Abstract
Due to the hostile conditions created by fly ash, its utilization in vegetation is restricted. Therefore, fly ash with soil amendment may offer a suitable combination to support plant growth, with reduced risk of metal toxicity. The present study evaluated different concentrations of fly ash with soil amendments on growth, photosynthesis, photosystem (PS) II activity and antioxidant defense in rice (Oryza sativa L.) seedlings to find out the optimum use of fly ash in rice cultivation. Low levels of fly ash (25%) amended soil improve the seedling growth parameters, CO2 photosynthetic rate and stomatal conductance in rice seedlings. Whereas leaf pigments and PS II activity remain unchanged under 25% fly ash treatment as compared to the plants grown in garden soil; however, these parameters declined under the treatments with higher levels of fly ash. Furthermore, the activities of some antioxidant enzymes and protein increased over control under low level of fly ash. The results showed maintenance of photosynthesis and PS II activity of rice seedlings under low levels of fly ash amendment, due to better antioxidative protection from oxidative damage. Taken together, soil amended at 25% fly ash improved the growth of rice seedlings; making fly ash a suitable component of plant growth substance. It can be concluded that a low level of fly ash can be used in amending rice soil for a short period of time but continuous use of fly ash can cause permanent soil contamination by increasing the load of toxic metals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fly ash, a coal combustion residue, with a matrix very similar to soil and its elemental composition varies due to types and sources of used coal. Globally, fly ash production approximates 600 million tonnes per year and may cover up to 3235 km2 of land area by 2015 [1, 2]. Despite its utilization in cement, sanitary and brick industries, huge quantities of fly ash remain dumped and unused. The management of the enormous quantity of fly ash generated every year has become a major problem. The presence of several macro and micronutrients in fly ash has encouraged agricultural researchers to use it in agriculture as a soil amendment [3]. Due to its physical properties and the presence of macro and micronutrients which are conducive for plant growth, fly ash has potential benefits for use in agronomy [2]. However, the augmented levels of non-essential and potentially toxic metals may pose a problem for plant growth [4]. Therefore, development of proper technologies for disposal of this solid waste in an eco-friendly manner becomes essential.
Several studies have been carried out using fly ash in combination with other organic amendments like press-mud and farmyard manure in soil which promotes plant growth and the yield [2, 3]. Lower amendment levels of fly ash caused enhancements of both growth and yield while adverse effects at higher levels were observed for several crops such as pigeon pea (Cajanus cajan), wheat (Tritiucm aestivum), alfalfa (Medicago sativa), barley (Hordeum vulgare), beans (Phaseolus vulgaris) [2]. Numerous reports suggest that the exposure of plants to toxic levels of fly ash triggers a wide range of physiological and metabolic alterations along with an effect on leaf gas exchange [5, 6]. The symptoms of fly ash toxicity are a reduction in plant growth, inhibition of antioxidative defense system with progressing senescence and plant death [7, 8]. The process of photosynthesis is known to be extremely sensitive to different stresses including heavy metal and fly ash [6, 9]. The toxic elements from fly ash can inhibit photosynthesis at several physiological levels: pigments, gas exchange, structure and function of chloroplasts [10, 11]. In vivo chlorophyll fluorescence has been used frequently in the past as a convenient informative tool for studying the effects of various environmental stresses on the process of photosynthesis; particularly on the function of PSII [12, 13]. However, the photosynthetic response of rice plant and its photosynthetic apparatus to different levels of fly ash has not yet been studied. Nevertheless, it is not clear what concentration of fly ash alters PS II activity and causes the inhibition of photosynthesis.
When plants are subjected to metal contaminated soil, the balance between the production of Reactive Oxygen Species (ROS) and their detoxification by the antioxidative system is altered [14]. To avoid this kind of cellular damage, plants possess a complex system of antioxidative enzymes such as superoxide dismutase, catalase, peroxidase and ascorbate peroxidase or the action of non-enzymatic antioxidants which help to scavenge ROS [15]. Unlike other heavy metal stress, relatively little information is published on fly ash induced oxidative stress in higher plants and less information is available in cereal crops, such as rice. The relationship of antioxidant defense with photosynthesis and PS II activity in rice leaves under fly ash amended soil has not yet been studied so far.
Keeping in view the above, the present study aims to evaluate the effects of fly ash amended soil on growth, photosynthesis and antioxidant response in two cultivars of rice and to find out the suitable concentration that could be recommended for sustainable rice cultivation.
Material and Methods
Collection and Characterization of Fly Ash and Garden Soil
The fly ash used in the study was collected from the fly ash deposits of National Aluminium Corporation Limited (NALCO), Koraput, Odisha, India (18°46′22″N–82°53′23″E). The garden soil was collected from the campus of Central University of Orissa, Koraput (82°44′54″E–18°46′47″N). Four (4) different amendments of fly ash and garden soil were prepared by mixing these two in different ratio in dry weight, and were placed in five-kg plastic pots (20 cm in diameter and 30 cm in height) i.e. garden soil (100%), fly ash (100%), fly ash: garden soil (25: 50%), and fly ash: garden soil (50: 50%). Pots were left at the experimental site for 15 days prior to plantation for physico-chemical stabilization and proper conditioning of treated soil. Analysis for different physicochemical properties such as pH, electrical conductivity (EC), nitrogen (N), available phosphorus (P), organic carbon (OC) and potassium (K) content of different fly ash and soil amendments were carried out in the laboratory as recently described by Bisoi et al. [16]. The data of elemental analysis (mg/kg) of fly ash was collected from NALCO, Koraput, India (Al2O3: 281,500; Fe2O3: 51,500; P2O5: 4500; SO3: 13,500; CaO: 8900; MgO: 3800; Na2O: 3700; MnO: 500; K2O: 800; ZnO: 100; CuO: 150).
Plant Material and Growth Condition
The study was conducted by taking two varieties of Indica rice (Oryza sativa L.), such as IR 64 and Kalajira. The seeds were collected from National Rice Research Institute (NRRI), Cuttack, India. Ten healthy seeds of both the varieties were selected, directly sown in plastic pots and the experiments were carried out in three replications in a randomized complete block design. Plants were grown in the campus of Central University of Orissa, Koraput, India (82°44′54″E–18°46′47″N, 880 m above the mean sea level), regularly irrigated with tap water and subjected to natural solar radiation, with daily maximum photosynthetic photon flux density (PPFD), air temperature and relative humidity being about 1460 ± 20 µ mol m−2 s−1, 33.6 ± 2 °C and 70–75%, respectively. The plants were maintained up to 60 days after sowing.
Growth Parameters
Plant growth was measured in terms of different growth parameters such as plant height, root length, fresh and dry weight; after 60 days of different treatments of five different plants, in each replication. Dry weight was obtained after drying at 80 °C until a constant weight was recorded.
Leaf CO2 Photosynthetic Rate (PN) and Stomatal Conductance (gs)
The measurements of PN and gs of 60-days-old rice seedlings were made on fully expanded leaves of five different plants using an open system photosynthetic gas analyzer (CI-304, CID, USA) under normal ambient environmental conditions. The second and third leaf from the top was selected and kept inside the chamber under natural irradiance until stable reading was recorded. Measurements were carried out at 33 ± 2 °C, 70% relative humidity, 1014 ± 33 μmol m−2 s−1 photosynthetic active radiation, 370 μmol CO2 m−2 s−1 and 21% O2.
Measurement of Chlorophyll Fluorescence
Chlorophyll fluorescence was measured on the same leaves used for gas exchange measurements using a portable chlorophyll fluorometer (JUNIOR-PAM, WALZ, Germany) at 10–12 h. Different fluorescence parameters like minimal fluorescence (Fo), maximal fluorescence (Fm), and maximum photochemical efficiency of PS II (Fv/Fm) were measured in 20 min dark-adapted leaves [13]. In light adapted leaves at a PPFD of 400 µmol m−2 s−1 (for 15 min), steady state fluorescence yield (Fs), maximal fluorescence (Fm’) after 0.8 s saturating white light pulse and minimal fluorescence (Fo’) were measured. Further, yield of PSII photochemistry (Y II), quenching value due to non-photochemical dissipation of absorbed light energy (NPQ) and the coefficient for photochemical quenching (qP) were calculated [13].
Measurement of Chlorophyll, Carotenoid and SPAD Index
The leaf Chl and carotenoid content was measured spectrophotometrically by taking absorbance at 663.6, 646.6 and 470 nm, as recently described in Bisoi et al. [16]. The total chlorophyll and carotenoid contents were calculated using the equations of Porra [17]. SPAD chlorophyll index of 30 days old plants were made on the fully expanded leaf of five (5) different plants using a SPAD 502 chlorophyll meter (KONIKA MINOTA SENSING, JAPAN).
Measurement of Antioxidant Enzyme Activity and Protein Content
After measurement of Chl fluorescence, the same leaf tissue was used for assay of antioxidant enzyme activity, as recently described, in the laboratory of authors [16]. In brief, superoxide dismutase (SOD; EC 1.15.1.1) activity was measured using the photochemical method followed by Giannopolitis and Ries [18] with modifications suggested by Choudhury and Choudhury [19]. Catalase (CAT; EC 1.11.1.6) activity was measured by monitoring the decomposition of H2O2 which followed at 240 nm [20]. Ascorbate peroxidase (APX; EC 1.11.1.11) was assayed following the method of Nakano and Asada [21] by monitoring the rate of ascorbate oxidation at 290 nm (E = 2.8 mM cm−1). Activity of guaiacol peroxidase (GPX; EC 1.11.1.7) was assayed as the increase in absorbance at 470 nm caused by guaiacol oxidation (E = 26.6 mM cm−1) following the method of Rao et al. [22]. An aliquot of the extract was used to determine protein content following Lowry et al. [23].
Statistical Analysis
Differences between various parameters were compared by ANOVA using CROPSTAT (International Rice Research Institute, Philippines). Mean values were compared by the least significant difference (LSD, P < 0.05), wherever the F test was significant. Correlation analysis and Duncan’s multiple range tests were done by the CROPSTAT software.
Results and Discussion
Physicochemical Parameters of the Garden Soil and Fly Ash
The nature of fly ash was alkaline (pH 8.10) and the fly ash was low in nitrogen (N) and organic carbon (OC) as compared to the garden soil (Table 1). The electrical conductivity (EC) was more in fly ash as compared to garden soil. The available phosphorus and potassium content was high in fly ash as compared to the garden soil. In addition, fly ash also contains some toxic levels of elements such as Al2O3, Fe2O3, P2O5, SO3, CaO, MgO, Na2O, MnO, ZnO and CuO. However, fly ash has some physical and chemical properties and the application of fly ash to soil significantly improves the pH, EC, N and OC that might be useful as soil amendment [1,2,3]. Fly ash had some lime equivalence due to which there was an increase of soil pH, but at low level of fly ash amendments, no visible adverse effect was observed as reported earlier [4, 5]. Increase of EC values due to fly ash application could suggest that it facilitates leaching of soluble salts from fly ash causing the eventual availability or entry of metal nutrients to growing plants, as reported earlier [4].
Growth Parameters
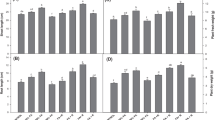
The shoot and root length of both varieties of the rice seedlings remarkably increased under 25% of fly ash-amended soil as compared to garden soil but significantly (*P < 0.05) decreased under 50 and 100% of fly ash treatments. Similarly, the fresh and dry weights of rice seedlings increased under low level of fly ash–amended soil (Table 2). The result suggests that 25% of fly ash in soil improves the growth of rice seedlings because, in low levels, fly ash increases the permeability of soil, moisture holding capacity, reduces acidity and heavy metal availability [3]. Maximum reduction of rice growth was observed under higher concentration of fly ash (100%) indicating elevated levels of metal in contaminated soils that can inhibit plant growth [16].
Leaf CO2 Photosynthetic Rate, Stomatal Conductance and Pigment Content
The CO2 photosynthetic rate (PN) of 60-days-old rice seedlings increased with 25% of fly ash-amended soil as compared to garden soil, along with increase of stomatal conductance (gs) (Fig. 1). However, these parameters significantly decreased under higher concentrations of fly ash (50, 100%). In addition, SPAD index, Chl and carotenoid content did not significantly change in 25% fly ash-amended soil as compared to the control but remarkably declined at higher levels of fly ash treatments (Table 3). Low level of fly ash in soil improves the leaf photosynthesis in rice that might be due to the improvement of soil’s physical and chemical parameters [3]. The rapid drop in PN under high concentration of fly ash was probably due to the structural damage suffered by the photosynthetic apparatus, as evident from the fall in the values of SPAD index, Chl and carotenoid contents (Table 3). Overall, lower photosynthetic efficiency occurred under elevated levels of fly ash in soil system due to the synergistic activities of different factors such as, decrease of Chl, carotenoid and oxidative load [24]. This is in agreement with earlier studies for other plant species [25, 26].
Changes of CO2 photosynthetic rate (PN) and stomatal conductance (gS) in rice leaves grown in different concentration of fly ash amended soil. The treatment 0, 25, 50 and 100 are different percentage of fly ash amended in soil. The measurements were carried out on fully expanded mature leaves at 30 ± 2 °C, 70% Relative humidity, 1114 ± 33 μmol m−2 s−1 photosynthetic active radiation, 370 μmol CO2 m−2 s−1 and 21% O2. Data are the mean of three replications and bars indicate the standard deviation of the means (n = 3). Means followed by a common letter are not significantly different at the 5% level by Duncan’s multiple range test
Chlorophyll Fluorescence
To clarify the alterations of PSII activity in rice seedlings under different levels of fly ash-amended soil, the authors measured different Chl fluorescence parameters such as Fo, Fm, Fv/Fm, Y (II), qP and NPQ. In the present study, under low levels of fly ash-amended soil (25%), Chl fluorescence parameters did not significantly change (*P < 0.05) as compared to the control seedlings. However, higher concentration of fly ash (50 and 100%) significantly declined the values of Fm, Fv/Fm, Y (II) and qP in rice seedlings (Fig. 2; Table S1). Fo,the minimal fluorescence level, remarkably increased in rice seedlings under high fly ash concentration. This may be due to disruption in energy transfer into the reaction centre and photo inhibition, as has been reported in other plants and grasses [26]. The Fm, Fv and Fv/Fm can be used to estimate the potential efficiency of PSII by taking dark adapted measurements [27]. In the present study, these parameters did not significantly change in low level of fly ash amendments but significantly decreased at elevated concentrations of fly ash. This indicates that fly ash in high concentration alters the PS II activity in rice seedlings.
Spider type visual plot showing quantitative extend of changes in various chlorophyll fluorescence parameters in rice seedlings grown in different concentration of fly ash amended soil. The black circle with radius 1 represents garden soil (0) and 25, 50 and 100 are different percentage of fly ash amendments. Data are the mean of three replications. Fo minimum fluorescence yield obtained with dark-adapted leaf, Fm maximum Chl fluorescence yield obtained with dark-adapted leaf, Fv variable fluorescence (Fm—Fo), Fv/Fm maximal photochemical efficiency of PS II, NPQ non-photochemical quenching, qP photochemical quenching, Y(II) yield of PSII photochemistry
The photosynthetic quenching (qP) that represents the energy consumed in photosynthesis and NPQ is the amount of dissipated irradiation into heat [28]. The qP and NPQ did not significantly chang in low level of fly ash amended soil but a decline in qP and increase of NPQ in rice seedlings were observed under high fly ash concentrations (Fig. 2). This suggests that there was a decrease in the quantum efficiency of PSII photochemistry; either due to decrease in the rate of primary charge separation or by increase of heat dissipation under elevated levels of fly ash. In the present study, the authors observed that there was an inverse relationship between qP and NPQ as well as between Y (II) and NPQ, in rice. This was in line with the results reported for other stress [13].
Levels of Antioxidant Enzyme Activity and Protein Content
To explain the oxidative protective mechanism in rice seedlings under different treatments of fly ash-amended soil, the authors measured the levels of the activities of some antioxidant enzymes. The activities of SOD, APX, GPX and CAT increased in both varieties of rice seedlings grown under low levels of fly ash (25%) amended soil as compared to control plants (Fig. 3; Table S2). This early rise of enzymes was the response to active oxygen species caused by metal ion present in fly ash. Possibly, increased levels of active oxygen stimulate the cellular protective mechanism to mitigate damages [14]. However, high concentration of fly ash significantly decreased the activities of SOD, APX, GPX and CAT; indicating the low activity of rice leaves to decompose into H2O2 and O2. This has been reported for other crops too [29]. Another possible cause of the reduction of these enzymes in rice seedlings under high fly ash concentration is the decrease of the production and/or activity of ROS [30]. In addition, marked increase of protein content was observed in low level (25%) of fly ash-amended soil and decline in further treatments (Fig. 3). This suggests that 25% of fly ash amended soil is not deleterious for rice growth and that heavy metals in fly ash induce the synthesis of stress proteins [30]. Further, high concentration of fly ash may decrease protease activities that seem to be a common feature involved in metal toxicity in plants, affecting total protein and growth of plants [7]. Our experiment showed a positive correlation between antioxidant enzymes activities with different photosynthetic parameters such as PN, gs, Fm, Fv/Fm, qP and Chl but significant negative association with Fo and NPQ (Table 4). The results suggest that the maintenance of photosynthetic activity in rice seedlings under low level of fly ash amendment might be due to better antioxidative protection from oxidatative damage. Based on the present findings, soil amended at 25% fly ash not only improved physical properties of the soil but also contributed to better growth and photosynthesis in rice. Higher levels of fly ash in soil amendments would be detrimental because micronutrients are required in lower doses and by increasing the levels of fly ash, the toxic heavy metals load may increase [5]. Further, it is also suggested that fly ash can be used in amending rice soils for a year or two in a field but the continuous use of fly ash in rice field, can cause leaching of toxic metals and lead to permanent soil contaminations [4].
Spider type visual plot showing quantitative extend of changes in different antioxidant enzymes activities and protein content in rice seedlings grown in different concentration of fly ash amended soil. The black circle with radius 1 represents garden soil (0) and 25, 50 and 100 are different percentage of fly ash amended soil. SOD superoxide dismutase, APX ascorbate peroxidase, GPX guaiacol peroxidase and CAT catalase. Data are the mean of three replications
Conclusion
The study showed that fly ash-amended soil in low concentration (25%) could be beneficial in rice seedlings as it improves growth and photosynthesis. It also helps in maintaining PS II activity. The decline in CO2 photosynthetic rate in rice seedlings under high concentration of fly ash was probably due to the structural and functional alteration of PS II, stomatal conductance and as well as chlorophyll damage. In addition, the results also suggest that the maintenance of photosynthesis in rice seedlings under low level of fly ash amendment might be due to better antioxidative protection from oxidative damage. Taken together, the finding is encouraging for an agro-friendly use of low levels of fly ash (25%) in rice cultivation. However, additional research is necessary to know the phytoaccumulation of metal in rice tissue. It could be concluded that low level of fly ash can be used in amending rice soil for a short period of time but continuous use of fly ash can cause permanent soil contamination by increasing the load of toxic metals.
References
Ram LC, Masto RE (2014) Fly ash for soil amelioration: a review on the influence of ash blending with inorganic and organic amendments. Earth Sci Rev 128:52–74
Verma SK, Singh K, Gupta AK, Pandey VC, Trivedi P, Verma RJ, Patra DD (2014) Aromatic grasses for phytomanagement of coal fly ash hazards. Ecol Eng 73:425–428
Pandey VC, Singh N (2010) Impact of fly ash incorporation in soil systems. Agric Ecosyst Environ 136:16–27
Padhy RN, Nayak N, Dash-mohini RR, Rath S, Sahu RK (2016) Growth, metabolism and yield of rice cultivated in soils amended with fly ash and cyanobacteria and metal loads in plant parts. Rice Sci 23(1):22–32
Mishra M, Sahu RK, Padhy RN (2007) Growth, yield and elemental status of rice (Oryza sativa) grown in fly ash amended soils. Ecotoxicology 16:271–278
Raja R, Nayak AK, Rao KS, Puree C, Shahid M, Panda BB, Kumar A, Tripathi R, Bhattacharyya P, Baig MJ, Lal B, Mohanty S, Gautam P (2014) Effect of fly ash deposition on photosynthesis, growth and yield of rice. Bull Environ Contam Toxicol 93:106–112
Sharma P, Dubey RS (2007) Involvement of oxidative stress and role of antioxidative defense system in growing rice seedlings exposed to toxic concentrations of aluminum. Plant Cell Rep 26:2027–2038
Gill M (2014) Heavy metal stress in plants: a review. Int J Adv Res 2:1043–1055
Panda D, Rao DN, Sharma SG, Strasser RJ, Sarkar RK (2006) Submergence effect on rice genotypes during seedling stage: probing of submergence driven changes of photosystem 2 by chlorophyll a fluorescence induction O-J-I-P transients. Photosynthetica 44:69–75
Reid RJ, Hayes JE, Post A, Stangoulis JCR, Graham RD (2004) A critical analysis of the causes of boron toxicity in plants. Plant Cell Environ 27:1405–1414
Gajić G, Pavlović P, Kostić O, Jarić S, ĐurĐević L, Pavlović D, Mitrović M (2013) Ecophysiological and biochemical traits of three herbaceous plants growing on the disposed coal combustion fly ash of different weathering stage. Arch Biol Sci 65:1651–1667
Sayed OH (2003) Chlorophyll fluorescence as a tool in cereal crop research. Photosynthetica 41:321–330
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence practical guide. J Exp Bot 51:659–668
Bhaduri MA, Fulekar MH (2012) Antioxidant enzyme responses of plants to heavy metal stress. Rev Environ Sci Biotechnol 11:55–69
Singh OV, Labana S, Pandey G, Budhiraja R, Jain RK (2003) Phytoremediation: an overview of metallicion decontamination from soil. Appl Microbiol Biotechnol 61:405–412
Bisoi SS, Mishra SS, Barik J, Panda D (2017) Effects of different treatments of fly ash and mining soil on growth and antioxidant protection of Indian wild rice. Int J Phytoremediat 19:446–452
Porra RJ (2002) The chequered history of the development and use of simultaneous equations for accurate determination of chlorophylls a and b. Photosynth Res 73:149–156
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: occurrence in higher plants. Plant Physiol 115:159–169
Choudhury SR, Choudhury MA (1985) Hydrogen peroxide metabolism as an index of water stress tolerance in jute. Physiol Plant 65:503–507
Cakmak I, Marschner H (1992) Magnessium deficiency and highlight intensity enhance activities of superoxide dismutase, ascorbate peroxidase and glutathione reductase in bean leaves. Plant Physiol 98:1222–1227
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Rao MV, Hale BA, Ormrod DP (1995) Amelioration of ozone-induced oxidative damage in wheat plants grown under high carbon dioxide: role of antioxidant enzymes. Plant Physiol 109:421–432
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Miteva E, Merakchiyska M (2002) Response of chloroplasts and photosynthetic mechanism of bean plants to excess arsenic in soil. Bulg J Agric Sci 8:151–156
Techer D, Laval-Gilly P, Bennasroune A, Henry S, Martinez-Chois C, D’Innocenzo M, Falla J (2012) An appraisal of Miscanthus giganteus cultivation for fly ash revegetation and soil restoration. Ind Crop Prod 36:427–433
Kostić O, Mitrović M, Knežević M, Jarić S, Gajić G, Djurdjević L, Pavlović P (2012) The potential of four woody species for the revegetation of fly ash deposits from the “Nikola Tesla –A” thermoelectric plant (Obrenovac serbia). Arch Biol Sci 64:145–158
Calatayud A, Roca D, Martínez PF (2006) Spatial-temporal variations in rose leaves under water stress conditions studied by chlorophyll fluorescence imaging. Plant Physiol Biochem 44:564–573
Pinnola A, Dall’Osto L, Gerotto C, Morosinotto T, Bassi R, Alboresi A (2013) Zeaxanthin binds to light-harvesting complex stress-related protein to enhance nonphotochemical quenching in Physcomitrella patens. Plant Cell 25:3519–3534
Arora A, Sairam RK, Srivastava GC (2002) Oxidative stress and antioxidative system in plants. Curr Sci 82:1227–1238
Rastgoo L, Alemzadeh A (2011) Biochemical responses of Gouan (Aeluropus littoralis) to heavy metals stress. Aust J Crop Sci 5:375–383
Acknowledgements
The authors are grateful to Dr. S. K. Palita, Head, Department of Biodiversity and Conservation of Natural Resources for providing necessary facilities for the work. The Director, NRRI, Cuttack is highly acknowledged for providing the rice seeds.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest to publish this manuscript.
Additional information
Significance statement The study showed that the fly ash-amended soil in low concentration (25%) could be beneficial in rice seedling as it improves growth and photosynthesis. The finding is encouraging for an agro-friendly use of low levels of fly ash (25%) in agriculture as a soil amendment.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Panda, D., Mandal, L., Barik, J. et al. Improvement of Growth, Photosynthesis and Antioxidant Defense in Rice (Oryza sativa L.) Grown in Fly Ash-Amended Soil. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 89, 853–860 (2019). https://doi.org/10.1007/s40011-018-0996-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-018-0996-7