Abstract
During alcoholic fermentation, most of the substrates supplied to the yeasts are converted into ethanol and carbon dioxide generating energy for cell maintenance. However, some of these substrates end up being diverted to other metabolic pathways generating by-products reducing the yield in ethanol production. Glycerol is the most important by-product quantitatively, and its production during fermentation is associated to the production of ethanol. The present study was carried out at a full scale in an industrial fermentation plant applied to sugar cane industry with bioreactors operated in fed-batch mode. Varying levels of the operating factors feeding time, temperature, and concentration of yeast were used in order to verify the interaction between ethanol and glycerol in the fermentative kinetics and how these factors can be optimized to increase ethanol production with reduced carbon losses during the formation of other products. The results obtained indicated that glycerol production is linearly associated with ethanol production and that this correlation is influenced by the process conditions. Feeding time had a significant effect and was inversely proportional to the glycerol/ethanol production ratio. Therefore, it can be said that a moderate feeding rate can reduce the production of glycerol in relation to the ethanol produced reducing losses and increasing the fermentation yield.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Petroleum products, which account for the largest portion of global energy, are non-renewable energy sources with limited resources that are located predominantly in regions of political territorial disputes. These factors result in price, supply, and distribution instability. Furthermore, these energy sources are responsible for a significant amount of greenhouse gas emissions into the atmosphere, a phenomenon that results in the increase of global warming and major climate changes [1]. Therefore, there has been a growing demand for biomass fuels to be used in internal combustion engines and that are capable of replacing fossil fuels partially or totally [2,3,4].
Ethanol is a renewable fuel, and it is widely used in Brazil. According to data of the National Supply Company [5], the sugar and alcohol sector in Brazil occupied 8.77 million hectares of sugarcane-planted area in the 2017/2018 harvest. The total amount of sugarcane bagasse in the 2017/18 harvest was 646.4 million tonnes, producing 26.12 billion liters of ethanol.
A large number of biotechnological processes, such as industrial fermentation, are conducted in fed-batch mode since it is the most effective operation mode for dealing with problems such as substrate inhibition and catabolite repression [6,7,8]. Most distilleries in Brazil use the Melle-Boinot fermentation process, which is based on the continuous feeding of the substrate to the bioreactor and on yeast recovery. The must that feeds the yeast can originate from sugarcane juice, from the molasses resulting from sugar production, or from a mixture of these components. The bioreactors are fed, fermented, and then centrifuged. After being separated from the wine, the cell mass is subjected to appropriate treatment and placed in the bioreactor as the inoculum for a new cycle [9].
The global reaction of glycolysis shows that 1 mol of glucose (180 g) produces 2 mol of ethanol (92 g), 2 mol of carbon dioxide (88 g), and 57 kcal of energy. Thus, the theoretical yield (YP/S) for the production of ethanol is 0.511 g/g. In practice, this value is not observed because part of glucose is used in the production of glycerol and fatty alcohols, and acids, substances necessary for the synthesis of cellular material and yeast maintenance [10,11,12,13], and glycerol is quantitatively the most important fermentation by-product when compared with other alcoholic fermentation by-products. Brumm and Hebeda [14] observed that the glycerol formed corresponded to 0.08 to 0.15 g per gram of ethanol, while Oura [15] found from 0.03 to 0.05 g glycerol per gram of ethanol. As for the production of ethanol in distilleries, the formation of glycerol is undesirable since it reduces the efficiency of fermentation [14, 16].
Considering carefully the reactions involved in the metabolic pathway of alcoholic fermentation, it can be seen that the yeast cell does not gain energy from the glycerol; however, it allows regeneration of NAD [17]. Thus, combined to the fact that this regeneration occurs before the oxidation of glyceraldehyde 3-phosphate when there is a consumption of NAD, it is clear that the yeast cell produces glycerol as an alternative to regenerate NAD [18]. This shows that the amount of NAD regenerated during the production of ethanol is not sufficient to meet the needs of the yeast, and therefore, glycerol production becomes important to maintain the metabolic balance of the yeast cells. Glycerol formation is increased if there is a negative effect on the reaction that converts acetaldehyde into ethanol. The production of glycerol from glucose is therefore a natural redox neutralizer of the process [19] and is increased in response to osmotic stress [20].
Many physical, chemical, and microbiological factors affect the efficiency of fermentation and the conversion of sugar into ethanol [11]. Glycerol formation is also affected by factors such as temperature, substrate feeding time, inoculum concentration, pH, bacterial contamination, nutrients, and inhibitors [16, 21,22,23].
The present study was developed to investigate the interaction between the production of ethanol and glycerol in fermentative kinetics and to analyze the effect of the variables (temperature, yeast concentration, and feeding time) on the production of glycerol and ethanol at a full scale in an industrial fermentation plant. Bioreactors in fed batch were used, aiming to have operational conditions that minimize carbon deviations from the substrate for other products than ethanol, increasing fermentation yields, optimizing the industrial plant, and reducing production costs.
Material and methods
Experimental conditions
The experiments were conducted in the fermentation unit of the “Catanduva sugar and ethanol plant” in the municipality of Ariranha, SP, Brazil, in the second semester of the 2012/2013 harvest.
The experiments were conducted in the plant bioreactor (fermentation vessel), which contains seven fermenters, each one with an average capacity of 1,200,000 L. The fermentation substrate used was the must from the mixture of sugarcane juice and molasses resulting from sugar production, and the yeasts Saccharomyces cerevisiae were those that were present in the industrial process during the assays.
The industrial process was adapted to the following experimental conditions: yeast concentration, temperature, and feeding time of the assays (as shown in Table 1).
The technological analysis of the wines obtained from fermentation in terms of ethanol [24] and glycerol concentration [25] was conducted in the Laboratory of Ethanol at the plant.
Experimental design
A two-level factorial design (variable maximum and minimum value) with three variables (yeast concentration, temperature, and bioreactor feeding time) was used, and 11 assays were carried out. The first eight assays corresponded to the linear model (23), followed by three replicates at the central point (the value of each variable was chosen based on the average values used in the industrial plant where the assays were conducted) to estimate the experimental error, as shown in Table 1.
Assays 9, 10, and 11 correspond to the central point (where three repetitions that determined the experimental error were performed); the variable values at the center point were the most frequently used in the industrial process of the plant analyzed when the assays started (feeding time of 6 h, temperature of 35.5 °C, and yeast concentration of 25%). In the other assays (1 to 8), the feeding time varied in ± 1 h, the temperature in ± 1.5 °C, and yeast concentration in ± 5% in relation to the central point.
Sample collection and analysis
In each assay, samples of the must and wine were collected throughout the entire fermentation process, from the first feeding up to the end of fermentation (before the wine was sent to the centrifugation step), with an interval of 1 h between collections. All fermentations were carried out for 9 h (sufficient time for the fermentations to be processed).The flow rates during collection, the initial volumes of treated yeast, and the final volume of the fermenters were determined.
The wine samples collected were transferred into 50-mL Falcon tubes and were cryopreserved in liquid nitrogen in order to stop the biological activity of yeasts and bacteria. Subsequently, analyses to determine ethanol concentration were conducted by densitometry [24], and glycerol concentration was determined using the enzymatic method and analyzed using a spectrophotometer [25].
Correlation coefficient of ethanol and glycerol mass (Y G/P) and effects of the variables on this coefficient
Based on the values of ethanol and glycerol concentration and the volumes obtained at each point studied, the masses of the products formed over time were determined, the YG/P coefficients of each assay were calculated using linear correlation of these variables, and the effect of the operating variables on YG/P was determined by statistical analysis.
Results
Fermentation profile
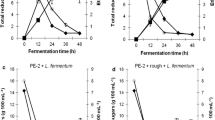
Figure 1 shows the concentration of the products as a function of time for each assay conducted.
Relationship between glycerol and ethanol production
Based on the masses of ethanol and glycerol, which are calculated with the concentration of the product and volume of the bioreactor at each central point, it was possible to determine the correlation coefficient of ethanol and glycerol mass (YG/P). This coefficient is obtained from the linear regression between the mass values of ethanol and glycerol for each assay.
Table 2 shows the linear regression equation obtained for each assay, the coefficient of determination (R2) from the linear regressions, and the YG/P coefficient.
Effect of the variables studied on the correlation between glycerol and ethanol production (Y G/P)
Table 3 shows the statistical analysis of the effect of the variables studied on the YG/P coefficient for a first-order model.
Discussion
Fermentation profile
The concentrations of the products in all assays increase over time, and the values tend to stabilize at the end of each fermentation assay (Fig. 1).
Relationship between glycerol and ethanol production
According to the coefficient of determination values (R2) shown in Table 2, it can be seen that the linear regression estimated the correlation between the masses of ethanol and glycerol as highly significant for all assays. This indicates that the production of glycerol is necessary for NAD regeneration during alcoholic fermentation since the regeneration provided by ethanol production alone is insufficient to meet the yeast needs [15, 17].
It was also observed that although the YG/P values obtained are constant in each assay, they are different between the assays, showing that different operating conditions can affect the formation of glycerol during alcoholic fermentation [16, 21,22,23].
The YG/P values obtained in the assays varied between 0.033 and 0.057 g glycerol/g ethanol, which is in accordance with data reported in the literature [15].
Effect of the variables studied on the correlation between glycerol and ethanol production (Y G/P)
It is possible to state with a confidence level of 95% (α = 0.05) that feeding time was the only variable that significantly affected YG/P. This effect was inversely proportional, i.e., YG/P decreased with increasing feeding time. Yeast concentration, although not significant at 95%, also had an inversely proportional effect on YG/P, which is evidenced by the high t value in Table 3. The variable temperature did not significantly affected YG/P. Both reduced feeding time (which increases the must feeding rate) and the lower concentration of cells may indicate higher substrate concentration, especially at the beginning of fermentation. Higher substrate concentration increases the osmotic pressure of the medium increasing the production of glycerol by the yeast during the fermentation process [16, 20].
Sucrose values ranged from assays 1 to 11 in the range of 183.18 (g L−1) to 197.57 (g L−1). The results indicated that the production of glycerol is linearly related to the production of ethanol and these two routes are linked to the regeneration of NAD by the yeast and that this correlation undergoes interference of process conditions. Feeding time had a significant effect and was inversely proportional to the production ratio between glycerol and ethanol. It is impossible to produce ethanol by alcoholic fermentation without producing concomitantly glycerol, and the relationship between the formations of these two products follows a linear regression. It is concluded that the control of operational parameters to reduce the formation of by-products and increase the production of ethanol is very important, especially carried out in real scale in an industrial fermentation plant.
References
Rodrigues RG (2017) Diversidade microbiana cultivável em processo industrial de produção de etanol. Brasília, Brasil. (M.Sc. Dissertation. Universidade de Brasília)
Gnansounou E, Dauriat A (2005) Ethanol fuel from biomass: a review. J Sci Ind Res 64:809–822
Wyman CE (2007) What is (and is not) vital to advancing cellulosic ethanol. Trends Biotechnol 25:153–157
Suhaimi SN, Phang LY, Maeda T, Abd-Aziz S, Wakisaka M, Shirai Y, Hassan MA (2012) Bioconversion of glycerol for bioethanol production using isolated Escherichia coli SS1. Braz J Microbiol 43(2):506–516
Conab – Companhia Nacional de Abastecimento (2013) Quarto levantamento cana-de-açúcar – Abril/2013. Disponível em: http://www.conab.gov.br/OlalaCMS/uploads/arquivos/13_04_09_10_30_34_boletim_cana_portugues_abril_2013_4o_lev.pdf. Acessado em: 04 de Jun de 2013
McNeil B, Harvey LM (1990) Fermentation – a practical approach, 1st edn. IRL PRESS at Oxford University Press
Lessmann WF (1993) Estudo do processo descontínuo alimentado para a produção de amiologlicosidase por Aspergillusawarnori NRRL3112 para concentrações de polissacarídeo de 40 e 80 g/L. São Paulo, Brasil. (M. Sc. Dissertation. Escola Politécnica. USP)
Costa AC, Lima EL, Alves TLM (1996) Otimização de processos fermentativos em batelada alimentada através da Teoria de Controle Singular. XI Simpósio Nacional de Fermentações, vol1
Keller R, Dunn J (1978) Computer of biomass production rate of cyclic fed batch continuous culture. J Appl Chem Biotechnol 28:784–790
Oura E (1974) Effect of aeration intensity on the biochemical composition of baker’s yeast. I factors affecting the type of metabolism. Biotechnol Bioeng 26(6):1197–1202
Lima UA, Basso LC, Amorim HV (2001) Biotecnologia Industrial: Processos Fermentativos e Enzimáticos. Edgard Blücher, São Paulo
Lima LR, Marcondes AA (2002) Álcool Carburante: Uma Estratégia Brasileira. UFPR, Curitiba
Barre P, Blondin B, Dequin S, Feuillat M, Sablayrolles JM, Basso LC (2004) Fisiologia e ecologia microbiana. I Workshop Tecnológico sobre Produção de Etanol, ESALQ/USP, Piracicaba
Brumm PJ, Hebeda RE (1988) Glycerol production in industrial alcohol fermentations. Biotechnol Lett 10(9):677–682
Oura E (1977) Reaction products of yeast fermentations. Process Biochem 12(19–21):35
Panchal CJ, Stewart GG (1980) The effect of osmotic pressure on the production and excretion of ethanol and glycerol by a brewing yeast strain. J Inst Brew 86:207–210
Biocontal (2013) Rendimento fermentativo por subproduto e suas distorções. Disponível em: http://www.biocontal.com.br/index_post.php?page=noticias/rendimentos_fermentativo.html. Acessado em 21 de jan de 2018
Amaral FS (2009) Influência conjunta do pH, temperatura e concentração de sulfito na fermentação alcoólica de mostos de sacarose. Uberlândia, Brasil. (M.Sc. Dissertation. Faculdade de Engenharia Química. UFU)
Basso LC (2004) Fisiologia e ecologia da fermentação alcoólica. I Workshop Tecnológico sobre Produção de Etanol, ESALQ/USP, Piracicaba
Nevoigt E, Stahl U (1997) Osmoregulation and glycerol metabolism in the yeast Saccharomyces cerevisiae
Neish AC, Blackwood AC (1950) Dissimilation of glucose by yeast at poised hydrogen ion concentrations. Can J Technol 29:123–129
Nordstrom K (1962) Formation of ethyl acetate in fermentation with brewer’s. III. Participation of coenzyme-A. J Inst Brew 68:398–407
Rankine BC, Bridson DA (1971) Glycerol in Australian wines and factors influencing its formation. Am J Enol Vitic 22:6–12
CTC – Centro de Tecnologia Canavieira (2011) Manual de Métodos de Análises para Açúcar e Álcool. Piracicaba – Álcool - CTC-LA-MT2–008 – versão 03. Disponível em CD ROM
Fermentec (2012) Métodos Analíticos para o Controle da Produção de Açúcar e Álcool. Métodos FTCQ 08/007 e FTCQ 08/001. Piracicaba, Brasil
Acknowledgements
The authors are grateful to the Catanduva industrial plant staff for carrying out the assays and analyses at their plant. To the Graduate Program in Agricultural Microbiology of FCAV/UNESP. To CAPES for the scholarship granted.
Funding
This research received a financial support from the Graduate Program in Agricultural Microbiology - FCAV/UNESP.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Eleni Gomes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mutton, M.J.R., Ferrari, F.C.S., Freita, L.A. et al. Interaction between the production of ethanol and glycerol in fed-batch bioreactors. Braz J Microbiol 50, 389–394 (2019). https://doi.org/10.1007/s42770-019-00051-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-019-00051-z