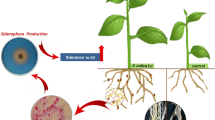
Abstract
The present study aimed on siderophore and ACC deaminase producing bacteria to mitigate salinity stress and simultaneous mobilization of nutrients. In this study, eight siderophore and ACCD producing bacterial strains selected from a previous study showed that four strains (CWTS 5, CWTS 10, KSBTS 12 and SBTS 12) enhanced mung bean and black gram plant growth in the plate assay (200 mM NaCl stress). Furthermore, evaluation in pot studies (10 g kg− 1 NaCl stress) revealed enhanced root (170.05-237.96% and 16.83-95.74%) and shoot length (139.91-196.96% and 19.87-77.26%) of mung bean and black gram, respectively, compared to the control. Inoculated plants also enhanced the uptake of nitrogen (36.62–41.47 mg g− 1 and 36.19–47.23 mg g− 1), phosphorous (3.29–3.98 mg g− 1 and 3.15–4.21 mg g− 1), Fe (44.36–56.21 µg g− 1 and 41.02–44.31 µg g− 1), Na+ ions (40.65–45.35 µg g− 1 and 20.18–22.11 µg g− 1), and chlorophyll content (12.71–29.33 mg g− 1 and 10.55–26.39 mg g− 1) of mung bean and black gram plants. The Cl− ion uptake by mung bean and black gram plants was below the toxic limit of 94.62–116.6 µg g− 1 and 117.6-150.2 µg g− 1 respectively. The inoculation of bacterial strains also increased the siderophore (259.53-556.05% and 267.26-304.77%) and ACCD (3.02–5.05 µmol mL− 1 and 2.84–4.41 µmol mL− 1) production in the soil and the enhancement was directly correlated with N, P, Fe and chlorophyll content of both the plants. In addition, the inoculated bacteria also enhanced the soil respiration activity such as dehydrogenase enzyme (112.53–167.40 µg g− 1 and 88.92-181.28 µg g− 1), FDA hydrolysis (31.66–44.51 µg g− 1 and 16.94–38.20 µg g− 1) and alkaline phosphatase enzyme (691.53–851.53 µg g− 1 and 511.53-811.53 µg g− 1) of mung bean and black gram soils. Overall, strains CWTS 5 (Bacillus subtilis), SBTS 12 (Rhodococcus sp.), and CWTS 10 (Bacillus albus) can be used to mitigate NaCl stress and mobilize nutrients and Fe in plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The food production largely depends on the use of chemical fertilizers to increase production. However, these fertilizers cause groundwater pollution via eutrophication of water bodies (Youssef et al. 2014). In addition, the salt concentration in soil increases because of salinized land near coastal areas, which poses a major threat to crop yield (Tomaz et al. 2020). Salinity affects 30% of irrigated land globally and 6% of the total land area (Chaves et al. 2009). Moreover, when plants are grown in saline soils with high Na+ concentrations, they affect plant growth, development, and availability of metal nutrients, particularly iron (Fe) deficiency (Abbas et al. 2015). Fe deficiency may affect many physiological processes in plants such as oxygen transport and metabolism, photosynthesis, respiration, and DNA and RNA synthesis (Chandwani et al. 2022). In addition to the above constraints in saline soils, salinity induces ethylene production and oxidative stress. Excessive ethylene production may hamper the seed germination and root development, thereby negatively affecting plants (Saravanakumar and Samiyappan 2007). Therefore, to mitigate environmental stressor such as salinity, bioorganic liquid fertilizers and plant nutrition enhancers such as the bacteria possessing ACCD activity and siderophore production are viable options (Turan et al. 2022).
Bacteria possessing ACCD hydrolyse ACC into ammonia and α-ketobutyrate, thereby preventing the over-production of the stress hormone ethylene and protecting the plant from its inhibitory effects (Gupta et al. 2021). Similarly, Fe is mainly found in its unavailable form in saline soil as Fe3+, which may form insoluble hydroxides that are inaccessible to plants. Under Fe-limiting conditions, siderophores helps in reducing insoluble complex of Fe3+ on the bacterial membrane to the soluble form Fe2+ making it readily available to plants. The siderophores are recycled for Fe transport once released inside the cells (Brock et al. 2003).
India is considered the largest producer and consumer of mung bean (V. radiata L.) and black gram (V. mungo L.). Mung bean and black gram are short duration pulse crops rich in proteins, fats, carbohydrates, micronutrients, antioxidants, and anthocyanins (Turan 2021). However, owing to several constraints, their yield has not been achieved at a level that can meet the public demand. The major constraints to achieve productivity is susceptibility to abiotic stressors, particularly salinity. The entire life cycle of mung bean and black gram is severely affected by saline stress leading to yield losses in both crops (Kumar et al. 2012; Jawad Hassan et al. 2020). Keeping this in view, this research focused on increasing the growth of mung bean and black gram under saline stress conditions.
Salinity is responsible for Fe deficiency and the production of the stress hormone ethylene, both of which affect root and shoot growth, chlorophyll content, biomass, and nutrient uptake. Therefore, the present study was conducted to (i) explore the role of siderophore and ACCD producing bacteria possessing salinity tolerance and alleviating salt stress in mung bean and black gram plants, (ii) study the effect of siderophore and ACCD producers on the mobilization of Fe content and Na+ and Cl− ions in the roots of mung bean and black gram plants, and (iii) evaluate the potential of bacteria on nutrient uptake (N and P), photosynthetic pigments, quantity of siderophore and ACCD in soil, and soil microbial activity.
2 Materials and Methods
2.1 Collection of Bacterial Strains
Bacillus altitudinis (CWN 5; accession no. MZ157033), B. subtilis (CWTS 5; accession no. MN715782), B. paramycoides (SBTS 18; accession no. MN382367 and KSBTS 12; accession no. MZ157031), B. albus (CWTS 10; accession no. MN715783), Rhodococcus sp (SBTS 12; accession no. ON377347), R. kroppenstedii (IPTS 7; accession no. ON377346), and Staphylococcus coagulans (CSN 17; accession no. MZ157032) strains with siderophore, ACCD and other plant growth promoting properties were collected based on our previous study (Chandwani et al. 2022).
2.2 Screening of Salt-tolerance Property
The above-mentioned bacterial strains were assessed for their salt tolerance on nutrient agar plates amended with 2–10% (2–10 g 100 mL− 1) NaCl. The growth observed after 48–72 h indicates that the organism was salt tolerant (Shultana et al. 2020). The experiment was conducted in triplicates.
2.3 Plant Growth Promotion in Petri Dish Method
The Petri dish method was used to test the efficacy of bacteria under 200 mM NaCl stress. The indigenous varieties of mung bean (variety GAM 5) and black gram (variety Usha-WBG-26) seeds were surface sterilized by dipping them in 0.8% NaOCl for 10 min, followed by 70% ethanol for 3 min, and 0.1% HgCl2 for 2–3 min respectively. Thereafter, the seeds were washed several times with double distilled water, soaked in bacterial cultures (1 × 108 CFU mL− 1) for 2 h and air-dried for half an hour. Seeds soaked in sterile distilled water were used as controls. Seeds were placed in Petri dishes containing a paper towel folded in half to create four layers. The paper towel was moistened with (1) water (uninoculated control), (2) NaCl solution (200 mM), and (3) NaCl solution + siderophore and ACCD producing bacteria (test). The seeds were allowed to germinate for 10 d. The paper towel was sprayed with 200 mM NaCl and watered every 2-day interval to maintain moisture and salinity conditions (Lu et al. 2022). Plant growth parameters (primary root and shoot length) of mung bean and black gram seedlings were measured after 10 d of incubation. The experiment was conducted in triplicates and repeated twice under saline conditions.
2.4 Plant Growth Promotion Under Greenhouse Condition
Based on the above experiments, four strains CWTS 5 (B. subtilis; accession no. MN715782), CWTS 10 (B. albus; accession no. MN715783), KSBTS 12 (B. paramycoides; accession no. MZ157031), and SBTS 12 (Rhodococcus sp; accession no. ON377347) were chosen for the pot culture study. The experiment was conducted in 12 inches’ plastic pots. The greenhouse conditions comprised a relative humidity (RH) of 75% at 34/28 ℃ day/night temperatures. The soil physicochemical parameters in which the pot study was carried out were as follows: pH 8, electrical conductivity 4.178 mmho cm− 1, available phosphorus 214 kg ha− 1, nitrogen 338 kg ha− 1, potassium 803 kg ha− 1, sulfur 4 kg ha− 1, and iron 8 µg g− 1. The above-mentioned indigenous varieties of mung bean and black gram seeds were surface sterilized as described above, and a completely randomized design was adopted. The treatments included (1) control (no stress), (2) control (10 g kg− 1 NaCl stress), and (3) siderophore and ACCD producers + NaCl stress. NaCl stress was created by dissolving 10 g NaCl in water and poured into the soil. The crop was irrigated with saline water (10 g NaCl) except for the control plants (no stress) and watered every three days interval with normal water to maintain moisture (Chandwani et al. 2022). After 30 days, plant growth parameters such as root length, shoot length, root wet weight, shoot wet weight, root dry weight, and shoot dry weight were recorded. The experiment was conducted in seven replicates and repeated twice.
2.5 Estimation of Nitrogen (N), Phosphorus (P), and Iron (Fe) Uptake
N content was estimated using the Kjeldahl assembly method, P was estimated using the gravimetric quimociac method, and Fe was estimated using the DTPA extractable iron method (Turan 2020) from the root samples of mung bean and black gram plants as described in our previous study (Chandwani et al. 2022).
2.6 Estimation of Sodium (Na+) and Chloride (Cl−) Ions
Five-gram root samples of mung bean and black gram plants were homogenized, oven-dried for 4 h, and reduced to ash in a furnace for 3 h at 400 ℃. The sample was then allowed to cool in a desiccator and dissolved in 5 mL of nitric acid with gentle heating. The final volume was made up to 50 mL using double distilled water and the mixture was filtered using filter paper. The filtrate was used for the quantitative estimation of Na+ ions using an atomic absorption spectrophotometer at a wavelength of 589 nm. Na+ ions were estimated using a standard curve (Harvey and Flowers 1978).
To estimate the Cl− ions from the plant roots, 0.2 g of the sample was dissolved in 40 mL double distilled water, 50 mL 0.1 N silver nitrate solution, and 5 mL concentrated nitric acid. Thereafter, nitrobenzene (0.5 mL) was added and the final volume of the mixture was made up to 100 mL using double distilled water. 50 mL of the solution was removed and 2 mL ferric ammonium sulphate was added, which was saturated with water and stabilized by the addition of 50 mL nitric acid. Excess silver nitrate was titrated with 0.1 N standard ammonium thiocyanate solution to a red blood colour end point (Tavakkoli et al. 2011). A blank test was carried out following the above procedure without using the plant material. Cl− ions were estimated using the following formula:
Where,
V1 = Volume (mL) of standard ammonium thiocyanate solution used in the blank determination;
V2 = Volume (mL) of standard ammonium thiocyanate solution used in the test with the plant material;
V = Normality of standard ammonium thiocyanate solution; and.
M = Mass in g of the dried prepared sample taken for the test (Harvey and Flowers 1978).
2.7 Estimation of Chlorophyll Content
The experiment was conducted in triplicates where one gram of leaf samples of mung bean and black gram plants were finely ground and mixed with 20 mL of 80% acetone and 0.5 g MgCO3. The samples were centrifuged at 5000 rpm for 10 min and the colour intensity of the collected supernatant was measured using a spectrophotometer at 645 and 663 nm. Total chlorophyll content was calculated using the formula: Total Chl = Chl a + Chl b, where Chl a = 11.75 × A663– 2.35 × A645; Chl b = 18.61 × A645– 3.96 × A663 (Hiscox and Israelstam 1979).
2.8 Estimation of Siderophore Production in Salinity Stressed Soil
Siderophore content in salinity stressed soil was estimated in triplicate using the CAS-Fe agar method with slight modifications. One gram of soil was inoculated into succinate medium and incubated on a shaker for 48 h. After two successive transfers of 1 mL of soil slurry were made into the succinate medium and left on a shaker for 48 h. Centrifugation was performed at 10,000 rpm for 10 min and 1 mL of the supernatant and 1 mL of CAS reagent were mixed thoroughly and incubated for 30 min. The absorbance was recorded at 630 nm to quantify siderophore production in the soil. The percentage increase in soil siderophore content was estimated by the formula- [(Ac– As)/ Ac] * 100, where Ac = Absorbance of control soil sample, and As = Absorbance of the test soil sample (Lewis et al. 2019).
2.9 Estimation of ACCD Production in Salinity Stressed Soil
Quantification of ACCD from salinity stressed soil was carried out in triplicates as described by Honma and Shimomura (1978) with minor modifications. One gram of the soil sample was inoculated into 20 mL of Luria-Bertani (LB) broth and incubated on a shaker for 48 h. Successive transfers of 1 mL of soil slurry were made to the LB broth and left on a shaker for 24 h. The soil slurry (1 mL) was transferred to 25 mL of Dworkin and Foster (DF) salt medium amended with 0.5 M ACC and incubated for 24 h on a shaker. Another 2 mL slurry was centrifuged at 10,000 rpm for 10 min and 1 mL of 0.1 M Tris-HCl (pH 7.6) was added to the pellet. The mixture was centrifuged again at 10,000 rpm for 5 min and 600 µL of 0.1 M Tris-HCl (pH 8.5) and 30 µL of toluene were added to the pellet and vortexed for 30 s. From the mixture, 200 µL was taken into the microcentrifuge tube and 20 µL of 0.5 M ACC was added, vortexed for 30 s and incubated for 20 min. After incubation, 1 mL of 0.56 N HCl was added and vortexed for 30 s. The reaction mixture was centrifuged at 10,000 rpm for 5 min and 1 mL of supernatant was mixed with 800 µL of 0.56 N HCl and vortexed for 30 s. Then, 300 µL of 2,4 Dinitrophenylhydrazine (0.2% in 2 N HCl) was added, vortexed for 30 s and incubated for 30 min. Finally, 2 mL of 2 N NaOH was added and mixed well. The absorbance at 540 nm was measured to quantify ACCD production using α-ketobutyrate as the standard (Agrawal et al. 2017).
2.10 Estimation of Soil Respiration Enzymes
The soil respiration enzymes were analyzed in triplicate. The dehydrogenase assay was performed using the 2-3-5-triphenyl tetrazolium chloride (TTC) reduction method as described by Casida et al. (1964) to measure the concentration of triphenyl formazan (TPF). Fluorescein diacetate (FDA) hydrolysis assay was performed as described by Adam and Duncan (2001) to measure the concentration of fluorescein. Alkaline phosphatase (Ap) was measured using the method described by Bessey et al. (1946) using para-nitrophenyl phosphate (pNPP) as a substrate for the production of para-nitrophenol (PNP) to detect phosphatase activity.
2.11 Statistical Analysis
All statistical analyses were performed using SPSS software version 23.0. Data were analyzed using one-way analysis of variance (ANOVA) with Duncan’s Multiple Range Test (DMRT) to determine the significance level among the treatments at the 95% confidence level (p ≤ 0.05). Data are presented as the mean ± standard error of the experimental replicates. Data symmetry was measured using Skewness and Kurtosis, and normality was determined by homogeneity of variance using Levene’s test. The relationship between PGP attributes was determined using the Pearson’s correlation coefficient. A correlation matrix based on the Pearson method was constructed using XLSTAT software.
3 Results
3.1 Screening of Salt-tolerance Property
Screening of eight siderophore and ACCD producing bacteria for salt tolerance revealed that all strains were able to tolerate up to 10% (10 g 100 mL− 1) of NaCl stress.
3.2 Plant Growth Promotion in Petri Dish Method
Inoculation with bacterial strains enhanced the root and shoot lengths of mung bean and black gram under 200 mM NaCl stress compared to the control. The enhancement in the root and shoot lengths of the mung bean plants was found to be 38.35-461.64% and 12.84-269.64%, respectively, whereas in the black gram plants, the enhancement was 15.05-340.86% and 15.56-269.64%, respectively (Fig. 1). The increase in secondary root numbers and the dry biomass of roots and shoots (data not shown) of both mung bean and black gram plants were also found in the treated plants compared to the control under salinity stress.
Effect of siderophore and ACCD producing bacteria on growth parameters under 200 mM NaCl stress (plate assay) (a) Plant growth parameters (root and shoot lengths) of mung bean plants under plate assay (b) Plant growth parameters (root and shoot lengths) of black gram plants under plate assay. Data analyzed using one-way analysis of variance (ANOVA). Values denoted are mean ± S.E. (n = 3) where lowercase letters denote the significance according to Duncan’s multiple range test (DMRT) (p ≤ 0.05)
3.3 Plant Growth Promotion Under Greenhouse Condition
Based on the greenhouse experiments, inoculation with CWTS 5, CWTS 10, KSBTS 12, and SBTS 12 enhanced the root and shoot lengths (170.05-237.96% and 139.91-196.96%) of mung bean and black gram (16.83-95.74% and 19.87-77.26%), respectively, in 10 g kg− 1 of NaCl stressed soil compared to the control (uninoculated plants). The inoculated bacteria also significantly enhanced the wet and dry biomass of roots and shoots (p ≤ 0.05) in both mung bean and black gram plants compared to the control under saline stress (Tables 1 and 2). Strains CWTS 5 (237.96% and 196.96%), followed by SBTS 12 (199.41% and 147.62%) increased the root and shoot lengths of mung bean plants under greenhouse conditions (Table 1). Similarly, in the case of black gram plants, strains CWTS 5 (95.74% and 77.26%) and CWTS 10 (85.84% and 45.96%) showed significant enhancement in root and shoot lengths under greenhouse conditions compared with uninoculated plants (Table 2).
3.4 Estimation of Nitrogen (N), Phosphorus (P), and Iron (Fe) Uptake
The uptake of N, P, and Fe by the roots of mung bean and black gram plants was significantly enhanced (p ≤ 0.05) in the NaCl stressed soil in the presence of siderophore and ACCD producing bacteria. The uptake of N by mung bean plants ranged from 36.62 to 41.47 mg g− 1 (Fig. 2a), P ranged from 3.29 to 3.98 mg g− 1 (Fig. 2b), and Fe ranged from 44.36 to 56.21 µg g− 1 (Fig. 2c) in the inoculated plants compared to the control (N– 30.62 mg g− 1, P– 2.36 mg g− 1, and Fe– 9.0 µg g− 1) in NaCl stressed soils. Similarly, the uptake of N by black gram plants ranged from 36.19 to 47.23 mg g− 1 (Fig. 2a), P ranged from 3.15 to 4.21 mg g− 1 (Fig. 2b), and Fe ranged from 41.02 to 44.31 µg g− 1 (Fig. 2c) in the inoculated plants compared to the control (N– 30.41 mg g− 1, P– 2.98 mg g− 1, and Fe– 12.21 µg g− 1) under NaCl stressed conditions. Under salinity conditions, the growth of both plants was reduced, and Fe uptake (9.0 µg g− 1 and 12.21 µg g− 1). However, in the bacteria inoculated plants, Fe uptake was enhanced (30–300 µg g− 1) in both plants in NaCl stressed soils. Overall, it was found that strains CWTS 5 and SBTS 12 showed tremendous effects in terms of N, P, and Fe uptake in mung bean plants, whereas strains CWTS 5 and CWTS 10 showed enhanced performance in black gram plants under NaCl stress (Fig. 2).
Effect of siderophore and ACCD producing bacteria on uptake of macronutrients and micronutrients in mung bean and black gram plants (a) N uptake by mung bean and black gram plants under NaCl stress (b) P uptake by mung bean and black gram plants under NaCl stress (c) Fe uptake by mung bean and black gram plants under NaCl stress. Data obtained are means of seven replicates (n = 7) and bars denotes standard error bars. Data analyzed using one-way analysis of variance (ANOVA). Mean values (± S.E.) denoted on the bars with different lowercase letters are significantly different according to Duncan’s multiple range test (DMRT) (p ≤ 0.05)
3.5 Estimation of Sodium (Na+) and Chloride (Cl−) Ions
The uptake of Na+ ions in the roots of mung bean and black gram plants enhanced to 40.65–45.35 µg g− 1 and 20.18–22.11 µg g− 1, respectively, in the inoculated plants compared to the control (36.36 µg g− 1 and 16.36 µg g− 1) (Fig. 3a). The Cl− ions uptake by roots of mung bean and black gram plants ranged from 94.62 to 116.6 µg g− 1 and 117.6–150.2 µg g− 1, respectively, in the inoculated plants compared to the control (172 µg g− 1 and 185.7 µg g− 1) (Fig. 3b). Cl− ion uptake was found to decrease in the roots of both mung bean and black gram plants inoculated with the bacterial strains in NaCl stressed soils compared to the control. In saline soils plant growth is affected by the toxicity caused by Cl− ions. In this study, Cl− ion uptake by both the plants in the control treatment was found to be in the toxic limit (172 µg g− 1 and 185.7 µg g− 1) in the NaCl stressed soils, whereas, with bacterial treatments, Cl− ion uptake was found to be in the normal range (70–140 µg g− 1) in both plants except for the SBTS 12 treatment in black gram plants (150.2 µg g− 1). Although, the concentrations of Na+ ions in the roots of both mung bean and black gram plants was enhanced in the PGPB treated plants (as the root biomass was found to be increased in the PGPB treated plants) but were below the toxic limits recommended (50 µg g− 1) in the inoculated plants (Flowers et al. 2015).
Effect of siderophore and ACCD producing bacteria on uptake of Na+ and Cl− ions in mung bean and black gram plants (a) Na+ uptake by mung bean and black gram plants under NaCl stress (b) Cl− uptake by mung bean and black gram plants under NaCl stress. Data obtained are means of seven replicates (n = 7) and bars denotes standard error bars. Data analyzed using one-way analysis of variance (ANOVA). Mean values (± S.E.) denoted on the bars with different lowercase letters are significantly different according to Duncan’s multiple range test (DMRT) (p ≤ 0.05)
3.6 Estimation of Chlorophyll Content
Inoculation of siderophore and ACCD producing bacteria significantly enhanced the chlorophyll content in the leaves of both mung bean and black gram plants in NaCl amended soils (p ≤ 0.05). The enhancement of chlorophyll content was ranged from 12.71 to 29.33 mg g− 1 (mung bean) and 10.55–26.39 mg g− 1 (black gram), respectively, in the inoculated plants compared to the control (1.29 mg g− 1 and 1.48 mg g− 1) (Fig. 4). This study revealed that the chlorophyll content in both mung bean and black gram plants were correlated with the N, P and Fe contents of both plants (Fig. 4). The strains CWTS 5 (B. subtilis) and SBTS 12 (Rhodococcus sp.) treatments in the mung bean and CWTS 5 (B. subtilis) and CWTS 10 (B. albus) treatments in the black gram were positively correlated with N, P, and Fe contents with the enhancement of chlorophyll content.
Determination of chlorophyll content from leaves of mung bean and black gram plants under NaCl stress. Data obtained are means of seven replicates (n = 7) and bars denotes standard error bars. Data analyzed using one-way analysis of variance (ANOVA). Mean values (± S.E.) denoted on the bars with different lowercase letters are significantly different according to Duncan’s multiple range test (DMRT) (p ≤ 0.05)
3.7 Estimation of Siderophore and ACCD Production in Salinity Stressed Soil
Siderophore and ACCD quantity in the salinized soils by inoculated bacteria revealed a significant enhancement (p ≤ 0.05). Siderophore quantity ranged from 259.53 to 556.05% in the bacterial inoculated NaCl stressed soils compared to the control (9.74%) in mung bean plants experiment. Whereas, in the black gram plant experiment, siderophore quantity ranged from 267.26 to 304.77% compared to the control (23.10%) (Fig. 5a). The simultaneous enhancement of ACCD activity in mung bean (3.02–5.05 µmol mL− 1) and black gram plants experiments (2.84–4.41 µmol mL− 1) compared to the control under NaCl stress (1.39 µmol mL− 1 and 0.81 µmol mL− 1) was also observed with the inoculated plants (Fig. 5b). In the present study, mung bean plants with high P, Fe, and chlorophyll contents were positively correlated with siderophore and ACCD quantity in the salinized soils whereas their N content was negatively correlated with siderophore and ACCD production in the salinized soils (Fig. 6a). The black gram plant with high Fe, and chlorophyll content positively correlated with siderophore and ACCD quantity in the salinized soils, whereas its N and P contents were negatively correlated with siderophore and slightly positively correlated with ACCD production in the salinized soil (Fig. 6b).
Effect of siderophore and ACCD producing bacteria on siderophore and ACCD production under NaCl stressed soils (10 g kg− 1 of NaCl stress) (a) Siderophore quantification from NaCl stressed soil (b) ACCD quantification from NaCl stressed soil. Data obtained are means of seven replicates (n = 7) and bars denotes standard error bars. Data analyzed using one-way analysis of variance (ANOVA). Mean values (± S.E.) denoted on the bars with different lowercase letters are significantly different according to Duncan’s multiple range test (DMRT) (p ≤ 0.05). ACCD denotes ACC deaminase
Pearson correlation coefficient between PGP attributes (Nitrogen, phosphorus, iron, chlorophyll, percentage of siderophore unit, ACC deaminase production, dehydrogenase, fluorescein diacetate hydrolysis, and alkaline phosphatase) (a) Mung bean plant (b) Black gram plant. Red and blue color of cells indicates negative and positive correlations, respectively (*) is statistically significant at p < 0.05 and (**) is significant at p < 0.01. N (Nitrogen), P (Phosphorus), Fe (Iron), Chl (Chlorophyll), PSU (Percentage of siderophore units), ACCD (ACC deaminase), DHA (Dehydrogenase assay), FDA (Fluorescein diacetate assay), and Ap (Alkaline phosphatase assay)
3.8 Estimation of Soil Respiration Enzyme Activities
Inoculation with siderophore and ACCD producing bacteria not only enhanced plant growth and nutrient uptake but also significantly increased microbial respiration activity in the salinized soils (p ≤ 0.05). In mung bean plants experiment, the dehydrogenase enzyme activity was ranged to 112.53–167.40 µg g− 1 in the inoculated plants compared to the control (11.15 µg g− 1) (Fig. 7a). Similarly, FDA hydrolysis (31.66–44.51 µg g− 1) (control 1.98 µg g− 1) (Fig. 7b), and Ap enzyme (691.53–851.53 µg g− 1) (control 64.87 µg g− 1) (Fig. 7c) was also enhanced in response to bacteria treatments. A similar enhancement was observed in the black gram plant experiment. The dehydrogenase enzyme activity was ranged to 88.92–181.28 µg g− 1 in the inoculated plants compared to the control (14.62 µg g− 1) (Fig. 7a), FDA hydrolysis (16.94–38.20 µg g− 1) (control 1.28 µg g− 1) (Fig. 7b), and Ap enzyme (511.53–811.53 µg g− 1) (control 80.87 µg g− 1) (Fig. 7c). Overall, strains CWTS 5, SBTS 12 and CWTS 10 showed enhanced soil respiration activities under salinized conditions in both plants.
Effect of siderophore and ACCD producing bacteria on soil respiration enzymes under NaCl stressed soils (10 g kg− 1 of NaCl stress) (a) Dehydrogenase assay from NaCl stressed soil (b) FDA hydrolysis assay from NaCl stressed soil (c) Alkaline phosphatase assay from NaCl stressed soil. Data obtained are means of seven replicates (n = 7) and bars denotes standard error bars. Data analyzed using one-way analysis of variance (ANOVA). Mean values (± S.E.) denoted on the bars with different lowercase letters are significantly different according to Duncan’s multiple range test (DMRT) (p ≤ 0.05)
3.9 Correlation Between PGP Attributes
Pearson’s correlation revealed a positive correlation between PGP attributes which was statistically significant (p < 0.01 and p < 0.05) (Fig. 6a and b). Iron content, chlorophyll content, percentage of siderophore units, ACCD production, and soil respiration enzymes (DHA, FDA, and Ap) were found to be strongly positively correlated with each other in both mung bean and black gram plants. Only the nitrogen content of mung bean plants was negatively correlated with siderophore and ACCD production and soil respiration enzymes. The nitrogen and phosphorus contents of black gram plants were also negatively correlated with the percentage of siderophore unit. An image of the correlation matrix was also drawn to determine the relationship between plant growth promoting attributes of PGPB under salinity stress (Fig. 6a and b).
4 Discussion
Salinity stress or salt concentration in soil is increasing day by day due to routine activities near coastal areas, which pose a major threat to agricultural systems, resulting in a reduction of crop yield (Tomaz et al. 2020). Globally, 30% of irrigated land and 6% of the total land area are affected by salinity (Chaves et al. 2009). Plants cultivated in soils with high Na+ concentrations reduce plant growth and yield, availability of micro and macronutrients, and cause iron (Fe) deficiency (Abbas et al. 2015). Therefore, the present investigation was undertaken to study the ability of siderophore and ACCD producing bacteria to mitigate salinity stress and to support plant growth and nutrient uptake in mung bean and black gram plants in saline soils with Fe limitation.
The present study examined the use of siderophore and ACCD producing bacteria for sustainable crop production under saline conditions. Siderophores have a strong affinity to chelate Fe, solubilize it, and make it available from the soil to plants to reduce Fe deficiency (Alam 2014). Under salinity conditions, siderophore chelate Fe, induce a bioremediation process of salinity (NaCl) stress, and enhance Fe content. Furthermore, siderophore production improves nutrient uptake and plant growth. Sultana et al. (2021) reported that the salt-tolerant PGP bacterium Bacillus aryabhattai MS3 showed maximum siderophore production under 200 mM NaCl stress by the activation of entD, which is responsible for siderophore biosynthesis. Therefore, promoting the growth of a salt-sensitive rice plant, enhanced IAA, availability of nutrients, Fe and chlorophyll content, proline accumulation and decreased malondialdehyde under 200 mM NaCl stress. Similarly, siderophore producing Streptomyces increased auxin production, solubilized tricalcium phosphate (TCP) and increased Fe content in the shoots of wheat plants under 300 mM NaCl stress (Sadeghi et al. 2012). The present study is in agreement with the above-mentioned studies, in which siderophore producing bacteria reduced salinity stress, promoted plant growth, and enhanced N, P, and Fe uptake in mung bean and black gram plants.
In addition to siderophore production, ACCD production by bacteria play a crucial role in reducing salinity stress. Salinity induces ethylene production and oxidative stress, which may hamper seed germination and root development and negatively affects plant growth and productivity by reducing photosynthetic pigments (carotenoids and chlorophylls), ionic imbalance, and changes in the morphological and anatomical features (Saravanakumar and Samiyappan 2007). Ethylene is synthesized from its precursor ACC under environmental stressors such as salinity stress. Therefore, bacteria possessing the ACCD enzyme hydrolyze ACC into ammonia and α-ketobutyrate, thereby reducing the surplus production of the stress hormone ethylene and protecting plants from its inhibitory effects (Gupta et al. 2021; Chandwani and Amaresan 2022). Gupta et al. (2022) investigated the ACCD producing Pseudomonas aeruginosa GKP KS2_7 and Bacillus subtilis MBD 133, where both degraded ACC into α-ketobutyrate by exhibiting more than 257 nmol of α-ketobutyrate (ACCD activity) in the presence of 3% (w/v) NaCl stress. The decline in ethylene levels increase the root and shoot length, fresh and dry weight, biomass, total sugar content, protein, chlorophyll and antioxidant enzymes in Pisum sativum to counteract the negative effects of soil salinity. Kang et al. (2019) studied the effects of ACCD producing Leclercia adecarboxylata MO1 on tomato plants and found that it lowered ethylene levels and improved tomato plant growth and nutrient uptake under salinity stress. Similarly, ACCD producing Arthrobacter protophormiae improved Pisum sativum growth and enhanced rhizobial nodulation and mycorrhizal colonization under salinized conditions (Barnawal et al. 2014). ACCD production by the bacteria in the present study may have reduced the overproduction of ethylene, thereby enhancing the growth of mung bean and black gram plants under saline conditions.
The bacteria identified in this study belong to the genera Bacillus, Staphylococcus, and Rhodococcus. Furthermore, these bacterial strains can form biofilms and exhibit various PGP properties such as IAA production, and phosphate solubilization (Chandwani et al. 2022). Therefore, inoculation with these bacterial strains significantly enhanced the growth of mung bean and black gram plants. IAA production directly promotes root development by inducing cell elongation in response to cell division (Vimal et al. 2019). In addition, IAA production associated with structural modifications in response to stressful conditions, enhances the germination process, root growth, root elongation, root hair production, lateral branching of plants, and photosynthetic pigments (Kaya et al. 2013). Under saline conditions, P uptake is significantly reduced, thereby limiting plant growth and development. It has been reported that siderophore and ACCD producing bacteria improve P uptake by releasing mineral-dissolving compounds such as CO2, protons, hydroxyl ions, and extracellular enzymes (Pick et al. 1990). This may be one of the reasons for the improved uptake of P observed in this study. Chandwani and Amaresan (2022) also reported that ACCD producing bacteria along with other PGP properties such as IAA, siderophore production, and phosphate solubilization helps in managing abiotic stresses such as salinity, drought, temperature and heavy metals.
In the present study, an in vivo study revealed that the uptake of essential nutrients such as N, P, Fe, and Na+ ions by the plants was lower in the control plants under 10 g kg− 1 of NaCl stress. Inoculation with siderophore and ACCD producing strains enhanced the uptake of N, P, Fe and Na+ ions in mung bean and black gram roots under NaCl stress. These results are in agreement with those reported by Sultana et al. (2021) and Sadeghi et al. (2012), in which siderophore producing bacteria mitigated salinity stress and combated Fe deficiency by enhancing the Fe content in rice and wheat plants, respectively. Na+ ion uptake in the roots of mung bean and black gram plants was enhanced in the inoculated plants below the toxic limits mentioned by the WHO (50 µg g− 1). Due to inoculation with PGPB, the root biomass was found to be higher in the treated plants than in the control. Therefore, the sodium ions were absorbed in greater amounts by the roots, but it was nontoxic to plants as the nutrient uptake (N and P uptake) by the plants was enhanced in the treated plants. Similar findings were reported by Desai et al. (2023), in which the salt-tolerant bacteria Cronobacter and Enterobacter mitigated salinity stress and enhanced the growth of mung bean plants, nutrient uptake, and mobilized Na+ ions below the toxic limit.
Salinity stress hinders plant growth by the generation of Cl− ions. The present study revealed that the Cl− ion uptake by both mung bean and black gram plants was in the toxic limit in uninoculated plants. The plants treated with bacterial strains showed Cl− ion uptake in the normal range (70–140 µg g− 1) in both plants under NaCl stressed soils. In salinized soils, Cl− ions accumulate in the plant tissues due to the passive movement of Cl− ions or their mobility or high-water solubility. However, salt-tolerant bacteria reduce Cl− ion concentrations inside the tissues under high salinity stress through an exclusion mechanism (Ebrahimi et al. 2011). Karlidag et al. (2013) reported that inoculation with five PGPRs (B. subtilis EY 2, B. atrophaeus EY 6, B. spharicus GC subgroup B EY 30, S. kloosii EY 37, and Kocuria erythromyxa EY 43) increased the growth, nutrient content, chlorophyll content, and yield and decreased the Cl− ions in the strawberry plants under saline stress.
Salinity stress inhibits photosynthesis and causes chlorosis. A previous study has demonstrated that the chloroplast structure is damaged by saline stress, leading to impaired energy transfer from PSII to PSI. This process lowers the synthesis of chlorophyll in pea crops under salinity stress, leading to the degradation of the chlorophyll content (Gupta et al. 2021). The present study showed enhanced chlorophyll content in both mung bean and black gram plants due to inoculation with siderophore and ACCD producing bacteria, which helps to combat saline stress. This enhanced photosynthetic activity may be responsible for the increased biomass of mung bean and black gram plants under NaCl stress conditions. Similarly, Wang et al. (2016) reported that inoculation with ACCD producing strains increased the photosynthetic pigments of the host plant (Pea) by reducing xylem equilibrium pressure. Plants with higher Fe content were also found to have a high chlorophyll content (Fig. 6a and b). The estimation of siderophore and ACCD production in the soil was also found to increase in response to these bacterial inoculations compared to the control.
The enhancement of soil respiration activity indicated that the inoculated bacteria played a major role in nutrient cycling under salinity stress. Omar et al. (2021) reported an increase in the soil respiration enzymes in the rice plant during bacterial inoculation under drought stress.Dehydrogenase activity indicates the decomposition of organic matter and nutrient recycling via mineralization and oxidation (Sritongon et al. 2022). The enhancement of the Ap (P cycling enzyme) and FDA hydrolysis activity of the treated soil was mainly due to the presence of organophosphate complexes or alterations in microbial action. These results agree with those of Sritongon et al. (2022), who reported an increase in soil respiration enzymes such as dehydrogenase, FDA hydrolysis, and Ap activity during bacterial inoculation under salinity stress. Plants with high N, P, Fe and chlorophyll content were correlated with the enhanced soil microbial activity.
5 Conclusions
The present study provides evidence that siderophore and ACCD producing bacteria not only mitigate salinity stress or salinity toxicity but also enhance macronutrients (nitrogen and phosphorus), micronutrients (iron) and mobilizing ions (sodium and chloride) below the toxic limits from soils to the plant roots. Furthermore, bacterial inoculation enhanced the chlorophyll content of the plants, siderophore, and 1-aminocyclopropane-1-carboxylic acid deaminase (ACCD) production in the soil, and soil respiration activity. Considering the beneficial effects of these siderophore and ACCD producing bacteria, the strains CWTS 5 (Bacillus subtilis), SBTS 12 (Rhodococcus sp.) and CWTS 10 (Bacillus albus) could be formulated as bio-inoculants for the mitigation of salinity stress, plant growth, and nutrient uptake in mung bean (Vigna radiata L.) and black gram (Vigna mungo L.) plants in saline soils. Future studies involving the type of siderophore production as well as the gene expression of siderophore and ACCD need to be conducted using advanced techniques to understand the role of these bacteria in remediating stress and fortifying essential nutrients to promote plant growth.
Data Availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- ACC:
-
1-aminocyclopropane-1-carboxylic acid
- ACCD:
-
ACC deaminase
- Na+ :
-
Sodium ions
- Cl− :
-
Chloride ions
- Fe:
-
Iron
- N:
-
Nitrogen
- P:
-
Phosphorus
- FDA:
-
Fluorescein diacetate
- TTC:
-
2-3-5-triphenyl tetrazolium chloride
- WHO:
-
World Health Organization
- Ap:
-
Alkaline phosphatase
- NaCl:
-
Sodium chloride
- PGPB:
-
Plant growth promoting bacteria
- CFU:
-
Colony forming unit
- CAS:
-
Chrome azural S
References
Abbas G, Saqib M, Akhtar J, Haq MAU (2015) Interactive effects of salinity and iron deficiency on different rice genotypes. J Plant Nutr Soil Sci 178:306–311. https://doi.org/10.1002/jpln.201400358
Adam G, Duncan H (2001) Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol Biochem 33:943–951. https://doi.org/10.1016/S0038-0717(00)00244-3
Agrawal T, Kotasthane AS, Kosharia A, Kushwah R, Zaidi NW, Singh US (2017) Crop specific plant growth promoting effects of ACCd enzyme and siderophore producing and cynogenic fluorescent Pseudomonas. 3 Biotech 7:27. https://doi.org/10.1007/s13205-017-0602-3
Alam A (2014) Soil degradation: a challenge to sustainable agriculture. Int J Sci Res Agric Sci 1:50–55. https://doi.org/10.12983/ijsras-2014-p0050-0055
Barnawal D, Bharti N, Maji D, Chanotiya CS, Kalra A (2014) ACC deaminase-containing Arthrobacter protophormiae induces NaCl stress tolerance through reduced ACC oxidase activity and ethylene production resulting in improved nodulation and mycorrhization in Pisum sativum. J Plant Physiol 171:884–894. https://doi.org/10.1016/j.jplph.2014.03.007
Bessey OA, Lowky OH, Brock MJ (1946) A method for the rapid determination of alkaline phosphatase with five cubic millimeters of serum. J Biol Chem 164:321–329
Brock TD, Madigan MT, Martinko JM, Parker J (2003) Brock biology of microorganisms. Prentice-Hall, Upper Saddle River (NJ)
Casida LE Jr, Klein DA, Santoro T (1964) Soil dehydrogenase activity. Soil Sci 98:371–376
Chandwani S, Amaresan N (2022) Role of ACC deaminase producing bacteria for abiotic stress management and sustainable agriculture production. Environ Sci Pollut Res 29:22843–22859. https://doi.org/10.1007/s11356-022-18745-7
Chandwani S, Chavan SM, Paul D, Amaresan N (2022) Bacterial inoculations mitigate different forms of iron (Fe) stress and enhance nutrient uptake in rice seedlings (Oryza sativa L). Environ Tech Innov 26:102326. https://doi.org/10.1016/j.eti.2022.102326
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103:551–560. https://doi.org/10.1093/aob/mcn125
Desai S, Mistry J, Shah F, Chandwani S, Amaresan N, Supriya NR (2023) Salt-tolerant bacteria enhance the growth of mung bean (Vigna radiata L.) and uptake of nutrients, and mobilize sodium ions under salt stress condition. Int J Phytorem 25:66-73. https://doi.org/10.1080/15226514.2022.2057419
Ebrahimi R, Bhatla SC (2011) Effect of sodium chloride levels on growth, water status, uptake, transport, and accumulation pattern of sodium and chloride ions in young sunflower plants. Commun Soil Sci Plant Anal 42:815–831. https://doi.org/10.1080/00103624.2011.552657
Flowers TJ, Munns R, Colmer TD (2015) Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Ann Bot 115:419–431. https://doi.org/10.1093/aob/mcu217
Gupta A, Bano A, Rai S, Kumar M, Ali J, Sharma S, Pathak N (2021) ACC deaminase producing plant growth promoting rhizobacteria enhance salinity stress tolerance in Pisum sativum. 3 Biotech 11:514. https://doi.org/10.1007/s13205-021-03047-5
Gupta A, Rai S, Bano A, Sharma S, Kumar M, Binsuwaidan R, Pathak N (2022) ACC deaminase produced by PGPR mitigates the adverse effect of osmotic and salinity stresses in Pisum sativum through modulating the antioxidants activities. Plants 11:3419. https://doi.org/10.1080/00103624.2011.552657
Harvey DM, Flowers TJ (1978) Determination of the sodium, potassium and chloride ion concentrations in the chloroplasts of the halophyte Suaeda maritima by non-aqueous cell fractionation. Protoplasma 97:337–349. https://doi.org/10.1007/BF01276291
Hiscox JD, Israelstam GF (1979) A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot 57:1332–1334. https://doi.org/10.1139/b79-163
Honma M, Shimomura T (1978) Metabolism of 1-aminocyclopropane-1-carboxylic acid. Agric Bio Chem 42:1825–1831. https://doi.org/10.1080/00021369.1978.10863261
Jawad Hassan M, Ali Raza M, Khan I, Ahmad Meraj T, Ahmed M, Abbas Shah G, Ansar M, Afzal Awan S, Khan N, Iqbal N, Peng Y (2020) Selenium and salt interactions in black gram (Vigna mungo L.): ion uptake, antioxidant defense system, and photochemistry efficiency. Plants 9:467. https://doi.org/10.3390/plants9040467
Kang SM, Shahzad R, Bilal S, Khan AL, Park YG, Lee KE, Lee IJ (2019) Indole-3-acetic-acid and ACC deaminase producing Leclercia adecarboxylata MO1 improves Solanum lycopersicum L growth and salinity stress tolerance by endogenous secondary metabolites regulation. BMC Microbiol 19:1–14. https://doi.org/10.1186/s12866-019-1450-6
Karlidag H, Yildirim E, Turan M, Pehluvan M, Donmez F (2013) Plant growth-promoting rhizobacteria mitigate deleterious effects of salt stress on strawberry plants (Fragaria× ananassa). HortScience 48:563–567. https://doi.org/10.21273/HORTSCI.48.5.563
Kaya C, Ashraf M, Dikilitas M, Tuna AL (2013) Alleviation of salt stress-induced adverse effects on maize plants by exogenous application of indole acetic acid (IAA) and inorganic nutrients-A field trial. Aust J Crop Sci 7:249–254
Kumar BS, Prakash M, Narayanan S, Gokulakrishnan J (2012) Breeding for salinity tolerance in mung bean. APCBEE Procedia 4:30–35. https://doi.org/10.1016/j.apcbee.2012.11.006
Lewis RW, Islam A, Opdahl L, Davenport JR, Sullivan TS (2019) Comparative genomics, siderophore production, and iron scavenging potential of root zone soil bacteria isolated from ‘Concord’grape vineyards. Microb Ecol 78:699–713. https://doi.org/10.1007/s00248-019-01324-8
Lu Y, Liu H, Chen Y, Zhang L, Kudusi K, Song J (2022) Effects of drought and salt stress on seed germination of ephemeral plants in desert of northwest China. Front Ecol Evol 10:1026095. https://doi.org/10.3389/fevo.2022.1026095
Omar SA, Fetyan NA, Eldenary ME, Abdelfattah MH, Abd-Elhalim HM, Wrobel J, Kalaji HM (2021) Alteration in expression level of some growth and stress-related genes after rhizobacteria inoculation to alleviate drought tolerance in sensitive rice genotype. Chem Biol Technol Agric 8:1–19. https://doi.org/10.1186/s40538-021-00237-4
Pick U, Rental M, Chitlaru E, Weiss M (1990) Polyphosphate-hydrolysis-a protective mechanism against alkaline stress. FEBS Lett 274:15–18. https://doi.org/10.1016/0014-5793(90)81318-I
Sadeghi A, Karimi E, Dahaji PA, Javid MG, Dalvand Y, Askari H (2012) Plant growth promoting activity of an auxin and siderophore producing isolate of Streptomyces under saline soil conditions. World J Microbiol Biotechnol 28:1503–1509. https://doi.org/10.1007/s11274-011-0952-7
Saravanakumar D, Samiyappan R (2007) ACC deaminase from Pseudomonas fluorescens mediated saline resistance in groundnut (Arachis hypogea) plants. J Appl Microbiol 102:1283–1292. https://doi.org/10.1111/j.1365-2672.2006.03179.x
Shultana R, Kee Zuan AT, Yusop MR, Saud HM (2020) Characterization of salt-tolerant plant growth-promoting rhizobacteria and the effect on growth and yield of saline-affected rice. PLoS ONE 15:e0238537. https://doi.org/10.1371/journal.pone.0238537
Sritongon N, Sarin P, Theerakulpisut P, Riddech N (2022) The effect of salinity on soil chemical characteristics, enzyme activity and bacterial community composition in rice rhizospheres in Northeastern Thailand. Sci Rep 12:20360. https://doi.org/10.1038/s41598-022-24902-2
Sultana S, Alam S, Karim MM (2021) Screening of siderophore-producing salt-tolerant rhizobacteria suitable for supporting plant growth in saline soils with iron limitation. J Agric Food Res 4:100150. https://doi.org/10.1016/j.jafr.2021.100150
Tavakkoli E, Fatehi F, Coventry S, Rengasamy P, McDonald GK (2011) Additive effects of Na+ and Cl– ions on barley growth under salinity stress. J Exp Bot 62:2189–2203. https://doi.org/10.1093/jxb/erq422
Tomaz A, Palma P, Alvarenga P, Gonçalves MC (2020) Soil salinity risk in a climate change scenario and its effect on crop yield. Climate change and soil interactions. Elsevier, Dutch, pp 351–396. https://doi.org/10.1016/B978-0-12-818032-7.00013-8
Turan V (2020) Potential of pistachio shell biochar and dicalcium phosphate combination to reduce pb speciation in spinach, improved soil enzymatic activities, plant nutritional quality, and antioxidant defense system. Chemosphere 245:125611. https://doi.org/10.1016/j.chemosphere.2019.125611
Turan V (2021) Arbuscular mycorrhizal fungi and pistachio husk biochar combination reduces Ni distribution in mungbean plant and improves plant antioxidants and soil enzymes. Physiol Plant 173:418–429. https://doi.org/10.1111/ppl.13490
Turan V, Aydın S, Sönmez O (2022) Production, cost analysis, and marketing of bioorganic liquid fertilizers and plant nutrition enhancers. Industrial microbiology-based entrepreneurship: making money from microbes. Springer Nature, Singapore, pp 193–198. https://doi.org/10.1007/978-981-19-6664-4_13
Vimal SR, Patel VK, Singh JS (2019) Plant growth promoting Curtobacterium albidum strain SRV4: an agriculturally important microbe to alleviate salinity stress in paddy plants. Ecol Indic 105:553–562. https://doi.org/10.1016/j.ecolind.2018.05.014
Wang Q, Dodd IC, Belimov AA, Jiang F (2016) Rhizosphere bacteria containing 1-aminocyclopropane-1-carboxylate deaminase increase growth and photosynthesis of pea plants under salt stress by limiting Na+ accumulation. Funct Plant Biol 43:161–172. https://doi.org/10.1071/FP15200
Youssef MMA, Eissa MFM (2014) Biofertilizers and their role in management of plant parasitic nematodes. A review. J Biotechnol Pharm Res 5:1–6
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
NA: designed the study; SC: performed the experiment and analyzed the data; SC and NA: drafted, reviewed, edited and finalized the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chandwani, S., Amaresan, N. Siderophore and ACC Deaminase Producing Bacteria Enhance the Growth of Vigna spp Under Iron Limited Saline Soils. J Soil Sci Plant Nutr 24, 3734–3748 (2024). https://doi.org/10.1007/s42729-024-01795-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-024-01795-w