa destructor. Parasitol Res 108:79–86
Joray M, del Rollán M, Ruiz G, Palacios S, Carpinella M (2011) Antibacterial activity of extracts from plants of Central Argentina—isolation of an active principle from Achyrocline satureioides. Planta Med 77(01):95–100
Albo GN, Henning C, Ringuelet JA, Reynaldi FJ, De Giusti MR, Alippi AM (2003) Evaluation of some essential oils for the control and prevention of American Foulbrood disease in honey bees. Apidologie 34:417–437
Fuselli SR, Garcia de la Rosa S, Gende LB, Eguaras MJ, Fritz R (2006) Antimicrobial activity of some Argentinean wild plant essential oils against Paenibacillus larvae, causal agent of American Foulbrood (AFB). J Apic Res 45:2–7
Gende LB, Bailac P, Maggi MD, Ponzi M, Fritz R, Eguaras MJ (2009) Antimicrobial activity of Pimpinella anisum and Foeniculum vulgare essential oils against Paenibacillus larvae. J Essent Oil Res 21:91–93
Santos RCV, Alves dos Santos CF, Schneider T, Lopes LQS, Aurich C, Giongo JL, Brandelli A, de Almeida Vaucher R (2012) Antimicrobial activity of Amazonian oils against Paenibacillus species. J Invertebr Pathol 109:265–268
Antúnez K, Harriet J, Gende L, Maggi M, Eguaras M, Zunino P (2008) Efficacy of natural propolis extract in the control of American Foulbrood. Vet Microbiol 131:324–331
Mărghitaş L, Dezmirean D, Chirilă F, Fiţ N, Bobiş O (2011) Antibacterial activity of different plant extracts and phenolic phytochemicals tested on Paenibacillus Larvae Bacteria. J Anim Sci Biotechno 44(2):94–99
Isidorov VA, Buczek K, Segiet A, Zambrowski G, Swiecicka I (2018) Activity of selected plant extracts against honey bee pathogen Paenibacillus larvae. Apidologie 49:687–704
Casero C, Machín F, Méndez-Álvarez S, Demo M, Ravelo AG, Pérez-Hernández N, Joseph-Nathan P, Estévez-Braun A (2015) Structure and antimicrobial activity of phloroglucinol derivatives from Achyrocline satureioides. J Nat Prod 78(1):93–102
Lorenzo D, Tai-Seraffini L, Santos AC, Frizzo CD (2000) Achyrocline satureioides essential oils from Southern Brazil and Uruguay. Planta Med 66:476–477
De Souza KCB, Bassani VL, Schapoval EES (2007) Influence of excipients and technological process on anti-inflammatory activity of quercetin and Achyrocline satureioides (Lam.) D.C. extracts by oral route. Phytomedicine 14:102–108
. Davies, Ph, Villamil, JJ (2004) Estudios en domesticación y cultivo de especies medicinales y aromáticas nativas. Serie FPTA-INIA Nº 11.
Giangulani RN (1976) Las especies argentinas del género Achyrocline (Compositae). Darwiniana 20:549–576
Retta D, Dellacassa E, Villamil J, Suárez SA, Bandoni AL (2012) Marcela, a promising medicinal and aromatic plant from Latin America: A review Ind. Crop Prod 38:27–38
Garcia D, Furlan MR, Diamante MS, Minatel IO, Borges CV, Wu Y, Pereira Lima GP, Ming LC (2019) Promising phytochemical responses of Achyrocline satureioides (Lam.) DC. under various farming conditions Ind. Crop Prod 129:440–447
Rivera F, Gervaz E, Sere C, Dajas FJ (2014) Toxicological studies of the aqueous extract from Achyrocline satureioides (Lam.) DC (Marcela). J Ethnopharmacol 95:359–362
González MJ, Marioli JM (2010) Antibacterial activity of water extracts and essential oils of various aromatic plants against Paenibacillus larvae, the causative agent of American Foulbrood. J Invertebr Pathol 104:209–213
González MJ, Beoletto VG, Agnese AM, Audisio M, Marioli JM (2015) Purification of substances from Achyrocline satureioides with inhibitory activity against Paenibacillus larvae, the Causal Agent of American Foulbrood in Honeybees’Larvae. Appl Biochem Biotechnol 175:3349–3359
Gende LB, Floris I, Fritz R, Eguaras MJ (2008) Antimicrobial activity of cinnamon (Cinnamonum zeylanicum) essential oil and its main components against Paenibacillus larvae from Argentina. Bull Insectology 61(1):1–4
Gende LB, Fernández N, Buffa F, Ruiu L, Satta A, Fritz R, Eguaras MJ, Floris I (2010) Susceptibility of Paenibacillus larvae isolates to a tetracycline hydrochloride and cinnamon (Cinnamomum zeylanicum) essential oil mixture. Bull Insectology 63:247–250
Reynaldi FJ, Rule R, Arauz S, Alippi AM (2010) Sensibilidad in vitro de Paenibacillus larvae frente a los antibióticos oxitetraciclina, tilosina, tilmicosina y lincomicina. Analecta Vet 30:25–29
Egbuna, C, Ifemeje, JC, Ma duako, MC, Tijjani, H, Udedi, SC, Nwaka, AC, Ifemeje, MO (2019), in Phytochemistry: Fundamentals, Modern Techniques, and Applications, vol 1: Phytochemical test methods: Qualitative, Quantitative and Proximate Analysis (Egbuna, C., Ifemeje, J.C., Udedi, S.C., Kumar, S., ed), Apple Academic Press, Canada, pp 381–426.
Iyengar MA (1995) Study of Crude Drugs, 8th edn. Manipal Power Press, Manipal, India, p p2
Alippi, AM (1990). Técnicas de laboratorio para el aislamiento e identificación de Bacillus larvae White, agente causal de la Loque Americana. Buenos Aires: Comisión de Investigaciones Científicas, Serie difusión, año 2, N° 6.
Lennette, S, Balows, R, Hansler, L, Shadony, E (1987) Manual de Microbiología Clínica. Ed. Panamericana, Buenos Aires, Argentina, pp. 244.
Mann CM, Markham JL (1998) A new method for determining the minimum inhibitory concentration of essential oils. J Appl Microbiol 84:538–544
Cugnata NM, Guaspari E, Pellegrini MC, Fuselli SR, Alonso-Salces RM (2017) Optimal concentration of organic solvents to be used in the broth microdilution method to determine the antimicrobial activity of natural products against Paenibacillus Larvae. J Apic Sci 61(1):37–53
García Damiano MC (1991) Normas de estudios clínicos de antimicrobianos (Parte II). Enfermedades Infecciosas Microbiología Clínica 3(3):17–28
Finelgold, S, Baron, E, Braily, S (1992) Diagnóstico microbiológico, aislamiento e identificación de microorganismos patógenos. Ed. Médica Panamericana, Bs. As., 36, 514- 533.
Fratini F, Mancini S, Turchi B, Friscia E, Pistelli L, Giusti G, Cerri D (2017) A novel interpretation of the fractional inhibitory concentration index: the case Origanum vulgare L. and Leptospermum scoparium J. R. et G. Forst essential oils against Staphylococcus aureus strains. Microbiol Res 195:11–17. https://doi.org/10.1016/j.micres.2016.11.005
Sabate DC, Gonzalez MJ, Porrini MP, Eguaras MJ, Audisio MC, Marioli JM (2012) Synergistic effect of surfactin from Bacillus subtilis C4 and Achyrocline satureioides extracts on the viability of Paenibacillus larvae World. J Microbiol Biotechnol 28:1415–1422
Rodríguez-Ramos F, Navarrete A (2009) Solving the confusion of gnaphaliin structure: gnaphaliin A and gnaphaliin B identified as active principles of Gnaphalium liebmannii with tracheal smooth muscle relaxant Properties. J Nat Prod 72:1061–1064
Kuete V (2010) Potential of Cameroonian plants and derived products against microbial infections: a review. Planta Med 76(14):1479–2149
Pimentel-Betancurt DC, Tonello NV, Padilla-Alvarez F, Paletti-Rovey MF, Oliva MM, Marioli JM (2021) Oral and contact toxicity of the extract obtained with hexane from Achyrocline satureioides on larvae and adult honey bees. Span J Agric Res 19(3):1–9
Harris R (2002) Synergism in the essential oil world. Int J Aromather 12(4):179–186
Wagner H, Ulrich-Merzenich G (2009) Synergy research: approaching a new generation of phytopharmaceuticals. Phytomedicine 16(2–3):97–110. https://doi.org/10.1016/j.phymed.2008.12.018
Tallarida R (2001) Drug Synergism: Its Detection and Applications. J Pharmacol Exp Ther 298(3):865–872
Delaquis PJ, Stanich K, Girard B, Mazza G (2002) Antimicrobial activity of individual and mixed fractions of dill, cilantro, coriander and eucalyptus essential oils. Int J Food Microbiol 74:101–109
Funding
This study was financially supported by Agencia Nacional de Promoción Científica and Secretaría de Ciencia y Técnica from the Universidad Nacional de Río Cuarto.
Author information
Authors and Affiliations
Contributions
Each author has contributed significantly to this work. NT performed the experiments and analyzed the data, DP and CH performed the experiments, JM designed the experiments, MM supervised the experiments, MO supervised the experiments and edited the manuscript, and FD wrote the manuscript and edited the manuscript. All authors have reviewed, discussed, edited, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
The authors consented to participate the current research.
Consent for publication
The authors approved the manuscript and agree to publish it in current form in Brazilian Journal of Microbiology.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Lucy Seldin
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tonello, N., Pimentel Betancurt, D., Huallpa, C.L. et al. Fractionation of hexane extracts from Achyrocline satureioides and their biological activities against Paenibacillus larvae. Braz J Microbiol 53, 1645–1655 (2022). https://doi.org/10.1007/s42770-022-00736-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-022-00736-y