Abstract
Municipal wastewater sludge can be pyrolyzed as biochars to better use nutrients and stabilize carbon compared with other typical technologies, such as landfill and incineration. However, sludge-derived biochars might contain large amounts of potentially toxic elements (PTEs), such as Zn, Cu, Cr, Ni, Pb, and As. The stability of PTEs in biochars might be improved by higher pyrolytic temperatures, which can be further improved by different modifications. Herein, PO4-modification at 300 °C and Cl-modification at 700 °C were carried out, respectively, to enhance the stability of PTEs. Various leaching tests have been performed to assess the stability of PTEs in biochars, including the synthetic precipitation leaching procedure (SPLP), toxicity characteristic leaching procedure (TCLP), diethylenetriamine pentaacetate (DTPA) extraction, and in vitro simple bioaccessibility extraction test (SBET). The morphological structure, elemental mapping, and mineral formation of the pristine and modified biochars were studied by scanning electron microscopy–energy-dispersive X-ray spectroscopy (SEM–EDS) and X-ray diffraction (XRD). Our results suggested that the leachability, mobility, plant-availability, and bioaccessibility of most PTEs were decreased by pyrolysis, yet the total contents of PTEs were elevated, especially at 700 °C. Generally, modification by phosphates and MgCl2 enhanced the stability of PTEs in biochars. Nevertheless, it should be noted that higher bioaccessibility of PTEs was observed in biochars of P-modification than Cl-modification, which is associated with the dissolution of phosphate precipitates under acidic conditions (pH<2). Additionally, Cl-modification leads to higher plant-available Zn and Cu and bioaccessible Zn compared with the unmodified biochar produced at 700 °C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sewage sludge is a typical solid waste produced by wastewater treatment plants. The generation of municipal sewage sludge has steadily increased worldwide, especially in some developing countries. The annual production of dry sludge was 6.5 million in 2015 in China, and was the largest producer of municipal solid waste worldwide [1,2,3]. Landfills, dumping into the ocean, incineration, composting, and utilization (e.g., building materials and fertilization) are common ways to treat sludge. Among them, landfills are the most frequently used technology in developing countries, occupy large land areas and might lead to the releas of large amounts of greenhouse gases, such as CH4 [3, 4]. Incineration is also frequently proposed because it can greatly reduce the mass and volume of sludge and generate power. However, unwanted NOX, SO2, CO2, PM2.5, and even dioxin might be generated if the incineration procedure is not well designed or controlled [5]. Sewage sludge contains certain amounts of biodegradable organic matter, dissolved carbon, and nutrients (e.g., P, N, and K); thus, it is a huge waste to directly discharge into landfills or burn in incineration [1]. Nevertheless, the direct use of sludge in soil can cause unintentionally adverse consequences to human beings, animals, and plants in the surroundings, because it contains large amounts of harmful inorganic and organic components, such as potentially toxic elements (PTEs), pesticides, herbicides, and pathogens [6].
Pyrolysis is a good way to reuse municipal sewage sludge by producing high-value products, i.e., biochar, bio-oil, and bio-gas, and avoiding the release of gaseous pollutants and particles [7]. For example, the pyrolytic temperature normally within 300–800 °C is high enough to greatly reduce the volume of sludge, kill pathogenic microorganisms, and decompose some organic contaminants. One of the products in pyrolysis, biochar, is a multifunctional material that sequesters carbon [7, 8]. Recently, it has been frequently reported to act as absorbents for removing pollutants in water treatments, for example, phosphate, PTEs, and other organic pollutants [9,10,11]. It can also act as soil amendment for removing or immobilizing organic or inorganic contaminants [12,13,14]. Previous studies showed that the sludge-derived biochars can be used to enhance the availability of nutrients, alkalinity, water holding capacity, and eventually fertility of soil [15,16,17]. For example, Ibrahim et al. [4] found that the application of sludge biochars produced at 550 °C enhanced soil pH, total N, total C, and dissolved organic carbon and successfully enhanced the production of Phaseolus vulgaris L.
However, biochars produced from sewage sludge contain larger amounts of PTEs than other plant-based feedstocks, such as As, Cd, Cr, Cu, Zn, and Se [8]. Herein, many studies have investigated the effects of application on plant-availability. Hossain et al. [18] found that the application (10 t ha−1) of a sludge biochar produced at 550 °C increased the yield of cherry tomato by 64% compared with soil samples with and without fertilizers, owing to the increased amounts of available nutrients, such as P and N. It should be noted that the PTEs, e.g., Cd, Cu, and Zn, released from the biochars are plant-available for cherry tomato, yet the amounts in the fruit were still below the maximum permitted concentrations of Australian food standard limitations.
After pyrolysis, the PTEs are enriched due to the loss of some organic components of the sludge and thermostability of the PTEs, which might lead to possible leaching or adverse consequences, especially under changed conditions in soil. Jin et al. [6] studied the speciation of Cu, Zn, Pb, Cr, Mn, and Ni in sludge-derived biochars via a sequential extraction proposed by the Commission of the European Communities Bureau of Reference (BCR). Their results suggested that the potential risks caused by PTEs can be significantly reduced owing to their transformation into stable residual fractions with increasing pyrolytic temperatures. A higher pyrolysis temperature, i.e., 600 °C in this study, is preferred for reducing the hazards of PTEs. The study also suggested that although the speciation of PTEs changed to more stable phases, long-term monitoring works should be performed when sludge-derived biochar is applied to soil. Wang et al. [1] studied the feasibility of combining hydrothermal treatment with pyrolysis to treat sewage sludge at 300–700 °C, using decomposed extracellular polymeric substances in the sludge to dewater. This work also suggested that a higher temperature of the combined hydrothermal treatment with pyrolysis can reduce the liable fractions of Cu, Zn, Cr, Ni, Pb, and Cd based on the results of BCR sequential extraction compared with direct pyrolysis.
However, the PTEs of sludge are enriched during pyrolysis, and their large amounts in biochars are still a problem. For this reason, sludge-based organic waste cannot be directly used in the soil because of the potential release of PTEs. Additionally, the speciation of PTEs could change under varied environmental conditions. Therefore, reducing the amounts of PTEs in sludge biochars during the pyrolysis process has become a promising option. Xia et al. [19] tried the addition of Cl-containing chemicals during pyrolysis, such as NaCl, KCl, MgCl2, and CaCl2, because of the successful reduction of PTEs in incineration by adding Cl-containing chemicals in previous studies. The addition of MgCl2 significantly removed Zn, Mn, Cu, and Pb in the process of pyrolysis, especially at 700 °C with a dosage of 80 g (Cl)/kg (dry sludge), and enhanced the availability of P. Likewise, Shi et al. [20] found that phosphate-assisted pyrolysis can enhance the stability of Pb by forming Pb phosphates supported by the results of X-ray diffraction (XRD) and sequential extraction. Both the bioavailability and leachability of Pb have been reduced by modified pyrolysis. Similarly, PTEs, especially for that can form stable phosphate precipitates can be readily immobilized in biochars.
Therefore, in the current study, two different modification methods, one using MgCl2 and the other using PO4-containing chemicals, were conducted. To distinguish the two methods well based on their mechanisms, PO4-modification was performed at 300 °C to avoid the gasification of PTEs, while Cl-modification was performed at 700 °C. Such a high temperature of 700 °C is beneficial for metal volatilization. We mainly focused on six PTEs in biochars, including Zn, Cu, Cr, Ni, Pb, and As, which are commonly found in municipal sludge. The leachability, mobility, plant-availability, and bioaccessibility will be systematically evaluated after the two prevalent modification methods are applied. The findings of this study may help establish a reference method for evaluating the stability of PTEs in biochar.
Materials and methods
Biochar production
The sludge was collected from a sewage wastewater treatment plant that used cyclic-activated system technology in Guangdong Province, China. The collected sewage sludge was air-dried at room temperature for 3 days to remove extra water content, and then dried in an oven at 105 °C for over 24 h until it reached a constant weight. The dried sludge samples were then ground and passed through a 0.15-mm sieve, referred as SS.
Two different modification methods were adopted to enhance the stability of PTEs of the sludge biochars in this study, i.e., MgCl2 and Ca(H2PO4)2, which were simplified as Cl-modification and PO4-modification, respectively. The Cl-modified biochar was produced by mixing the sludge powder with MgCl2 at a ratio of 80 g (Cl):kg (dry sludge), and then pyrolyzed at 700 °C with a heating rate of 5 °C min−1 for 4 h under N2 flushing (0.4 L min−1) in a tube furnace, referring as Cl-700BC [19]. The PO4-modified biochar was prepared by mixing the SS with Ca(H2PO4)2 at 1:10 by weight for 24 h [20]. In brief, 10 g dry sludge was added to the 1 mol L−1 Ca(H2PO4)2 solution and stirred for 12 h, referring as P-300BC. The mixture was then dried at 100 °C until dry and pyrolyzed at 300 °C under N2 flushing (0.25 L min−1) for 3 h. For a better comparison, two additional sludge biochars were produced under the same pyrolytic conditions without further modification, i.e., one was produced at 700 °C for 4 h (similar to Cl-700BC) and the other is produced at 300 °C for 3 h (similar to P-300BC), which were referred as 700BC and 300BC, respectively. All the biochars were homogeneously mixed, ground, passed through a 0.15-mm sieve, and stored in sealed bags after cooling to room temperature for subsequent experiments.
Leaching experiments
The stability of PTEs of SS and biochars was evaluated from four aspects: mobility, leachability, plant-availability, and bioaccessibility. The mobility of PTEs was evaluated by the synthetic precipitation leaching procedure (SPLP, USEPA Method 1312). The SPLP solution was prepared by concentrated H2SO4 and HNO3 at 60%/40% (in weight) at pH 4.20±0.05 adjusted by 0.1 mol L−1 NaOH [21]. The SS and biochars were mixed with the SPLP solution at a solid-to-liquid ratio of 1:20 g mL−1 for 18 h in an end-over-end rotator at 30 rpm.
The leachability of SS and biochars was studied by the toxicity characteristic leaching procedure (TCLP, USEPA Method 1311). The extractant was prepared by 0.1 mol L−1 glacial acetic acid with pH adjustment to 2.88±0.05 by 0.1 mol L−1 NaOH owing to the slightly high alkalinity of biochars [22]. Similar to SPLP, the extractant was mixed with SS or biochars at a solid-to-liquid ratio of 1:20 g mL−1 for 18 h in an end-over-end rotator at 30 rpm.
The plant-availability of PTEs in the SS and biochar samples was studied by mixing a reagent comprised of 0.005 mol L−1 diethylenetriamine pentaacetic acid (DTPA), 0.1 mol L−1 triethanolamine, and 0.01 mol L−1 CaCl2 at pH 7.30±0.05 with the samples at a solid-to-liquid ratio of 1:2 mL g−1. The mixing was conducted for 2 h in an end-over-end rotator at 30 rpm [23, 24].
The bioaccessibility of PTEs in the SS and biochars was evaluated by the in vitro simple bioaccessibility extraction test (SBET). The extractant was prepared with 0.4 mol L−1 glycine (at pH=1.50, adjusted by concentrated HCl). The samples were mixed with the SBET extractant at a solid-to-liquid ratio of 1:50 mL g−1 in a pre-heated orbital shaker at 100 rpm for 1 h at 37 °C [25].
All the obtained mixtures of the above leaching experiments were centrifuged at 4000 rpm for 15 min, and then filtered by 0.45-μm MCE (mixed cellulose ester) membrane filters with disposal syringes. The filtered supernatant was then stored at 4 °C and analyzed by an inductively coupled plasma optical emission spectroscopy (ICP-OES, PerkinElmer, Avoi 200, USA) to quantify PTEs.
Characterization of biochar
The SS and biochar were digested first to analyze the total contents of PTEs. After comparing several different digestion methods, a modified microwave digestion method using inverse aqua regia was adopted [26, 27]. Briefly, 0.1-g oven-dried (at 105 °C) sludge or biochar samples were completely soaked with 8 mL inverse aqua regia (6 mL HNO3 and 2 mL HCl) for 1 h at room temperature and then heated in a microwave digestor (CEM MARS-6, USA) at 200 °C and 1000 W for 30 min. Detailed progress of pre-heating and retention time of microwave digestion are documented in Table S1 of Supporting Information. After cooling to room temperature, the digested samples were filtered and diluted to 50 mL with 5% HNO3 for the determination of PTEs (Zn, As, Cr, Cu, Ni, and Pb) by ICP-OES.
Scanning electron microscopy (SEM) (Prox, Phenom-World, USA) with energy-dispersive X-ray spectroscopy (EDS) system was used to observe the morphological structure and elemental composition of the sludge and biochars. XRD (Rigaku Ultima IV, Japan) equipped with Cu Kα radiation was used to identify minerals in the biochars, in the angle range (2θ) of 8°–80° and a scanning rate of 5° min−1.
Statistical analysis
All experiments were conducted in triplicate with chemicals at analytical grade. All the data are presented as averages with standard deviations in the figures. Statistical analysis of data was conducted with SPSS 23.0 (one-way ANOVA with Duncan tests) to compare the significant differences in the leachability, mobility, plant-availability, and bioaccessibility of the PTEs in the SS and pristine and modified biochars.
Results and discussion
Total contents of PTEs in SS
The total contents of major PTEs in SS were in the order of Zn>Cu>Cr>Ni>Pb≈As (Table 1). These elements are also frequently found in municipal sludge in many studies, from several to several hundred mg kg−1 [5, 6]. Although the total concentration of As and Pb in the digested SS samples were lower than the detection limits of the ICP-OES we used, the two elements were still included in this study owing to their high toxicity. Previous studies have shown that municipal sludge generally contains larger amounts of Zn, which could be associated with the widespread use of galvanized pipes (a way to coat pipes with Zn to prevent rust) for conveying wastewater in cities and the complicated sources of wastewater [6]. Lu et al. [28] studied the total contents of PTEs in sludge samples of three different sewage treatment plants in the same city as our study in 2013. The total Zn (629.1–1237.9 mg kg−1) was significantly higher than our result (444.91 mg kg−1). The total Cu was in the range of 401.0–611.3 mg kg−1, nearly 2–3 times that of the sludge in our study (173.83 mg kg−1). They also observed high contents of Pb in the sludge, which was in a range of 136.5–224.5 mg kg−1. Generally, the higher total contents of PTEs determined in their study compared with ours might be associated with two reasons: (1) the management and techniques of the wastewater plants and the awareness of PTEs for government and residents have been improved in the past ten years; and (2) the wastewater plants they collected sludge from included mainly domestic wastewater and minor industrial wastewater from electroplating, chemical industry, machining, tanning, etc.
Total contents of PTEs of the pristine and modified biochars
The total contents of all six PTEs in both pristine and modified biochars under various temperatures were remarkably higher than those in SS (Table 1). This is possibly due to the intense decomposition of organic matter (to volatilize as gaseous carbon products) in SS, higher thermal stability of PTEs in general, and a corresponding mass loss during pyrolysis at 300–700 °C, resulting in the enrichment of PTEs in biochars [5, 15, 18, 23]. The SEM–EDS results showed that a porous structure has been developed in biochars, compared with SS (Figs. 1 and 2). Notably, the total contents of As and Pb are in the range of maximum allowed thresholds for biochars, which are 13–100 mg kg−1 and 121–300 mg kg−1, respectively [29]. However, the total contents of Zn, Cu, Cr, and Ni in the pristine and modified biochars are higher than the minimum values of the maximum allowed thresholds (Zn: 416–7400 mg kg−1, Cu: 143–6000 mg kg−1, Cr: 93–1200 mg kg−1, and Ni: 47–420 mg kg−1) [29], even after Cl-modification.
Both modifications by MgCl2 and phosphates and the pyrolytic temperatures affected the total contents of PTEs in biochars, and the latter is the dominant reason. Similarly, Lu et al. [8] studied the change of total contents of PTEs in biochars, and found similar results that total Zn, Ni, As, and Cu in biochars produced at 700 °C were higher than those produced at 300 °C. Higher temperatures in pyrolysis often lead to higher ash contents and lower H/C ratios owing to the increased proportion of concentrated and remaining inorganic constituents. A lower molar H/C ratio of biochars indicates a higher aromatic degree in the biochar, which suggested a higher stability. Previous studies also suggests that the addition of phosphate salts, such as Ca(H2PO4)2 [30, 31], can lead to a lower C/H ratio at the same temperature, indicating higher stability.
At 300 °C, the total contents of Pb and Ni were slightly reduced by the PO4-modification, yet the total Zn, Cu, and Cr were marginally changed, compared with 300BC. One of the major removal mechanisms for PO4-modification is that Zn, Cd, Cu, and Pb can form stable compounds with phosphoric acids that generated by decomposing of Ca(H2PO4)2 [30, 32], without significant reductions in their total contents. Gu et al. [30] suggested that phosphate-containing minerals have been formed in sludge biochars produced at 450 °C, 550 °C, and 650 °C, respectively, with the addition of Ca(H2PO4)2 in the pyrolysis, including Cd(PO3)2, Cu(OH)3PO4, and Fe2Pb3(PO4)4 supported by XRD results. The equations of the related mechanisms of Zn3(PO4)2 and Zn2P2O7 are described by Eq. 1–4 [30]. We expected Pb, Cu, and Ni to be stabilized under similar mechanisms of Zn. In our study, although P-300BC was produced at 300 °C, Zn2P2O7 and Cu2P2O7 were observed in the XRD characterizations (Fig. 3c), while only Si-containing minerals were found for 300BC and 700BC (Fig. 3a and b). Similar mechanisms might occur for Pb, yet its total content in SS is too low to be detected by XRD.
Under the same pyrolysis temperature of 700 °C, the Cl-modification was expected to directly reduce the total contents of metallic PTEs in biochars. Under such a high temperature, the related reactions of possible mechanisms are described below as Eq. 5–8 [19, 33]. Metallic PTEs, Pb, Zn, and Ni, might undergo similar reactions as Cu. The PTEs were chlorinated by the addition of chloride salts which are more volatile under a high pyrolysis temperature. Interestingly, our XRD results suggested that Zn3(PO4)2 formed after modification, indicating that phosphate was available in the SS. Additionally, it might be associated with the high contents of Zn in the sludge (Fig. 3d). Needle-like minerals that are Cl-associated were found via SEM–EDS on the surface of Cl-700BC, which are probably newly formed chlorides (Fig. 2b). However, the total contents of most of the evaluated PTEs were insignificantly changed, compared with 700BC, except for Cr (Table 1). It should be noted that the generation of Cl2 and volatile chlorides of PTEs in the production should be carefully captured and treated before discharge during pyrolysis, especially for large-scale production.
Although the total amounts of PTEs of all pristine and modified biochars were enriched, their speciation was changed and tested via different leaching tests.
Leachability and mobility of PTEs in the pristine and modified biochars
After pyrolysis, the leachability of Zn, As, Cu, and Ni of pristine and modified biochars was markedly reduced, compared with SS, except for Cu of 700BC (Fig. 4). It is proposed that PTEs in the form of minerals and hydroxides in sludge can be converted into more stable oxides or sulfides during pyrolysis [8]. Raj et al. [15] also reached similar results that the leachability of Ni, Pb, Cr, Cu, and Zn of biochars produced at 350–500 °C were notably reduced compared with SS, yet the leachable Cd increased approximately twofold. They suggested that biochars can form a more well-developed porous structure, a larger specific surface area, and functional groups (metal–organic complexes), which may lead to less leachability of PTEs in biochars than SS.
Leachability (evaluated by TCLP, pH=2.88±0.05) of Zn, As, Cr, Cu, Ni, and Pb in sewage sludge (SS) and biochars (300BC: sludge biochar produced at 300 °C; P-300BC: modification by phosphate at 300 °C; 700BC: sludge biochar produced at 700 °C; Cl-700BC: modification by Cl at 700 °C. The same letters above the bars indicate that the results are not significantly different according to one-way ANOVA with the Duncan’s test (p<0.05)). TCLP toxicity characteristic leaching procedure
Without further modification, however, 700BC has the highest leachability of As, Cu, and Ni among all the biochars, while with the addition of Cl, the leachability of the three PTEs can be markedly reduced, especially for Cu. This suggested that a high pyrolytic temperature of 700 °C might lead to slightly higher leachable PTEs compared with 300 °C. The possible reasons are (1) enrichment of the PTEs by the enhanced pyrolytic temperatures (Table 1), and (2) increased surface hydrophilicity by the higher pyrolytic temperature leading to a higher ability to react with extractants of the TCLP test. Specifically, Shi et al. [20] suggested that the hydrophilicity of biochar surface is enhanced by a higher pyrolytic temperature, which is associated with the increase of ash contents (inorganic minerals), compared with those produced at low temperatures. However, Lu et al. [8] supported that the relationship between temperature and the leaching behaviors of PTEs of sludge biochars is complicated and non-linear. Their results showed that the respective leaching rates (leachable fractions of total contents of PTEs) of Pb, Ni, Cd, Cr, and Zn showed limited differences under pyrolytic temperatures of 400–700 °C, while mobile and easily available amounts of Cu and As were reduced by increasing temperature. However, some studies have suggested that biochars produced at 300 °C showed lower stability, as the temperature is not high enough to stabilize PTEs [34].
Notably, Zn has the highest leachability among the six PTEs of the SS and biochars, which is greatly associated with its high contents in the feedstock. The pyrolysis at 300 °C, even with the addition of phosphate, only slightly reduced the leachable Zn, while pyrolysis at 700 °C reduced approximately 50% of the leachable Zn. Besides, the addition of Cl can further reduce the leachable Zn in 700BC.
Although Shi et al. [20] suggested that phosphate-assisted pyrolysis reduced the leaching potential of Pb in biochars produced at different temperatures, our SS, the feedstock of biochars, contained low Pb. It should also be noted that all the leachability of PTEs in both SS and biochars in this study are lower than the standards, which are 5, 15, 5, 5, 5, and 5 mg L−1 for Zn, Cu, Cr, Ni, Pb, and As, respectively (USEPA Method 1311).
The mobility of PTEs in SS was higher than that of both pristine and modified biochars, except for Cr (Fig. 5). Such a high pyrolytic temperature of 700 °C greatly enhanced the mobility of Cr for 700BC; nevertheless, with the addition of MgCl2, it sharply decreased, which is similar to the variation of total Cr in 700BC and Cl-700BC (Table 1). It is possible that the Cr of SS existed as CrO4-containing salts at 700 °C, and CrO42− can be easily exchanged by the anions of the extractant of SPLP (mainly SO42− and NO3−), resulting in high mobility in 700BC. Kirk et al. [35] also found enhanced Cr solubility in a heated fly ash of municipal solid waste at 990±10 °C resulting from the formation of chromates, with the help of CaO. In the ash content of biochar, especially for those induced by sewage sludge, CaO is frequently detected [36]. It should be noted that the fly ash was heated with O2, while the pyrolysis in our study was fluxed with N2. However, it is still possible to generate chromate with limited O2. In contrast, in Cl-modified biochar, the addition of MgCl2 and Cl-containing by-products in pyrolysis might react with CaO to generate CaCl2, and hinder the possible formation of chromate [33, 37]. As a result, Cr extracted by the SPLP solution was significantly decreased by the addition of MgCl2. This might indicate a risk that high pyrolytic temperature could lead to high mobility of Cr without Cl-modification.
Mobility of Zn, As, Cr, Cu, Ni, and Pb in sewage sludge (SS) and biochars (300BC: sludge biochar produced at 300 °C; P-300BC: modified biochar by phosphate at 300 °C; 700BC: sludge biochar produced at 700 °C; Cl-700BC: modified biochar by Cl at 700 °C; The same letters above the bar indicate that the results are not significantly different according to the one-way ANOVA with the Duncan’s test (p<0.05))
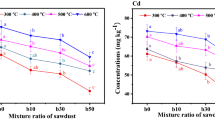
Plant-availability and bioaccessibility of PTEs in the pristine and modified biochars
The DTPA can extract water-soluble, exchangeable, organically bound, and partially oxidized PTEs in sludge; therefore, it can better reflect the plant-availability of PTEs than their total contents. In vitro SBET is frequently used for evaluating the potential bioaccessibility of PTEs in soil remediation. Generally, the plant-availability and bioaccessibility of PTEs in sludge were reduced by pyrolysis and further decreased by modification (Figs. 6 and 7). In PO4-modified biochar, phosphate precipitates most metallic PTEs and forms stable phosphate products [20]. Consequently, the PTEs of PO4-modified biochar can hardly be extracted by DTPA. However, the formed precipitates can be dissolved and re-released under strong acid conditions, for example, the extractants of bioaccessibility of PTEs (pH=1.50). That is why, in general, Cl-700BC had lower bioaccessible PTEs than P-300BC. This result is similar to the findings of Xu et al. [38] that at pH=2 which is close to SBET, the release of Pb from PO4-modified biochar was higher than that of unmodified biochars. Furthermore, the PO4-modification cannot reduce the bioaccessible As and Cr of the biochars, even slightly enhanced the bioaccessibility of Cr, because they mainly exist as anions that cannot form stable phosphate precipitates (Fig. 7). The Cl-modification converts PTEs into a more active chlorinated state, which can be volatilized easily compared with other forms. Compared with the PO4-modification, it markedly decreased the total contents of Cr, and consequently decreased the bioaccessible fraction. However, the addition of MgCl2 can greatly enhance the plant-available Zn and Cu and bioaccessible Zn compared with 700BC and SS, which should be carefully noted. The adverse effects should be carefully investigated in future studies.
Plant-availability of Zn, As, Cr, Cu, Ni, and Pb in sewage sludge (SS) and biochars (300BC: sludge biochar produced at 300 °C; P-300BC: modified biochar by phosphate at 300 °C; 700BC: sludge biochar produced at 700 °C; Cl-700BC: modified biochar by Cl at 700 °C; The same letters above the bar indicate that the results are not significantly different according to one-way ANOVA with the Duncan’s test (p<0.05))
Bioaccessibility of Zn, As, Cr, Cu, Ni, and Pb in sewage sludge (SS) and biochars (300BC: sludge biochar produced at 300 °C; P-300BC: modified biochar by phosphate at 300 °C; 700BC: sludge biochar produced at 700 °C; Cl-700BC: modified biochar by Cl at 700 °C; Same letters above the bar indicate that the results are not significantly different according to the one-way ANOVA with the Duncan’s test (p<0.05))
Conclusions
This study used two methods with different mechanisms to enhance the stability of PTEs in sludge biochars. Our results suggested that the total contents of PTEs in biochars were enriched by pyrolysis, yet the leachability, mobility, plant-availability, and bioaccessibility of PTEs were mostly reduced at different levels, compared with SS. The increased pyrolytic temperature led to higher leachability and mobility for some PTEs, which can be further reduced by Cl-modification. Notably, a higher bioaccessibility of PTEs was observed in P-300BC than in Cl-700BC, owing to the dissolution of phosphate precipitates by acidic extractants of SBET. However, the Cl-modification might cause unwanted effects on the stability of PTEs in sludge biochars. For example, it enhanced the plant-available Zn and Cu and bioaccessible Zn, compared with 700BC, which should be carefully investigated in future studies. To avoid possible adverse effects, sludge biochars should be circumspectly treated and evaluated for risks before application. Those biochars that cause high risks can possibly be used in other areas rather than as soil amendments, such as the cement industry.
Availability of data and materials
The authors declare that all data and materials supporting the results of this study are available within the article.
References
Wang, X., Chi, Q., and Liu, X. 2019. Influence of pyrolysis temperature on characteristics and environmental risk of heavy metals in pyrolyzed biochar made from hydrothermally treated sewage sludge. Chemosphere 216: 698–706.
Yang, G., Zhang, G., and Wang, H. 2015. Current state of sludge production, management, treatment and disposal in China. Water Research 78: 60–73.
Havukainen, J., Zhan, M., Dong, J., et al. 2017. Environmental impact assessment of municipal solid waste management incorporating mechanical treatment of waste and incineration in Hangzhou, China. Journal of Cleaner Production 141: 453–461.
Ibrahim, M., Li, G., Khan, S., et al. 2017. Biochars mitigate greenhouse gas emissions and bioaccumulation of potentially toxic elements and arsenic speciation in Phaseolus vulgaris L. Environmental Science and Pollution Research International 24: 19524–19534.
Zhang, Z., Ju, R., Zhou, H., et al. 2021. Migration characteristics of heavy metals during sludge pyrolysis. Waste Management 120: 25–32.
Jin, J., Li, Y., Zhang, J., et al. 2016. Influence of pyrolysis temperature on properties and environmental safety of heavy metals in biochars derived from municipal sewage sludge. Journal of Hazardous Materials 320: 417–426.
Bolan, N., Hoang, S.A., Beiyuan, J., et al. 2021. Multifunctional applications of biochar beyond carbon storage. International Materials Reviews 67 (2): 150–200.
Lu, T., Yuan, H., Wang, Y., et al. 2015. Characteristic of heavy metals in biochar derived from sewage sludge. Journal of Material Cycles and Waste Management 18 (4): 725–733.
Liang, J., Ye, J., Shi, C., et al. 2022. Pyrolysis temperature regulates sludge-derived biochar production, phosphate adsorption and phosphate retention in soil. Journal of Environmental Chemical Engineering 10 (3): 107744.
He, M., Xu, Z., Hou, D., et al. 2022. Waste-derived biochar for water pollution control and sustainable development. Nature Reviews Earth & Environment 3: 444–460.
Xu, M., Qin, Y., Huang, Q., et al. 2022. Arsenic adsorption by different Fe-enriched biochars conditioned with sulfuric acid. Environmental Science and Pollution Research. https://doi.org/10.1007/s11356-022-23123-4.
Yoo, J.C., Beiyuan, J., Wang, L., et al. 2018. A combination of ferric nitrate/EDDS-enhanced washing and sludge-derived biochar stabilization of metal-contaminated soils. Science of the Total Environment 616–617: 572–582.
Liu, J., Ren, S., Cao, J., et al. 2020. Highly efficient removal of thallium in wastewater by MnFe2O4-biochar composite. Journal of Hazardous Materials 401: 123311.
Beckers, F., Awad, Y.M., Beiyuan, J., et al. 2019. Impact of biochar on mobilization, methylation, and ethylation of mercury under dynamic redox conditions in a contaminated floodplain soil. Environment International 127: 276–290.
Raj, A., Yadav, A., Arya, S., et al. 2021. Preparation, characterization and agri applications of biochar produced by pyrolysis of sewage sludge at different temperatures. Science of the Total Environment 795: 148722.
Khan, S., Chao, C., Waqas, M., et al. 2013. Sewage sludge biochar influence upon rice (Oryza sativa L) yield, metal bioaccumulation and greenhouse gas emissions from acidic paddy soil. Environmental Science and Technology 47 (15): 8624–8632.
Chen, H., Ma, J., Wei, J., et al. 2018. Biochar increases plant growth and alters microbial communities via regulating the moisture and temperature of green roof substrates. Science of the Total Environment 635: 333–342.
Hossain, M.K., Strezov, V., Yin Chan, K., et al. 2010. Agronomic properties of wastewater sludge biochar and bioavailability of metals in production of cherry tomato (Lycopersicon esculentum). Chemosphere 78 (9): 1167–1171.
Xia, Y., Tang, Y., Shih, K., et al. 2020. Enhanced phosphorus availability and heavy metal removal by chlorination during sewage sludge pyrolysis. Journal of Hazardous Materials 382: 121110.
Shi, L., Wang, L., Zhang, T., et al. 2017. Reducing the bioavailability and leaching potential of lead in contaminated water hyacinth biomass by phosphate-assisted pyrolysis. Bioresource Technology 241: 908–914.
Beiyuan, J., Lau, A.Y.T., Tsang, D.C.W., et al. 2018. Chelant-enhanced washing of CCA-contaminated soil: Coupled with selective dissolution or soil stabilization. Science of the Total Environment 612: 1463–1472.
Beiyuan, J., Tsang, D.C.W., Valix, M., et al. 2017. Selective dissolution followed by EDDS washing of an e-waste contaminated soil: Extraction efficiency, fate of residual metals, and impact on soil environment. Chemosphere 166: 489–496.
Yuan, H., Lu, T., Huang, H., et al. 2015. Influence of pyrolysis temperature on physical and chemical properties of biochar made from sewage sludge. Journal of Analytical and Applied Pyrolysis 112: 284–289.
He, J., Strezov, V., Kan, T., et al. 2019. Effect of temperature on heavy metal(loid) deportment during pyrolysis of Avicennia marina biomass obtained from phytoremediation. Bioresource Technology 278: 214–222.
Beiyuan, J., Awad, Y.M., Beckers, F., et al. 2017. Mobility and phytoavailability of As and Pb in a contaminated soil using pine sawdust biochar under systematic change of redox conditions. Chemosphere 178: 110–118.
Long, Y.-Y., Hu, L.-F., Fang, C.-R., et al. 2009. An evaluation of the modified BCR sequential extraction procedure to assess the potential mobility of copper and zinc in MSW. Microchemical Journal 91 (1): 1–5.
Han, H.J., Lee, J.U., Ko, M.S., et al. 2020. Comparison of five extraction methods for evaluating cadmium and zinc immobilization in soil. Environmental Geochemistry and Health 42 (12): 4203–4212.
Lu, H., Zhang, W., Wang, S., et al. 2013. Characterization of sewage sludge-derived biochars from different feedstocks and pyrolysis temperatures. Journal of Analytical and Applied Pyrolysis 102: 137–143.
International Biochar initiative. 2015. Standardized Product Definition and Product Testing Guidelines for Biochar that Is Used in Soil. Available at: https://biochar-international.org/characterizationstandard/. Accessed 9 Sep 2022.
Gu, W., Guo, J., Bai, J., et al. 2022. Co-pyrolysis of sewage sludge and Ca(H2PO4)2: Heavy metal stabilization, mechanism, and toxic leaching. Journal of Environmental Management 305: 114292.
Du, J., Zhang, L., Liu, T., et al. 2019. Thermal conversion of a promising phytoremediation plant (Symphytum officinale L.) into biochar: Dynamic of potentially toxic elements and environmental acceptability assessment of the biochar. Bioresource Technology 274: 73–82.
Xu, Q., Ye, B., Mou, X., et al. 2019. Lead was mobilized in acid silty clay loam paddy soil with potassium dihydrogen phosphate (KDP) amendment. Environmental Pollution 255 (Pt 1): 113179.
Yu, J., Sun, L., Ma, C., et al. 2016. Mechanism on heavy metals vaporization from municipal solid waste fly ash by MgCl2⋅6H2O. Waste Management 49: 124–130.
Chen, T., and Yan, B. 2012. Fixation and partitioning of heavy metals in slag after incineration of sewage sludge. Waste Management 32 (5): 957–964.
Kirk, D.W., Chan, C.C.Y., and Marsh, H. 2002. Chromium behavior during thermal treatment of MSW fly ash. Journal of Hazardous Materials 90 (1): 39–49.
Fan, J., Li, Y., Yu, H., et al. 2020. Using sewage sludge with high ash content for biochar production and Cu(II) sorption. Science of the Total Environment 713: 136663.
Li, Z., Huang, Y., Zhu, Z., et al. 2023. Co-pyrolysis of sewage sludge with polyvinyl chloride (PVC)/CaO: Effects on heavy metals behavior and ecological risk. Fuel 333: 126281.
Xu, X., Hu, X., Ding, Z., et al. 2017. Effects of copyrolysis of sludge with calcium carbonate and calcium hydrogen phosphate on chemical stability of carbon and release of toxic elements in the resultant biochars. Chemosphere 189: 76–85.
Acknowledgements
The authors appreciate the financial supports by the National Natural Science Foundation for Young Scientists of China (No. 42007142), the Guangdong Basic and Applied Basic Research Foundation (No. 2019A1515110927), and the Key Scientific and Technological Project of Foshan City (No. 2120001008392).
Author information
Authors and Affiliations
Contributions
JB and FL: contributed to the study conception and design. Material preparation, data collection and analysis were performed by QH, SC, JL, XW, and YW. The first draft of the manuscript was written by QH. JB, JW, JL, WY, and CN: critically revised and commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Not applicable.
Ethical approval
Not applicable.
Consent to participate
Not applicable. The authors all agreed to participate and publish our data here.
Consent to publish
Not applicable. The authors all agreed to participate and publish our data here.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, Q., Chen, S., Lin, J. et al. Stability of potentially toxic elements in municipal sludge biochars modified by MgCl2 and phosphate. Waste Dispos. Sustain. Energy 5, 13–23 (2023). https://doi.org/10.1007/s42768-022-00128-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42768-022-00128-w