Abstract
This study aimed to determine the survival and antibacterial activity of Lactobacillus plantarum TISTR1465 encapsulated with Eleutherine americana oligosaccharide extract. Capsules were stored at 4 °C for 0, 2, and 4 weeks. The encapsulated cells were evaluated for their survival after sequential exposure to simulated gastric and intestinal juices, then evaluated in terms of their antibacterial activity. Survival of the encapsulated cells was higher than that of free cells at weeks 2 and 4. Highest levels of viable cells were observed with encapsulation in E. americana oligosaccharide extract. No surviving free cells were found in week 4. Yoghurt prepared with encapsulated cells showed less acidification than with free cells. Antibacterial activity of L. plantarum TISTR1465 before pH neutralisation against Clostridium perfringens ATCC13124, Staphylococcus aureus ATCC 25923, Escherichia coli ATCC 25922, and Salmonella Typhimurium ATCC13311 was higher than after pH neutralisation. Encapsulation by extrusion enhanced antibacterial activity of the cells against enteropathogenic bacteria. The antibacterial activity of encapsulated cells against Gram-positive bacteria was higher than that against Gram-negative bacteria. Results indicates that L. plantarum TISTR1465 encapsulated with E. americana oligosaccharide extract showed potential for application as a functional food additive.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Functional foods provide health benefits beyond traditional nutrients. Probiotics and/or prebiotics are natural additions that can enhance the functionality of food products. The market share of probiotics has been 30% of the worldwide functional market, which is estimated to be resulting in a $50 billion market [1]. Probiotics are live microorganisms which, when ingested in adequate numbers, confer beneficial effects to the host. Many lactobacilli fall into the category of Generally Regarded as Safe (GRAS) bacteria, and are used in probiotic foods. Moreover, lactobacilli produce substances that inhibit pathogens [2]. It is known that there are various factors that affect the viable number of probiotics, including gastrointestinal conditions, the food processing, and the storage temperature. International Dairy Federation (IDF) has suggested that the survival of probiotics should be at least 107 CFU/g (of the food product) at the time of consumption [3]. There is an increasing demand for probiotic-containing dairy products, and there are many studies of incorporating probiotics in yoghurt [4, 5] or in cheese [6, 7]. Yoghurts are the most popular dairy foods for the delivery of probiotics, due to their excellent nutritional values. However, the production of lactic acid by Lactobacillus delbrueckii subsp. bulgaricus or by Streptococcus thermophilus is the major factor reducing survival of probiotics in yoghurt [3].
Encapsulation by extrusion has been developed to protect and maintain live probiotics in food products and during gastrointestinal transit. Sodium alginate is the most commonly used encapsulating agent for probiotics. However, the effectiveness of sodium alginate is limited due to its porous nature that allows diffusion of other substrates into the beads. To overcome these problems, a combination of sodium alginate with prebiotic oligosaccharides may better protect probiotics in adverse conditions due to synbiosis [8]. Prebiotics refer to non-digestible oligosaccharides that improve the host health by increasing the number of bacteria in the colon [9]. Many commercial prebiotic oligosaccharides are synthesised by chemical and/or enzyme reactions. The potential use of oligosaccharides from Eleutherine americana bulb instead of commercial oligosaccharides is of high interest, since most consumers tend to prefer new prebiotics from natural sources [10]. E. americana bulb extracts have been used in foods [11, 12]. Moreover, an E. americana oligosaccharide extract has been used as a prebiotic that stimulates growth of the intestinal microbiota [10]. E. americana oligosaccharide extract has demonstrated resistance to low pH and partial tolerance to human α-amylase [13]. It has been previously found that encapsulation of Bifidobacterium longum with E. americana oligosaccharide extract increased its survival in simulated gastrointestinal conditions [13], in fresh milk tofu, and in pineapple juice [14]. It has been documented that encapsulation of probiotic lactobacilli with chitosan [15], pectin and whey protein [16],, or inulin [17] increases survival of those probiotics in gastrointestinal conditions and in yoghurt. Adding probiotic lactobacilli encapsulated with calcium alginate or chitosan increased antibacterial activity significantly more than by adding free cells [18, 19]. However, there has been no prior study on both survival and antibacterial activity of L. plantarum TISTR1465 encapsulated with a prebiotic oligosaccharide extract.
Therefore, the objectives of this study were to evaluate survival and antibacterial activity of L. plantarum TISTR1465 encapsulated with E. americana oligosaccharide extract.
Materials and methods
Probiotic bacteria
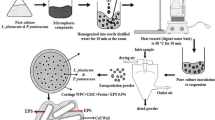
Lactobacillus plantarum TISTR1465 (Thailand Institute of Scientific and Technological Research) was cultivated in 50 mL of MRS broth (Merck, Darmstadt, Germany) and incubated at 37 °C for 24 h. The test samples were centrifuged at 10,000×g for 10 min, at 4 °C, and the pellets were washed with 0.85% (w/v) normal saline solution. The cell suspensions were adjusted to a final concentration of approximately 1 × 1010 CFU/mL in 10 mL of 0.1% (w/v) peptone. These samples were divided into two groups: one was used for encapsulation and the other as free cells (non-encapsulated L. plantarum TISTR1465).
Eleutherine americana oligosaccharide extract
Bulbs of E. americana were collected from Songkhla, Thailand. The extract was obtained by hot water extraction. Briefly, the bulbs were extracted with distilled water in ratio of 1:10 (w/v) at 80 °C for 1 h, and the filtrates were dried by freeze dryer (Flexi Dry, Germany). E. americana oligosaccharide extract was obtained using Saccharomyces cerevisiae BCC 12652 and 80% ethanol at 4 °C for 12 h [10]. A commercial fructo-oligosaccharide preparation (Sigma-Aldrich, Steinheim, Germany) was used as a reference.
Encapsulation of Lactobacillus plantarum TISTR1465 with Eleutherine americana oligosaccharide extract
Encapsulation procedure
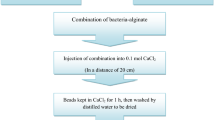
The encapsulation was done by extrusion as described previously by Krasaekoopt et al. [20] Briefly, two millilitres of cell suspension (1 × 1010 CFU/mL) was mixed with 16 mL of sterile 2% (w/v) sodium alginate solution (Fluka, Switzerland). Two millilitres of E. americana oligosaccharide extract (or of the commercial fructo-oligosaccharides) was added to the above mixtures to make the final concentration 1% (w/v). The final concentration of cells in this mixture was about 1 × 109 CFU/mL. This mixture was injected through a syringe needle size 23 G (Nipro, Japan) into sterilised 0.1 M CaCl2 solution (Difco, Dickinson, USA) and allowed to harden for 30 min. The capsules formed were washed twice with 0.85% (w/v) normal saline solution and stored in 0.1% (w/v) peptone solution (pH 6) at 4 °C until use. The free cells were used as a control culture. The capsule diameter was measured using a vernier calliper.
Encapsulation yield (%) and cell release (%)
One gram of capsules was transferred to 9 mL of 0.1 M phosphate buffer (pH 7.4) and homogenised in a stomacher for 5 min. The samples were then centrifuged at 10,000×g for 10 min, at 4 °C, and the supernatant was plated on MRS agar. Viable cell counts after incubation are shown as log colony-forming units per gram (log CFU/g). The encapsulation yield, which is a combined measurement of the efficacy of entrapment and the survival of viable cells during encapsulation, was calculated as proposed by Chavarri et al. [21]
where N is the number of viable entrapped cells released from the capsules, and No is the number of free cells added to the biopolymer mix during formation of the capsules.
One gram of capsules was added to 9 mL of 0.1 M phosphate buffer (pH 7.4) followed by incubation at 37 °C for 1 h. The number of viable cells in the suspension was then determined by the plate count as described above [22].
where N is the number of viable cells in the suspension released from the capsules, and No is the number of free cells added to the biopolymer mix during the formation of the capsules.
Textural properties
The capsules were subjected to a texture profile analyser (TPA), Stable Micro Systems model TA-XT (UK). Thirty grams of capsules was placed on a fixed bottom glass plate under the cylindrical steel probe. Automatic detection of contact by the probe with the capsules used threshold force of 0.005 N. The textural properties of hardness, cohesiveness, and springiness were obtained using the equipment’s Texture Expert Software [ 23].
Survival of encapsulated Lactobacillus plantarum TISTR1465 after sequential incubation in simulated human gastric and intestinal juices
The encapsulated L. plantarum TISTR1465 and the free cells were stored in a peptone solution (pH 6) at 4 °C for 0, 2, and 4 weeks, and their survival was determined after exposure to simulated gastric and intestinal juices as described by Sandoval-Castilla et al. [24] The gastric juice was mimicked by a HCl buffer of pH 2 containing the following: NaCl, 8 g; KCl, 0.2 g; Na2HPO4.2H2O, 8.25 g; NaH2PO4, 14.35 g; CaCl2.2H2O, 0.1 g; MgCl2.6H2O, 0.18 g; pepsin (Sigma-Aldrich), 3 g/L. The encapsulated L. plantarum (1 g) or the free cells (1 mL) were added to 9 mL of simulated gastric juice and incubated at 37 °C, and 150 rpm for 3 h in an orbital shaker incubator (Forma, Scientific, USA). The samples were added with 0.1 M phosphate buffer (pH 7.4), and the viable cell counts were obtained as described above. The samples after exposure to simulated gastric juice were centrifuged, washed with a 0.85% (w/v) normal saline solution, transferred in 9 mL of simulated intestinal juice (pH 7.4) containing NaCl, 6.50 g; KCl, 0.84 g; CaCl2, 0.22 g; NaHCO3, 1.39 g; bile salt (Difco), 3 g; and pancreatin (Sigma-Aldrich), 1 g/L, and incubated at 37 °C for 3 h in a rotary shaker. The samples were centrifuged, washed with normal saline solution, and the viable cell counts were determined as described above. Three replications of the experiment were done.
Survival of encapsulated Lactobacillus plantarum TISTR1465 and starter cultures in yoghurt
Preparation of yoghurt
Yoghurts were prepared following a method modified from Kailasapathy [25]. Five litres of ultra-high-temperature plain milk (UHT, Nongpho, Ratchaburi, Thailand) and skim milk powder were mixed with high-speed stirring, to make the total solids 180 g/L in yoghurt. Heating was continued at a constant 85 °C for 20 min. The sample was then cooled to 45 °C and 2% yoghurt starter culture (Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus) was added. Fermentation was carried out at 45 °C for 3 h until pH 4.8 was reached. After cooling to 4 °C, the mixture was divided into four equal fractions. Then, L. plantarum TISTR1465 encapsulated with E. americana oligosaccharide extract, or with commercial fructo-oligosaccharides, free cells, or control culture (without L. plantarum TISTR1465) were added aseptically into the mixture. The capsules and free cells were added to concentrations of about 1 × 109 CFU/g and 1 × 109 CFU/mL, respectively. The ratio of capsules or free cells to yoghurt was 1:10. The yoghurt samples were distributed to sterile plastic cups and packed with sterile plastic lids.
Physicochemical parameters of encapsulated Lactobacillus plantarum TISTR1465 during refrigerated storage
Water holding capacity
The water holding capacity (WHC) of the yoghurt was measured by centrifugation (K241R Medium Prime Centrifuge, Chichester, UK) of at 4500 rpm for 30 min at 10 °C for 10 g yoghurt sample. The following formula for WHC [26] uses as inputs w1 = weight of co-encapsulating agents after centrifugation, and w2 = yoghurt weight.
The water holding capacity was measured during the refrigerated storage at 0, 2, and 4 weeks.
Lactic acid determination
Production of lactic acid in yoghurt containing encapsulated L. plantarum TISTR1465, free cells, or control culture (without L. plantarum TISTR1465 and co-encapsulating agents) during the refrigerated storage for 0, 2, and 4 weeks was determined by the method of Holdeman and Moore [27] with slight modifications. The samples were centrifuged at 10,000×g for 15 min, at 4 °C, and the supernatant was subjected to high-performance liquid chromatography (Agilent 1100, Germany). The concentration of lactic acid was quantified by comparing the peak area to the standard. All experiments were performed in triplicates.
pH measurement
pH of the yoghurts was determined with a pH meter (Science analyst, Thailand) during storage at 4 °C for 0, 2, and 4 weeks.
Survival of encapsulated Lactobacillus plantarum TISTR1465 during refrigerated storage
Survival of encapsulated L. plantarum TISTR1465 and starter cultures while in refrigerated storage was studied by the method of Brinques and Ayub with slight modifications [28]. Briefly, encapsulated L. plantarum TISTR1465 (10 g) or free cells (10 mL) were added to 90 mL of 0.1% (w/v) peptone solution with pH 6. The samples were stored in a refrigerator at 4 °C for 0, 2, and 4 weeks. Cell counts were determined after depolymerisation in 0.1 M phosphate buffer as described above. All the experiments were performed in triplicates.
Antibacterial activity of encapsulated Lactobacillus plantarum TISTR1465
Agar well diffusion method
Encapsulated L. plantarum TISTR1465 and the free cells were stored in a peptone solution at 4 °C for 0 and 4 weeks. Their antibacterial activity against enteropathogenic bacteria was determined using an agar well diffusion method. The experiments mainly followed from previous reports [19, 29]. Aliquots (60 μL) of encapsulated L. plantarum TISTR1465 (1 × 109 CFU/g), free cells (1 × 109 CFU/mL), or control culture (without L. plantarum TISTR1465) were placed in wells 6 mm in diameters punched in cooled MRS agar plates, and were tested against 20 μL of Clostridium perfringens ATCC13124 (1 × 107 CFU/mL), Staphylococcus aureus ATCC25923 (1 × 107 CFU/mL), Escherichia coli ATCC25922 (1 × 107 CFU/mL), and Salmonella Typhimurium ATCC13311 (1 × 107 CFU/mL). The plates were incubated at 37 °C both under aerobic and anaerobic conditions for 24 h and 48 h, respectively. The sizes of the inhibition zones were measured. The same procedure was carried out using aliquot samples after pH neutralisation. All experiments were performed in triplicates.
Broth co-culture assay
Encapsulated L. plantarum TISTR1465 and the free cells were kept at 4 °C for 0 and 4 weeks. Their antibacterial activities against enteropathogenic bacteria were determined by broth co-culture assay. The experiments were mainly followed from previous reports [30]. The brain–heart infusion broth (BHIB) and pre-reduced BHIB supplemented with 0.5% yeast extract were used to culture facultatively anaerobic bacteria and anaerobic bacteria, respectively. One millilitre of enteropathogenic bacteria (1 × 107 CFU/mL), namely C. perfringens ATCC13124, S. aureus ATCC25923, E. coli ATCC25922, and S. Typhimurium ATCC13311 was co-cultured with aliquots (1 mL) of encapsulated L. plantarum TISTR1465 (1 × 109 CFU/g), or free cells (1 × 109 CFU/mL). The samples were incubated at 37 °C in both aerobic and anaerobic conditions for 24 h and 48 h, respectively. Controls without L. plantarum TISTR1465 were set up under the same conditions. The same procedure was carried out using the aliquot samples after pH neutralisation. The experiments were performed in triplicates and mean bacterial numbers in terms of colony forming units (CFU) were reported. The inhibitory effects of L. plantarum TISTR1465 on enteropathogenic bacteria were measured and compared with the control culture.
Statistical analysis
The data sets are reported as mean ± standard deviation (S.D.). Differences between treatments were examined for statistical significance by analysis for variance (ANOVA). The criterion for statistical significance was p < 0.05.
Results
Properties of L. plantarum TISTR1465 encapsulated with Eleutherine americana oligosaccharide extract
Encapsulation by an extrusion was applied to a combination of sodium alginate with E. americana oligosaccharide extract or with commercial fructo-oligosaccharides. The properties of the capsules are shown in Table 1. The capsules appeared white and round in shape. The type of co-encapsulating agents had no influence on the size of the capsules, encapsulation yield, or cell release. The diameters of the capsules made with alginate-E. americana oligosaccharide extract and with alginate-fructo-oligosaccharides were 1.59 and 1.47 mm, respectively. The encapsulation yields of capsules made with alginate-E. americana oligosaccharide extract and alginate-fructo-oligosaccharides were 93.52% and 94.21%, respectively. About 93.87–94.15% of the cells in the capsules was released within 1 h.
The textural properties of the capsules were affected by the biopolymer composition. The results show that the capsules made with alginate-E. americana oligosaccharide extract indicated significantly higher hardness than those made by alginate-fructo-oligosaccharides (p < 0.05), but these had comparable cohesiveness and springiness. The hardness of the capsules made with alginate-E. americana oligosaccharide extract was 2.10 N (Table 1).
Survival of Lactobacillus plantarum TISTR1465 encapsulated with Eleutherine americana oligosaccharide extract after sequential exposure to simulated human gastric and intestinal juices
Survival of L. plantarum TISTR1465 encapsulated with E. americana oligosaccharide extract, or with commercial fructo-oligosaccharides, and of free cells in peptone solution (pH 6), after sequential exposure to simulated gastric and intestinal juices, is shown in Table 2. The capsules made by extrusion were stored at 4 °C for 0, 2, and 4 weeks. Initially, the viable count of L. plantarum TISTR1465 was in the range 9.35–9.51 log CFU/g. The survival of encapsulated cells before and after sequential incubation in the simulated gastric and intestinal juices was higher than that of the free cells at weeks 0, 2, and 4 (p < 0.05).
The number of viable cells decreased with refrigerated storage for 4 weeks. The highest count of viable cells at week 4 was observed for encapsulation with E. americana oligosaccharide extract. The survival of L. plantarum TISTR1465 encapsulated with E. americana oligosaccharide extract declined in the peptone solution from 9.51 to 8.26 log CFU/g, respectively, when exposed to the simulated juices. However, none of the free cells survived at week 4 when exposed to the simulated juices.
Physicochemical parameters of Lactobacillus plantarum TISTR1465 encapsulated with Eleutherine americana oligosaccharide extract in yoghurt
The physicochemical parameters in yoghurt (water holding capacity, production of lactic acid, and pH) of L. plantarum TISTR1465 encapsulated with E. americana oligosaccharide extract, with commercial fructo-oligosaccharides, and of free cells are presented in Fig. 1. The capsules were kept in yoghurt at 4 °C for 0, 2, and 4 weeks. The water holding capacity (WHC) of yoghurt was in the range of 62–64% during 4 weeks of refrigerated storage. The initial concentration of lactic acid in yoghurt ranged from 1.43 to 1.50 g/L. The yoghurt containing free cells had significantly higher acidity than the samples with encapsulated L. plantarum TISTR1465. The amount of lactic acid in the samples containing encapsulated L. plantarum TISTR1465 remained constant at 4 °C for 0, 2, and 4 weeks. The highest production of lactic acid was observed in yoghurt containing free cells, increasing over the range 1.50–2.53 g/L. The concentration of lactic acid in yoghurt containing encapsulated L. plantarum TISTR1465 ranged from 1.43 to 1.55 g/L. The pH of yoghurt containing encapsulated L. plantarum TISTR1465 remained constant due to the high buffering capacity of the samples. However, pH of the samples with free cells decreased during 4 weeks of refrigerated storage.
Physicochemical parameters of Lactobacillus plantarum TISTR1465 encapsulated with Eleutherine americana oligosaccharide extract in yoghurt during refrigerated storage. (a) Water holding capacity, (b) production of lactic acid, and (c) pH. L. plantarum TISTR1465 encapsulated with E. americana oligosaccharide extract (A-E), L. plantarum TISTR1465 encapsulated with commercial fructo-oligosaccharides (A-C), free cells, and control (without L. plantarum TISTR1465). Bars represent standard deviations of the means according to repeated experiments
Survival of Lactobacillus plantarum TISTR1465 encapsulated with Eleutherine americana oligosaccharide extract and starter cultures in yoghurt
The effects of refrigerated storage on the survival of L. plantarum TISTR1465 encapsulated with E. americana oligosaccharide extract, or with commercial fructo-oligosaccharides, the free cells, and the starter cultures are shown in Table 3. The number of starter cultures decreased during refrigerated storage for 4 weeks (p < 0.05). High cell viability in yoghurt (pH 4.8) in the range 9.51–9.70 log CFU/g was achieved with the encapsulation by extrusion during storage at 4 °C, for 0, 2, and 4 weeks. The viable counts of encapsulated cells were higher than those of the free cells at weeks 0, 2, and 4 (p < 0.05). The highest levels of viable cells were observed for encapsulation with E. americana oligosaccharide extract, with a decrease from 9.70 to 8.45 log CFU/g, at week 4. However, no free cells survived at week 4.
Antibacterial activity of encapsulated Lactobacillus plantarum TISTR1465
Agar well diffusion method
Aliquots of L. plantarum TISTR1465 encapsulated with E. americana oligosaccharide extract, or with commercial fructo-oligosaccharides, and free cells were tested for antibacterial activity by using an agar well diffusion method (Fig. 2). The capsules were kept in a peptone solution at 4 °C for 0 and 4 weeks. The antibacterial activity of the samples before pH neutralisation against C. perfringens ATCC 13124 and S. aureus ATCC25923 was better than that against E. coli ATCC 25922 and S. Typhimurium ATCC13311. The encapsulated cells had significantly larger inhibition zones than the samples with free cells (p < 0.05). The encapsulated cells inhibited the growth of C. perfringens ATCC 13124 with an inhibition zone 12.8 mm in diameter, and inhibited also S. aureus ATCC25923 (13.0 mm). However, the free cells did not inhibit the growth of enteropathogenic bacteria at week 4. The inhibition zones of aliquots of the encapsulated cells after pH neutralisation were smaller than before neutralisation. The encapsulated cells inhibited the growth of C. perfringens ATCC 13124 with inhibition zone diameter 7.5 mm and the growth of S. aureus ATCC25923 (7.4 mm). However, the encapsulated and free cells did not inhibit the growth of E. coli ATCC 25922 or S. Typhimurium ATCC13311 at week 4.
Antibacterial activity of Lactobacillus plantarum TISTR1465 before (a) and after (b) pH neutralisation against Clostridium perfringens ATCC13124, Staphylococcus aureus ATCC25923, Escherichia coli ATCC 25922, and Salmonella Typhimurium ATCC13311 by agar well diffusion method. Alginate-E. americana oligosaccharide extract (A-E; white bars), alginate-commercial fructo-oligosaccharides (A-C; bar with diagonal stripes), free cells (white bar with black dots), and control (without L. plantarum TISTR1465; black bars). Means ± standard deviation for triplicates are illustrated. Different lowercase letters in co-encapsulating agents indicate significant differences (p < 0.05)
Broth co-culture assay
L. plantarum TISTR1465 encapsulated with E. americana oligosaccharide extract, or with commercial fructo-oligosaccharides, and free cells were tested for antibacterial activity by broth co-culture assay (Fig. 3). The capsules were stored in a peptone solution at 4 °C for 0 and 4 weeks. The antibacterial activity of the samples before pH neutralisation against C. perfringens ATCC 13124 and S. aureus ATCC25923 was better than that against E. coli ATCC25922 and S. Typhimurium ATCC13311. The encapsulated cells had significantly higher antibacterial activity than the samples with free cells (p < 0.05). The encapsulated cells inhibited the growth of C. perfringens ATCC 13124 which was estimated by the count reduction from 8.55 to 6.40 log CFU/mL, and S. aureus ATCC25923 counts decreased from 9.52 to 6.47 log CFU/mL. However, the free cells did not inhibit the growth of enteropathogenic bacteria at week 4. The antibacterial activity of the samples after pH neutralisation against C. perfringens ATCC 13124 and S. aureus ATCC25923 slightly decreased, compared with control. However, free cells did not inhibit the growth of enteropathogenic bacteria at week 4.
Antibacterial activity of Lactobacillus plantarum TISTR1465 before (a) and after (b) pH neutralisation against Clostridium perfringens ATCC13124, Staphylococcus aureus ATCC25923, Escherichia coli ATCC 25922, and Salmonella Typhimurium ATCC13311 by broth co-culture assay. Alginate-E. americana oligosaccharide extract (A-E; white bars), alginate-commercial fructo-oligosaccharides (A-C; bar with diagonal stripes), free cells (white bar with black dots), and control (without L. plantarum TISTR1465; black bars). Means ± standard deviation for triplicates are illustrated. Different lowercase letters in co-encapsulating agents indicate significant differences (p < 0.05)
Discussion
Encapsulation is recognised as a powerful way to improve survival of probiotic lactic acid bacteria [8]. Here, capsules were retained resistant to disintegration in simulated gastric juice (pH 2). Phosphate ions in simulated intestinal juice (pH 7.4) degrade the capsule membrane density and this enhances permeability through the outer membrane and release of the bacteria. Addition of oligosaccharide extract positively influenced the number of cells released at an appropriate time. [31] Our results indicate contrasting cross-linking reactions between alginate-E. americana oligosaccharide extract and alginate-fructo-oligosaccharide polymeric networks, resulting in different responses to mechanical deformation.
The compacted alginate layer can enhance the permeability of hydrogels and increase the survival of encapsulated cells. In addition, probiotics encapsulated with prebiotic oligosaccharides extract that tend to decrease the pore sizes have a favourable environment for viability of the cells [32]. The layered capsules constructed by extrusion may have afforded additional physical protection against simulated gastric and intestinal juices. The results showed that the 2% sodium alginate and 1% E. americana oligosaccharide extract concentrations gave the maximum protection to encapsulated cells against these adverse environmental conditions. It is expected that factors such as the concentration of encapsulating agents affect the survival of encapsulated cells [33]. The good resistance of E. americana oligosaccharide extract to simulated gastric and intestinal juices might be due to α and –β glycosidic linkages in the oligosaccharide extract [10]. The acid and enzyme tolerances of probiotic cells depend on the pH profile of H+-ATPase in composition of cytoplasmic cell membranes [20]. Accordingly, encapsulating probiotics with hi-maize and chitosan [34], with xanthan and chitosan [35],, and with inulin and chitosan [36] have improved their survival over that of free cells after exposure to simulated digestive juices.
The type of co-encapsulating agents had no influence on the survival characteristics. The WHC indicates the ability of the proteins and fat globules to retain water within the yoghurt structure [26]. The yoghurt starter cultures with Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus could ferment lactose into lactic acid. The yoghurts are susceptible to pH decay that causes whey separation, and this also affects probiotic survival [25]. Therefore, encapsulation could decrease such problems from acidification and could maintain encapsulated probiotics during refrigerated storage. This could be due to the slow uptake of nutrients and the slow release of metabolites across the co-encapsulating agent shells of the capsules [37]. Accordingly, Lactobacillus acidophillus encapsulated with pectin and whey protein caused significantly less acidity in yoghurt than free cells during refrigerated storage [5, 16].
The formation of a hydrogel around the cell pellet provided probiotic protection. The diminished pore sizes hindered the interactions of bacterial cells and yoghurt during storage. At low temperatures, enzyme activity in the free cells slows and the fluidity of cell membrane is decreased, and this interferes with the transport mechanisms [38]. Moreover, hydrogen peroxide produced by probiotic bacteria, low pH, fermentation temperature, starter culture, and availability of growth factors might be affecting the survival of probiotic bacteria in yoghurt. Partially damaged capsules were broken in the yoghurt permitting the simulated juices access into the capsules [39]. Several authors have found that different probiotic strains, encapsulation techniques, and co-encapsulating agents could improve the survival rates of encapsulated probiotic bacteria over that of free cells [16, 24, 28]. Accordingly, encapsulated probiotics with chitosan [28] and with pectin [24] have improved survival over that of free cells in yoghurt during refrigerated storage.
The survival of L. plantarum TISTR1465 in the simulated gastric and intestinal juices or yoghurt positively correlated with the mechanical strength of capsules. The capsules with higher mechanical strength could better resist the osmotic effects, prevent access by digestive juices, and reduce cell damage [31]. The capsules provide a favourable environment for cell growth in which higher biomass yield can be obtained as a result of substrate digestion to heat [40]. Accordingly, previous studies have demonstrated that probiotic cell survival in simulated gastric and intestinal juices was positively correlated with the mechanical properties of capsules [24, 41].
The antibacterial activity was enhanced by the cell protection that increased the production of inhibitory substances. The antimicrobial metabolites produced by probiotic lactic acid bacteria can be classified into two groups: organic acids with a broad spectrum activity, and bacteriocins with a narrow spectrum activity [29]. In this study, the antibacterial activity of aliquots of encapsulated cells before and after pH neutralisation was evaluated to determine if the inhibitory effect was due to organic acid production or due to other antimicrobial compounds. Co-encapsulating agents such as E. americana oligosaccharides and commercial fructo-oligosaccharides have potential use as prebiotics to produce growth stimulation of lactobacilli and increase organic acid productions. The main antibacterial activity resulted from lactic acid and acetic acid causing low pH [10]. Accordingly, lactobacilli encapsulated with sodium alginate [18], or with chitosan [19], have improved antibacterial activity against several pathogenic bacteria. The antibacterial activity was due to the production of organic acids.
There are observations on the capacity of heterofermentative probiotics lactic acid bacteria to ferment glucose via 6-phosphogluconate/phosphoketolase (6-PG/PK) pathway. Undissociated forms of organic acids are known to penetrate and dissociate it inside the cytoplasm of pathogens, thus diminishing the intracellular with accumulation of ionised organic acids, eventually leading to the death of the pathogens [29].
In conclusion, L. plantarum TISTR1465 encapsulated with E. americana oligosaccharide extract was more efficacious than the free cells, after sequential exposure to simulated gastric and intestinal juices and refrigerated storage in yoghurt. The encapsulated cells caused less acidification during refrigerated storage than the free cells. The encapsulation with alginate and E. americana oligosaccharide extract improved the antibacterial activity of L. plantarum TISTR1465 far beyond that of the free cells. Further studies in animal models would be required before application as a functional food additive for human consumption.
References
Stanton C, Ross RP, Fitzgerald GF, Sinderen DV (2005) Fermented functional foods based on probiotics and their biogenic metabolites. Curr Opin Biotechnol 16:198–203
Food and Agriculture Organization of the United Nations and World Health Organization Report (2001) Evaluation of health and nutritional properties of probiotics in food including powdered milk with live lactic acid bacteria. http://www.fao.org/es/esn/probio/probio.htm; Accessed 15.08.15
Shah NP (2007) Functional cultures and health benefits. Int Dairy J 17:1262–1277
Li C, Wang CL, Sun Y, Li AL, Liu F, Meng XC (2016) Encapsulation of Lactobacillus rhamnosus GG by transglutaminase cross-linked soy protein isolate to improve survival in simulated gastrointestinal conditions and yoghurt. J Food Sci 81(7):M1726–MM734
Shoji AS, Oliveira A, Balieiro JC et al (2013) Viability of Lactobacillus acidophilus capsules and their application to buffalo milk yoghurt. Food Bioprod Process 91(2):83–88
Amine KM, Campagne CP, Raymond Y et al (2014) Survival of microencapsulated Bifidobacterium longum in cheddar cheese during production and storage. Food Control 37:193–199
Rodriguez-Huezo ME, Estrada-Fernandez AG, Garia-Almendarez BE et al (2014) Viability of Lactobacillus plantarum entrapped in double emulsion during Oaxaca cheese manufacture, melting and simulated intestinal conditions. LWT Food Sci Technol 59(2):768–773
Nazzaro F, Fratianni F, Coppola R, Sada A, Orlando P (2009) Fermentative ability of alginate-prebiotic encapsulated Lactobacillus acidophilus and survival under simulated gastrointestinal conditions. J Funct Foods 1:319–323
Gibson GR, Probert HM, Van Loo J, Rastall RA, Roberfroid MB (2004) Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev 17:259–275
Phoem AN, Voravuthikunchai SP (2013) Eleutherine americana as a growth promotor for infant intestinal microbiota. Anaerobe 20:14–19
Ifesan BOT, Siripongvutikorn S, Hutadilok TN, Voravuthikunchai SP (2009) Evaluation of the ability of Eleutherine americana crude extract as natural food additive in cooked pork. J Food Sci 74(7):M352–M357
Ifesan BOT, Siripongvutikorn S, Voravuthikunchai SP (2009) Application of Eleutherine americana crude extract in homemade salad dressing. J Food Prot 72:650–655
Phoem AN, Chanthachum S, Voravuthikunchai SP (2015) Preparation of Eleutherine americana-alginate complex capsules and application in Bifidobacterium longum. Nutrients 7:831–848
Phoem AN, Chanthachum S, Voravuthikunchai SP (2015) Applications of microencapsulated Bifidobacterium longum with Eleutherine americana in fresh milk tofu and pineapple juice. Nutrients 7:2469–2484
Krasaekoopt W, Watcharapoka S (2014) Effect of addition of inulin and galactooligosaccharides on the survival of microencapsulated probiotics in alginate beads coated with chitosan in simulated digestive system, yogurt and fruit juice. LWT Food Sci Technol 57(2):761–766
Ribeiro MC, Chaves KS, Gebara C, Infante FNS, Grosso CRF, Gigante ML (2014) Effect of encapsulation of Lactobacillus acidophilus LA-5 on physicochemical, sensory and microbiological characteristics of stirred probiotic yoghurt. Food Res Int 66:424–431
Pinto SS, Fritzen-Freire CB, Munoz IB, Prudencio ES, Amboni RD (2012) Effects of the addition of microencapsulated Bifidobacterium BB-12 on the properties of frozen yogurt. J Food Eng 111(4):563–569
Brachkova MI, Duarte MA, Pinto JF (2010) Preservation of viability and antibacterial activity of Lactobacillus spp. in calcium alginate beads. Eur J Pharm Sci 41(5):589–596
Trabelsi I, Ayadi D, Bejar W, Bejar S, Chouayekh H, Ben Salah R (2014) Effects of Lactobacillus plantarum immobilization in alginate coated with chitosan and gelatin on antibacterial activity. Int Bio Macromole 64:84–89
Krasaekoopt W, Bhandari B, Deeth H (2004) The influence of coating materials on some properties of alginate beads and survivability of microencapsulated probiotic bacteria. Int Dairy J 14:737–743
Chavarri M, Maranon I, Ares R, Ibanez FC, Marzo F, Villaran MDC (2011) Encapsulation of a probiotic and prebiotic in alginate-chitosan capsules improves survival in simulated gastro-intestinal conditions. Int J Food Microbiol 142:185–189
Rao A, Shiwnarain N, Maharaj I (1989) Survival of microencapsulated Bifidobacterium pseudolongum in simulated gastric and intestinal juices. Can Inst Food Sci Technol 22:345–349
Bourne MC (2002) Food texture and viscosity: concept and measurement, 2nd edn. Academic Press, New York Texture, viscosity, and food
Sandoval-Castilla O, Lobato-Calleros C, Garcia-Galindo HS, Alvarez-Ramirez J, Vernon-Carter EJ (2010) Textural properties of alginate-pectin beads and survivability of entrapped Lactobacillus casei in simulated gastrointestinal conditions and in yoghurt. Food Res Int 43:111–117
Kailasapathy K (2006) Survival of free and encapsulated probiotic bacteria and their effect on the sensory properties of yoghurt. LWT Food Sci Technol 39:1221–1227
Ranadheera CS, Evans CA, Adams MC, Baines SK (2012) Probiotic viability and physic-chemical and sensory properties of plain and stirred fruit yoghurts made from goat’s milk. Food Chem 135:1411–1418
Holdeman LV, WEC M (1977) Anaerobe laboratory manual. Virginia Polytechnic Institute and State University, Blacksburg 132 pp
Brinques GB, Ayub MAZ (2011) Effect of encapsulation on survival of Lactobacillus plantarum in simulated gastrointestinal conditions, refrigeration, and yogurt. J Food Eng 103:123–128
Tejero-Sariñena S, Barlow J, Costabile A, Gibson GR, Rowland I (2012) In vitro evaluation of the antimicrobial activity of a range of probiotics against pathogens: evidence for the effects of organic acids. Anaerobe 18(5):530–538
Schoster A, Kokotovic B, Permin A, Pedersen PD, Bello FD, Guardabassi L (2013) In vitro inhibition of Clostridium difficile and Clostridium perfringens by commercial probiotic strains. Anaerobe 20:36–41
Zhao M, Qu F, Wu Z, Nishinari K, Phillips GO, Fang Y (2017) Protection mechanism of alginate capsules with different mechanical strength for Lactobacillus plantarum ST-III. Food Hydrocoll 66:396–402
Shi LE, Li Z, Li D, Xu M, Chen H, Zhang Z (2013) Encapsulation of Lactobacillus bulgaricus in carrageenan-locust bean gum coated milk microspheres with double layer structure. LWT Food Sci Technol 54(1):147–151
Chandramouli V, Kailasapathy K, Peiris P, Jones M (2004) An improved method of encapsulation and its evaluation to protect Lactobacillus spp. in simulated gastric conditions. J Microbiol Methods 56(1):27–35
Etchepare MDA, Raddatz GC, Moraes Flores EMD et al (2016) Effect of resistant starch and chitosan on the survival of Lactobacillus acidophilus microencapsulated with sodium alginate. LWT Food Sci Technol 65:511–517
Argin S, Kofinas P, LoY M (2014) The cell release kinetics and swelling behavior of physically crosslinked xanthan-chitosan hydrogels in simulated gastrointestinal conditions. Food Hydrocoll 40:138–144
Darjani P, Nezhad MH, Kadkhodaee R, Milani E (2016) Influence of prebiotic and coating materials on morphology and survival of a probiotic strain of Lactobacillus casei exposed to simulated gastrointestinal conditions. LWT Food Sci Technol 73:162–167
Homayouni A, Azizi A, Ehsani MR, Yarmand MS, Razavi SH (2008) Effect of microencapsulation and resistant starch on the probiotic survival and sensory properties of synbiotic ice cream. Food Chem 111:50–55
Corcoran B, Stanton C, Fitzgerald G, Ross R (2008) Life under stress: the probiotic stress response and how it may be manipulated. Curr Pharm Des 14:1382–1399
Dave RI, Shah NP (1997) Viability of yoghurt and probiotic bacteria in yoghurts made from commercial starter cultures. Int Dairy J 7(1):31–41
Ma J, Qi WT, Yang LN, Yu WT, Xie YB, Wang W, Ma XJ, Xu F, Sun LX (2007) Microcalorimetric study on the growth and metabolism of microencapsulated microbial cell culture. J Microbiol Methods 68:172–177
Zhao M, Qu F, Cai S, Fang Y, Nishinari K, Phillips GO (2015) Encapsulation of Lactobacillus acidophilus CGMCC1.2686 correlation between bacteria survivability and physical properties of capsules. Food Biophys 10(3):292–299
Acknowledgments
We thank Natural Product Research Center of Excellence, Prince of Songkla University, for providing E. americana oligosaccharide extract. We acknowledge Peter Charge, George William Gibson, and Associate Professor Dr. Seppo Karrila for assistance with manuscript preparation.
Funding
This research has been supported by Research Fund of Songkhla Rajabhat University, Fiscal Year 2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Susana Saad
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Phoem, A.N., Mayiding, A., Saedeh, F. et al. Evaluation of Lactobacillus plantarum encapsulated with Eleutherine americana oligosaccharide extract as food additive in yoghurt. Braz J Microbiol 50, 237–246 (2019). https://doi.org/10.1007/s42770-018-0017-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-018-0017-2