Abstract
Ethanol was produced by sole glycerol and glycerol-glucose fermentation using Enterobacter aerogenes TISTR 1468. The initial experiment was set up with waste glycerol as a sole carbon source with initial concentration ranged from 0, 8.7, 23.4, 43.4, to 63.1 g L−1 in 100-mL serum bottles under controlled condition at 30°C for 240-hr fermentation. Liquid samples, during the fermentation, were collected to quantitate concentrations of volatile fatty acids and glycerol, whilst gaseous samples were collected to quantitate CO2 and CH4 constituent. E. aerogenes TISTR 1468 produced maximum ethanol concentration and yield of 215.1 mM and 0.71 mol mol−1 after 72-hr fermentation with the initial waste glycerol concentration of 63.1 g L−1 (equivalent to 79.11 g COD L−1). The ethanol production from glycerol-glucose fermentation was set up based on the initial COD concentration equivalent to 79.11 g COD L−1 with varying glycerol-glucose ratios of 1:1, 10:1, and 100:1. At the ratio of 100:1, maximum ethanol concentration and yield were 232.8 mM and 0.59 mol mol−1 after 24-hr fermentation. Addition of glucose as co-carbon did improve ethanol concentration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Increasing energy demand and environmental problems from fossil fuels in Thailand has continuously directed the country’s energy policy toward more sustainable and renewable energy sources. Environmentally friendly renewable energy such as biodiesel, bioethanol, and biogas can be generated from biomass resources abundant in the agricultural sector. The increase of biodiesel production generates the considerable amount of glycerol by-product of about 10% by weight of biodiesel [1]. The global glycerol market size was valued at USD 2.6 billion in 2019 and is expected to grow at a compound annual growth rate (CAGR) of 4.0% from 2020 to 2027 [2]. In small-scale biodiesel manufacturers, the glycerol by-product typically becomes waste. The waste glycerol obtained from biodiesel production contains impurities including ethanol or methanol, water, sodium chloride, organic compounds, and free fatty acids. The high-purity glycerol can be used as a raw material for soap, drug, cosmetic, food, and beverage.
Bioethanol is most commonly produced from agricultural products such as sugarcane, rice, millet, corn, and cassava via fermentation [3]. Ethanol with 99.5% purity by volume is used as fuel and/a mixture in fuel for vehicles. It may be used as a replacement for benzene or used in other industries such as food, cosmetics, beverages, and pharmaceuticals. The global ethanol production was 111.7 billion litters in 2019, whilst Thailand is 6th rank with 1.6 % share on the global ethanol production [4]. The local production, with molasses, cassava, and cane juice, is heavily dependent on the demand for liquid fuels on domestic and export market. Thailand government’s renewable policy effectively influences over the demand of ethanol fuel by specifying the quality of ethanol required to mix with regular patrol to yield E10, E20, and E85 fuels.
Ethanol production costs from conventional feedstocks such as molasses, sugarcane, and corn are relatively high due the costs of feedstock and operation. Abundant agricultural lignocellulose is sustainable feedstock for ethanol fermentation, but its pre-treatment prior to fermentation is required. When the glycerol waste is used as the feedstock, the ethanol production costs will be 40% less than that from corn [5].
In the glycerol fermentation, type of glycerol and mirobial stain, initial glycerol concentration, pH, temperature, fermentation process mode, and nutrient sources have been extensively investigated (Table 1). Previous studies [1, 6,7,8,9,10,11,12,13,14,15,16,17,18,19,19] reported the initial glycerol concentrations appropriate for ethanol production were in the range of 10 to 126 g L−1, in which each bacterial strain can convert glycerol to ethanol [6, 7]. The increase of initial waste glycerol up to 37 g L−1 using E. aerogenes KKU-S1, resulted the incrase of both ethanol concentration and yield [17]. Vikromvarasiri and Pisutpaisal [10] and Varrone et al. [16, 18] found that mixed culture can produced ethanol when the initial glycerol is in the range of 7.3 to 26 g L−1 with the glycerol optimum concentration of 20 g L−1. Klebsiella [1, 9, 11,12,13, 15] has been widely used for ethanol production from glycerol both batch-and fed-batch fermentation systems. K. pneumoniae showed better conversion of waste glycerol than pure glycerol to ethanol at concentration of 20 g L−1 [1, 9] in fed-batch reactor. The increase of glycerol concentrations from 30-126 g L−1, resulted significantly increased ethanol concentration and yield [11, 13, 15]. Thes results were consistent with the previouse studies using E. aerogenes TISTR 1468 [8], E. coli SS1, and mixed cultures [10]. Low ethanol yield and concentration in the fermentation are assocaited to the presence of other metabolite products such as 1,3-propanediol, acetic-, propionic-, and butyric acids resulting in decreased ethanol production. The current work aimed to improve glycerol fermentation to raise ethanol production with respect to concentration and yield by using glucose as the co-carbon source for E. aerogenes TISTR 146.
2 Materials and methods
2.1 Pure culture and medium
E. aerogenes TISTR 1468 was purchased from the Thailand Institute of Scientific and Technological Research (TISTR). E. aerogenes was a cultured and increased cell in nutrient broth (NB) 500 mL by using 10% bacterial (50 mL), incubated at 30 °C, 180 rpm for 24 hr by measuring the growth of bacteria every hour by spectrophotometer (OD 600 nm). Growth curve of E. aerogenes showed late log phase (OD 600 nm ~ 1.2) at 18 hr before reaching stationary phase (Fig. 1). The culture medium was centrifuge at 4°C, 180 rpm after 15-hr culturation to harvest the bacterial cells. The cells were washed with simple medium (SM) [10] prior to use in the fermentation.
2.2 Carbon sources
Waste glycerol used in this research is obtained from the partial biodiesel purification process from Trang Palm Oil Co., Ltd. (Trang, Thailand). The waste glycerol obtained from the manufactures contained 728.2 g glycerol L−1 and total COD of 642,127 mg L−1 (Table 2). D-glucose (QReC, New Zealand), was used as a carbon source in glycerol-glucose fermentation by Enterobacter aerogenes TISTR 1468.
2.3 Ethanol fermentation
The ethanol fermentations were conducted with and without glucose. The waste glycerol concentration was varying at 0, 8.7, 23.4, 43.4, and 63.1 g L−1 (Table 3), and the ratio of waste glycerol to glucose is 1: 1, 10: 1, and 100: 1, waste glycerol (63.1 g L-1) and glucose (73.32 g L-1) as shown in Table 4. All components were prepared in the serum bottles (working volume 50 mL) that consist of Enterobacter aerogenes TISTR 1468 2.5 mL (TVS 11.4 g L−1) and SM medium, adjusted the pH to 7. The rubber stopper and aluminium cap were closed and flowed with nitrogen gas to occur anaerobic condition, incubated at 30 °C by shaking 180 rpm. Liquid samples were collected at 0, 24, 40, 56, 72, 96, 168, and 240 h to analyse ethanol, volatile fatty acids, glycerol concentration, glucose concentration, chemical oxygen demand (COD), and collecting gas samples for gas composition analysis.
2.4 Analytical techniques
The concentrations of ethanol and volatile fatty acids was quantitated by gas chromatography-flame ionization detector (Shimadzu GC-2010, Japan), and gas composition was detected by gas chromatography-thermal conductivity detectors (Shimadzu GC-2014, Japan) [20]. Total gas volume was measured by the water displacement method. Glycerol and glucose concentrations were determined according to chromatropic acid method [21] and dinitrosalicylic acid (DNS) method [22]. Chemical oxygen demand (COD) was analysed by the closed reflux colorimetric method. Total solids (TS) and total volatile solids (TVS) were determined according to standard methods for the examination of water and wastewater [23]. pH was analysed by pH electrode LE 409 (Mettler Toledo, USA).
3 Results and discussions
3.1 Ethanol production from glycerol fermentation
Waste glycerol content rapidly decreased after 24-hr fermentation and slowly decreased until the end of the experiment at the initial waste glycerol concentration of 8.7, 23.7, and 63.1 g L−1. For the initial glycerol concentration of 43.4 g L−1, the amount of waste glycerol was rapidly decreased at the fermentation time of 40 hr and then slowly decreased until the end of the fermentation period (Fig. 2a). The glycerol utilization is almost 100%, at the initial waste glycerol of 8.7 and 23.7 g L−1 after 24-hr fermentation (Fig. 2b). The initial waste glycerol was 43.4 g L−1 which had the percentage of glycerol utilization rapidly increased at the 40-h fermentation period, after which there was a relatively stable trend. The initial glycerol concentration was 63.1 g L−1, and glycerol was used less than 60% throughout the fermentation. The initial waste glycerol obviously affected the amount of glycerol used by E. aerogenes TISTR 1468 to produce ethanol.
Utilization of the waste glycerol after 72-hr fermentation fluctuated when the initial glycerol concentration of 8.7–43.4 g L−1. At the initial glycerol concentration above 43.4 g L−1, the trend of glycerol utilization was inversely with the increase of glycerol concentrations. High lycerol concentration can raise osmotic pressure inside and outside of microbial cells [24] and exterted toxic impact on the microbial growth and decreased glycerol utilization rates [6, 25]. The current results exhibited finding in the accordance with Vikromvarasiri and Pisutpaisal [10] and Nwachukwu et al. [14] studies.
Ethanol production increased sharply in the fermentation period of 24–72 hr. The tendency of ethanol production increased with the initial waste glycerol concentration in the range 8.7–63.1 g L−1 (Fig. 3a) corresponding to the amount of acetic acid that tends to increase during the 72 hr and continues constantly until the end of the fermentation process (Fig. 3b). Our findings contraciicted with Nwachukwu et al. [26] indicated that ethanol fermentation will be deducted significantly after 48 hr when using E. aerogenes S012. Lactic acid production occurs after the 24-hr fermentation period; the maximum lactic acid was 6.4 mM (Fig. 3c). The concentration of propionic acid, butyric acid, and valeric acid had a very small amount (data not shown). The appearance of fermented acids has directly resulted in the continuously decreased pH during the 240-hr fermentation period (Fig. 3d). The results demonstrated that E. aerogenes TISTR 1468 can convert waste glycerol into volatile fatty acids by ethanol as the main product. The ethanol concentration increased according to the concentration of wastes glycerol in the range 8.7–63.1 g L−1, the initial glycerol concentration at 43.4 and 63.1 g L−1 had the mole ethanol decrease. In the presence of acetic acid coupled with ethanol via acetyl P, waste glycerol was used as a carbon source for fermentation through acetaldehyde to produce only ethanol [27], the main factors that support the pathway of ethanol production such as glycerol concentration, pH, temperature, oxygen demand, and culture medium.
In the metabolism of glycerol through the glycolytic pathway, hydrogen and carbon dioxide can be the products [6, 7, 11, 18] their minimal amount (<3.5 mM); does not affect the ethanol fermentation. The results consistent with the research of Rossi et al. [15] and Choi et al. [25] found that by-products of waste glycerol fermentation are acetic- and lactic acids that lower ethanol production. Therefore, it not only increased the concentration of ethanol but also reduced the concentration of by-products (lactic-, formic-, succinic-, acetic acids, CO2, and H2) in the glycerol fermentation, which is the key success to ethanol fermentation.
Ethanol yield increased rapidly during 24-hr fermentation and gradually declined until the end of the experiment. The highest ethanol production is equal to 1.04 mol ethanol mol−1 glycerol, when setting up the initial waste glycerol concentration of 63.1 g L−1 (Fig. 4a). The initial wastes glycerol concentration of 8.7-43.4 g L−1 exhibited the inverse ethanol production with glycerol concentration (Fig. 4b). The main product of fermentation with waste glycerol as a carbon source under anaerobic conditions is ethanol, when the waste glycerol concentration begins to increase, resulting in inhibiting the production of ethanol and the production of volatile fatty acids instead. Wu et al. [11] found that when the initial glycerol concentration up to 70 g L−1 results in decreased the ethanol production and increased of 1,3-propanediol, 2,3-butanethiol, lactic- and acetic acids. The maximum of ethanol production was observed at 63 g L−1waste glycerol.
Ethanol yield (a) during 240-hr fermentation period and ethanol yield (b) at 72-hr fermentation period to produce ethanol from the glycerol glycerol fermentation at initial concentrations of 0 (  ), 8.7 (
), 8.7 (  ), 23.7 (
), 23.7 (  ), 43.4 (
), 43.4 (  ), and 63.1 (
), and 63.1 (  ) g L−1 by E. aerogenes TISTR 1468. The data points and histograms show the average values (n=3). I-bars represent standard deviations
) g L−1 by E. aerogenes TISTR 1468. The data points and histograms show the average values (n=3). I-bars represent standard deviations
3.2 Ethanol production from glycerol-glucose fermentation
The glycerol in the glycerol-glucose fermentation at the ratio of 10:1 and 100:1 was rapidly decreased during the fermentation of 24–40 hr and slowly declined after then. For the glycerol fermentation (63.1 g L−1), glycerol decreases rapidly within the first 24 hr, while the glycerol decreased slowly from the beginning until the end of the fermentation for the glycerol-glucose fermentation at the ratio of 1:1 (Fig.5a). The glycerol utilization slowly increased in all experiment conditions until the end of the experiment with greater 80% (Fig. 5b). The glucose concentration in the glycerol-glucose fermentaion at the ratio of 1:1 decreased rapidly within 24 hr and stabilized until the end of the fermentation (Fig. 5c). At glycerol-glucose ratios of 1:1 and 100:1, glucose utilization (51.5%) were similar during the first 24-hr. Less than 20% of glucose was consumed throughout the fermentation with the presence of sole glucose (73.3 g L−1) (Fig. 5d). The presence of glucose in the glycerol fermentation obviously enhanced the glycerol utilization by E. aerogenes TISTR 1468. The results of this experiment are different from the finding by Metsoviti et al. [13] in which the addition of glucose to culture medium does not enhancd the glycerol utilization.
Glycerol concentration (a), glycerol utilization (b), glucose concentration (c), and glucose utilization (d) from the glycerol-glucose fermentation at the ratio of 1:1 (  ), 10:1 (
), 10:1 (  ), 100:1 (
), 100:1 (  ) glycerol (
) glycerol (  ), and glucose (
), and glucose (  ) by E. aerogenes TISTR 1468. Data points show the average values (n=3). I-bars represent standard deviations
) by E. aerogenes TISTR 1468. Data points show the average values (n=3). I-bars represent standard deviations
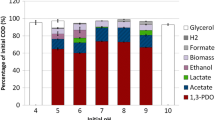
The soluble COD concentration at the glycerol-glucose ratios of 1:1, 10:1, and 100:1 were similar and gradually decreased until the end of the experiment. For glucose fermentation (73.3 g L−1), the CODs concentration decreased rapidly at the 24-hr fermentation period (Fig. 6a). The CODs removal tended to increase slowly until the end of the fermentation in all experimental conditions. The CODs removal during the 240-hr fermentation was found in the range of 50–54% (Fig. 6b). The results showed that the CODs removal after 24-hr fermentation was in the accordance to the glucose and glycerol utilization (Fig. 5b, d) and ethanol production (Fig. 7a).
Concentration (a) and percentage (b) of soluble chemical oxygen demand during the 240-hr fermentation from the glycerol-glucose fermentation at the ratio of 1:1 (  ), 10:1 (
), 10:1 (  ), 100:1 (
), 100:1 (  ), and glucose (
), and glucose (  ) by E. aerogenes TISTR 1468. Data points show the average values (n=3). I-bars represent standard deviations
) by E. aerogenes TISTR 1468. Data points show the average values (n=3). I-bars represent standard deviations
Ethanol production increased rapidly during the first 24-hr fermentation period. The highest ethanol concentration was 232.8 mM obtined from the glycerol-glucose fermentation at the ratio of 100: 1 (Fig. 7a). Ethanol concentration tends to increase with concentrations of waste glycerol-glucose at 1:1, 10:1, and 100:1, respectively. In contrast, ethanol produced from the fermenation with single waste glycerol (63.1 g L−1) and single glucose (73.3 g L−1) tended to decrease and acetic- and lactic acids were accumulated after 24-hr fermentation. The maximum acetic acid concentration was 27.7 mM (Fig. 7b). The highest concentration of lactic acid was 8.5 mM (Fig. 7c). Propionic-, butyric-, and valeric acids were found negligible (data not shown). The experiment shows that ethanol concentration varies with the proportion of waste glycerol-glucose increased. The ethanol concentration corresponds to the utilization of the initial waste glycerol. In a single fermentation, ethanol concentration from waste glycerol (63.1 g L−1) is greater than glucose (73.3 g L−1), consistent with Oh et al. [9] which found that K. pneumonia GEM167 was able to produce ethanol from glycerol as a carbon source more than glucose as a carbon source. The finding by Metsoviti et al. [12] indicated that the ethanol production from culture media with initial glycerol 20–40 g L−1 is a carbon source greater than culture media with glucose as a source carbon.
The initial pH tends to increase with the amount of initial glycerol concentration in the range of 6.8–7.8. Waste glycerol fermentation (63.1 g L−1) and glucose fermentation (73.3 g L−1) caused the pH to be decreased as 4.73, but the glycerol-glucose fermentation at the ratio of 10:1 and 100:1 helps maintain the pH value appropriate for ethanol production during 240-hr fermentation period (Fig. 7d). The results are consistent with the Lee et al. [24] and Ciptanto [8] ‘s report at pH range of 5–8 where E. aerogenes (strain ATCC. 29007 and TISTR 1468) were able to grow and produce ethanol.
Ethanol yield (Fig. 8a, b) tends to be similar, with the highest ethanol yield at 24 h of fermentation. Yield ethanol per mole glycerol (Fig. 8a) tends to decrease when the amount of waste glycerol is more than 32.46 g L−1; the best ratio for produce ethanol was 1:1 (Fig. 8c). The ethanol yield per mole glucose tends to decrease when the glucose concentration is more than 0.62 g L−1 (Fig. 8b). The highest ethanol yield was found in the ratio of 100:1 (Fig. 8d). When considering the results of the experiment, we found that the production of ethanol by E. aerogenes TISTR 1468 does not come from using glucose as the main source of carbon, but waste glycerol as the main source of carbon causes ethanol yield (mol ethanol mol−1 glucose) higher than ethanol yield (mol ethanol mol−1 glycerol), which corresponds to the maximum ethanol concentration (Fig. 7a). When comparing the results with previous studies, it was found that E. aerogenes TISTR 1468 can produce ethanol from waste glycerol up to 9.91 g L−1 (Table 5) and ethanol concentration increased when co-fermented with glucose (10.72 g L−1) more than single glycerol [17, 30]. Thapa et al. [29] reported the overexpression of the alcohol dehydrogenase (adhE) gene increased in the mutant strain resulting in the ethanol production greater than that produced by its wild type.
Yield of ethanol per mol glycerol (a) and yield of ethanol per mol glucose (b) during 240-hr fermentation period and histograms of ethanol yield per mol glycerol (c) and ethanol yield per mol glucose (d) at 24-hr fermentation period to produce ethanol from the glycerol-glucose fermentation at the ratio of 1:1 (  ), 10:1 (
), 10:1 (  ), 100:1 (
), 100:1 (  ), glycerol (
), glycerol (  ), and glucose (
), and glucose (  ) by E. aerogenes TISTR 1468. The data points and histograms show the average values (n=3). I-bars represent standard deviations
) by E. aerogenes TISTR 1468. The data points and histograms show the average values (n=3). I-bars represent standard deviations
3.3 Comparative ethanol production from glycerol and glycerol-glucose fermentation
E. aerogenes TISTR 1468 can produce ethanol from single glycerol fermentation and glycerol-glucose fermentation. At the ratio of 100:1, E. aerogenes could convert waste glycerol 36.5 g L−1 (57%) to ethanol concentration 232.8 mM greater than the single fermentation with the initial waste glycerol concentration of 63.1 g L−1 (44.5% and ethanol concentration of 215.1 mM). The ethanol yield from the glycerol fermentation with the initial glycerol concentration of 63.1 g L−1 was 0.71 mol ethanol mol−1 glycerol, which is greater than that from the glycerol-glucose at a ratio of 100:1 (0.59 mol ethanol mol−1 glycerol). All conditions of ethanol fermentation from glycerol fermentation and glycerol-glucose fermentation generated acetic and lactic acid, H2, and CO2 as negligible by-products (data not shown). The result indicated the presence of glucose in the glycerol fermentation improves ethanol concentration. The pathway of glycerol and glucose fermentation are through pyruvate before becoming ethanol according to Eq. 1 and 2.
Comarative ethanol production between the single glycerol fermentation and glycerol-glucose fermentation after 72-hr fermentation indicated the presence of glucose did improve glycerol utilization compared to the experiment with single glycerol fermentation. The glycerol-glucose fermentation in the ratio of 100:1 exhibited maximum ethanol concentration in the short fermentation period (Table 6). The glycerol-glucose fermentation demonstrated that E. aerogenes TISTR 1468 can convert glycerol and glucose into ethanol as the main product in the glycerol-glucose fermentation. Glucose is used as a carbon source for fermentation that can be oxidized to glyceraldehydes-3-phosphate and finally convert into volatile fatty acids such as acetic-, lactic-, and succinic acids, including H2 and CO2. The best condition to produce ethanol from glycerol-glucose is 100:1. The results of the glycerol showed that the highest waste glycerol concentration in this study that did not inhibit the growth and metabolism of E. aerogenes TISTR 1468 was 63.26 g L−1, consistent with Szymanowska-Powałowska [32] demonstrated bacteria tolerant the maximum concentrations of waste glycerol up to 70 g L−1. Glycerol is cheap and has a greater degree of reduction than sugars, resulting in higher ethanol yield from glycerol higher than ethanol obtained from sugar [33]. This study, E. aerogenes TISTR 1468 consumed glycerol very rapidly within 24 hr in the presence of glucose.
4 Conclusions and future directions
This study successfully demonstrated ethanol production from waste glycerol by E. aerogenes TISTR 1468 using glucose as a co-carbon source. Glucose boosted the ethanol production from the waste glycerol. With respect to the ethanol concentration and yield, the ethanol production from the glycerol-glucose fermentation improves the ethanol concentration and ethanol yield. At the ratio of 100:1, waste glycerol can be used up to 36.5 g L−1 (57.7%) with ethanol yield up to 0.59 mol ethanol mol−1 glycerol and the maximum ethanol concentration of 232.8 mM (86.9%) after 24-hr fermentation. The ethanol obtained from the experiment can be used as alternative energy to reduce the amount of oil imported from foreign countries and help solve the problem of glycerol prices. The knowledge of the ratio of the co-carbon source that can adapt to the pilot-scale to improve the quality and quantity of ethanol before scaling up to industrial scale.
Abbreviations
- CODS :
-
soluble chemical oxygen demand
- E. aerogenes :
-
Enterobacter aerogenes
- EtOH:
-
ethanol
- Glu:
-
glucose
- Gly:
-
glycerol
- g L-1 :
-
gramme per litre
- g mol-1 :
-
gramme per mole
- HAc:
-
acetic acid
- HLa:
-
lactic acid
- MLD:
-
million litres per day
- OD:
-
optical density
- SM:
-
simple medium
References
Aghbashlo M, Tabatabaei M, Jazini H, Ghaziaskar HS (2018) Exergoeconomic and exergoenvironmental co-optimization of continuous fuel additives (acetins) synthesis from glycerol esterification with acetic acid using Amberlyst 36 catalyst. Energy Convers Manag 165:183–194
Grand View Research (2020) Market analysis report: glycerol market size, share & trends, analysis report.
Panahi HKS, Dehhaghi M, Aghbashlo M, Karimi K, Tabatabaei M (2020) Conversion of residues from agro-food industry into bioethanol in Iran: an under-valued biofuel additive to phase out MTBE in gasoline. Renew Energy 145:699–710
Tunpaiboon N (2019) Thailand Energy Outlook 2019-21: Ethanol Industry. Krungsri Research, 1-7
Cheah WY, Sankaran R, Show PL, Ibrahim T, Baizura TN, Chew KW, Chang JS (2020) Pretreatment methods for lignocellulosic biofuels production: current advances, challenges and future prospects. Biofuel Res J 7:1115–1127
Ito T, Nakashimada Y, Senba K, Matsui T, Nishio N (2005) Hydrogen and ethanol production from glycerol containing wastes discharged after biodiesel manufacturing process. J Biosci Bioeng 100:260–265
Sakai S, Yagishita T (2007) Microbial production of hydrogen and ethanol from glycerol-containing wastes discharged from a biodiesel fuel production plant in a bioelectrochemical reactor with thionine. J Biotechnol Bioeng 98:340–348
Ciptanto A (2009) Optimization on ethanol production from glycerol using Enterobacter aerogenes TISTR 1468. Master of Science in Biotechnology Prince of Songkla University.
Oh BR, Seo JW, Heo SY, Hong WK, Luo LH, Joe MH, Park DH, Kim CH (2011) Efficient production of ethanol from crude glycerol by a Klebsiella pneumonia mutant strain. Bioresour Technol 102:3918–3922
Vikromvarasiri N, Pisutpaisal N (2011) Fermentative conversion of glycerol to ethanol using mixed consortium. J Biotechnol 6:5–12
Wu KJ, Lin YH, Lo YC, Chen CY, Chen WM, Chang JS (2011) Converting glycerol into hydrogen, ethanol and diols with a Klebsiella sp. HE1 strain via anaerobic fermentation. J Taiwan Inst Chem Eng 42:20–25
Metsoviti M, Paramithiotis S, Drosinos EH, Galiotou-Panayotou M, Nychas GJE, Zeng AP, Papanikolaou S (2012) Screening of bacterial strains capable of converting biodiesel-derived raw glycerol into 1, 3-propanediol, 2, 3-butanediol and ethanol. J Eng Life Sci 12:57–68
Metsoviti M, Paraskevaidi K, Koutinas A, Zeng AP, Papanikolaou S (2012) Production of 1, 3- propanediol, 2, 3-butanediol and ethanol by a newly isolated Klebsiella oxytoca strain growing on biodiesel-derived glycerol based media. J Process Biochem 47:1872–1882
Nwachukwu RE, Shahbazi A, Wang L, Ibrahim S, Worku M, Schimmel K (2012) Bioconversion of glycerol to ethanol by a mutant Enterobacter aerogenes. AMB Express 2:1–6
Rossi DM, da Costa JB, de Souza EA, Peralba MDCR, Ayub MAZ (2012) Bioconversion of residual glycerol from biodiesel synthesis into 1, 3-propanediol and ethanol by bacteria from environmental consortia. Renew Energy 39:223–227
Varrone C, Giussani B, Izzo G, Massini G, Marone A, Signorini A, Wang A (2012) Statistical optimization of biohydrogen and ethanol production from crude glycerol by microbial mixed culture. J Inst J Hydrog Energy 37:16479–16488
Reungsang A, Sittijunda S, Angelidaki I (2013) Simultaneous production of hydrogen and ethanol from waste glycerol by Enterobacter aerogenes KKU-S1. J Hydrogen Energy 38:1813–1825
Varrone C, Liberatore R, Crescenzi T, Izzo G, Wang A (2013) The valorization of glycerol: economic assessment of an innovative process for the bioconversion of crude glycerol into ethanol and hydrogen. J Appl Energy 105:349–357
Adnan NAA, Suhaimi SN, Abd-Aziz S, Hassan MA, Phang LY (2014) Optimization of bioethanol production from glycerol by Escherichia coli SS1. Renew Energy 66:625–633
Haosagul S, Boonyawanich S, Pisutpaisal N (2019) Biomethane production from co-fermentation of agricultural wastes. Int J Hydrog Energy 44:5355–5364
Van Handel E (1961) Suggested modification of the micro determination of triglycerides. Clin Chem 7:249–251
Saqib AAN, Whitney PJ (2011) Differential behaviour of the dinitrosalicylic acid (DNS) reagent towards mono- and di-saccharide Sugars. Biomass Bioenergy 35:4748–4750
APHA, AWWA, WEF (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association, Washington
Lee SJ, Kim SB, Kang SW, Han SO, Park C, Kim SW (2012) Effect of crude glycerol-derived inhibitors on ethanol production by Enterobacter aerogenes. Bioprocess Biosyst Eng 35:85–92
Choi WJ, Hartono MR, Chan WH, Yeo SS (2011) Ethanol production from biodiesel-derived crude glycerol by newly isolated Kluyvera cryocrescens. J Appl Microbiol Biotechnol 89:1255–1264
Nwachukwu RE, Shahbazi A, Wang L, Worku M, Ibrahim S, Schimmel K (2013) Optimization of cultural conditions for conversion of glycerol to ethanol by Enterobacter aerogenes S012. AMB Express 3:12
Ward B, (2015) Bacterial energy metabolism. In Molecular Medical Microbiology Academic Press, 201-233.
Jitrwung R, Yargeau V (2015) Biohydrogen and bioethanol production from biodiesel-based glycerol by Enterobacter aerogenes in a continuous stir tank reactor. Int J Mol Sci 16:10650–10664
Thapa LP, Lee SJ, Yang X, Lee JH, Choi HS, Park C, Kim SW (2015) Improved bioethanol production from metabolic engineering of Enterobacter aerogenes ATCC 29007. Process Biochem 50:2051–2060
Sunarno JN, Prasertsan P, Duangsuwan W, Cheirsilp B, Sangkharak K (2019) Biodiesel derived crude glycerol and tuna condensate as an alternative low-cost fermentation medium for ethanol production by Enterobacter aerogenes. Ind Crop Prod 138:111451
Sunarno JN, Prasertsan P, Duangsuwan W, Cheirsilp B, Sangkharak K (2020) Improve biotransformation of crude glycerol to ethanol of Enterobacter aerogenes by two-stage redox potential fed-batch process under microaerobic environment. Biomass Bioenergy 134:105503
Szymanowska-Powałowska D (2015) The effect of high concentrations of glycerol on the growth, metabolism and adaptation capacity of Clostridium butyricum DSP1. Electron J Biotechnol 18:128–133
Da Silva GP, Mack M, Contiero J (2009) Glycerol: a promising and abundant carbon source for industrial microbiology. Biotechnol Adv 27:30–39
Acknowledgements
The authors are grateful to Trang Palm Oil Co., Ltd. (Trang, Thailand), for supporting the waste glycerol throughout the study.
Funding
The authors would like to thank King Mongkut's University of Technology North Bangkok for the financial support (grant no. KMUTNB-62-KNOW-08 and KMUTNB-63-KNOW-17).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Boonyawanich, S., Haosagul, S. & Pisutpaisal, N. Ethanol production from waste glycerol using glucose as co-carbon source. Biomass Conv. Bioref. 13, 2769–2778 (2023). https://doi.org/10.1007/s13399-021-01325-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01325-z


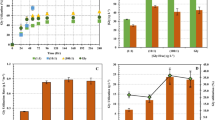


 ), 8.7 (
), 8.7 (  ), 23.7 (
), 23.7 (  ), 43.4 (
), 43.4 (  ), and 63.1 (
), and 63.1 (  ) g L−1 by E. aerogenes TISTR 1468. Data points show the average values (n=3). I-bars represent standard deviations
) g L−1 by E. aerogenes TISTR 1468. Data points show the average values (n=3). I-bars represent standard deviations
 ), 8.7 (
), 8.7 (  ), 23.7 (
), 23.7 (  ), 43.4 (
), 43.4 (  ) and 63.1 (
) and 63.1 (  ) g L-1 by E. aerogenes TISTR 1468. Data points show the average values (n=3). I-bars represent standard deviations.
) g L-1 by E. aerogenes TISTR 1468. Data points show the average values (n=3). I-bars represent standard deviations.



 ), 10:1 (
), 10:1 (  ), 100:1 (
), 100:1 (  ), glycerol (
), glycerol (  ), and glucose (
), and glucose (  ) by E. aerogenes TISTR 1468. The data points and histograms show the average values (n=3). I-bars represent standard deviations
) by E. aerogenes TISTR 1468. The data points and histograms show the average values (n=3). I-bars represent standard deviations