Abstract
Beekeeping is an important agricultural and commercial activity globally practiced. Honey bee is attacked by certain infectious pathogens. Most important brood diseases are bacterial including American Foulbrood (AFB), caused by Paenibacillus larvae (P. larvae), and European Foulbrood (EFB) by Melissococcus plutonius (M. plutonius) in addition of secondary invaders, e.g. Paenibacillus alvei (P. alvei) and Paenibacillus dendritiformis (P. dendritiformis). These bacteria cause the death of larvae in honey bee colonies. In this work, antibacterial activities of extracts, fractions, and isolated certain compounds (nominated 1–3) all originated from moss, Dicranum polysetum Sw. ( D. polysetum), were tested against some honey bee bacterial pathogens. Minimum inhibitory concentration, minimum bactericidal concentration, and sporicidal values of methanol extract, ethyl acetate, and n-hexane fractions ranged between 10.4 and 18.98, 83.4-303.75 & 5.86–18.98 µg/mL against P. larvae, respectively. Antimicrobial activities of the ethyl acetate sub-fractions (fraction) and the isolated compounds (1–3) were tested against AFB- and EFB-causing bacteria. Bio-guided chromatographic separation of ethyl acetate fraction, a crude methanolic extract obtained from aerial parts of D. polysetum resulted in three natural compounds: a novel one, i.e. glycer-2-yl hexadeca-4-yne-7Z,10Z,13Z-trienoate (1, dicrapolysetoate; given as trivial name), in addition to two known triterpenoids poriferasterol (2), and γ-taraxasterol (3). Minimum inhibitory concentration ranges were 1.4-60.75, 8.12-65.0, 2.09–33.44 & 1.8-28.75 µg/mL for sub-fractions, compounds 1, 2, and 3, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Finding new bioactive substances of natural sources is a continuous research for therapeutic uses. About 30% of medicines sold worldwide based on natural products (Grabley and Thiericke 1999). In Turkey, the Eastern Black Sea is one of the most important regions for the common medicinal plant diversity and endemic plant richness due to different geographical conditions, high-elevation plateaus, and climate (Kaya and Raynal 2001). The prevalence of medicinal plants has a significant potential for both beekeeping applications and natural product/products development.
The American Foulbrood (AFB) agent, Paenibacillus larvae (P. larvae), and the European Foulbrood (EFB) one, Melissococcus plutonius (M. plutonius), are the main pathogens of honey bee larvae (Evans and Schwarz 2011). Both bacterial pathogens lead to larval death and hive collapse, and the beekeeper suffers great economic losses (Genersch 2010). Ingestion of at least ten P. larvae spores by larvae with contaminated food is sufficient to develop AFB disease (Hitchcock et al. 1979; Brødsgaard et al. 1998). P. larvae only infects unsealed young honey bee larvae, then larval death occurs depending on the ERIC I-V genotype, within 3–12 days, from the beginning of the infection (Hornitzky and Wilson 1989; Ellis and Munn 2005; Beims et al. 2020).
In EFB infection, M. plutonius is ingested with contaminated food and multiply rapidly with asymptomatic colonization in the midgut of larvae. Some other bacteria are frequently associated with EFB, including P. dendritiformis, P. alvei, Enterococcus faecalis, Bacillus pumilus, Achromobacter eurydice, and Brevibacillus laterosporus as secondary invaders agents (Forsgren et al. 2013; Gaggia et al. 2015; Erler et al. 2018). P. dendritiformis is a facultative anaerobic bacterium with rod-shaped, Gram-positive, motile, and round, including cylindrical or oval spores formed in swelling sporangia. Moreover, P. alvei is a saprophytic, aerobic bacterium that can usually be isolated from diseased larvae obtained from honey bee colonies infected with M. plutonius (Forsgren 2010; Djukic et al. 2012).
The illegal global use of antibiotics by beekeepers against these diseases triggers antibiotic resistance in these bacteria and resistance genes are spreading into the environment (Genersch 2010; Evans 2003). The use of antibiotics in the treatment of AFB is prohibited in European Union countries due to antibiotic residues detected in honey and the spread of antibiotic resistance (Genersch 2010; Forsgren et al. 2018). Oxytetracycline hydrochloride (OTC) is a bacteriostatic antibiotic used in many countries (USA, Canada, and Australia) that inhibits the growth of M. plutonius (Thompson and Brown 2001; Richards et al. 2021; Masood et al. 2022). OTC resistance of P. larvae increased as a function of the antibiotic concentration applied (Miyagi et al. 2000). Antibiotics are not a sustainable strategy as they only masks the clinical symptoms and signs of AFB and they are ineffective against the bacterial spores that cause the disease (Oldroyd et al. 1989; Stephan et al. 2019). The demands for alternative natural products (e.g. essential oils, plant extracts, and bee propolis) that can prevent and control AFB are increasing (Alonso-Salces et al. 2017). Extracts of many plants are being investigated for their antibacterial and biochemical effects on bees and larvae infected with P. larvae (Basile et al. 1998; Akmaz 2001; Chaimanee et al. 2017; Giménez-Martínez et al. 2019; Alpay Karaoğlu et al. 2022).
Dicranum polysetum Sw. (D. polysetum) is a moss belonging to the genus Dicranum, family Dicranaceae, and the division Bryophyta, a group of non-vascular plant species distributed worldwide (Hedenäs and Bisang 2004; Záveská Drábková et al. 2015). Fourteen species of Dicranum have been registered from Turkey (Ros et al. 2013; Erata and Batan 2020). Natural compounds, e.g. organic acids and phenolics isolated from some bryophyte species, i.e. of Sphagnum, Dicranum, Polytrichum, Atrichum, and Mnium genera, show antibiotic properties and are effective on certain bacteria (Nikolajeva et al. 2012), and reported to have high biological activity (Krzaczkowski et al. 2009; Üçüncü et al. 2010; Cheng et al. 2012). Therefore, extracts and fractions of mosses could be an important source as new effective pharmaceutical agents (Wang et al. 2005; Asakawa 2007). However, the potential of the crude extract of D. polysetum and the compounds fractionated from the extract for use against bee diseases has not yet been investigated.
The aim of the present study was to evaluate the effects of D. polysetum extract, fractions, and relevant certain purified compounds obtained by different solvents against the pathogens of AFB and EFB disease in honey bee larvae. In addition, certain novel secondary metabolites were isolated from D. polysetum fractions by means of spectroscopic methods (i.e. NMR, UV, FT-IR, and LC-QTOF-MS).
Materials and methods
General procedures
NMR spectra were recorded on a Bruker spectrometer in CDCl3 using tetramethylsilane as an internal standard at 400 MHz for 1 H NMR and 100 MHz for 13 C NMR spectra. Chemical shifts were expressed in δ (ppm) and coupling constants (J) were reported in hertz (Hz). The assistance of ACD NMR programs was also used for the elucidation of isolated compounds. LC-QTOF-MS was obtained on an Agilent 6230 A instrument. UV spectra were obtained with a Spectrostar nano BMG labtech spectrometer. Infrared spectra were obtained with a PerkinElmer 1600 FT-IR (ATR) (4000 − 400 cm− 1) spectrometer. Melting points were determined using Thermovar apparatus fitted with a microscope and are uncorrected. Column chromatography was performed with silica gel 60 (320–400 mesh). Thin-layer chromatography was performed with TLC Silica gel 60 F254 aluminum TLC plates, visualized by UV or spraying with vanillin/H2SO4 solution followed by warming (Erik et al. 2021a, b).
Moss material
D. polysetum samples were collected from Sis Mountain, 40º52ʹ28.89ʺ N, 39º06ʹ53.32ʺ E, altitude 1931 m on Oct26th, 2018 at Trabzon province, Turkey. After laboratory identification, the moss samples were cleaned and dried under appropriate conditions (Smith 1980; Hedenäs and Bisang 2004; Ros et al. 2013; Özdemir and Batan 2017; Erata and Batan 2020). The plant material was identified by one of the co-authors (Prof. Dr. Nevzat Batan) following the protocol of Hedenäs and Bisang (2004). A voucher specimens (Voucher number: KTUB 1613) were stored in the bryophyte collections of the Biology Department, Faculty of Science, Karadeniz Technical University, Turkey.
Extraction and isolation
Dried powdered sample of D. polysetum (~ 1500 g) was macerated with methanol (5 L) three times for 72 h at room temperature (24 ± 1 °C) on stirring. Total extract was evaporated under vacuum at a temperature not exceeding 40 °C using a chiller (-10 °C) to yield crude extract (34.20 g). The crude methanol extract (30 g) was dissolved in MeOH-H2O (2:8 v/v, 100 mL) and then successively fractionated with n-hexane (500 mL x 3), ethyl acetate (500 mL x 3), and water (250 mL) to yield 7.56, 9.57, and 13.25 g fractions, respectively (Narayan et al. 2010). Crude ethyl acetate fraction was used for further phytochemical tests. This fraction (9.57 g) was subjected to CC (Kieselgel 60, 230–400 mesh) using increased polarity of n-hexane (100 mL), n-hexane:chloroform (1:1, 100 mL), chloroform (100 mL), chloroform-ethyl acetate (1:1, 100 mL), ethyl acetate (100 mL), ethyl acetate-methanol (1:1, 100 mL), and methanol (100 mL) mixture to get six sub-fractions (~ 120 mL, each, DPE1-6, respectively). After the TLC analysis, the DPE-2 fraction afforded compound 1 (353 mg) given a trivial name dicrapolysetoate.
The DPE-3 fraction (2.49 g) was subjected to CC (Kieselgel 60, 320–400 mesh) using increased polarity of n-hexane-chloroform (100:0, 80:20, 60:40, 20:80, and 0:100 mL each), chloroform-ethyl acetate (100:0, 80:20, 60:40, 20:80, and 0:100 mL, each), and ethyl acetate-methanol (100:0, 80:20, 60:40, 20:80, and 0:100 mL each) mobile phase to get 26 fractions (~ 50 mL each). After TLC control, DPE-3 Fr 5 afforded compound 2 (23.8 mg). After TLC analysis, DPE-4 and DPE-5 fractions were combined and subjected to CC (Kieselgel 60, 320–400 mesh) using increased polarity of n-hexane-chloroform (100:0, 95:5, 90:10, 85:5, 80:20, 75:25, 70:30,65:35, 60:40, 50:50, 40:60, 20:80, and 0:100 mL, each), chloroform-ethyl acetate (100:0, 90:10, 80:20,70:30, 60:40, 50:50,40:60, 30:70, 20:80, and 0:100 mL each), and ethyl acetate-methanol (100:0, 80:20, 60:40, 20:80, and 0:100 mL, each) mobile phase to get 41 fractions (~ 40 mL each). After TLC control, Fr 8–15 (0.71 g) were combined and again was further purified with CC (Kieselgel 60, 320–400 mesh) with increasing polarity of n-hexane-ethyl acetate (100:0, 98:2, 96:4,92:8,90:10, 88:12, 86:14, 82:18, 80:20, 75:25, 70:30, 65:35, 60:40, 50:50, 40:60, 30:70, 20:80,10:90, and 0:100 mL, each) to get 30 sub-fractions (SFr) (~ 30 mL, each). After TLC control, SFr 18 afforded compound 3 (6.2 mg).
The most active ethyl acetate fractions of D. polysetum were separated into six sub-fractions with column chromatography using different solvents of increasing polarity. These six obtained sub-fractions were kept in the refrigerator until it was time to test them for antimicrobial activity. These sub-fractions (1–6) were weighed in specific amounts and tested for activity against honey bee pathogens.
Glycer-2-yl hexadeca-4-yne-7Z,10Z,13Z-trienoate (1)
Colorless oily; Rf: 0.82 (n-hexane-AcOEt, 9.5:0.5); UV (Methanol) λmax (nm): 216, 245, 280. FT-IR (ATR, cm− 1): FT-IR (ATR, cm− 1): 3405 (-OH), 2934, 2860 (-CH), 2148 (C ≡ C), 1705 (C = O), 1450, 1373 (C = C) 1268, 1141, 1055, 1033 (C-O); C19H28O4, LC-QTOF-MS: m/z (%) [M-H]+ 319.1960 (15), calc 319.1910. NMR data of new compound 1 are in Table 1.
Poriferasterol (2)
Rf: 0.70 (Chloroform-EtOAc, 7:3); NMR data of known compound 2 are in Table 2 (Matin Yekta and Alavi 2008; Yekta et al. 2018).
γ-Taraxasterol (3)
Rf: 0.68 (Chloroform-EtOAc, 7:3); NMR data of known compound 3 are in Table 2 (Matin Yekta and Alavi 2008; Yekta et al. 2018).
Antimicrobial activities
Bacteria and cultures
Bacterial strains causing AFB and EFB disease were isolated from honey bees (Apis mellifera anatoliaca); workers, larvae, and honey. These samples were characterized by conventional methods (de Graaf et al. 2013). Bacterial DNA samples were amplified by PCR (Bio-Rad Model T100, CA, USA) using universal specific primers (27 F and 1492R) targeting the 16 S rRNA genes. The PCR conditions were adapted according to the study of Demirci et al. (2013). After the nucleotide sequencing of the PCR products, closely related strains were searched from the NCBI GenBank databases (Baş and Karaoğlu 2015; Pınarbaş and Karaoğlu 2017). The NCBI GenBank accession numbers of the identified bacteria strains were listed in Table 3. In addition, the P. larvae genotype reference strains P. larvae ERIC-I (ATCC 9545), ERIC-II (DSM 25,430), and ERIC-III (LMG 16,252) used in the study were obtained from Samsun Veterinary Control Institute. P. alvei (Clone M6) and M. plutonius (clone I1) were obtained from Sophia Antipolis, Honeybee Pathology Laboratory Unit (France). MYPGP (Mueller Hinton Broth (1%), yeast extract (1.5%), K2HPO4 (0.3%), Glucose (0.2%), Sodium pyruvate (0.1%), and agar (15%) (Merck, Germany) medium was used for the culture of all bacteria (Dingman and Stahly 1983; Sevim et al. 2021). Cultures were inoculated on MYPGP agar and incubated with a 5% CO2 incubator at 36 ± 1 °C for three days under microaerophilic conditions (Alpay Karaoğlu et al. 2022).
Antimicrobial activities of D. polysetum extract, fractions, and isolates
Potential antimicrobial activities of D. polysetum extracts and fractions were tested against honey bee tested pathogens using the agar well diffusion method (Perez et al. 1990; CLSI 2015). The bacterial culture was grown in a shaking incubator overnight to produce a bacterial turbidity of 1 × 108 CFU/mL in MYPGP broth. McFarland 0.5 bacterial turbidity was prepared and spread with a sterile cotton swab over the surface of MYPGP agar in 90 mm diameter petri-dishes. Wells of 5 mm diameter at 3 cm intervals were made in MYPGP agar petri-dishes using sterile glass tubes. A 50 µL of moss extract and fractions were dropped into each well. The inhibition zone diameters were measured with a ruler after the petri-dishes were incubated for three days at 36 ± 1 °C with a 5% CO2 incubator (Nordström and Fries 1995; Sevim et al. 2021). Tetracycline (CT0054B, Oxoid, England) (30 µg) was used as a standard drug control (Mejias 2020) as well as solvents.
Minimum inhibition (MIC) and minimum bactericidal concentrations (MBC)
MIC and MBC tests were performed for the moss extract, fractions, and compounds (1–3) that could prevent the development of AFB and EFB agents. Bacteriostatic and Bactericidal concentrations (µg/mL) were determined by applying the micro-dilution method in ELISA (96 well) plates (CLSI 2015). Bacterial turbidity was prepared as 0.5 McFarland or 1 × 108 CFU/mL. The 10 µL bacterial solution was added to each well and incubated for 48 h for P. alvei and P. dendritiformis, and 72 h for P. larvae and M. plutonius at 36 ± 1 °C with a 5% CO2 incubator (Arai et al. 2012). The MIC value was taken from the no-growth diluted well, i.e. the lowest antimicrobial agent concentration. For MBC, 10 µL dilutions were taken from non-growing wells and were dropped onto MYPGP agar plates. The concentration of the substance in the well with no growth was determined as the MBC value (Table 4).
Sporicidal activity
The effectiveness of moss extracts, i.e. methanol, ethyl acetate, and n-hexane fractions in stopping germination of spore form of P. larvae was investigated. Spore-producing P. larvae PB31B was tested. This strain was incubated on MYPGP agar medium for 5 days and kept at 4 °C for 15–20 days. Spore harvest from the culture was done in cold, sterile distilled water using sterile swabs and placed in a hot water bath (72 ± 1 °C) for 10 min to kill vegetative bacterial cells. Serial dilutions were prepared to determine the number of viable spores per mL, and 100 µL was plated on MYPGP agar. To obtain a concentration of 2.0 × 106 spores/mL, a stock spore suspension was made for bacterial strain (Okayama et al. 1997). The sporicidal activities of D. polysetum extract and fractions against the spore form of P. larvae PB31B strain were analyzed by the microdilution technique (Table 4).
Results
Antimicrobial activities of D. polysetum against certain honey bee pathogens
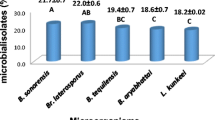
Mosses could be considered as a source of novel biologically active compounds. Compared to plants, phytochemical and biological activities of mosses in bee diseases have been less studied. Determination of antibiotic susceptibility and resistance characteristics of the strains used is important in evaluating the efficacy of D. polysetum extracts, fractions, and individual compounds against AFB and EFB agents. In this study, the potential antimicrobial activities of D. polysetum extract, fractions, and isolated compounds (nominated 1–3) (Fig. 1) were investigated. D. polysetum was found to have strong antimicrobial activity against P. larvae, P. dendritiformis, P. alvei, and M. plutonius. Antibiotic resistance profile of bacteria causing AFB and EFB are listed in Table 3. Antimicrobial resistance in bacteria has been a growing problem in recent years. The tested moss extract was found to have an 18-mm zone of inhibition against P. larvae SV27B and P. larvae PB31B, strains resistant to antibiotics (tylosin, tetracycline, and oxytetracycline) used in bee diseases control. Remarkable antimicrobial activities of moss extracts, fractions, and compounds against antibiotic-sensitive and -resistant strains were observed (Table 4).
Noticeably, methanol extract of D. polysetum exhibited antimicrobial activities against AFB and EFB agents (Table 3). MIC and MBC values of the extracts against high antibiotic- resistant strains, i.e. PB31B and SV27B were 6.91 & 13.83 µg/mL, respectively, highest values were observed for PB35 and ERIC-II strains. M. plutonius was the most sensitive strain (MIC and MBC were 6.91 & 13.83 µg/mL, respectively). Activities against other tested EFB agents were similar to those of the ERIC-II and ERIC-III strains. Obtained results suggest potent activity of the extract regardless of the strains or antibiotic resistance. The elucidation of the mechanism of action of tested extracts or purified novel compounds will be subjected in further studies.
Antimicrobial activities of aqueous, ethyl acetate, and n-hexane fractions of the methanolic extract of D. polysetum against P. larvae was determined (Table 4). Aqueous fraction was not effective, but ethyl acetate and n-hexane fractions were strong antimicrobial (11.72 & 18.99 µg/mL, respectively) and sporicidal (5.86–11.72 & 18.98 µg/mL, respectively) agents. PB35 strain was found to be less susceptible (inhibition zones ranged 10–12 mm) than other tested strains (19–25 mm) (Table 4).
The six sub-fractions of the ethyl acetate exhibited potent antimicrobial activities against P. larvae (MIC range 1.4–60.78 µg/mL), while was between 2.8–92.5 µg/mL for other strains (Table 3). All subfractions showed antimicrobial activity values less than 100.0 µg/mL as well as strong anti-Paenibacillus effectiveness.
Subfraction 5 gave the highest MIC values, range 1.4–5.6 µg/mL, against all tested bacteria (Table 5) regardless the antibiotic-resistance profiles of the strains.
Subfraction 2 was effective at 7.6 µg/mL in ERIC-II reference strain or M. plutonius, while was between 15.25 and 60.78 µg/mL against AFB agents. Compound 1 has high activity against P. larvae PB31B (8.12 µg/mL), ERIC-I reference strain (16.25 µg/mL) and P. dendritiformis PB31A (16.25 µg/mL), while range was 32.5–130.0 µg/mL against other tested bacteria. Compound 2 showed strong antimicrobial activity against AFB and EFB agents (range 2.09–33.44 µg/mL) (Table 5) as well as PB31B (2.09 µg/mL), resistant to tetracycline and tylosin. MIC (8.36 µg/mL) was recorded for M. plutonius, P. dendritiformis PB31A, and reference strain (ERIC-I and ERIC-III genotype). Sub-fraction 3 (DPE-3) was active against AFB and EFB agents (range 4.57–36.56 µg/mL). DPE-4 and DPE-5 fractions were combined, while compound 3 showed strong antimicrobial activity (range 1.8–28.75 µg/mL) against AFB and EFB agents (Table 5), highest antimicrobial activity was observed against P. larvae PB31B, P. larvae SV27B, and P. dendritiformis PB31A. Contrarily, fraction DPE-4 has low antimicrobial activity, while fraction DPE-5 has very high antimicrobial activity (1.4–5.6 µg/mL) against tested microorganisms.
Structure elucidation of 1 isolated from ethyl acetate fraction of D. polysetum
The methanol extract of the aerial part of D. polysetum was successively fractionated with n-hexane, EtOAc, and water. Repeated column chromatography for the most active EtOAc fraction led to the isolation of three compounds (Fig. 1). The structures of the isolated compounds were determined by spectroscopic methods, including NMR, UV, FT-IR, and LC-QTOF-MS. Compound 1 was obtained as oily. The molecular formula was determined as C19H28O4 from the LC-QTOF-MS data (m/z 319.1960 [M-H]+, calc 319.1910). The FT-IR (ATR) spectrum of compound 1 gave the presence of a hydroxyl group (3405 cm− 1), an acetylenic (2148 cm− 1), C = C (1450, 1373 cm− 1), and a carbony1 group (1705 cm− 1). The UV spectrum of 1 showed absorption maxima at 216, 245, 280 nm typical of a higher unsaturated acetylene-containing fatty acid derivative (Paul and Fenical 1980; Ichikawa 1984). The 1 H NMR spectrum of 1 showed six olefinic protons at δ 5.36 ppm (H-7,8, H-10,11, and H-13,14) and 13 C NMR spectrum revealed six olefinic CH peaks at δ 126.3-131.9 ppm, two acetylenic carbons at δ 78.5 (C-4) and 79.2 (C-5), six methylene, and one ester carbonyl at δ 172.8 ppm. The presence of the glyceryl moiety was indicated by 1 H NMR resonances of one oxymethine proton at δ 5.36 ppm (m, 1 H, H-1’), together with two sp3 oxymethylene protons at δ 4.23 ppm (m, 4 H, H-2’, 1’’) and carbon peaks at δ 68.9 (C-1’), and 62.1 (C-2’, 1’’) which was substituted by ester linkage at the C-1 position of a fatty acid moiety of compound 1. The NMR data of 1 (Table 1) were very similar to those of the higher unsaturated acetylene-containing fatty acid (16:4, n-3 and 18:4, n-3) derivative but differed from known compounds with regard to the glyceryl moiety (Paul and Fenical 1980; Borel et al. 1993; Klavina et al. 2015). In the present work, compound 1 (a novel substance) was elucidated as glycer-2-yl hexadeca-4-yne-7Z,10Z,13Z-trienoate. In addition, structures of two known triterpenoids (poriferasterol, 2, and γ-taraxasterol, 3) from the ethyl acetate fraction of D. polysetum were also isolated.
Discussion
The antimicrobial activities of the ethanol extract of three moss species (Dicranum polysetum, Calliergonella cuspidata, and Hypnum cupressiforme) against various bacteria were different, e.g. Altuner et al. (2014) assumed weak activity. However, in 13 different mosses, including D. polysetum, zone of inhibition ranged 9–15 mm against Bacillus cereus, Pseudomonas aeruginosa, Staphylococcus aureus, and Escherichia coli (Borel et al. 1993; Gahtori et al. 2011) found that petroleum ether, methanol, and chloroform extracts of D. undulatum had effective MIC (range 0.65–2.50 µg/mL) and MBC (range 1.25-5.00 µg/mL) against M. plutonius strains. These findings reveal the importance of selecting solvents in extraction process. All the three substances used in our study that purified by crude methanol extract, ethyl acetate, and hexane fractions of D. polysetum were antimicrobial against AFB- and EFB-causing bacteria, even at low concentrations.
On the other hand, antibiotic-resistant isolates of P. larvae have been reported in the United States, Canada, and Argentina (Miyagi et al. 2000; Evans 2003; Alippi et al. 2007). Antibiotic use in controlling foulbrood is permitted in many countries (Thompson and Brown 2001). Consequently, resistant bacterial strains complicate treatment, prolong duration of infection, and adversely affect other types of native bacteria (Miyagi et al. 2000; Evans 2003).
Antimicrobial activity of tested fractions against antibiotic-sensitive P. larvae was effective at low concentration (0.36–0.73 µg/mL), but in antibiotic-resistant P. larvae, concentration increased to 6.91–27.65 µg/mL (Tables 4 and 5). Paenibacillus spp. are spore-forming bacteria and the MIC value was determined at the same or similar concentrations as the inhibitory value of spore germination. MBC of crude methanol extract at low concentration (range 0.72 to 55.28 µg/mL) is promising to prevent bacterial diseases in honey bee larvae by inhibiting spore germination.
In the agar-well diffusion experiment, compounds 1–3 inhibited the growth of Paenibacillus larvae, P. alvei, P. dendritiformis, and M. plutonius. Use of extracts or molecules, e.g. antimicrobial fatty acid derivatives or triterpenoids is an alternative way in preserving honey bee larvae (Beuchat 1989). Supporting our finding for Compound 1, a fatty acid derivative, plant extracts including essential fatty acids were in vitro effective against P. larvae (Mărghitaş et al. 2011; Özkırım et al. 2012; Boligon et al. 2013). Conversely, six chemotypes (acetates) of Thymus vulgaris didn’t inhibit bacterial growth of EFB (Wiese et al. 2018). The effect of alpha-terpineol against M. plutonius was undetected, but MIC values; 292.2 ppm for P. alvei and 489.5 ppm for P. dendritiformis were reported. Poriferasterol and γ-taraxasterol belong to organic compounds known as triterpenoids. γ-Taraxasterol is a pentacyclic-triterpene and is found in a few plants, including chicory, arnica, and dandelion, Taraxacum officinale. Pharmacological effects of taraxasterol, e.g. antitumor, anti-inflammatory, antimicrobial (Zhang et al. 2012); antioxidant, hepatoprotective (You et al. 2010; Aggarwal et al. 2016), and anti-allergic (Mabona et al. 2013) activities have been described. The variation in antimicrobial activity between the pure compound and the subfraction may be due to the synergistic effect.
Conclusion
Recently, new strategies in combating diseases are highly requested, particularly with the use of natural products. The present study aimed to investigate potent efficacy of the moss, Dicranum polysetum; extracts, fractions, and isolates against antibiotic-sensitive and -resistant bacterial strains infecting honey bee. Bio-guided chromatographic separation yielded three natural compounds, including a novel one, i.e. glycer-2-yl hexadeca-4-yne-7Z,10Z,13Z-trienoate (1), and two known triterpenoids poriferasterol (2), and γ-taraxasterol) (3). As far as we know these compounds were isolated and identified for the first time from D. polysetum. Crude methanol extract was observed to have significant anti-P. larvae activity in the ethyl acetate fractions. The lowest concentration exhibited antimicrobial activity of methanolic extract was 10.4 µg/mL, while the highest one was 18.98 µg/mL for n-hexane fraction. This MIC value is a sporicidal dose as well. The crude methanol extract of D. polysetum, ethyl acetate/ n-hexane fractions, and EtOAc sub-fractions were found to have potent antimicrobial activity against AFB and EFB-causing bacteria. Ethyl acetate fractions and sub-fractions showed potent antimicrobial activities against tested honey bee larvae pathogens in the concentration range of 1.8–130.0 µg/mL. However, compounds 2 and 3 were the most effective in a range 1.8 to 33.44 µg/mL MIC. In a conclusion, the current study can contribute to the unveiling of novel alternative extracts and compounds that can be used to protect honey bee larvae from infectious bacterial diseases.
References
Aggarwal D, Gautam D, Sharma M, Singla S (2016) Bergenin attenuates renal injury by reversing mitochondrial dysfunction in ethylene glycol induced hyperoxaluric rat model. Eur J Pharmacol 791:611–621. https://doi.org/10.1016/j.ejphar.2016.10.002
Akmaz Ö (2001) The prevalence of american foulbrood disease in Adana region. Pendik Vet Mikrobiyol 32(1–2):55–60
Alippi AM, López AC, Reynaldi FJ, Grasso DH, Aguilar OM (2007) Evidence for plasmid-mediated tetracycline resistance in Paenibacillus larvae, the causal agent of American foulbrood (AFB) disease in honey bees. Vet Microbiol 125(3–4):290–303. https://doi.org/10.1016/j.vetmic.2007.05.018
Alonso-Salces RM, Cugnata NM, Guaspari E, Pellegrini MC, Aubone I, De Piano FG, Antunez K, Fuselli SR (2017) Natural strategies for the control of Paenibacillus larvae, the causative agent of American foulbrood in honey bees: a review. Apidologie 48(3):387–400. https://doi.org/10.1007/s13592-016-0483-1
Alpay Karaoğlu Ş, Yayli N, Erik İ, Korkmaz B, Akpinar R, Bozdeveci A, Suyabatmaz Ş, Batan N, Yeşilyurt A, Kaya S, Nisbet C, Güler A (2022) Biological activity and phytochemical analysis of Dicranum scoparium against the bacterial disease for honey bee. Chem Biodivers 19(7):e202100887. https://doi.org/10.1002/cbdv.202100887
Altuner EM, Canli K, Akata I (2014) Antimicrobial screening of Calliergonella cuspidata, Dicranum polysetum and Hypnum cupressiforme. J Pure Appl Microbiol 8(1):539–545
Arai R, Tominaga K, Wu M, Okura M, Ito K, Okamura N, Onishi H, Osaki M, Sugimura Y, Yoshiyama M, Takamatsu D (2012) Diversity of Melissococcus plutonius from honeybee larvae in Japan and experimental reproduction of european foulbrood with cultured atypical isolates. PLoS ONE 7(3):e33708. https://doi.org/10.1371/journal.pone.0033708
Asakawa Y (2007) Biologically active compounds from bryophytes. Pure Appl Chem 79(4):557–580. https://doi.org/10.1351/pac200779040557
Basile A, Vuotto M, Ielpo M, Moscatiello V, Ricciardi L, Giordano S, Cobianchi RC (1998) Antibacterial activity in Rhynchostegium riparioides (hedw.) card. extract (bryophyta). Phytother Res 12(S1):S146–S148. https://doi.org/10.1002/(SICI)1099-1573(1998)12:1+<S146::AID-PTR278>3.0.CO;2-4
Baş Y, Karaoğlu SA (2015) Characterization and antimicrobial susceptibility of spore-forming bacilli isolated from honeycomb. Recep Tayyip Erdogan University, Graduate School of Natural and Applied Sciences, Department of Biology, Rize, p 123
Beims H, Bunk B, Erler S, Mohr KI, Spröer C, Pradella S, Günther G, Rohde M, von der Ohe W, Steinert M (2020) Discovery of Paenibacillus larvae ERIC V: phenotypic and genomic comparison to genotypes ERIC I-IV reveal different inventories of virulence factors which correlate with epidemiological prevalences of american Foulbrood. Int J Med Microbiol 310(2):151394. https://doi.org/10.1016/j.ijmm.2020.151394
Beuchat LR (1989) Antimicrobials occurring naturally in foods. Food Technol 134–142
Boligon AA, de Brum TF, Zadra M, Piana M, dos Santos Alves CF, Fausto VP, Júnior VdSB, de Almeida Vaucher R, Santos RCV, Athayde ML (2013) Antimicrobial activity of Scutia buxifolia against the honey bee pathogen Paenibacillus larvae. J Invertebr Pathol 112(2):105–107. https://doi.org/10.1016/j.jip.2012.11.009
Borel C, Welti DH, Fernandez I, Colmenares M (1993) Dicranin, an antimicrobial and 15-lipoxygenase inhibitor from the moss Dicranum scoparium. J Nat Prod 56(7):1071–1077. https://doi.org/10.1021/np50097a010
Brødsgaard CJ, Ritter W, Hansen H (1998) Response of in vitro reared honey bee larvae to various doses of Paenibacillus larvae larvae spores. Apidologie 29(6):569–578. https://doi.org/10.1051/apido:19980609
Chaimanee V, Thongtue U, Sornmai N, Songsri S, Pettis JS (2017) Antimicrobial activity of plant extracts against the honeybee pathogens, Paenibacillus larvae and Ascosphaera apis and their topical toxicity to Apis mellifera adults. J Appl Microbiol 123, 1160–1167. https://doi.org/10.1111/jam.13579
Cheng X, Xiao Y, Wang X, Wang P, Li H, Yan H, Liu Q (2012) Anti-tumor and pro-apoptotic activity of ethanolic extract and its various fractions from Polytrichum commune L. ex Hedw in L1210 cells. J Ethnopharmacol 143(1):49–56. https://doi.org/10.1016/j.jep.2012.05.054
CLSI (2015) (Clinical and Laboratory Standards Institute) Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard-Tenth Edition. CLSI document M07-A10 (ISBN 1-56238-987-4 [Print] (2015)
De Graaf DC, Alippi AM, Antunez K, Aronstein KA, Budge G, De Koker D, De Smet L, Dingman DW, Evans JD, Foster LJ, Fünfhaus A, Garcia-Gonzalez E, Gregore A, Human H, Murray KD, Nguyen BK, Poppinga L, Spivak M, Engelsdorp D, Wilkins S, Genersch E (2013) Standard methods for american foulbrood research. J Apicult Res 52(1):1–26. https://doi.org/10.3896/IBRA.1.52.1.11
Demirci M, Sevim E, Demir İ, Sevim A (2013) Culturable bacterial microbiota of Plagiodera versicolora (L.) (Coleoptera: Chrysomelidae) and virulence of the isolated strains. Folia microbiol 58(3):201–210. https://doi.org/10.1007/s12223-012-0199-1
Dingman DW, Stahly DP (1983) Medium promoting sporulation of Bacillus larvae and metabolism of medium components. Appl Environ Microbiol 46(4):860–869
Djukic M, Becker D, Poehlein A, Voget S, Daniel R (2012) Genome sequence of Paenibacillus alvei DSM 29, a secondary invader during european foulbrood outbreaks. J Bacteriol 194(22):6365. https://doi.org/10.1128/JB.01698-12
Ellis JD, Munn PA (2005) The worldwide health status of honey bees. Bee World 86(4):88–101. https://doi.org/10.1080/0005772X.2005.11417323
Erata H, Batan N (2020) New and remarkable bryophyte records from Turkey and South-West Asia. Plant Biosyst 154(3):376–383. https://doi.org/10.1080/11263504.2019.1635219
Erik İ, Coşkunçelebi K, Makbul S, Yayli N (2021a) New chlorogenic acid derivatives and triterpenoids from Scorzonera aucheriana. Turk J Chem 45:199–209. https://doi.org/10.39 06/kim-2009-17
Erik İ, Yaylı N, Coşkunçelebi K, Makbul S, Karaoğlu ŞA (2021b) Three new dihydroisocoumarin glycosides with antimicrobial activities from Scorzonera aucheriana. Phytochem Lett 43:45–52. https://doi.org/10.1016/j.phytol.2021.02.010
Erler S, Lewkowski O, Poehlein A, Forsgren E (2018) The curious case of Achromobacter eurydice, a Gram-variable pleomorphic bacterium associated with european Foulbrood disease in honeybees. Microb Ecol 75(1):1–6. https://doi.org/10.1007/s00248-017-1007
Evans JD (2003) Diverse origins of tetracycline resistance in the honey bee bacterial pathogen Paenibacillus larvae. J Invertebr Pathol 83(1):46–50. https://doi.org/10.1016/s0022-2011(03)00039-9
Evans JD, Schwarz RS (2011) Bees brought to their knees: microbes affecting honey bee health. Trends Microbiol 19(12):614–620. https://doi.org/10.1016/j.tim.2011.09.003
Forsgren E (2010) European foulbrood in honey bees. J Invertebr Pathol 103(l):5–9. https://doi.org/10.1016/j.jip.2009.06.016
Forsgren E, Budge GE, Charrière JD, Hornitzky MA (2013) Standard methods for european foulbrood research. J Apic Res 52(1):1–14. https://doi.org/10.3896/IBRA.1.52.1.12
Forsgren E, Locke B, Sircoulomb F, Schäfer MO (2018) Bacterial diseases in honeybees. Curr Clin Microbiol Rep 5:18–25. https://doi.org/10.1007/s40588-018-0083-0
Gaggia F, Baffoni L, Stenico V, Alberoni D, Buglione E, Lilli A, Di Gioia D, Porrini C (2015) Microbial investigation on honey bee larvae showing atypical symptoms of european foulbrood. Bull Insectol 68(2):321–327
Gahtori D, Chaturvedi P, Singh S (2011) Using bryophytes as a tool to cure european foulbrood disease of honey bee: an eco-friendly approach. Curr Sci 101(3):420–423
Genersch E (2010) American Foulbrood in honeybees and its causative agent, Paenibacillus larvae. J Invertebr Pathol 103:10–19. https://doi.org/10.1016/j.jip.2009.06.015
Giménez-Martínez P, Cugnata N, Alonso-Salces RM, Arredondo D, Antunez K, De Castro R, Fuselli SR (2019) Natural molecules for the control of Paenibacillus larvae, causal agent of american foulbrood in honey bees (Apis mellifera L). Span J Agric Res 17(3). https://doi.org/10.5424/sjar/2019173-14740
Grabley S, Thiericke R (1999) Bioactive agents from natural sources: trends in discovery and application. Adv Biochem Engin/Biotechnol 64:101–154. https://doi.org/10.1007/3-540-49811-7-4
Hitchcock J, Stoner A, Wilson W, Menapace D (1979) Pathogenicity of Bacillus pulvifaciens to honey bee larvae of various ages (Hymenoptera: Apidae). J Kans Entomol Soc 238–246. https://www.jstor.org/stable/25083900
Hornitzky MA, Wilson S (1989) A system for the diagnosis of the major bacterial brood diseases of honeybees. J Apic Res 28(4):191–195. https://doi.org/10.1080/00218839.1989.11101183
Ichikawa T (1984) New cyclopentenonyl fatty acids from Japanese mosses (Proceedings of the World Conference of Bryology, Tokyo, Japan, May 23–28, 1983-2-) (Chemistry). J Hattori Bot Lab 56:209–213
Kaya Z, Raynal DJ (2001) Biodiversity and conservation of turkish forests. Biol Conserv 97(2):131–141
Klavina L, Springe G, Nikolajeva V, Martsinkevich I, Nakurte I, Dzabijeva D, Steinberga I (2015) Chemical composition analysis, antimicrobial activity and cytotoxicity screening of moss extracts (moss phytochemistry). Molecules 20(9):17221–17243. https://doi.org/10.3390/molecules200917221
Krzaczkowski L, Wright M, Rebérioux D, Massiot G, Etiévant C, Gairin JE (2009) Pharmacological screening of bryophyte extracts that inhibit growth and induce abnormal phenotypes in human HeLa cancer cells. Fundam Clin Pharmacol 23(4):473–482. https://doi.org/10.1111/j.1472-8206.2009.00698.x
Mabona U, Viljoen A, Shikanga E, Marston A, Van Vuuren S (2013) Antimicrobial activity of southern african medicinal plants with dermatological relevance: from an ethnopharmacological screening approach, to combination studies and the isolation of a bioactive compound. J Ethnopharmacol 148(1):45–55. https://doi.org/10.1016/j.jep.2013.03.056
Mărghitaş L, Dezmirean D, Chirilă F, Fiţ N, Bobiş O (2011) Antibacterial activity of different plant extracts and phenolic phytochemicals tested on Paenibacillus larvae bacteria. J Anim Sci Biotechnol 44(2):94–99
Masood F, Thebeau JM, Cloet A, Kozii IV, Zabrodski MW, Biganski S, Liang J, Marta Guarna M, Simko E, Ruzzini A, Wood SC (2022) Evaluating approved and alternative treatments against an oxytetracycline-resistant bacterium responsible for european foulbrood disease in honey bees. Sci Rep 12(1):5906. https://doi.org/10.1038/s41598-022-09796-4
Matin Yekta M, Alavi SHR (2008) New triterpenoids from Peucedanum ruthenicum. Iran J Pharm Sci 4(4):289–294
Mejias E (2020) American Foulbrood and the Risk in the Use of Antibiotics as a Treatment. Modern Beekeeping - Bases for Sustainable Production https://doi.org/10.5772/intechopen.90303
Miyagi T, Peng CY, Chuang RY, Mussen EC, Spivak MS (2000) Verification of oxytetracycline-resistant american foulbrood pathogen Paenibacillus larvae in the United States. J Invertebr Pathol 75(1):95–96. https://doi.org/10.1006/jipa.1999.4888
Narayan GR, Viswas K, Pathak M, Singh PS, Gupta A (2010) Antibacterial activities of ethanolic extracts of plants used in folk medicine. Int J Ayurveda Res (IJRAP) 1(2):529–535
Nikolajeva V, Liepina L, Petrina Z, Krumina G, Grube M, Muiznieks I (2012) Antibacterial activity of extracts from some bryophytes. Adv Microbiol 2(03):345. https://doi.org/10.4236/aim.2012
Nordström S, Fries I (1995) A comparison of media and cultural conditions for identification of Bacillus larvae in honey. J Apic Res 34(2):97–103
Oldroyd B, Goodman R, Hornitzky M, Chandler D (1989) The effect on american foulbrood of standard oxytetracycline hydrochloride treatments for the control of european foulbrood of honeybees (Apis mellifera). Aust J Agric Res 40(3):691–697
Okayama A, Sákogawa T, Nakajima C, Hayama T (1997) Sporicidal activities of disinfectants on Paenibacillus larvae. J Vet Med Sci 59(10):953–954. https://doi.org/10.1292/jvms.59.953
Özdemir T, Batan N (2017) The bryophyte checklist of Trabzon province of Turkey. Arctoa 26(1):58–67. https://doi.org/10.15298/arctoa.26.05
Özkırım A, Keskin N, Kürkçüoğlu M, Başer KHC (2012) Evaluation of some essential oils as alternative antibiotics against american foulbrood agent Paenibacillus larvae on honey bees Apis mellifera L. J Essent Oil Res 24(5):465–470. https://doi.org/10.1080/10412905.2012.703504
Paul VJ, Fenical W (1980) Palisadins A, B and related monocyclofarnesol-derived sesquiterpenoids from the red marine alga Laurencia cf. palisada. Tetrahedron Lett. 21(29):2787–2790. https://doi.org/10.1016/S0040-4039(00)78607-2
Perez C, Pauli M, Bazerque P (1990) An antibiotic assay by agar well diffusion method. Acta Biol Med Ger 15:113–115
Pınarbaş M, Karaoğlu A (2017) Characterization and antibiotic sensitiveness of Gram positive bacteria isolated from american foulbrood suspect bee (Apis mellifera) and bee products, with genetic diversity of Paenibacillus larvae isolates. Recep Tayyip Erdogan University, Graduate School of Natural and Applied Sciences, Department of Biology, p 117
Richards ED, Tell LA, Davis JL, Baynes RE, Lin Z, Maunsell FP, Riviere JE, Jaberi-Douraki M, Martin KL, Davidson G (2021) Honey bee medicine for veterinarians and guidance for avoiding violative chemical residues in honey. J Am Vet Med Assoc 259(8):860–873. https://doi.org/10.2460/javma.259.8.860
Ros RM, Mazimpaka V, Abou-Salama U, Aleffi M, Blockeel TL, Brugués M, Cros RM, Dia MG, Dirkse GM, Draper I (2013) Mosses of the Mediterranean, an annotated checklist. Cryptogamie Bryologie 34(2):99–283. https://doi.org/10.7872/cryb.v34.iss2.2013.99
Sevim E, Bozdeveci A, Pınarbaş Çetin M, Kekeçoğlu M, Akpınar R, Keskin M, Kolaylı S, Alpay Karaoğlu Ş (2021) Antibacterial effects of Anatolian propolis on Paenibacillus larvae. U Bee J 21(2):177–186. https://doi.org/10.31467/uluaricilik.976536
Stephan JG, Lamei S, Pettis JS, Riesbeck K, de Miranda JR, Forsgren E (2019) Honeybee-specific lactic acid bacterium supplements have no effect on American foulbrood-infected honeybee colonies. Appl Environ Microbiol 85(13):e00606-e00619. https://doi.org/10.1128/AEM.00606-19
Smith AJE (1980) The moss flora of Britain and Ireland. Cambridge university press
Thompson HM, Brown MA (2001) Is contact colony treatment with antibiotics an effective control for european foulbrood? Bee World 82(3):130–138. https://doi.org/10.1080/0005772X.2001.11099515
Üçüncü O, Cansu TB, Özdemir T, Karaoğlu A, Yaylı N (2010) Chemical composition and antimicrobial activity of the essential oils of mosses (Tortula muralis Hedw., Homalothecium lutescens (Hedw.) H. Rob., Hypnum cupressiforme Hedw., and Pohlia nutans (Hedw.) Lindb.) from Turkey. Turk J Chem 34(5):825–834. https://doi.org/10.3906/kim-1002-62
Wang XN, Yu WT, Lou HX (2005) Antifungal constituents from the chinese moss Homalia trichomanoides. Chem Biodivers 2(1):139–145. https://doi.org/10.1002/cbdv.200490165
Wiese N, Fischer J, Heidler J, Lewkowski O, Degenhardt J, Erler S (2018) The terpenes of leaves, pollen, and nectar of thyme (Thymus vulgaris) inhibit growth of bee disease-associated microbes. Sci Rep 8(1):14634. https://doi.org/10.1038/s41598-018-32849-6
Yekta SS, Hedenström M, Stehr JE, Dario M, Hertkorn N, Björn A (2018) Pretreatment of anaerobic digester samples by hydrochloric acid for solution-state 1H and 13 C NMR spectroscopic characterization of organic matter. Chemosphere 199:201–209. https://doi.org/10.1016/j.chemosphere.2018.02.015
You Y, Yoo S, Yoon HG, Park J, Lee YH, Kim S, Oh KT, Lee J, Cho HY, Jun W (2010) In vitro and in vivo hepatoprotective effects of the aqueous extract from Taraxacum officinale (dandelion) root against alcohol-induced oxidative stress. Food Chem Toxicol 48(6):1632–1637. https://doi.org/10.1016/j.fct.2010.03.037
Záveská Drábková L, Dobrev PI, Motyka V (2015) Phytohormone profiling across the Bryophytes. PLoS ONE 10(5):e0125411. https://doi.org/10.1371/journal.pone.0125411
Zhang X, Xiong H, Liu L (2012) Effects of taraxasterol on inflammatory responses in lipopolysaccharide-induced RAW 264.7 macrophages. J Ethnopharmacol 141(1):206–211. https://doi.org/10.1016/j.jep.2012.02.020
Funding
Author Ş.A.K. has received research support from Recep Tayyip Erdogan University Research Fund (RTEÜ-FBA-2017-807) and Scientific and Technological Research Council of Turkey (TUBITAK-TOVAG-118O415). Thanks for their financial supports.
Author information
Authors and Affiliations
Contributions
All authors contributed significantly to this article. Conceptualization; ŞAK, NY, RA, AG, Methodology; ŞAK, RA, NY, AB, NB, ŞS, İE, BK, SK, CN, and AG, Draft-manuscript; ŞAK, NY, AB, İE, CN, Funding Acquisition; ŞAK, Writing–Final Version; All authors have reviewed, discussed, edited, and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Ethical approval
Not applicable, because the research was not on humans or animals.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karaoğlu, Ş.A., Yayli, N., Akpinar, R. et al. Phytochemicals, antimicrobial, and sporicidal activities of moss, Dicranum polysetum Sw., against certain honey bee bacterial pathogens. Vet Res Commun 47, 1445–1455 (2023). https://doi.org/10.1007/s11259-023-10094-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-023-10094-1