Abstract
Purpose
Fly ash (FA) is a waste byproduct produced in large quantities by coal-fired power stations. Its accumulation causes environmental issues, so it needs safe disposal of FA to reduce its accumulation. Herbal medicines like Mentha arvensis are being investigated worldwide for the prevention and treatment of a wide range of disorders because of their remarkable therapeutic benefits and absence of side effects when compared to current medications.
Methods
The aim of the study was to determine the effect of different concentrations of fly ash on growth, biochemical parameters, and constituents of essential oils of M. arvensis.
Results
The findings demonstrated that FA improved some important physical and chemical properties of soil. The use of FA-amended soil (10%) significantly improved the growth performance, photosynthetic pigments, protein, proline, antioxidant activity, and mineral contents. Conversely, the higher fly ash doses (25%) resulted in oxidative stress by increasing lipid peroxidation and electrolytic leakage levels, which negatively affected all of the aforementioned parameters. A confocal microscopic examination of the roots of M. arvensis revealed that fly ash at concentration of 25% resulted in membrane damage. In addition, alcohols, phenols, allenes, ketenes, isocynates, and hydrocarbons were among the functional groups found in the control and 10% of fly ash. Gas chromatography–mass spectrometry analysis of essential oils of M. arvensis treated with 10% fly ash revealed the presence of 32 bioactive components.
Conclusions
It is possible to use the 10% FA concentrations to increase plant growth and decrease the accumulation of FA that pollutes the environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
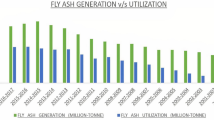
Fly ash is a waste byproduct that coal-fired power stations have created in enormous quantities. As a result, it has developed into a significant problem, and immediate action is required to decrease its accumulation as well as enhance the safe disposal of FA (Ahmad et al. 2021). By the end of 2031, FA production in India is anticipated to rise from the current level of 300 million tons per year to approximately 700 million tons (Qadir et al. 2022). In India, fly ash was produced at a rate of 270.82 million tons in 2021–2022, however, 259.86 million tons were used (Yadav et al. 2022).
Fly ash is one of the more cost-effective waste products in forestry and agriculture that, when applied in small amounts, has a big potential to repair degraded areas (Ansari et al. 2023). Fly ash can be used to increase soil fertility since it includes useful elements (Haris et al. 2021); (Bhat et al. 2023). Additionally, the soil's pH, porosity, water-holding capacity, and carbon content have all increased (Bhat et al. 2023). FA is a better source with large potential uses in the Indian agronomic sector due to the availability of necessary nutrients that are fostering plant health development and growth progression. Numerous studies have demonstrated that using fly ash could enhance the physico-chemical characteristics of soil, enhancing plant development, mineral uptake, and yield (Ahmad et al. 2021); (Ansari et al. 2022; Ansari et al. 2023).
Fly ash negatively impacts soil and crops at greater levels because it also contains heavy metals including lead, nickel, arsenic, chromium, cadmium, etc. (Varshney et al. 2021). Therefore, proper disposal of the leftover fly ash is required. Use in the cement and brick industries, land reclamation, and soil amendment in agriculture are among the main applications (Qadir et al. 2022). These harmful metals and metalloids interfere with physiological, photosynthetic, and gas exchange activities and disrupt the structural and functional integrity of chloroplasts, which results in the production of reactive oxygen radicals, or ROS in the form of hydrogen peroxide (H2O2), hydroxyl radicals (OH), and superoxide radicals (O2), which generated under adverse environmental conditions and cause oxidative stress in various plant tissues (Kumar et al. 2021); (Ansari et al. 2022).
According to (Pandey and Bhattacharya 2018), the generation of reactive oxygen species (ROS) would unavoidably impair the activity of vital cellular enzymes, interfere with the antioxidant defence system, cause problems with membrane fusion, break down protein and chlorophyll, halt cellular metabolism, and potentially even result in cell death. Numerous studies have found that plants exposed to high quantities of hazardous metals show reduced plant growth, impaired photosynthetic processes, and altered food metabolism. Increased or unmetabolized ROS will inevitably have an adverse effect on important macromolecules, such as lipids and proteins, which will stop cellular metabolism (Shakeel et al. 2020). Moreover, (Anjum et al. 2015) suggest that reactive carbonyls and malondialdehyde (MDA), which are byproducts of lipid peroxidation, may serve as markers for the existence of ROS-induced oxidation of lipids and proteins, respectively.
In particular, plants possess both enzymatic and non-enzymatic antioxidants that regulate increased and/or unmetabolized ROS and maintain optimum cellular metabolism (Aly et al. 2013); (El-Mahdy et al. 2021). Enzymatic antioxidants that scavenge reactive oxygen species (ROS) include superoxide dismutase (SOD), catalate (CAT), and ascorbate peroxidase (APX); on the other hand, peroxidase (POX) is involved in peroxide-cleaving redox processes (Abd El-Rahman et al. 2012); (Abu-Shahba et al. 2022). However, the effects of most heavy metals on plant systems have been evaluated taking into account the reactions of carbonic anhydrase (CA), a metalloenzyme localized in the chloroplast, nitrate reductase (NR), a rate-limiting enzyme of nitrogen metabolism, and the cellular level of proline (a nitrogenous osmolyte) (Jan et al. 2014).
One of the main causes of many metabolic problems, such as obesity, diabetes, and cardiovascular diseases, is a poor diet that results in malnutrition (Ansari et al. 2023). Mentha species have long been used in herbal therapy and are well-known for their medicinal properties. Its products, which include natural herbal formulations, medications, and herbal treatments, have seen a sharp rise in demand recently (Saqib et al. 2022). Due to their exceptional therapeutic benefits and lack of side effects compared to modern drugs, herbal remedies are now being explored globally to prevent and treat various illnesses. It is predicted that two-thirds of the world's population will rely on plant-based products for their basic medical needs. Furthermore, roughly 30% of all medications sold worldwide contain active compounds derived from plants (Ansari et al. 2023).
A significant crop, Mentha piperita (peppermint), generates essential oils that contain menthol, menthofuran, menthone, 1,8-cineole, and other compounds (Kumar and Patra 2012). It is an aromatic plant that belongs to the Lamiaceae family (Aqeel et al. 2023). Several investigations have shown that Mentha plants contain very high concentrations of phenolic chemicals, specifically phenols, flavonoids, terpenes, quinines, and polysaccharides (Bouyahya et al. 2020); (Saqib et al. 2022). These phytochemicals paved the way for their substantial application in the food and beverage industry's manufacturing of pharmaceuticals (Saqib et al. 2022). The oil is in high demand both domestically and abroad due to its significant application in medicinal and nutraceutical goods (Nabi et al. 2020). In India, essential oil (EO) crops are among the most popular non-food crops. Provided that the pollutants do not considerably reduce yield, these oil-bearing crops may be produced on heavily polluted land and in polluted air. Different techniques are used to extract essential oils from plants, but they all seem to stop the transport of heavy metals from the herbage to the oil (Kumar and Patra 2012).
Topical use of diluted mentha-derived EO helps protect against ringworm infections, burns, itching, and other skin-related discomforts (Thakur et al. 2021). Additionally, EO is known to have astringent, antipyretic, antiviral, antifungal, and antimicrobial qualities (Hussain et al. 2021). The EO has additional carminative, antibacterial, diuretic, stimulant, skin-soothing, and refrigerant characteristics because of the presence of a significant amount of menthol (40–50% of EO yield) (Thawkar et al. 2016). Menthyl acetate also gives the essential oil exceptional antifungal and insect-repellent characteristics (Hussain et al. 2021). Applications of essential oil constituents are anticipated to boost consumer preferences in the near future, and the global market for EOs is anticipated to grow significantly (Choudhary et al. 2020).
The aim of the present study was to evaluate the effects of different concentrations of FA amendments soil on M. arvensis as a biofertilizer. To achieve this, a pot experiment was conducted to evaluate the effects of different FA concentrations on growth traits, photosynthetic measurements, antioxidant enzymes, oxidative stress metabolism, mineral content, Fourier transform infrared spectrophotometer, and essential oil constituents of M. arvensis.
2 Materials and Methods
2.1 Plant Material and Experimental Site
For this experiment, menthol mint (Mentha arvensis Linn. cv. Kushal) was chosen as the plant material. The authorized suckers (stoten) are collected from the Central Institute of Medicinal and Aromatic Plants, Lucknow, India. The suckers were then surface sterilized with 0.2% HgCl2 for 6 min before being washed again with distilled water. Suckers were cut to 3–5 cm in length and directly planted in earthen clay pots with different amended treatments of garden soil (GS) and fly ash (FA). Plants were grown in the experimental site green house of the Department of Botany (N latitude, 27.91499°; E longitude, 78.07274°; and elevation of 192.92 m) at AMU Aligarh.
2.2 Collection and Analysis of Soil and Fly Ash
2.2.1 Soil Collection
The soil was obtained from a clean and uncontaminated garden on a neighboring University Campus.
2.2.2 Fly Ash Sampling and Analysis
The fly ash used in the experiments came from the Harduaganj Thermal Power Plant (HTPP) in Aligarh, U.P., India. The thermal power plant has a power capacity of 610 megawatts (M.W.) with ten active units and accounts for approximately 7.889 (Lac MT) of FA annually. The combined samples were dried in the sun and kept in plastic bags at 28 °C. Physical and chemical analyses were carried out by grounding the sample and sieving it through a 0.2-mm mesh. Using these methods, (Chatterjee et al. 2009) investigated the physicochemical properties of FA and soil. Before planting, pots were placed in the experimental area for 14 days to ensure physicochemical stability. (Panda et al. 2018a; Panda et al. 2018b) determined the chemical characteristics of fly ash and garden soil additions in the research lab. These parameters included electrical conductivity (EC), pH, organic carbon (OC), P, N, K, Mn, Al, Cr, Fe, Zn, and Cu concentrations. FA particle size was characterised by semi-quantitative elemental analysis and scanning electron microscopy (SEM) and energy dispersive x-ray (EDX).
2.3 Determination of Physiochemical Characteristics of Fly Ash, Soil, and Fly Ash Amended Soil
FA amendment can enhance the physio-chemical and biological aspects of acidic soils. Fly ash could be used as an acidic soil ameliorator depending on its qualities, such as acidity or alkalinity. The physico-chemical parameters of soil, FA, and an enhanced substrate containing 10% FA were investigated. Double-distilled water was used to dilute the soil samples, and the results were analysed to determine the pH, bulk density, electrical conductivity, and water holding capacity of the air-dried soil, FA, and FA-treated soil samples. A digital pH device is used for measuring a set quantity of FA and GS in distilled water and recording the results to calculate the pH. An electrical conductivity meter was used to calculate the soil suspension's electrical conductivity. Fly ash, which could be either acidic or alkaline based on the source, can be used to stabilize soil. Applying alkaline fly ashes to cultivated soils may increase pH. (Jackson 1973) pH buffers were used to measure and calibrate the soil pH using a pH meter. (Walkley and Black 1934) determined the organic matter and organic carbon (O.C.) content. Adding fly ash to the soil changes the texture of the soil. Various soils containing up to 10% fly ash were studied to evaluate their physical properties.
The soil textural triangle approach examines soil types based on sand, silt, and clay proportions estimated by (Chopra and Kanwar 1991). The percentage of the soil volume that is encased in pore spaces is known as porosity (St), and this definition is in line with the methodology established by (Carter et al. 1993). When fly ash is added to soil, it increases the workability and porosity of the soil while also reducing bulk density. The addition of fly ash improved the water-retention capacity of sandy and loamy soils. This was followed by a rise in hydraulic conductivity, which promoted the breakdown of the surface layer. (Priha and Smolander 1999) were used to calculate the water holding capacity. The (Jackson 1973) technique was used to determine the cation exchange capacity (CEC) of soil samples. The electrical conductivity (EC) technique was determined according to the method of (Rayment and Higginson 1992).
To determine phosphorus (P) from an extract solution used as a calorimetric after HClO4, the (Dickman and Bray 1940) equation was used. Potassium (K) was measured with a flame photometer (ME 882) in soil and fly ash samples. For each sample, the nitrogen content was determined using (Kjeldahl 1883) method. Chopra and Kanwar (1982) performed measurements of magnesium (Mg) concentration. (Richard 1954) technique was used to determine carbonates and bicarbonates in samples. The amount of phosphorus present in the extraction solution was calculated using a spectrophotometer set at 660 and 440 nm ranges. The elements K, Na, Ca, Mg, Fe, Mn, Zn, and Cu were determined in the diluted digests using atomic absorption spectroscopy (AAS, 3690-Perkin Elmer, Massachusetts, United States) according to (Jackson 1973) method.
2.4 Experimental Design and Treatments
The experimental pots were planted in early February. The studies were conducted using 36 earthen clay pots, with each treatment having five replications. The complete randomization block design (CRBD) approach was used in their creation and planting for each treatment. Each clay pot contains 4.5 kg of soil-amended fly ash to aid in the plant's development. Plants were grown in a green net house to imitate agricultural conditions. For the duration of this experiment, plants were watered to their maximum capacity to ensure the soil was moist in their pots in order to prevent water from leaking from them. With the effects of soil and fly ash, plants were grown to the age of 105 days (in the flowering stage) and used to measure many different parameters.
Following collection, the fly ash (FA) and garden soil (GS) were allowed to air dry for seven days before being used to make six separate fly ash and garden soil additions in 4.5 kg earthen clay pots (w/w). There are six treatments: T1: (control) garden soil + FA (100% + 0%), T2: garden soil + FA (95% + 5%), T3: garden soil + FA (90% + 10%), T4: garden soil + FA (85% + 15%), T5: garden soil + FA (80% + 20%), and T6: garden soil + FA (75% + 25%).
2.5 Analysis of Scanning Electron Microscopy (SEM)
Using the methodology described by (Bertrand and Poirier 2005), the number and size of stomata were estimated using the scanning electron microscope (SEM). Fresh leaves of M. arvensis samples were mounted for four hours in 0.1 M phosphate buffer (pH 7.0) with 2.5% glutaraldehyde and 2% para-formaldehyde. Phosphate buffer (pH 7.0) was used to treat the leaves for 50 min with 2% osmium tetroxide before thoroughly washing them for 10 min. After fixing, the samples were dehydrated with a series of alcohols (50% to 100%). The leaf was sputter-coated with gold and palladium before being examined under a high-voltage (15 kV) scanning electron microscope (JEOL JSM-6510 LV Japan). Using methods described by (Ashfaque and Inam 2019); (Ansari et al. 2022), the leaf's stomatal area was measured at high magnification.
2.6 Analysis of Energy-Dispersive X-Ray (EDX)
For the EDX analysis, fresh leaves were examined. Consider the small leaf part that was taken out of the middle of the midrib. Leaf samples were taken for each treatment. Round aluminium stubs were used to attach the dissected samples to adhesive carbon tape. A scanning electron microscope (JEOL JSM-6510 LV, Japan) equipped with an EDX (JEOL JFC-1600, Japan) instrument was used to investigate the garden soil and fly ash air dried powder samples for elemental analysis. The chamber pressure and accelerating voltage were set at 30 pA and 15 kV, respectively. Elemental mapping was examined using the INCA X-Act mapping programme (Oxford Instruments).
2.7 Determination of Growth Characteristics
The plant is carefully uprooted from the clay pot, and the soil that was stuck to it is washed with double-distilled water. For measuring the morphological changes, the lengths of the shoots and roots (SL and RL) and their fresh and dry weights (FW and DW) were measured. The plants were then delicately removed, and the root and shoot lengths were measured in centimeters (cm) using a scale in metric units. The fresh mass of each plant's root and shoot was estimated using an electronic balance. After placing the root and shoot samples in an oven that was maintained at 70 °C for 72 h, the dry mass of the samples was then measured. The leaf area index was calculated for each treatment as determined by the Watson method (1958).
2.8 Determination of Physiological and Biochemical Activities
2.8.1 Determination of Photosynthetic Pigments
The chlorophyll concentration of photosynthetic pigments in fresh mint leaves was calculated using (Maclachlan and Zalik 1963). A healthy tissue plant leaf was crushed and ground with an 80% aqueous acetone solution using a mortar and pestle. Using a spectrophotometer, the chlorophyll extract's optical density (O.D.) was measured at 663 nm for chl a, 645 nm for chl b, and 450 nm for carotenoid content. The results of these measurements were recorded. The total chlorophyll content was computed by summing both concentrations and is expressed in mg g−1 F.W.
2.8.2 Determination of Proline Content
The procedure described by (Bates et al. 1973) was used to calculate the proline content. A half gram of fresh leaves and 5 mL of a sulfosalicylic acid solution at 3% were mixed. The solution was then centrifuged for 10 min at 10,000 × g. After that, 2 mL of the supernatant was mixed with 1 mL of acid ninhydrin solution and 1 mL of glacial acetic acid and then heated in a water bath for one hour at 100 °C. After cooling, 3 mL of toluene was added, and after shaking the mixture for 20 to 25 s, the reaction was stopped. The toluene layer was read at 520 nm using a spectrophotometer (Shimadzu UV-1700, Tokyo, Japan).
2.8.3 Determination of Protein, Malondialdehyde Content and Electrolyte Leakage (EL)
The Folin–Ciocalteu reagent was used to quantify the amount of protein in accordance with the method described by Lowery and Newman (1951) The optical density was read at 750 nm using a spectrophotometer (Shimadzu UV-1700, Tokyo, Japan), bovine serum was employed as the reference material.
Heath and Packer 1968) method was used to determine lipid peroxidation (MDA) content by using 0.5% thiobarbituric acid (TBA), and the solution's absorbance was measured at 532 nm and 600 nm using a spectrophotometer (Shimadzu UV-1700, Tokyo, Japan). The absorbance coefficient of malondialdehyde was determined by using an extinction value of 155 mM−1 cm−1 as the basis for the calculation. The electrolyte leakage was calculated by following the specific procedures outlined in (Dionisio-Sese and Tobita 1998) study.
Using a modified method outlined by (Awasthi et al. 2018), the amount of MDA in leaves and roots was assessed. Prior to being washed with a sulfite solution for 10 min, leaf and root tip specimens subsequently immersed in Schiff's reagent for 1 h. Following a double-distilled water (DDW) wash to remove any last stains, the samples were dried. The chlorophyll was then removed from the samples by heating them at 100 °C while they were dissolved in pure ethanol. After being chilled, the samples were then added to a 20% glycerol solution. Images were taken with the help of a digital camera.
2.9 Determination of Oxidative Stress Markers
2.9.1 Visualization and Estimation of Superoxide (O2 −●) Content
To assess the visual delectability of superoxide (O2−), the leaves were stained with nitro blue tetrazolium (NBT) chemical, as described in the methodology provided by (Scarpeci et al. 2008). The leaves were allowed to dip in a 10 mL of 0.2% NBT solution and included 50 mM of sodium phosphate buffer (pH 7.4) for 8 h at room temperature. After that, any remaining chlorophyll was removed by boiling the discolored leaves in pure alcohol at 100 °C. Several leaves with blue dots on them were photographed.
The method developed by (Liu et al. 2007) was used to accurately estimate the number of spots representing free radical O2 content. After allowing the leaf samples to cool, they were placed in a 20% glycerol solution. The camera (Nikon D3500) was used to capture photos.
2.9.2 Visualization and Estimation of Hydrogen Peroxide (H2O2) Content
According to the method described by (Scarpeci et al. 2008), hydrogen peroxide (H2O2) was detected by dyeing the leaves with 3,3′-diaminobenzidine (DAB) chemical, allowing for the visually detectable presence of hydrogen peroxide. The leaves were dipped in 10 ml of a 1% DAB solution (DAB solution, 20 mM sodium phosphate, pH 3.8) at 37 °C for 8 h at room temperature. In order to remove the chlorophyll from the colored leaves, the stained tissues were dipped in pure alcohol and then boiled at 100 °C until the chlorophyll was completely removed. After the leaf samples were allowed to cool, they were transferred to 20% glycerol. The camera (Nikon D3500) was used to capture photos.
2.9.3 ROS Detection
The fluorescent probes used were 2,7-dichlorodihydroflurescein diacetate (DCF-DA /H2DCFDA). The method described by Rodríguez-Serrano et al. (2009) was used to detect ROS in roots. The roots of M. arvensis were thoroughly washed and subjected to tests to detect ROS. The accumulation of ROS (H2O2) inside the plant roots was examined using DCFDA/H2DCFDA probing. To get rid of the excessive stains, the small pieces of root tip were dried for five minutes in the dark with either a 20 M solution of DCFDA/H2DCFDA. Confocal microscopy (Zeiss, LSM 780, Tokyo, Japan) was utilized to detect the concentration of ROS (H2O2) as green fluorescence intensity.
2.10 Analysis of Confocal Laser Scanning Microscopy (CLSM)
2.10.1 Cell Viability
Propidium iodide (PI) was utilized as the fluorescence probes, respectively. The standardized method proceed by (Rattan et al. 2017) was utilized in order to determine the level of cell viability present in the root tissues. The roots of M. arvensis were thoroughly washed and subjected to tests to detect ROS and cell viability. The roots were dipped in a propidium iodide solution to study cell viability. With the due carefully, root tips were prepared and cleansed 3–4 times with double-distilled water (DDW). Root tips were then immersed for 30 min in stained solutions before being washed with DDW. Frequent washing might eliminate a slightly reduced stain. After being placed on glass slides, the samples were topped with cover slips and looked at using a confocal microscope (Zeiss, LSM 780, Tokyo, Japan).
2.10.2 Cell Death Estimation (Tryphan blue)
Staining the leaves with trypan blue in the procedure outlined by (Mason et al. 2016) provided for the investigation of the visual detection of cell death. Using this method, the leaves were put into a solution of ethanol and 0.04% lactophenol-trypan blue (1:1 ratio) and left there for a while. Staining solution-containing samples were heated for one minute and then allowed to cool for ten minutes. After the samples were taken out of the staining solution and soaked overnight in de-staining solutions (2.5 g mL−1 chloral hydrate), they were photographed.
2.11 Enzymatic Antioxidant Defense System Quantification
2.11.1 Preparation of Enzyme Extract
The enzymatic study was carried out with 0.5 g of fresh M. arvensis leaves mashed in liquid nitrogen (LN2) at 4 °C with a mortar and pestle. As a result, 0.5 g of coarse powder was mixed with 1% polyvinylpyrrolidone (w/v), 100 mM potassium phosphate buffer (pH 7.8) and 0.5% Triton-X-100 (v/v). The samples were centrifuged for 5 min at 4 °C at 10,000 rpm. (Kuo et al. 1982) examined the anti-oxidative activity of enzymes using the supernatant from centrifugation.
2.11.2 Activity of Superoxide Dismutase (SOD; E.C. 1.15.1.1)
The approach of (Beauchamp and Fridovich 1971) was carried out to determine SOD activity. The freshly prepared enzyme sample was added with 40 mL of NBT (55 mM), Methionine (9.9 mM), Triton-X-100 (0.02%), Riboflavin (1 mM), and EDTA (2 mM). The mixture was then lit and maintained at 30 °C for an hour. A spectrophotometer (Shimadzu UV-1700, Tokyo, Japan) was used to measure the absorbance at 560 nm of the reaction mixture. The units of SOD represent the quantity of enzyme necessary to reduce nitroblue tetrazolium’s reactivity by 50% at the specified wavelengths. Additionally, it was observed that the sample solution used methyl red as a marker in a titration over 0.05 N HCl.
2.11.3 Activity of Catalase (CAT; EC 1.11.1.6)
An approach that slightly modified Chandlee and Scandalios (1984) method was used to assess the activity of CAT. To 0.04 mL of enzyme extract, H2O2 (15 mM) and potassium phosphate buffer (50 mM, pH 7) were added. The mixture was centrifuged at 12,500 × g for 20 min at 4˚C. The effect of H2O2 at 240 nm was recorded for 2 min, with a 5-s break in the middle, in order to determine an enzyme's level of activity.
2.11.4 Activity of Ascorbate Peroxidase (APOX; EC 1.11.1.1)
The (Nakano and Asada 1981) method was used to determine the enzyme activity of APX. The mixture (0.1 mM) is made by heating the enzyme extract with ascorbate (0.5 mM), buffer (50 mM with pH 7), EDTA (0.1 mM), and hydrogen peroxide (H2O2). The decrease in substrate wavelength at 290 nm was used to calculate the enzyme's activity. The optimal duration of time per minute to break down one mole of medium at 25 °C is one APX unit.
2.11.5 Activity of Peroxidase (POX; EC 1.11.1.7)
The activity of peroxidase was measured using pyrogallol and the (Kar and Mishra 1976) method. The peroxidase activity assay mixture included 1 mL of the 20-fold diluted enzyme extract, 50 M of pyrogallol, 50 M of H2O2, and 125 M of phosphate buffer at pH 6.8. This was stopped with 0.5 mL of 5% (v/v) H2SO4 after incubation for 5 min at 25 °C. The absorbance of the mixture was read at 420 nm to calculate the quantity of purpurogallin produced.
2.11.6 Activity of Guaiacol Peroxidase (GPX; EC 1.11.1.7)
Guaiacol peroxidase was evaluated with guaiacol using the (Mazhoudi et al. 1997) procedure. The reaction mixture for GPX contained 25 mM of K-phosphate buffer (pH 7.0), 9 mM of guaiacol, 10 mM of H2O2, and enzyme solution. The enzymatic activity was determined by observing the increment in wavelength at 470 nm.
2.11.7 Activity of Nitrate Reductase (NR; EC 1.7.1.2)
A known weight of fresh leaves (0.2 g) ground and mixed with 2 mL of isopropanol (5%), 0.5 mL of potassium nitrate, and 2 mL of phosphate buffer 0.1 M (stock solution) (pH 7.5) to determine nitrate reductase (NR) according to the method of (Jaworski 1971). The mixture was incubated for two hours at 30 °C. Then, 0.5 mL of the aliquant was added to each test tube, along with 0.3 mL (0.02%) of N-(1-Naphthyl) ethylenediamine dihydrochloride and 0.3 mL (1%) of sulfanilamide for the azoo-coupling with NED-HCl and sulfanilamide that resulted in the production of nitrite. Further diluting to a final amount of 5 mL was performed using deionized water. The absorbance of the solution was read at 540 nm using a spectrophotometer (Shimadzu UV-1700, Tokyo, Japan).
2.12 Estimation of the N, P, and K Contents in Leaves
The quantity of N, P, and K in the leaves was later determined by digesting leaf samples collected from each treatment. In an oven that has been set to 100 °C, the leaves are dried at room temperature. With the use of a mortar and pestle, the dried leaves were crushed, and samples were then put through a mesh sieve. The concentrations of N, P, and K were calculated using the dried leaves. A digestion flask was filled with 100 mg of dried leaf powder and 2 mL of analytical-grade sulfuric acid-digesting leaf samples collected from each treatment.. On a Kjeldahl thermostat, the mixture was heated for approximately 2 h at 100 °C before cooling for around 15 min at room temperature. This procedure was repeated until the combination in the test tube remained colourless.
The nitrogen content was determined by (Lindner 1944) and modified by (Novozamsky et al. 1983) using Nessler's reagent. A spectrophotometer is used to measure the optical density (OD) of the substance at 525 nm. To determine the phosphorus content of the peroxide-digested leaf tissue, the procedure of (Fiske and Subba Row 1925) and (Rorison et al. 1993) was used using 2.5% molybdic acid and 2-Hydroxy-4-sulfo-1-naphthylamine. At 620 nm, spectrophotometry measures the sample's absorbance. According to (Hald 1947), a flame-photometer (Model C150, AIMIL, India) was used to determine potassium content in the peroxide-digested material using the absorption spectrum and certain filters.
2.13 Fourier Transform Infrared Spectrophotometer (FT-IR)
Fourier Transform transform infrared (FT-IR) spectrophotometer is the most powerful tool for identifying the types of chemical bonds and functional groups present in compounds. The wavelength of light absorbed is characteristic of the chemical bond. By interpreting the infrared absorption spectrum, the chemical bonds in a molecule can be determined. For FT-IR analysis, dried powders of M. arvensis methanolic leaf extracts from the control and 10% FA-amended plant samples were used. 10 mg of the dried leaf extract powder was encapsulated in 100 mg of KBr pellets in order to prepare translucent sample discs. The powdered sample of each plant specimen was loaded into an FTIR spectrophotometer (Perkin Elmer Spectrum 2 Instrument) with a scan range of 400 to 4000 cm−1 and a resolution of 4 cm−1.
2.14 Essential oil (EO) Extraction Analysis
The extraction of the essential oil of M. arvensis was obtained by utilizing Clevenger's equipment (Borosil, India) in conjunction with the hydro-distillation procedure described by (Guenther 1972), and the results were then estimated gravimetrically. Leaves of this plant, weighing about 250 g, were plucked from each treatment plant and then chopped up into smaller pieces. The oil was obtained by a distillation method that continued for 3 h. After everything was said and done, the oil that had been extracted was dried with white crystalline anhydrous sodium sulphate (Na2SO4) (Kumari et al. 2009) and then assessed using GC–MS.
2.14.1 GC–MS Analysis of Essential Oil
The phytochemical extract was evaluated with GC–MS (Shimadzu QP 2010 Ultra, Japan) to identify the dynamic components of EO at the Jawaharlal Nehru University in Delhi, India. A PerkinElmer® Clarus® 680 Gas Chromatograph (GC) interfaced with a Clarus sq 8 mass spectrometer (MS) and was outfitted with a TG-WAX MS fused silica capillary GC column inner diameter and film thickness SH-5 capillary, 30 m × 0.32 mm × 0.25 µm, to perform GC–MS. The temperature of the injector was set at 260 °C, and the split ratio was set at 110. The temperature in the column oven was 50 °C, and the pressure was 69.0 kPa. A column flow of 1.21 mL/min and a linear velocity of 39.9 cm/sec were maintained by the use of helium as the carrier gas. The energy used is decreased (< 70 eV), and the mass scan range (m/z) is between 40 m/z and 650 m/z. The interface temperature was 270 °C, whereas the ion source was 220 °C.
2.14.2 Identification of Components
To determine the retention index for all of the volatile elements, a similar sequence of n-alkanes was used in the calculation. The constituents of the oil were identified by comparing their mass spectra to those that were saved in a computer library such as the Wiley mass spectroscopy library. Additionally, their retention indices (RI) were compared either with authentic compounds or with data that was published in the literature and was based on the retention indices of the components. By comparing the retention indices and mass spectra from the NIST computer library database, the chemicals in plant extracts could be analyzed.
2.15 Statistical Analysis
The variance analysis in a one-way ANOVA was used to analyze the experimental data in R (version 64.2.14.1). The average values of the FA treatments were assessed in order to find differences between them using Duncan's multiple range test at p ≤ 0.05. Using the mean of five different replications (n = 5), each value was computed. Utilizing Origin Pro (2023b) software, data correlation research was conducted utilizing principal component analysis (PCA) and Pearson's correlation coefficient.
3 Results
3.1 Characterization of Fly Ash and Garden Soil
Fly ash particles were shown by scanning electron microscopy (SEM) to be spherical glassy particles (Fig. 1 A, D, G), whereas garden soil particles were shown to be flaky (Fig. 1 B, E, H). Tables 1 and 2 give general physico-chemical characteristics of fly ash, garden soil, and fly ash-enhanced soils. The garden soil, fly ash, and soils with 10% fly ash were all silt loam, sandy loam, and silt loam, respectively, in terms of physicochemical texture.
The enhancement in soil characteristics following 10% FA amendment is shown in Table 1. The soil's electrical conductivity (EC) increases by 95.9%, cation exchange capacity (CEC) by 101.9%, porosity by 28.2%, water holding capacity by 15.6%, and moisture content increases by 25.2% with the addition of 10% FA. In contrast, 10% FA added to garden soil reduces relative density by 19.4%.
Table 2 displays the chemical characteristics of soil and soil supplemented with 10% FA. The pH of the amended soil with 10% FA increased from 6.8 to 7.7. In addition, the content of sulfate, chloride, carbonate, and bicarbonate was also improved with 10% FA amendment to the soil by 54.0%, 3.2%, 16.8% and 11.0%, respectively. Moreover, the content of Ca, Na, K, Zn, and Mn was also increased in soil amended with 10% FA by about 122.3%, 91.3%, 44.0%, and 370.4%, respectively. However, due to the absence of the N element in FA, the N content reduced by 11.2%, and the content of P and Mg also decreased by 3.2% and 10.3, respectively. The elemental compositions of garden soil, FA, and 10% fly ash amended soils were displayed in Fig. 1 C, F, I.
3.2 Effect of Soil Amended With FA on Growth Parameters
The results shown in Table 3 and supplemented Fig. 1 illustrated the effects of various FA levels amended to the soil on M. arvensis growth performance. In comparison to other treatments, soil supplements with 10% fly ash demonstrated improved plant growth, whereas soil amendments with 25% fly ash demonstrated decreased plant growth performance in M. arvensis.
The most notable improvements in shoot length (21.9%), root length (79.0%), fresh weight of shoots and roots (57.9 and 79.4%), dry weight of shoots and roots (159.0 and 124.0%), root circumference (80.5%), number of branches (70.1%), number of leaves (52.9%), leaf area (60.0%), and stomatal area (64.8%) were noted in plants grown in soil amended with 10% FA as compared to the control plants.
3.3 Effect of Soil Amended With FA on Photosynthetic Pigments and Biochemical Markers
In M. arvensis, the photosynthetic pigments content such as chlorophyll 'a', chlorophyll 'b', and carotenoid increased significantly at 10% FA amended soil but declined significantly at 25% FA in comparison to the control (Table 4).
In comparison to the control, the levels of chlorophyll 'a' and 'b' and carotenoid increased by 34.5%, 79.2%, and 57.1%, respectively. The stomatal width (4.28 µm) and length (14.36 µm) increased in plants grown in 10% FA as compared to width (1.51 µm) and length (12.08 µm) of control plants (Fig. 2).
Compared to control plants, M. arvensis plants cultivated in soil amended with 10% FA had a substantial increase in protein content of about 38.4% and decreased by about 20% in plants cultivated in soil amended with 25% FA. In comparison to control plants, different FA concentrations considerably boosted the proline content of M. arvensis leaves. Plants growing in soil that had 25% FA added to it had the greatest proline content, which was found to be around 116.5% (Table 4).
3.4 Effect of Soil Amended With FA on Oxidative Stress and ROS Production
Different tissues of M. arvensis exposed to fly ash treatments showed an increase in MDA and electrolytic leakage (EL) % levels when compared to plants grown in garden soil. With increasing concentrations of FA amended into garden soil, MDA and EL content increased significantly and reached the highest levels at 25% FA by 94.6% and 16.8%, respectively (Table 4).
The findings in Table 4 show that adding varying FA levels to the soil directly affects the amount of O2− anions and H2O2 that are produced in M. arvensis leaves. With rising FA levels, the concentration of O2− radicals and H2O2 increased substantially. However, compared to control plants, the 25% FA level had the highest O2− (160.8%) and H2O2 (145.5%) content. The findings indicate that high concentrations of (25%) FA may damage cellular membranes and generate H2O2.
The findings were further supported by the histochemical localization of O2− (Fig. 3 A) and H2O2 (Fig. 3 B) in M. arvensis leaves, where the unstressed plants had minimal staining while the plants treated with greater levels of 25% FA displayed extensive staining. NBT and DAB staining was used to further examine the ROS production in leaves, with blue and brown spots denoting the localization of O2− and H2O2, respectively (Fig. 3 A and B). In 25% of FA plants, these spots were more noticeable, indicating that there was an excess ROS generation (Fig. 3 Af and Bf).
(A) Superoxide anion content and histochemical localization of O2− and (B) Hydrogen peroxide content and histochemical localization of H2O2 in the leaves of 105-day-old M. arvensis treated with different level of fly ash amended soil. (C) Reactive oxygen species (ROS) localization in the roots of 90-day-old M. arvensis treated with different level of fly ash amended soil. (a) Control, (b) 5% FA, (c) 10% FA, (d) 15% FA, (e) 20% FA, and (f) 25% FA
The DCF-DA fluorescent dye was used to find ROS in the roots, which were then examined under a confocal microscope. DCF-DA reacts with ROS molecules in cells and produces green fluorescence. More ROS formation in cells is indicated by maximum green fluorescence. According to Fig. 3 C, plants cultivated with 25% FA exhibit the highest levels of green fluorescence in their root samples (Fig. 3 C f), whereas plants produced in control garden soil exhibit the lowest levels of green fluorescence (Fig. 3 C a).
3.5 Effect of Soil Amended With FA on Cell Death
Trypan blue staining was used to histochemically detect cell death in the leaves. As shown in Fig. 4 A, the density of blue spots was higher in the leaves of plants grown in soil that had been amended by 25% FA (Fig. 4 Af), indicating that more dead cells were present there. In contrast, the control plants (Fig. 4 Aa) had the fewest blue spots.
Propodium iodide was used to detect cell death in the roots. The detection of dead cells increased with the quantity of red fluorescent nuclei. The plants cultivated on soil supplemented with 25% FA (Fig. 4 BF) show the reddest fluorescent nuclei, whereas the plants not treated (Fig. 4 Ba) show the least amount of red fluorescent nuclei.
3.6 Effect of Soil Amended With FA on Antioxidant Activity
The enzyme antioxidants SOD, CAT, POX, APX, GPX, and NR were found to be present in greater quantities in the plants growing in soil supplemented with FA. By increasing the concentrations of FA, their actions were further enhanced (Table 5). The plants treated with 25% FA showed the greatest increase in antioxidant activity, which was greater than that of the control by 202.2% in SOD, 78.0% in CAT, 102.0% in POX, 157.0% in APX, 128.5% in GPX, and 108.6% in NR content.
3.7 Effect of Soil Amended With FA on Nutrient Composition
The amounts of essential and some non-essential elements differed in the soil supplemented with different FA levels. The findings showed that lower levels of FA (10%) markedly increased the concentration of N (66.5%), P (182.4%), K (79.3%), Ca (55.5%), Mg (80.0%), Cu (108.3%), Mn (100%), Zn (118.8%), and Fe (19.3%) in plants compared to the control (Table 6). However, lower levels of FA (10%) markedly decreased Na (42.9%). Plants grown with 25% FA had the lowest mineral content than the control, according to the present study (Fig. 5).
Through SEM–EDX mapping at 10% FA level, several elements, including C, O, N, Fe, Cl, K, Mg, Ca, Si, Na, Cu, S, and P, were localized in dried leaves of M. arvensis as opposed to control plants, which include C, O, N, K, Mg, Ca, Na, Cu, S, and Cl (Fig. 4). It is significant to note that M. arvensis did not accumulate any of the potentially harmful heavy metals at the 10% FA level. Therefore, this concentration of fly ash was suggested to be the optimum concentration of fly ash supplemented in M. arvensis.
3.8 Effect of Soil Amended With FA on Functional Group Identification by FT-IR
Based on the peak values in the IR radiation band, the functional groups of the active components found in the extract were identified using the FT-IR spectrum. The phytocompounds of the methanolic leaf extract of M. arvensis were examined by using biomolecule functional groups. The control and 10% FA amended plants sampled major peaks in the 400 to 4000 cm−1 range (Fig. 6). Functional groups and peaks of the biomolecules like alcohols and phenols at 3439 and 3449 (O–H stretching and H–bonded), allenes, ketenes, isocyanates, Isothiocyanate 2084 and 2968 (N = C = S stretching and C–H stretching), Hydrocarbon part of molecule, alkenes, isothiocyanates 1633 and 2094 (C = C stretching and N = C = S stretching), aldehyde, amide II band of proteins, primary amines 1556 and 1741 (C = O stretching and N–H bending), aromatics, nitro compound, alkene 1415 and 1636 (Nitro (R– NO2), alcoholic carboxylic acid, esters and ethers functional sites of biomolecules, nitro compound 1209 and 1552 (C–O stretching and N–O stretching), sulfones, sulfonyl chloride, aromatics, nitro compound 1021 and 1415 (S = O stretching Nitro, (R– NO2), alkenes, sulfonate 924 and 1367 (C = C bending and S = O stretching), alkyl halides, fluoro compound 803 and 1213 (C–Cl stretching and C–F stretching), Alkyl halides, alkenes 631 and 1018 (C–Cl stretching and C = C bending), alkyl halides, aromatic 517 and 927 (C–Br stretching and C–H bending), Alkyl halides 806 (C–Cl stretching), alkynes 634 (C≡C bending), and alkyl halides 520 (C–Br stretching) were identified respectively (Table 7 and Fig. 6).
3.9 Effect of Soil Amended With FA on Essential Oil (EO) Content and Its Active Constituents
The essential oil content and its bioactive components in M. arvensis plants were also assessed. The chromatogram of the GC–MS spectrum analysis, which is shown in Table 8 and 9, reveal the peaks of a number of compounds from the GC fractions of the methanol extract of M. arvensis plants grown in garden soil and in soil amended with 10% FA. The highest number of components was obtained in plants grown in soil amended with 10% FA (32 compounds) (Table 9). The lowest number of components was obtained in control plants without any treatments (29 compounds) (Table 8). Increase the concentration of FA in the soil (10%) caused an increased number of compounds and increased percentage of essential oil. The main components of M. arvensis essential oil were limonene (3.77%), menthone (5.97%), isomenthone (5.54%), menthol (61.74), and menthyl acetate (10.16%). In addition, the low levels of FA (10%) in the soil caused a significant increase in limonene (1.1%), menthone (1.9%), isomenthone (1.5%), menthol (0.3%), and menthyl acetate (1.9%) content as compared to control plants (Table 10 and supplemented Figs. 2 and 3). In addition, the highest level of FA (25%) caused a significant decrease in limonene, menthone, isomenthone, menthol, and menthyl acetate content as compared to control plants.
3.10 Principal Component Analysis (PCA) and Pearson Correlation Coefficient
According to Fig. 7 principal component analysis (PCA), differing FA levels have a sizable impact on the plant growth and photosynthetic pigments of M. arvensis. The first (PC-1) and second (PC-2) principal component scores, which indicate 74.5% and 22.6%, respectively, suggest a significant collection of data that was analyzed. The score and loading plot of PCA showed a maximum (97.1%) variation across all the parameters studied. There were shown to be strong positive relationships between plant growth, photosynthetic pigments characteristics, antioxidant activity, oxidative stress markers, essential oil content and mineral content.
Principal Component Analysis (PCA) showing loading plots demonstrating the relationship between Photosynthesis parameters, Growth and Yield parameters, Antioxidant activity, Oxidative Stress Markers, Essential oil content, and Mineral content of leaves of M. arvensis grown under different treatments of Fly ash amended Soil. Different abbreviations used in the figure are as follows: SL; Shoot length, SFW; Shoot fresh weight, SDW; Shoot dry weight, RL; Root length, RFW; Root fresh weight, RDW; Root dry weight, RC; Root circumference, NOL; Number of leaves, LA; Leaves area, NOB; Number of branches, SA; Stomata area, RWC; Relative water content, Chl a; Chlorophyl a, Chl b; Chlorophyl b, Caro; Carotenoids, T. Chl; Total Chlorophyl, SOD; Superoxide dismutase, CAT; Catalase, APX; Ascorbate peroxidase; POX peroxidase; GPX; Guaiacol peroxidase, NR; Nitrate reductase, Protein, Lime; Limonene, Menth; Menthone, Isome; Iso menthone, Mentho; Menthol, Mace; Menthyl acetate, N; Nitrogen, P; Phosphorus, K; Potassium, Ca; Calcium, Mg; Magnesium, Na; Sodium, Cu; Copper, Mn; Manganese Zn; Zinc, Fe; Iron, MDA; Malondialdehyde, H2O2; Hydrogen Peroxide, O2-; Superoxide ion, and EL; Electrolyte leakage, Proline
On the other hand, PC1 and PC2, particularly PC2, contributed to parameters like MDA, H2O2, O2−, EL, and proline. In the case of the score plot, T3 (10% FA + 90% GS) had a maximum addition to PC1, representing a strong negative correlation to T6 (25% FA + 75% GS). The treatments (T5–T6) contributed maximally to PC2, whereas a small contribution was represented by treatments (T1 and T4). In contrast, T3 (10% FA + 90% GS) showed a positive relationship with PC1. On the other hand, various photosynthetic pigments, growth and yield parameters, essential oil content, and mineral content negatively contributed to high levels of fly ash. Furthermore, Fly ash elevated the ROS, MDA, and EL in plants, indicating a strong correlation between the two; however, it negatively related to T5 and T6 (25% FA + 75% GS and 25% FA + 75% GS). In addition, the application of a higher level of fly ash (T6: 25%FA + 75%GS) enhanced the enzymatic and non-enzymatic antioxidants, proline levels, and related metabolic enzymes, all of which are positively correlated to both PC1 and PC2.
Pearson's correlation plot was used to evaluate the correlation of the studied parameters. For correlation analysis between all the studied parameters of this study, the Pearson correlation coefficient was used. The correlation plot is represented in Fig. 8, and the analysis was executed using the mean value of each variable. The values of oxidative stress markers (MDA; malondialdehyde; H2O2; hydrogen peroxide; O2; superoxide ion; EL; electrolyte leakage; and Pro; proline) show a negative correlation with all other parameters, indicating a positive relationship. Additionally, all the studied antioxidant parameters (SOD, APX, CAT, and POX) are positively associated with the majority of elemental concentrations in the plant leaves. This suggests that M. arvensis growth is beneficial at a 10% FA level and mitigates the oxidative stress caused by heavy metals found in FA.
Pearson correlation coefficient. The correlation matrix heatmap shows the values of the Pearson correlation coefficient for all studied parameters, the positive values in red, negative in blue. It ranges from − 1 to 1, whereby − 1 means a perfect negative linear relationship between variables, 1 indicates a perfect positive linear relationship between variables and 0 indicates that there is no relationship between studied variables. The studied parameters were SL; Shoot length, SFW; Shoot fresh weight, SDW; Shoot dry weight, RL; Root length, RFW; Root fresh weight, RDW; Root dry weight, RC; Root circumference, NOL; Number of leaves, LA; Leaves area, NOB; Number of branches, SA; Stomata area, RWC; Relative water content, Chla; Chlorophyl a, Chlb; Chlorophyl b, Caro; Carotenoids, T.Chl; Total Chlorophyl, SOD; Superoxide dismutase, CAT; Catalase, APX; Ascorbate peroxidase; POX; peroxidase, GPX; Guaiacol peroxidase, NR; Nitrate reductase, Prot; Protein, MDA; Malondialdehyde, H2O2; Hydrogen Peroxide, O2−; Superoxide ion, and EL; Electrolyte leakage Prol; Proline, Lime; Limonene, Menth; Menthone, IM; Iso menthone, Mentl; Menthol, MA; Menthyl acetate, N; Nitrogen, P; Phosphorus, K; Potassium, Ca; Calcium, Mg; Magnesium, Na; Sodium, Cu; Copper, Mn; Manganese Zn; Zinc, and Fe; Iron
4 Discussion
Global agricultural production is decreased as a result of rapid urbanization and industrialization, which cause varying degrees of toxic contamination in agricultural soils (El-Mahdy et al. 2021). Using FA amendments as fertilizers to boost the growth and yield of a variety of crops is a growing area of interest in agricultural soil science research (Ahmad et al. 2021); (Ansari et al. 2022; Ansari et al. 2023).
The data in this investigation demonstrated that soil characteristics improved after being amended with 10% FA. Additionally, the soil's EC, CEC, porosity, water-holding capacity (WHC), and moisture content are all enhanced by the addition of 10% FA. The 10% FA amendment raised the pH of the soil, bringing it up to 7.7 from 6.8. The "master soil variable" is thought to be soil pH because it has a significant impact on the physical, chemical, and biological characteristics of the soil (Shakeel et al. 2021). It is essential for the availability and uptake of nutrients by plants, which impacts plant development and crop productivity (Prasad et al. 2020). According to (Jan et al. 2014), the neutralization of H + by alkali salts and the solubilization of the basic metallic oxides of fly ash in the soil may both be to blame for the rise in soil pH. Fly ash may have increased EC values, which could mean that metal ions bound to soil particles more quickly, increasing the amount of metal nutrients available to plants (Jan et al. 2014). The improvement in WHC was due to higher space between the soil particles (Gond et al. 2013).
The addition of 10% FA to the soil also enhanced the levels of sulfate, chloride, carbonate, bicarbonate, Ca, Na, K, Zn, and Mn. The outcomes are in agreement with (Panda et al. 2018a). Due to the fly ash's alkaline nature, the pH of soil that has been modified with it has increased. The FA's CaO and sulfur contents are responsible for the soil's pH improvement (Shakeel et al. 2021). Similarly, (Panda et al. 2018b) reported that the EC and P of the soil amendments are greatly increased by the fly ash, which is beneficial for plant growth. A greater concentration of inorganic compounds in FA, better accessibility of macro- and micronutrients, and metals released into the soil with the ash could all be contributing factors to the higher EC (Ahmad et al. 2021). Numerous studies have also documented the use of FA to enhance soil properties as pH, electric conductivity, soil texture, porosity, bulk density, and water holding capacity (Varshney et al. 2022; Varshney et al. 2023). FA improves the possibility of water-holding capacity by altering the soil's structure and texture, which increases the porosity structure, particle density, and particle size storage efficiency (Ahmad et al. 2021).
According to the results of the current investigation, fly ash at a 10% concentration is efficient, non-phytotoxic, and appears to improve M. arvensis growth characteristics, while 25% of fly ash soil amended demonstrated poorer plant growth performance. The improvement in the soil's nutritional status (Ca, Mg, K, P, and S) and the soil's physical properties, such as soil pH, may be the cause of this improvement (Shakeel et al. 2020). An important aspect that enhances the availability of vital plant nutrients is an adjustment in the soil pH (Prasad et al. 2020). Because calcium functions as a secondary messenger and promotes cell wall production, calcium enrichment significantly contributes to growth improvement (Shakeel et al. 2021). Although phosphorous is only found in trace amounts in FA, it may promote more efficient cell division to increase leaf area. The addition of S also significantly improves plant growth since it is necessary for secondary metabolism, electron transport, sulfur-containing proteins like methionine, and the creation of Fe-S clusters in the photosynthetic apparatus (Shakeel et al. 2020). In addition, FA contains some vital micronutrients including Mn, Cu, and Fe that also contribute to the enhancement of plant performance (Shakeel et al. 2020). Potassium promotes and controls ATPase in the plasma membrane to provide acid stimulation, which subsequently causes cell wall permeabilization and hydrolase activation thus stimulating cell growth (Oosterhuis et al. 2014). Potassium is highly mobile in plants and is essential for maintaining the proper balance of cations and anions in the cytoplasm and controlling cell osmotic pressure (Osakabe et al. 2013). Therefore, the FA soil amendment, which adds K ion to the soil, may be the cause of the observed improvement in plant growth, yield, and stomatal characteristics.
The increased soil pH that resulted from higher FA application rates may have decreased several essential mineral components' availability and extractability, resulting in decreased plant performance (Jan et al. 2014). The compaction of fly ash particles, which likely acted as a physical barrier to root elongation, may be the cause of the inhibition of root growth. High levels of heavy metals found in fly ash may prevent root growth by slowing down cell division (Ahmad et al. 2021). The majority of the harmful metals in soil might be deposited in the root tissues, which are the first organs to come into touch with them. Metal buildup in the root may prevent hazardous metals from reaching the leaves and other shoot organs (Ansari et al. 2023), thus partially controlling how the shoot reacts to the ingredients of fly ash. Our findings showed that growth was reduced when fly ash concentrations were higher. Induced changes in the soil's chemical composition could also be to blame for this decline (Ansari et al. 2022).
The primary physiological constraint on crop output is photosynthesis. The ability of individual leaves to perform photosynthetic functions is a key component in determining crop production and dry matter (Jan et al. 2014). In comparison to the control, M. arvensis' photosynthetic pigment levels significantly raised at 10% FA amended soil and dramatically decreased at 25% FA. This may be caused by adding Mg to the soil, which is the centre atom of the chlorophyll molecule surrounded by four N atoms (Ansari et al. 2022). Additionally, it stabilizes the nucleic acids for certain enzymes needed for plant growth processes and serves as an activator for others (Gerendas and Fuhrs 2013). The enrichment of soil with K ion is another explanation for the enhancement in M. arvensisplant growth and stomatal aperture. It is the most prevalent cationic element, which is crucial for plant growth and development (White and Karley 2010). Moreover, the availability of micronutrients like potassium and iron in FA may have had a significant impact because potassium is connected to enzyme activities that are essential for growth, and iron is implicated in the photosynthetic pathway (Ashfaque and Inam 2019).
The decline of chlorophyll pigments under fly ash may be caused by oxidative chlorophyll breakdown by ROS and increased metal concentrations that hinder chlorophyll synthesis (Pandey 2013). Chlorophyll reduction can happen when precursor levels are restricted or when metal-induced aminolevulinic acid dehydratase is targeted for -SH groups. Chlorophyll content decreases as a result of the buildup of heavy metals, which prevents the production of chlorophyll (El-Mahdy et al. 2021). Metal ions including Cu2+, Zn2+, Cd2+, Pb2+, and Ni2+ may replace Mg2+ ions in chlorophyll molecules, leading to the degradation of photosynthetic pigment (Jana et al. 2017).
Our findings indicate that the carotenoid content declined with increasing fly ash concentration, and that an increase in carotenoid content with the application of 10% fly ash may have taken place to shield the plant from the damaging effects of free radicals. Carotenoids are a class of naturally occurring, non-enzymatic antioxidants that act as secondary pigments for photosynthesis in plants. By using a variety of mechanisms, such as reducing photodynamic processes and preventing peroxidation, they protect the chlorophyll molecule from damage caused by environmental stressors (Jan et al. 2014).
In comparison to control plants, the protein content of M. arvensis plants grown in soil amended with 10% FA was significantly higher, but declined by about 20% at 25% FA. In comparison to control plants, different FA concentrations substantially improved the proline content of M. arvensis leaves. Numerous crucial enzymes involved in crucial biochemical activities like protein synthesis, sugar transport, N and C metabolism, and plant photosynthesis are activated by K, which is present in fly ash and soil (Jan et al. 2014). Overall, enhanced photosynthesis, electrical conductivity, and soil pH levels led to higher protein content (Ansari et al. 2023).
Furthermore, the presence of a high amount of FA lowers the protein content, which may be caused by enhanced protein degradation, decreased de novo cell cycle, or amino acid destruction of subsequent proteins (Ahmad et al. 2021). Our findings are in agreement with those of (Haris et al. 2021), who found that proline can reduce the oxidative stress caused by higher FA concentrations, safeguard cellular functioning by scavenging ROS, and maintain osmotic stability or cell turgor. Proline accumulation may be caused by reduced protein hydrolysis, reduced de novo synthesis, and decreased protein buildup.
The adaptable amino acid proline is an excellent osmolyte, metal chelator, and antioxidant molecule that appear to perform a number of functions under stressful conditions, including stabilizing subcellular structures, membranes, and proteins (Ansari et al. 2023). Moreover, it has been documented that fly ash, which contains high levels of heavy metals, causes plants to produce reactive oxygen species. Through oxidative alteration and an increase in proteolytic activity, these free radicals harm the photosynthetic system and break down proteins (Jan et al. 2014).
With increasing concentrations of FA amended in garden soil, MDA and EL content considerably increased in the current study. Malondialdehyde, a byproduct of lipid peroxidation, is used to measure membrane damage in the presence of oxidative stress (Jana et al. 2017). The Ricinus communis cell membrane may be damaged by higher metal concentrations in fly ash, which could also hinder metabolic processes and, eventually, stunt growth (Panda et al. 2020). In addition, ROS production under FA stress increases the formation of MDA, disrupting the fluidity and integrity of the membrane (Qadir et al. 2022), preventing protein channeling (Panda et al. 2020), increasing electrolyte leakage (Ahmad et al. 2021), and decreasing enzyme activities (Qadir et al. 2022).
Increased FA amendment in garden soil has resulted in increasing rates for O2− anions and H2O2 in M. arvensis leaves. The histochemical localization of O2− and H2O2 detected intensive staining in the plants cultivated in garden soil with higher levels of 25% FA. In addition, (Ansari et al. 2023) revealed that the higher FA levels increased the amounts of malondialdehyde and hydrogen peroxide in Withania somnifera plants because higher concentrations of FA contain heavy metals which may contribute to oxidative stress. These outcomes are consistent with (Shakeel et al. 2021) who discovered that the levels of FA were directly correlated with the quantity of O2 radicals. In comparison to the control, the O2− concentration increased at 15% FA level. However, the control plants had the lower amount of O2− content while the 25% FA level had the higher amounts. In addition, (Aqeel et al. 2023) who discovered that the generation of ROS in Mentha arvensis leaves was examined further using NBT and DAB staining, with blue and brown dots denoting the locations of O2 and H2O2, respectively. Cu-stressed plants had these spots more noticeably, indicating excessive ROS generation.
Trypan blue staining was used in histochemistry to detect cell death in the leaves. In the roots of plants growing in 25% FA, dead cells were found to be more abundantly represented by red fluorescent nuclei. These outcomes are consistent with (Ansari et al. 2023) who found that plants grown in soil with 15% FA had higher root cell viability than plants grown in soil with 25% FA. This is because 25% FA contains high concentrations of heavy metals like Al and Au, which affect cell viability and result in cell damage (Ahmad et al. 2021).
An increase in the amounts of enzymatic antioxidants (SOD, CAT, POX, APX, GPX and NR) was detected in the plants cultivated on FA amended soil. Their activities were further improved by increasing the concentrations of FA. The same outcomes are reported by (Panda et al. 2020) who found that there was an increase in the levels of APX, CAT, GPX, and SOD enzyme activities in plants cultivated in soil containing fly ash compared to controls. Higher APX, GPX, and SOD activity were observed in leaf tissues. These enzymes' stimulation may be interpreted as a response to the oxidative stress caused by the metals found in fly ash, and it demonstrated a metal tolerance response.
SOD and CAT are crucial antioxidant enzymes that help plants maintain a healthy amount of potentially harmful radicals (Ahmad et al. 2021). As the initial line of defence, SOD scavenges O2− radicals by converting them to O2 and H2O2. This H2O2 is converted into H2O by the action of CAT, and strong APX activities in response to FA stress indicate an efficient H2O2 scavenging potential (Panda et al. 2020). Also, (Shakeel et al. 2020) detected a significant increase in antioxidant activity in plants grown in soils containing fly ash.
Many authors have shared their evaluations of the various aspects of antioxidant enzymes, which are essential for the production of numerous significant molecules, the transport system, and, most importantly, stress tolerance. (Qadir et al. 2019) observed an increase in the antioxidant enzymes such as ascorbate peroxidase (APX), catalase (CAT), peroxidase (POD), and superoxidase dismutase (SOD) in Pithecellobium dulce grown on fly ash as the content of FA in the soil increased.
ROS are primarily produced by a number of important metabolic and physiological processes that are present in plants. On the other hand, ROS buildup in stressful conditions may result in membrane damage, membrane lipid peroxidation, and even cell death. Over time, plants have evolved a complex redox balancing system based on antioxidant systems, both enzymatic and nonenzymatic. Several environmental perturbations in plants lead to higher antioxidant enzyme levels correspondingly a substantial surge in CAT, POD, and SOD, activities were perceived in plants after exposure to fly ash stress (Qadir et al. 2019).
In addition, (Ansari et al. 2023) demonstrated that when 15% FA was amended into the soil, greater NR activity was found in W. somnifera leaves plants. Lower FA concentrations increased nitrate reductase activity, allowing it to produce N precursors for the synthesis of amino acids and proteins more quickly, increasing the amount of protein in the treated plants (Ansari et al. 2022). Higher fly ash concentrations resulted in a drop in NR activity, which may have been caused by the enzyme molecule decomposing. Fly ash has low nitrogen content; therefore either a decrease in the protein's content or a change in the substrate's availability may have inhibited NR activity. At greater levels of fly ash, Carthamus tinctorius also showed NR activity inhibition (Jan et al. 2014). The ability of these metals to bind to the -SH groups of enzymes may also be the cause of NR inhibition (Jan et al. 2014). The nitrogen status of the plant can be determined by looking at the NR activity of the leaves. Because nitrogen is an essential part of the structure of phytochelatin, the enhanced activity of NR in the presence of FA may eventually strengthen the heavy metal detoxifying system that is found in FA and result in increased plant growth and development (Ansari et al. 2022).
The results demonstrated that lower level of FA (10%) considerably increased the N, P, K, Ca, Mg, Cu, Mn, Zn and Fe content but decreased Na in plants compared to the control. The minimum minerals content was observed in plants grown with 25% FA less than the control. Similar results are in accordance with (Ansari et al. 2022) who discovered that adding N to soil with low FA levels (5, 10, and 15%) led to a significant increase in the minerals N, P, K, Ca, Na, Mg, Cu, Mn, Zn, and Fe in radish plants compared to control plants, whereas treatment with high FA levels caused a significant decrease in all of the above minerals with the exception of Na, which showed a significant increase. Similar findings were made in pumpkin crops, where it was found that there was an increase in NPK content with lower levels of fly ash (Ahmad et al. 2021).
The functional groups of the components were divided based on the ratio of the peak created by the FT-IR analysis of the leaf extract. In the 800 to 4000 cm−1 range, the control and 10% FA-amended plants contain about 11 to 14 peaks, respectively. The outcomes of this investigation align with those of (Ansari et al. 2023); (Aryal et al. 2020).
M. arvensis plants grown in control soil showed the presence of 29 different bioactive compounds on the chromatogram of GC–MS spectra analysis, whereas plants cultivated in 10% FA showed the presence of 32 bioactive compounds. The chemicals limonene, menthone, isomenthone, menthol, and menthyl acetate were the most prevalent in the methanolic extract of essential oil. The concentration of limonene, menthone, isomenthone, menthol, and menthyl acetate significantly increased over the corresponding control plants due to the low levels of FA (10%) in the soil. At concentrations less than 15%, FA treatment had no appreciable impact on the M. piperita plant's essential oil content. These results are in accordance with (Kumar and Patra 2012) discovered that the usage of FA at concentrations greater than 50% had a negative impact on the amount of oil in the M. piperita plant's essential oils, though a minor improvement was seen when FA was applied at concentrations less than 50%. The glandular trichomes found on leaves of Lamiaceae plants have an impact on the enhancement in the generation of secondary metabolites (essential oil) (Karray-Bouraoui et al. 2009). An increase in essential oil production was connected with the growth of these glandular trichomes. FA addition to soil growth medium becomes harmful at larger levels, as seen by plant development stunting and leaf burning. This is likely caused by the decrease in photosynthetic pigments and the limited soil nitrogen and phosphate availability at higher FA levels (Kumar and Patra 2012).
The principal component analysis (PCA) and Pearson's correlation plot was revealed that different FA levels significantly impact the plant growth, and photosynthetic pigments of M. arvensis. The score and loading plot of PCA showed a maximum (97.1%) variation among all the parameters studied, of which the first (PC-1) and second (PC-2) principal component scores, which indicate 74.5% and 22.6%, combined, show a significant set of data that was investigated. Our results provide credence to the observed variability of 70% of the data, which was first proposed by (Sneath and Sokal 1973) research as an indicator of the fitness state of the data.
5 Conclusion
The results of the current study indicate that supplementing garden soil with lower levels of FA (10%) improved its physical, chemical, and nutritional qualities, which led to an improvement in M. arvensis growth and yield, as shown in Supplemented Fig. 4. In M. arvensis leaves, this study indicated that increasing the FA concentration increased the antioxidant activity and oxidative stress markers; however, FA 10% was shown to be the most effective for plant growth and yield measurements. Lower FA concentrations were recommended for usage in agricultural systems to render them harmless and free of hazardous FA components. Therefore, more research is required to determine the important elements found in FA and the conformational mechanisms by which certain elements are connected to various plant biochemical activities. Therefore, using FA soil can increase the supply and solve the waste disposal issue.
Data availability
Not applicable.
References
Abd El-Rahman SS, Mazen MM, Mohamed HI, Mahmoud NM (2012) Induction of defence related enzymes and phenolic compounds in lupin (Lupinus albus L.) and their effects on host resistance against Fusarium wilt. Eur J Plant Pathol 134:105–116. https://doi.org/10.1007/s10658-012-0028-z
Abu-Shahba MS, Mansour MM, Mohamed HI, Sofy MR (2022) Effect of biosorptive removal of cadmium ions from hydroponic solution containing indigenous garlic peel and mercerized garlic peel on lettuce productivity. Sci Hortic 293:110727. https://doi.org/10.1016/j.scienta.2021.110727
Adams RP (1995) Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy. Allured Publishing Corporation, IL, USA
Ahmad G, Khan AA, Mohamed HI (2021) Impact of the low and high concentrations of fly ash amended soil on growth, physiological response, and yield of pumpkin (Cucurbita moschata Duch. Ex Poiret L.). Environ Sci Pollut Res 28:17068–17083. https://doi.org/10.1007/s11356-020-12029-8
Aly AA, Mohamed HI, Mansour MT, Omar MR (2013) Suppression of Powdery Mildew on Flax by Foliar Application of Essential Oils. J Phytopathol 161:376–381. https://doi.org/10.1111/jph.12080
Anjum NA, Sofo A, Scopa A, Roychoudhury A, Gill SS, Iqbal M, Lukatkin AS, Pereira E, Duarte AC, Ahmad I (2015) Lipids and proteins – major targets of oxidative modifications in abiotic stressed plants. Environ Sci Pollut Res 22:4099–4121. https://doi.org/10.1007/s11356-014-3917-1
Ansari MS, Ahmad G, Khan AA, Mohamed HI, Elhakem A (2022) Coal fly ash and nitrogen application as eco-friendly approaches for modulating the growth, yield, and biochemical constituents of radish plants. Saudi J Biol Sci 29:103306. https://doi.org/10.1016/j.sjbs.2022.103306
Ansari MS, Ahmad G, Khan AA, Mohamed HI (2023) Coal fly ash application as an eco-friendly approach for modulating the growth, yield, and biochemical constituents of Withania somnifera L. plants. Environ Sci Pollut Res 30:87958–87980. https://doi.org/10.1007/s11356-023-28318-x
Aqeel U, Parwez R, Aftab T, Khan MM, Naeem M (2023) Silicon dioxide nanoparticles suppress copper toxicity in Mentha arvensis L. by adjusting ROS homeostasis and antioxidant defense system and improving essential oil production. Environ Res 236:116851. https://doi.org/10.1016/j.envres.2023.116851
Aryal S, Shrestha S, Devkota A, Bhandari NL, Jha RN (2020) FTIR, GC-MS analysis and bioactivity studies of Withania somnifera L. of Nepalese origin. J Nepal Chem Soc 41(1):36–45
Ashfaque F, Inam A (2019) Interactive effect of potassium and flyash: a soil conditioner on metal accumulation, physiological and biochemical traits of mustard (Brassica juncea L.). Environ Sci Pollut Res 26:7847–7862. https://doi.org/10.1007/s11356-019-04243-w
Awasthi JP, Saha B, Chowardhara B, Devi SS, Borgohain P, Panda SK (2018) Qualitative analysis of lipid peroxidation in plants under multiple stress through schiff’s reagent: a histochemical approach. Bio Protoc 8: e2807. https://doi.org/10.21769/BioProtoc.2807
Bates L, Waldren R, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287. https://doi.org/10.1016/0003-2697(71)90370-8
Bertrand M, Poirier I (2005) Photosynthetic organisms and excess of metals. Photosynthetica 43:345–353. https://doi.org/10.1007/s11099-005-0058-2
Bhat AA, Shakeel A, Kesba HH, Handoo ZA, Khan AA (2023) Elucidating the role of fly ash in root-knot nematode (Meloidogyne incognita) suppression on okra (Abelmoschus esculentus L.): Insights into cellular viability and host defence system. Physiol Mol Plant Pathol 126:102042. https://doi.org/10.1016/j.pmpp.2023.102042
Bouyahya A, Lagrouh F, El Omari N, Bourais I, El Jemli M, Marmouzi I, Salhi N, Faouzi ME, Belmehdi O, Dakka N, Bakri Y (2020) Essential oils of Mentha viridis rich phenolic compounds show important antioxidant, antidiabetic, dermatoprotective, antidermatophyte and antibacterial properties. Biocatal Agric Biotechnol 23:101471. https://doi.org/10.1016/j.bcab.2019.101471
Carter MR, Ball B (1993) Soil porosity. In: Carter MR (ed) Soil sampling and methods of analysis. Canadian Society of Soil Science, Lewis Publishers, CRC Press, Boca Raton, pp 581–588
Chandlee JM, Scandalios JG (1984) Analysis of variants affecting the catalase developmental program in maize scutellum. Theor App Gene 69:71–77. https://doi.org/10.1007/BF00262543
Chatterjee T, Saha T, Ghosh PB (2009) Impact of fly ash on soil from coal fired power station in west bengal. Environ Ecol 27:1435
Chopra SL, Kanwar JS (1991) Analytical agricultural chemistry. Kalyani Publishers, New Delhi-Ludhiana
Chopra SL, Kanwar JS (1982) Analytical Agriculture Chemistry. Kalyani Publishers, New Delhi, India, pp 191–205
Choudhary S, Zehra A, Naeem M, Khan M, Aftab T (2020) Effects of boron toxicity on growth, oxidative damage, antioxidant enzymes and essential oil fingerprinting in Mentha arvensis and Cymbopogon flexuosus. Chem Biolog Tech Agr 7:1–11. https://doi.org/10.1186/s40538-019-0175-y
Dickman SR, Bray RH (1940) Colorimetric determination of phosphate. Ind Eng Chem Anal Ed 12:665–668
Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9. https://doi.org/10.1016/S0168-9452(98)00025-9
El-Mahdy OM, Mohamed HI, Mogazy AM (2021) Biosorption effect of Aspergillus niger and Penicillium chrysosporium for Cd-and Pb-contaminated soil and their physiological effects on Vicia faba L. Environ Sci Pollut Res 28:67608–67631. https://doi.org/10.1007/s11356-021-15382-4
Fiske CH, Subba Row Y (1925) The colorimetric determination of phosphorus. J Biol Chem 66:375–400
Gerendas J, Fuhrs H (2013) The significance of magnesium for crop quality. Plant Soil 368:101–128. https://doi.org/10.1007/s11104-012-1555-2
Gond DP, Singh S, Pal A, Tewary BK (2013) Growth, yield and metal residues in Solanum melongena grown in fly ash amended soils. J Environ Biol 34:539–544
Guenther E (1972) The essential oils: history, origin in plants, production analysis, vol I. Robert E. Kriger Publishing Co., Malabar, p 427 Clevenger's equipment (Borosil, India)
Hald PM (1947) The flame photometer for the measurement of sodium and potassium in biological materials. J Biol Chem 167:499–510. https://doi.org/10.1016/S0021-9258(17)31003-7
Haris M, Ansari MS, Khan AA (2021) Supplementation of fly ash improves growth, yield, biochemical, and enzymatic antioxidant response of chickpea (Cicer arietinum L.). Horti Environ Biotechnol 62:715–724. https://doi.org/10.1007/s13580-021-00351-0
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Bioph 125:189–198. https://doi.org/10.1016/0003-9861(68)90654-1
Hussain S, Tanvir M, Ahmad M, Munawar KS (2021) Phytochemical composition of mint (Mentha), its nutritional and pharmacological potential. Lahore Garr Univ J Life Sci 5: 241–258. https://doi.org/10.54692/lgujls.2021.0504188
Jackson ML (1973) Soil chemistry analysis. Prentice-Hall, New Delhi
Jan S, Khan R, Siddiqi TO (2014) Soil amendments of fly ash: effects on function and biochemical activity of Carthamus tinctorius L. plants. Israel J Plant Sci 61:12–24
Jana A, Ghosh M, De A, Sinha S, Jothiramajayam M, Mukherjee A (2017) Comprehensive analysis of fly ash induced changes in physiological/growth parameters, DNA damage and oxidative stress over the life cycle of Brassica juncea and Brassica alba. Chemosphere 8:35–45. https://doi.org/10.1016/j.chemosphere.2017.08.023
Jaworski EG (1971) Nitrate reductase assay in intact plant tissues. Biochem Biophys Res Commun 43:1274–1279. https://doi.org/10.1016/S0006-291X(71)80010-4
Kar M, Mishra D (1976) Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiol 57: 315–319. https://www.jstor.org/stable/4264341
Karray-Bouraoui N, Rabhi M, Neffati M, Baldan B, Ranieri A, Marzouk B, Lachaal M, Smaoui A (2009) Salt effect on yield and composition of shoot essential oil and trichome morphology and density on leaves of Mentha pulegium. Ind Crop Prod 30:338–343. https://doi.org/10.1016/j.indcrop.2009.06.003
Kjeldahl C (1883) A new method for the determination of nitrogen in organic matter. Z Anal Chem 22:366–382. https://doi.org/10.1007/BF01338151
Kumar KV, Patra DD (2012) Alteration in yield and chemical composition of essential oil of Mentha piperita L. plant: effect of fly ash amendments and organic wastes. Ecol Engin 47:237–241. https://doi.org/10.1016/j.ecoleng.2012.06.019
Kumar R, Thangaraju MM, Kumar M, Thul ST, Pandey VC, Yadav S, Singh L, Kumar S (2021) Ecological restoration of coal fly ash-dumped area through bamboo plantation. Environ Sci Pollut Res 28:33416–33432. https://doi.org/10.1007/s11356-021-12995-7
Kumari R, Agrawal SB, Sarkar A (2009) Evaluation of changes in oil cells and composition of essential oil in lemongrass (Cymbopogon citratus (DC) Stapf.) due to supplemental ultraviolet-B irradiation. Curr Sci 9:137–1. https://www.jstor.org/stable/24111955
Kuo TM, Warner RL, Kleinhofs A (1982) In vitro stability of nitrate reductase from barley leaves. Phytochem 21:531–533. https://doi.org/10.1016/0031-9422(82)83134-8
Lindner RC (1944) Rapid analytical methods for some of the more common inorganic constituents of the plant tissues. Plant Physiol 19:76–89. https://doi.org/10.1104/pp.19.1.76
Liu JX, Srivastava R, Che P, Howell SH (2007) An endoplasmic reticulum stress response in Arabidopsis is mediated by proteolytic processing and nuclear relocation of a membrane-associated transcription factor, bZIP28. Plant Cell 19:4111–4119. https://doi.org/10.1105/tpc.106.050021
Lowery GH, Newman RJ (1951) Notes on the ornithology of southeastern San Luis Potosi. The Wilson Bull 63:315–322
Maclachlan S, Zalik S (1963) Plastid structure, chlorophyll concentration, and free amino acid composition of a chlorophyll mutant of barley. Cana J Bot 41:1053–1062. https://doi.org/10.1139/b63-088
Mason GA, Lemus T, Queitsch C (2016) The mechanistic underpinnings of an ago1-mediated, environmentally dependent, and stochastic phenotype. Plant Physiol 170:2420–2431. https://doi.org/10.1104/pp.15.01928
Mazhoudi S, Chaoui A, Ghorbal MH, El Ferjani E (1997) Response of antioxidant enzymes to excess copper in tomato (Lycopersicon esculentum, Mill.). Plant Sci 127:129–137. https://doi.org/10.1016/S0168-9452(97)00116-7
Nabi A, Naeem M, Aftab T, Khan M (2020) Intimidating effects of heavy metals on Mentha species and their mitigation using scientific approaches. In: Contaminants in Agriculture. Springer, Cham, pp. 305–325
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Novozamsky I, Houba VJG, van Eck R, van Vark W (1983) A novel digestion technique for multi-element plant analysis. Commun Soil Sci Plant Anal 14:239–248. https://doi.org/10.1080/00103628309367359
Oosterhuis DM, Loka DA, Kawakami EM, Pettigrew WT (2014) The physiology of potassium in crop production. Adv Agron 126:203–233. https://doi.org/10.1016/B978-0-12-800132-5.00003-1
Osakabe Y, Arinaga N, Umezawa T, Katsura S, Nagamachi K, Tanaka H, Yamaguchi-Shinozaki K (2013) Osmotic stress responses and plant growth controlled by potassium transporters in Arabidopsis. Plant Cell 25(609–624):10. https://doi.org/10.1105/tpc.112.105700
Panda D, Mandal L, Barik J, Padhan B, Bisoi SS (2020) Physiological response of metal tolerance and detoxification in castor (Ricinus communis L.) under fly ash-amended soil. Heliyon 6:e04567. https://doi.org/10.1016/j.heliyon.2020.e04567
Panda D, Panda D, Pradhan B, Biswas M (2018a) Growth and physiological response of lemongrass (Cymbopogon citratus (D.C.) Stapf.) under different levels of fly ash amended soil. Int J Phytoremed 20:538–544. https://doi.org/10.1080/15226514.2017.1393394
Panda D, Mandal L, Padhan B, Mishra SS, Barik J (2018b) Improvement of growth, photosynthesis and antioxidant defense in rice (Oryza sativa L.) grown in fly ash amended Soil. Proc Natl Acad Sci India B Biol Sci 89:853–860. https://doi.org/10.1007/s40011-018-0996-7
Pandey SK, Bhattacharya T (2018) Effect of two biodegradable chelates on metals uptake, translocation and biochemical changes of Lantana camara growing in fly ash amended soil. Int J Phytoremed 20:214–224. https://doi.org/10.1080/15226514.2017.1365350
Pandey VC (2013) Suitability of Ricinus communis L. Cultivation for phytoremediation of fly ash disposal sites. Ecol Eng 57:336–341. https://doi.org/10.1016/j.ecoleng.2013.04.054
Prasad M, Chrysargyris A, McDaniel N, Kavanagh A, Gruda NS, Tzortzakis N (2020) Plant nutrient availability and pH of biochars and their fractions, with the possible use as a component in a growing media. Agronomy 10:10. https://doi.org/10.3390/agronomy10010010
Priha O, Smolander A (1999) Nitrogen transformations in soil under Pinus sylvestris, Picea abies and Betula pendula at two forest sites. Soil Biol Biochem 31:965–977. https://doi.org/10.1016/S0038-0717(99)00006-1
Qadir SU, Raja V, Siddiqui WA, Mahmooduzzafar, Abd_Allah EF, Hashem A, Alam P, Ahmad P (2019) Fly-Ash Pollution Modulates Growth, Biochemical Attributes, Antioxidant Activity and Gene Expression in Pithecellobium Dulce (Roxb) Benth. Plants 8:528https://doi.org/10.3390/plants8120528
Qadir SU, Raja V, Siddiqui WA, Shah T, Alansi S, El-Sheikh MA (2022) Ascorbate glutathione antioxidant system alleviates fly ash stress by modulating growth physiology and biochemical responses in Solanum lycopersicum. Saudi J Biol Sci 29:1322–1336. https://doi.org/10.1016/j.sjbs.2021.12.013
Rattan S, Zhou C, Chiang C, Mahalingam S, Brehm E, Flaws JA (2017) Exposure to endocrine disruptors during adulthood: consequences for female fertility. J Endocrinol 233:R109–R129. https://doi.org/10.1530/JOE-17-0023
Rayment GE, Higginson FR (1992) Australian Laboratory Handbook of Soil and Water Chemical Methods. Inkata Press Pty Ltd., Sydney
Richard LA (1954) Diagnasis and improvement of saline and alkali soils. Agric. Hand book, 60, U.S. Dept, Agric. Washington DC. p:160
Rodríguez-Serrano M, Romero-Puertas MC, Pazmino DM, Testillano PS, Risueño MC, Del Río LA, Sandalio LM (2009) Cellular response of pea plants to cadmium toxicity: cross talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiol 150:229–243. https://doi.org/10.1104/pp.108.131524
Rorison IH, Spencer RE, Gupta PL (1993) Chemical analysis. In: Hendry GAE, Grime JP (eds) Methods in Comparative Plant Ecology. Chapman and Hall, New York, pp 156–161
Saqib S, Ullah F, Naeem M, Younas M, Ayaz A, Ali S, Zaman W (2022) Mentha: nutritional and health attributes to treat various ailments including cardiovascular diseases. Molecules 27:6728. https://doi.org/10.3390/molecules27196728
Scarpeci TE, Zanor MI, Carrillo N, Mueller-Roeber B, Valle EM (2008) Generation of superoxide anion in chloroplasts of Arabidopsis thaliana during active photosynthesis: a focus on rapidly induced genes. Plant Mol Biol 66:361–378. https://doi.org/10.1007/s11103-007-9274-4
Shakeel A, Khan AA, Alharby HF, Bamagoos AA, Tombuloglu H, Hakeem KR (2021) Evaluation of coal fly ash for modulating the plant growth, yield, and antioxidant properties of Daucus carota (L.): a sustainable approach to coal waste recycling. Sustainability 13:5116. https://doi.org/10.3390/su13095116
Shakeel A, Khan AA, Hakeem KR (2020) Growth, biochemical, and antioxidant response of beetroot (Beta vulgaris L.) grown in fly ash-amended soil. SN Appl Sci 2:1378. https://doi.org/10.1007/s42452-020-3191-4
Sneath PH, Sokal RR (1973) Numerical taxonomy. The principles and practice of numerical classification. Taxon 12:190–199. https://doi.org/10.2307/1217562
Thakur S, Walia B, Chaudhary G (2021) Mentha arvensis (Pudina): a review based upon its medicinal properties. Res J Pharmacogn Phytochem 13: 143–148. https://doi.org/10.52711/0975-4385.2021.00024.
Thawkar BS, Jawarkar AG, Kalamkar PV, Pawar KP, Kale MK (2016) Phytochemical and pharmacological review of Mentha arvensis. Int J Green Pharm 10: 71–76. https://doi.org/10.22377/ijgp.v10i2.643.
Varshney A, Dahiya P, Mohan S (2023) Growth, biochemical, and antioxidant response of pot marigold (Calendula officinalis L.) grown in fly ash amended soil. Int J Phytoremed 25:115–124. https://doi.org/10.1080/15226514.2022.2063794
Varshney A, Dahiya P, Sharma A, Pandey R, Mohan S (2022) FA application in soil for sustainable agriculture: an Indian overview. Energy Ecol Environ 7:340–357. https://doi.org/10.1007/s40974-022-00241-w
Varshney A, Mohan S, Dahiya P (2021) Growth and antioxidant responses in plants induced by heavy metals present in fly ash. Energy Ecol Environ 6:92–110. https://doi.org/10.1007/s40974-020-00191-1
Walkley A, Black IA (1934) Estimation of soil organic carbon by chromic acid titration method. Soil Sci 37:29–38
Watson DJ (1958) The dependence of net assimilation rate on leaf-area index. Annals Bot 22: 37–54. https://www.jstor.org/stable/42907407
White PJ, Karley AJ (2010) Potassium. In Cell Biology of Metals and Nutrients; Springer: Berlin/Heidelberg, Germany, pp.199–224
Yadav VK, Gacem A, Choudhary N, Rai A, Kumar P, Yadav KK, Islam S (2022) Status of coal-based thermal power plants, coal fly ash production, utilization in India and their emerging applications. Minerals 12:1503. https://doi.org/10.3390/min12121503
Funding
None.
Author information
Authors and Affiliations
Contributions
Moh Sajid Ansari, Gufran Ahmad, Abrar A. Khan and Heba I. Mohamed: Conceptualization, Investigation, Writing – original draft, Review and Editing, Data curation, Formal analysis, Visualization, Writing – original draft.
Moh Sajid Ansari, Gufran Ahmad, Abrar A. Khan and Heba I. Mohamed: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology.
Heba I. Mohamed and Abrar Ahmad Khan: Supervision; Validation; Visualization; Roles/Writing – original draft. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ansari, M.S., Ahmad, G., Khan, A.A. et al. Evaluation of Coal Fly Ash for Reinforcing the Plant Growth, Antioxidant Properties, and Essential Oil Content of Mentha arvensis L.: A Sustainable Approach to Coal Waste Management. J Soil Sci Plant Nutr 24, 1369–1393 (2024). https://doi.org/10.1007/s42729-024-01642-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-024-01642-y