Abstract
Purpose
Compared with mineral fertilizers, the use efficiency of nitrogen (N), phosphorus (P), and potassium (K) from organomineral matrices increases as biochar is mixed with acidulated apatite to produce slow-release NPK-biochar-based fertilizers (BBFs). The BBFs reduce P losses while improving plant nutrition in Oxisols. Thus, the aim of this study was to synthetize and evaluate the effects of slow-release BBFs produced by mixing coffee waste-derived biochar with acidulated apatite.
Methods
The agronomic efficiency of BBFs was evaluated through the cultivation of maize in greenhouse conditions. The availability of NPK in whole soil and solution and the kinetics of P released by fertilizers in water and citric acid leachates were evaluated as well.
Results
Compared with mineral fertilizers, N and K supplied by BBFs did not improve the nutrition of maize plants. Triple superphosphate (TSP) released nearly all the total P, while the BBFs released less than 10% of the P released by the TSP in the first 24 h. Biochar based-fertilizers, depending on the synthesis route adopted, produced a greater or similar maize biomass. The acquisition of P from BBFs was greater than that of NPK supplied via mineral fertilizers. Thus, BBFs can slow the release of P in water leachates and, simultaneously, release high levels of P in citric acid leachates.
Conclusions
The BBFs formulated with coffee husk-derived biochar and acidulated apatite are a sustainable and efficient approach to producing NPK sources with higher agronomic effectiveness than soluble mineral fertilizers. The BBFs improved the nutritional status and growth of maize and the residual available soil P content in the Oxisols as well (Ferralsols).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Tropical soils naturally contain low levels of nitrogen (N), phosphorus (P), and potassium (K), which reduce plant growth and yield (FAO 2017; Lopes and Guilherme 2016). In addition, P is specifically and highly adsorbed in Fe and Al oxides and kaolinite, reducing the availability of soil P to crops (Fink et al. 2016). Phosphorus fertilization is essential to increasing crop yield in Oxisols. Soluble mineral fertilizers are the main P sources used to nourish crops, although the efficiency with which plants utilize soluble P-fertilizer is generally low (5–25%) (Maluf et al. 2018; Raniro et al. 2023; Withers et al. 2018). One of the strategies used to increase the efficiency of P fertilization is to increase the soil organic matter (SOM) content in tropical soils. Increase of SOM content is a difficult task only achieved in the long term in a few cropland areas, which is partially explained by low amounts of crop residues and C inputs, scarcity and poor distribution of precipitation, high mean temperature, and elevated SOM decomposition rates in most tropical crop fields (Fink et al. 2016; Gmach et al. 2020; Raniro et al. 2023). Tropical soils exhibit a reduced capacity to retain N as ammonium and K+, which increases the leaching of soluble N and K chemical species from the surface to the highly weathered subsoil layers.
The use of organic fertilizers in replacement of mineral fertilizers is one strategy to increase the efficiency with which crops utilize and uptake P, although organic fertilizers contain a low P concentration, besides N in forms that need to be mineralized before being uptake by crops, and low levels of available K as well; thus, high organic fertilizer rates are required to fulfill the NPK plant requirement (Mumbach et al. 2020). Organomineral fertilizers (OMFs) are a class of fertilizers that combine the best properties of mineral fertilizers (high, balanced and readily available nutrient concentrations) with those of organic fertilizers (nutrient gradual release, organic ligands capable of blocking soil phosphate fixation sites, complexation of metals) (Bai et al. 2022; Gwenzi et al. 2018; Piash et al. 2022; Suwanree et al. 2022). Different synthesis routes and organic and mineral sources can be used to produce OMFs. Among the fertilizer formulation processes currently used, pyrolysis and its product (biochar) are a promising route and matrix to formulate novel fertilizers that exhibit a higher agronomic performance than mineral soluble fertilizers (Barbosa et al. 2022; Fachini et al. 2022; Gwenzi et al. 2018; Johan et al. 2021; Piash et al. 2022; Suwanree et al. 2022).
Biochars are stable and rich C matrices produced through the pyrolysis of renewable organic residues or wastes without or with a limited supply of oxygen (Singh et al. 2017). During pyrolysis, N, sulphur (S), oxygen (O), and some carbon (C) acidic compounds are volatilized; thus, commonly, the biochar alkaline character is not a suitable matrix to promote full apatite solubilization, which requires a strong acidic media to release readily available P to crops. Potassium is not volatilized during pyrolysis; in contrast, potassium is relatively enriched in charred matrices, mainly those produced at high (>600 °C) pyrolysis temperatures. Expensive and strong acids such as sulfuric and phosphoric acids are normally employed in the apatite solubilization process, although the whole process in the industry takes a long time to convert apatite-P into available P forms for crops, besides generating by-products such as dehydrated calcium sulfate (gypsum). We have invented a new fertilizer synthesis route in which the pyrolysis of coffee residues produced a K-enriched biochar, which was mixed with an acidulated and fully solubilized apatite in a fast way and without the generation of gypsum (Silva and Morais 2022). The mixture of biochar with the solubilized apatite at room temperature generated N and P-soluble fertilizers, which were mixed with coffee residue-derived biochars (K source) to produce novel NPK composites, which are hereafter called biochar-based fertilizers (BBFs). In addition, there were high levels of N in the BBFs because the source of N in the BBFs was added after pyrolysis; thus, there were no significant losses of N during pyrolysis.
In biochar-based fertilizers, the myriad nutrient pools control the kinetics of P release, in addition to gradually releasing K and N to plants (Shi et al. 2020; Suwanree et al. 2022). When mineral-soluble N is mixed with biochar, part of the N interacts with the pyrolyzed organic matrix, forming organic N-containing compounds, while the remaining N could be adsorbed on the surface of the charred matrices, thus becoming part of the biochar organic N pool (Barbosa et al. 2022; Phillips et al. 2022; Shi et al. 2020). Therefore, in BBFs, mineral and organic N pools in which the mineralization rate (organic N) and availability of mineral N forms control the amount of N supplied to crops (Phillips et al. 2022). Potassium interacts with biochar through weak electrostatic bonds through negative charges present in the charred organic functional groups, K diffusion into biochar micropores, and being trapped in charred hydrophobic radicals, which decreases K dissolution and release rate to plants (Fachini et al. 2022; Hossain et al. 2020).
When P interacts with the biochar organic matrix, it is gradually released by BBFs, and P losses due to specific adsorption and precipitation are reduced; consequently, the availability of P in soil is increased (Bai et al. 2022; Ghodszad et al. 2021). A more gradual release of P is revealed by the amounts in fertilizer of P soluble in water and by the kinetics rate of P release in water by the P source (Carneiro et al. 2021; Suwanree et al. 2022). In addition, other fertilizer-P availability indices, such as P soluble in citric acid, which dissolves phosphate chemical species in OMFs, show potential to be used as a P eluting solution in P kinetics release studies (Johan et al. 2021). In organomineral fertilizers, P is complexed in organic forms; consequently, it is less prone to precipitation with Ca and other cations found in soils. Additionally, organic ligands found in OMFs are capable of blocking phosphate adsorption sites that are massively present in tropical soils, thus increasing the P content in soil solution (An et al. 2021; Bai et al. 2022; Ghodszad et al. 2021; Johan et al. 2021). Fertilizers that gradually release nutrients to crops, besides reducing nutrient losses, may exhibit greater agronomic performance than that of soluble mineral P fertilizers (Guelfi et al. 2022).
Nutrient pools and properties of BBFs are influenced by the adopted synthesis route and pyrolysis conditions as well (An et al. 2021; Lustosa Filho et al. 2019; Piash et al. 2022). When low-grade phosphate rocks were mixed with plant residues, the synthesized BBFs did not have a greater agronomic value than the raw igneous phosphate rock (Tumbure et al. 2020). Thus, for BBF synthesis routes that include poorly soluble phosphate rocks, apatite must be acidulated before the pyrolysis to produce composites in which soluble mineral P fertilizers prevail (United Nations Industrial Development Organization Staff 1998). When low-grade phosphate rocks are acidulated and mixed with charred matrices, the solubilized P chemical species react with C, forming P-organic complexes with higher agronomic performance and availability than those of non-complexed soluble P mineral fertilizers (Erro et al. 2012).
When the right pyrolysis conditions are employed, the biochar yield is greater than 50% and a mean K2O content of 16% in the final coffee husk-derived biochar is achieved (Domingues et al. 2017). At the same time, post-harvest coffee residues, which are massively produced in Brazil, are poorly used as K sources; on the contrary, they are burned by farmers in the process of coffee bean drying (Hoseini et al. 2021; IBGE 2021). Due to low levels, N volatilization is not a serious issue when coffee residues are pyrolyzed. By combining solubilized apatite with coffee residue-derived biochar to produce composites, e.g., BBFs, a more agronomic cost-effective and friendly option is provided to minimize the specific adsorption and precipitation of P, leaching of K, and volatilization of N in highly weathered soils. Despite the several benefits of biochar (cation exchange capaacity (CEC), water holding capacity, interaction of organic matrix with soil, plant, water and biota, long C persistence in soils, etc.), the application of biochar to add nutrients to soils and nourish plants is limited due to low NPK levels and losses (volatilization) of N, S, O, and some C compounds during pyrolysis (Bai et al. 2022; Munera-Echeverri et al. 2018; Piash et al. 2022).
Thus, we hypothesize that the use of a feedstock rich in K, the optimization of pyrolysis conditions, and the mixing of K-enriched biochars with low-cost, solubilized P compounds, and available N chemical species produced during apatite solubilization are a suitable route to create slow-release, cost-effective, high agronomic value, and environmentally eco-friendly fertilizers, e.g., BBFs. The aims of this study were the following: (i) create a novel and effective synthesis route of NPK based on the mixing of acidulated apatite with a postharvest coffee-derived biochar; (ii) characterize the novel BBFs and evaluate their kinetics of NPK release; and (iii) evaluate the effect of BBFs on soil NPK availability and maize growth.
2 Material and Methods
2.1 Synthesis and Biochar-Based Fertilizer Characterization
Biochar-based fertilizers were formulated using different routes protected by the patent BR1020220127840 deposited at the Instituto Nacional da Propriedade Industrial (INPI) (National Institute of Industrial Property) (Silva and Morais 2022), following the registration procedure regulated by Brazilian laws. Briefly, BBFs synthesis includes the preparation of two materials that were later mixed in sequential steps, the first material is characterized as biochar samples, and the second one consist of acidulated phosphate rock.
The first material is biochar produced with coffee postharvest residues pyrolyzed at low (300°C) or high (500°C) temperatures. Pyrolysis was performed in a muffle furnace at a heating rate of 10 °C min-1 and the feedstock was kept in the pyrolysis chamber (retention time) at the target temperature for 60 min. The acidulated phosphate rock was produced as follows: (i) the acidulation of Araxá phosphate rock (APR) was performed using two proportions of a strong inorganic acid (nitric acid 65%, - Reagent grade, Synth)-apatite; (ii) mixtures of apatite and inorganic acid were left to rest for 30 min; and (iii) the apatite-acidulated mixtures were shaken at 120 rpm for 1 h in a horizontal shaker.
After the acidulation process, samples of the modified apatite were immediately mixed with coffee husk-derived biochars. After the mixture of the acidulated phosphate rock with biochar, the final synthesized BBF was left to rest for 30 min. In sequence, the BBFs samples were dried in an oven at 60° C until constant weight. Therefore, the use of different strong acid-apatite stoichiometric ratios and the production of two contrasting coffee residue-derived biochars produced four BBFs whose main properties are shown in Table 1.
The biochar-based fertilizers synthesized and conventional sources of NPK were fully characterized using three replicates. Mineral sources used were ammonium nitrate (N source), triple superphosphate (TSP) (P source), and potassium chloride (KCl) (K source). The fertilizer samples (BBFs and mineral) were passed through a 1 mm sieve, dried, and stored for further analysis. The pH of BBFs was determined in a 0.01 mol L-1 CaCl2 solution at a ratio of 1:10 (m/v) (Brazil 2017; Singh et al. 2017). Nitrogen as ammonium (N-NH4+) and nitrate (N-NO3-) contents in fertilizers was extracted using a 2 mol L-1 KCl solution and, in sequence, determined using the distillation (Kjeldahl method) procedure (Singh et al. 2017).
Total nitrogen content in fertilizers was determined using the Kjeldahl method after digesting the samples with concentrated sulfuric acid (Brazil 2017; Phares et al. 2020). Total N content was determined through the Kjeldahl method; thus, the fertilizer organic matrix was digested, and, in sequence, the acid digested samples were distilled to convert N chemical species into ammonium. It should be taken into consideration that the nitrate content was not determined in the fertilizer samples (Bremner 1965). Thus, due to the high content of N-NO3- in some BBFs, the contents of N determined by the Kjeldahl method and N-NO3- were summed to obtain the total N content of each fertilizer.
Samples of mineral fertilizers and BBFs were digested using the nitric-perchloric acid analytical procedure to assess their total contents of P and K (Brazil 2017; Enders and Lehmann 2012). Phosphorus and K soluble in water in fertilizer samples were determined through the successive washing of 1 g of sample with distilled water until a final volume of 250 ml was reached (Brazil 2017). One gram of fertilizer sample was stirred with 100 ml of a 2% citric acid (CA) solution for 30 min to assess the soluble contents of P and K in CA (Brazil 2017). One gram of fertilizer samples was boiled in a 50 ml of neutral ammonium citrate plus water (NAC+H2O) solution to assess the soluble contents of P and K in NAC+H2O (Brazil 2017). To assess the soluble contents of P and K in formic acid (FA), the recommended method for assessing nutrient availability in BBFs, 0.35 g fertilizer samples were mixed with 35 ml of a 2% acid formic solution, ultrasonicated for 10 min, and then shaken for 30 min (Singh et al. 2017). The contents of P in extracts (total and soluble methods) were determined by the vanadomolybdate method (Kitson and Mellon 1944), and the contents of K were determined by the photometry method (Fox 1951). The main properties, contents, and pools of NPK in the BBFs and mineral fertilizers are shown in Table 2.
Nitrogen forms (mineral and organic N) and soluble contents of P and K in fertilizer samples were normalized in relation to total NPK contents in order to calculate N, K, and P indices according to Eq. 1 (N index), Eq. 2 (P index), and Eq. 3 (K index). This approach is suitable to compare fertilizers with different total nutrient contents, considering that it eliminates (through the normalization procedure) the effect of the increase or decrease of NPK concentration in contrasting nutrient sources (Morais et al. 2023). Therefore, the NPK indices allow comparing the agronomic value of nutrient sources, besides guiding crop fertilization management.
2.2 Infrared Spectroscopy
Two biochars produced at low or high pyrolysis temperatures and used in the BBF synthesis, and four BBF samples were scanned in the medium infrared region to identify the main peaks and the fertilizer spectroscopic signatures. The infrared spectra were obtained at 4000 to 650 cm-1 wavenumber range with a resolution of 4 cm-1 through the Attenuated Total Reflectance Fourier transform infrared (FTIR) spectroscopy technique using an Agilent® Cary 630 spectrometer. Pre-processing (normalization) of the infrared dataset was performed for all samples to compare the spectrum (Morais et al. 2023). The main peaks in infrared spectra were identified according to the main infrared features and organic functional groups assigned to BBFs and fertilizer samples, following libraries already described in Janu et al. (2021), Lustosa Filho et al. (2017), Morais et al. (2023), and Singh et al. (2017). The main peaks and infrared spectra features of biochars are shown in Supplementary Figure S1.
2.3 Maize Growth Conditions
The agronomic value of BBFs over NPK mineral sources was assessed through the cultivation of maize in a Sandy Loam Oxisol (Ferralsol). Maize plants were cultivated for 25 days under greenhouse conditions in a novel container in which plants were grown in a mini-lysimeter filled with 0.550 kg of soil (soil particles < 2 mm) (Supplementary Figure S2). The cultivation time was delimited by the maximum plant growth without compromising plant growth and nutrition in the pot filled with the Ferralsol. Initially, 5 seeds were sown per pot, and after 5 days, thinning was performed, with the subsequent cultivation of one plant per pot during 25 days (V5 growth stage). Six treatments were tested in a completely randomized design with three replicates. The treatments evaluated are described as follows: four synthesized BBFs, positive control (NPK added to the soil via single mineral fertilizers), and a negative control (maize cultivated without the addition of NPK fertilizers). The main properties of the soil used in maize cultivation are shown in Table 3.
Phosphorus fertilization was homogeneously added to the whole soil at 200 mg kg-1 P. The phosphorus rate was based on the fertilizer-soluble contents of P in NAC+H2O as proposed by Morais et al. (2023). There was no standardization of the amount of N and K supplied to maize plants by BBFs. Thus, when BBFs were used to fertilize plants, no additional fertilization with N and K was provided to maize plants. The amounts of N and K added to the Oxisol via mineral fertilizers were based on the average amounts of N and K added to soil by BBFs, considering fertilizer recommendations for plants grown in pots filled with Oxisols (Novais et al. 1991). Therefore, concentrations of 200 and 150 mg kg-1, respectively, of N and K were supplied to maize through ammonium nitrate and potassium chloride. Amounts of N and K were divided; thus, 20% of the N and K rate was added to the soil at sowing fertilization and the remaining 80% as topdressing fertilization (13 days after sowing). The amounts of mineral N and soluble contents of K and P in different fertilizer tests are shown in Supplementary Table S1. In sowing fertilization, it was also considered the fertilization recommendation for plants grown in experiment carried out in pots filled with Oxisols (Novais et al. 1991). The following nutrient rates were supplied to maize: 30, 40, 0.81, 2, 7.3, 0.15, 5, and 10 mg kg-1, respectively, of Mg, S, B, Cu, Mn, Mo, Zn, and Fe, which were supplied as MgSO4.7H2O, H3BO3, CuSO4.5H2O, MnCl2.4H2O, (NH4)6Mo7O24.4H2O, ZnSO4.7H2O, and FeCl3.6H2O (Reagent grade, Synth).
Before and after maize cultivation (25 days), 15 g of the whole soil were sampled in each experimental unit (pot filled with soil plus treatments). Soil samples were dried and passed through a 2-mm sieve. In the whole soil samples, pH was determined in water at a ratio of 1:2.5 (w/v) (Sparks et al. 2020); N-NH4+ and N-NO3- were extracted using a 1 mol L-1 KCl solution (10 mL of the extractant solution: 1 g of soil). Soil mineral N content was determined through the distillation method, followed by the titration procedure (mineral N in the soil) (Bremner and Keeney 1966; Sparks et al. 2020). Available contents of K were determined through the Mehlich-1 soil test and quantified in a flame photometer (Sparks et al. 2020). The available contents of P in the soil were assessed by the resin soil test and determined through the molybdenum blue method (Raij et al. 1986).
After maize cultivation, plants were harvested, separated into shoot and root, and dried at 60 °C until constant weight. In sequence, shoot (SDM) and root (RDM) dry matter were weighed. Total dry matter production was obtained by adding SDM to RDM. The shoot tissue samples were ground through a 1 mm sieve in a Wiley Mill. In shoot tissues, the content of N was determined by the Kjeldahl method, and the contents of P and K were determined by digestion of samples in a nitric perchloric acid solution, followed by the determination of the content of P by the molybdenum blue method, while the contents of K were determined in a flame photometer (Kalra 1998). The accumulation of N, P, and K (NuAc) in maize shoot was calculated as follows (Eq. 4):
2.4 Kinetics of P Release
Based on an equal amount of P added to Oxisol through P fertilization, the kinetics of P release for the four BBFs and TSP were carried out using water or a 2% citric acid solution as extractant solutions (Morais et al. 2023). The extractors used in the kinetics of P release allowed the determination of readily soluble P in the fertilizer (P-water) and P potentially available to plants (P-citric acid solubility) (Morais et al. 2023). The mini-lysimeter packing and sequence of layers, washed sand, and other materials are shown in detail in Morais et al. (2023). The method consists, briefly, of a mixture of fertilizer samples with sand (pre-washed with hydrochloric acid) and incubation in sequence in a 250-ml mini-lysimeter. After the initial leaching time (0 h), 50 ml of distilled water or citric acid solution were used to leach the sand-fertilizer mixtures packed in the mini-lysimeters. In sequence, P was leached from each lysimeter in a cumulative volume of water or citric acid solution equivalent to 7 mL h-1 at the following leaching times: 0, 4, 12, 24, 48, 72, 120, 168, 216, and 264 h. The contents of P in the leachates were determined by the vanadomolybdate method (Kitson and Mellon 1944). The amounts of P released from each experimental unit were calculated by measuring the volume of leachate and the content of P in it (Morais et al. 2023).
2.5 Statistical Analysis
All statistical analysis was carried out through the R software (R Core Team 2020), using the base, stats, corrplot nlstools, tidyverse, Metrics, emmeans packages (Baty et al. 2015; Hamner and Frasco 2018; R Core Team 2020; Russel 2021; Wei and Simko 2017; Wickham et al. 2019). The means of the N, P, and K fertilizer indices were compared by the Dunnett test (p<0.05), after the basic assumptions of analysis variance (normality, homoscedasticity, additivity, and independence of residuals) were attained, and the significance of the analysis of variance (ANOVA) was reached (p<0.05). The dataset of maize cultivation (biomass production, nutrient accumulation, and whole soil) was also compared by the Duncan test (p<0.05), after ANOVA showed a significant difference (p<0.05), and the basic assumptions of analysis variance attended; however, the dataset of soil solution was analyzed as a split-plot experiment design (4 times of soil solution collection×6 fertilizer treatments).
In the kinetics of P release studies, different nonlinear models were adjusted to explain the P released for each fertilizer (Liang et al. 2014; Lustosa Filho et al. 2017). The chosen mathematical model of the kinetics of P released was based on the highest value of the coefficient of determination (R2), the lowest value of root-mean-square error (RMSE), and the Akaike information criterion (AIC) (Akaike 1979). Mathematical models adjusted to the kinetics of P release of each fertilizer were compared by a confidence interval generated through a 95% bootstrap confidence interval using 1000 bootstrap interactions. The mathematical models tested were the Elovich model (Eq. 5), the simple exponential model (Eq. 6), the power function (Eq. 7), and the hyperbolic model (Eq. 8).
where Pt, fraction of P released from fertilizer in each leaching time evaluated; a, initial content of P released from fertilizer; b, P release rate constant for each fertilizer; t, leaching nutrient release time (hour); N0, maximum amount of P released from fertilizer during the whole kinetics study.
The dataset from plants cultivated without NPK fertilization was removed from the whole dataset, and then, the maize biomass production and NPK accumulated in maize shoot were correlated with the respective available contents of nutrients in the whole soil and soil solution through the Pearson correlation matrix (p<0.05).
3 Results
3.1 Fertilizer Characterization
The BBFs had a lower (~32%) proportion of mineral N (ammonium+nitrate) according to total N content compared with mineral fertilizers. All BBFs had a lower proportion of P soluble in water and FA as related to fertilizer total P content compared with TSP. Compared with TSP, BBF1, BBF3, and BBF4 reduced the soluble P in CA (−32%) and NAC+H2O (−31%). Similar proportions of soluble P in CA and NAC+H2O according to total P content were observed for BBF2 and TSP. The soluble contents of K in water and in CA (% of total K in fertilizer) were lower for BBFs compared with KCl, on average, a reduction of 23% and 26%, respectively. However, the soluble contents of K in NAC+H2O were only reduced in BBF4 (−11%) compared with KCl, while BBF1, BBF2, and BB3 had a similar K soluble content in NAC+H2O compared with KCl (Table 4).
3.2 Infrared Spectroscopy
Infrared spectra of raw coffer residue biochars used in the BBF synthesis showed peaks related to OH stretching, C–H stretching of aliphatic groups, COO- carboxylate anions, C–H deformation from CH2 groups, C–H bending, OH (phenolic groups), and C–O stretching (Supplementary Figure S1A). Araxá phosphate rock showed peaks related to CO3 groups and P–O bonds (Supplementary Figure S1B). Infrared spectra of BBFs had peaks related to OH stretching (3415 cm-1) and carbon groups assigned as C–H stretching of aliphatic groups (2905 cm-1), C–H deformation from CH2 groups (1380 cm-1). It was also assigned for BBFs groups related to N–H bonds (3240 cm-1), N–H flexural acid amides (1634 cm-1), and NO3 asymmetric stretching (1340 cm-1). Phosphorus peaks found in BBFs spectra were related to P–O bonds (740, 820, 950, and 1040 cm-1), P=O bonds (1235 cm-1), ionic bond P+-O− in the structure of acid phosphate esters (1080 cm-1) and P–H in organophosphorus groups (2353 cm-1) (Fig. 1). The peaks observed and assigned for organic and mineral compounds in all BBFs were similar; however, the intensity of the peaks varied among BBFs.
Compared with the raw biochar used in the BBF synthesis, the spectra of BBFs reduced the intensity of aliphatic groups. Peaks related to COO-carboxylate anions and OH from phenolic groups verified in biochar samples were not recorded in the spectra of BBFs. The differences in BBFs spectral signature were related to the intensity of NO3 asymmetric stretching peaks in the following decreasing order: BBF3 > BBF2 > BBF4 > BBF1. The increased absorbance in the FTIR range between 1500 cm-1 and 1740 cm-1 was assigned to N–H flexural acid amides in replacement for NO3 asymmetric stretching. Peaks related to P+-O− in acid phosphate esters and P=O bonds increased in BBFs as follows: BBF3 > BBF4 = BBF2 > BBF1. Peaks related to P–O groups were higher in BBF1 than in the other BBFs. However, peaks related to P–H organophosphorus and aliphatic carbon groups were higher in the BBF1 and BBF4 spectra compared with the FTIR spectral signatures of BBF2 and BBF3.
3.3 BBFs Agronomic Value
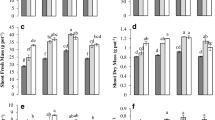
The pH of soil is shown in Supplementary Figures S3a and S3b, and the main difference was verified at 25 days of cultivation in soil solution, when BBFs-treated soils had a higher pH in soil solution compared with mineral fertilization. The no application of fertilizers reduced the contents of NPK in the whole soil and its soil solution (Fig. 2a-e), and consequently, there was a reduction of SDM, RDM, and TDM and an accumulation of NPK in the shoot (Fig. 3). Among the fertilizers used, BBF4 had a higher content of mineral N in the whole soil (Fig. 2a), and BBF3 had the highest available content of K (Fig. 2e). The available contents of soil P resin (Fig. 2c) increased by applying BBF1 (+28%), BBF3 (+22%), and BBF4 (+7%) compared with TSP. The available contents of P after maize cultivation were higher for BBF1 (+29%), BBF2 (+15%), and BBF3 (+43%) compared with mineral fertilizers. Compared with BBFs, the use of KCl increased (+79%) the content of K in the whole soil after maize cultivation.
Contents of mineral N (N-NH4++ N- NO3-) (a) and available contents of phosphorus (P) (c) and K (c) in the whole soil before and after the maize cultivation; and contents of mineral N (b), P (d), and K (e) in soil solution at different times of collect of soil solution as function of fertilizer treatments. In the whole soil ((a), (c), and (e)), the bars with standard error followed by the same uppercase letter mean did no differ fertilizer treatments regarding in the whole soil evaluated before or after cultivation according to the Duncan test (p<0.05). In the soil solution ((b), (d), and (f)), the bars with standard error followed by the same uppercase letter mean did no differ fertilizer treatments regarding in soil solution collected at 1, 7, 15, and 25 after maize sowing according to the Duncan test (p<0.05) and the bars with standard error followed the same lowercase letter did not differ the time evaluated in soil solution in each fertilizer treatment tested according to the Duncan test (p<0.05). BBF: biochar-based fertilizer; +NPK: NPK added via mineral fertilizers; -NPK: maize cultivation without NPK application
Biomass production (a) and accumulation of nitrogen (N) (b), phosphorus (P) (c), and potassium (K) (d) in the maize shoot. The bars with standard error followed by the same bold italic, uppercase, and lowercase letter did not differ the fertilizer treatments, respectively for total (full bar), shoot, and root dry matter production according to the Duncan test (p<0.05). The bars with standard error followed by the same uppercase letter did not differ the fertilizer treatments for accumulation of N or P or K in the maize shoot according to the Duncan test (p<0.05). BBF: biochar-based fertilizer; +NPK: NPK added via mineral fertilizers; -NPK: maize cultivation without NPK application
In the soil solution phase, among BBFs, BBF4 increased the N in soil solution at 1 (+24%) and 7 (+20%) days (Fig. 2b) as well as the K in soil solution (Fig. 2f) at 1 day (+23%) and BBF3 at day 7 (+20%). The K in the soil solution at 15 days was higher for mineral fertilization (+25%) compared with BBFs. BBFs, compared with TSP at 1 and 7 days, initially released a lower content of P in the soil solution (Fig. 2d), an average reduction of 85%. However, at 15 days, compared with TSP, the contents of P in soil solution were similar for BBFs, but at 25 days, some BBFs (BBF1, BBF2, and BBF3) had higher contents in soil solution, an average increase of 105%.
Some biochar-based fertilizers (BBF1, BBF3, and BBF4) increased ~24% SDM, compared with TSP (Fig. 3a). All BBFs in comparison with TSP increased TDM (BBF1 +25%, BBF2 +16%, BBF3 +33%, and BBF4 +25%) and RDM (an average of +37%). The accumulation of N in shoot did not differ between BBFs and TSP (Fig. 3b). However, P in maize shoot was higher for all BBFs compared with TSP (Fig. 3c), with a higher accumulation of P for BBF3, an increase ~46%, compared with TSP. The K accumulated in maize shoot was similar for BBF3, BBF4, and TSP-fertilized plants (Fig. 3d); this group increased ~35%, compared with the average of BBF1 and BBF2.
3.4 Kinetics of P Release
In the study of the kinetics of P release, the equivalent amount used for maize cultivation was 110 mg P per pot based on the contents of P soluble in NAC+H2O; thus, the total P content applied was different among fertilizers, and the total contents of P by mini-lysimeter were 151.0, 111.4, 179.4, 148.8, and 114.6 mg, respectively, by BBF1, BBF2, BBF3, BBF4, and TSP. The power model was the best model fitted for P released in all fertilizers using water as an extractor solution (Supplementary Table S2). For the contents of P released in CA, the power model was the best model fitted to BBF2, BBF4, and TSP, while the hyperbolic model was the best model fitted to BBF1 and BBF3 (Supplementary Table S2).
Triple superphosphate released the highest contents of P in water (Fig. 4a) compared with BBFs. In TSP, about 81.2 mg of P was released up to 12 h, with a constant release rate from 12 h to 264 h, represented ~71% of the total content of P added to mini-lysimeters (Fig. 4a). Among BBFs, at the end of the kinetics study (264 h), BBF4 and BBF2 had similar patterns of P released in water leachates, close to 74 mg P, equivalent to 67% and 49% of the total content of P added, but P from BBF2 were gradually released compared with BBF4. The P released in water by BBF2 and BBF4 was lower compared with TSP and other BBFs. Initially, BBF1 and BBF3 had similar contents of P released in water; however, after 24 h, BBF1 had a higher release of water; thus, at 254 h, BBF1 released 31.5 mg (21% of the total P added) and BBF3 released 26.7 mg (15% of the total P added).
Compared with TSP, BBF2, and BBF4, the kinetics of P release in CA (Fig. 4b) showed that BBF1 and BBF3 initially released the lowest contents of P in CA; however, at 264 h, they had higher contents of P released, reaching 129.3 mg (86% of the total P added) and 135.0 mg (75% of the total P added). Between TSP, BBF2, and BBF4, initially, the contents of P released in CA were similar (~45.1 mg P), whereas, as the time of the kinetics study evolved, TSP released P at a faster pace and in higher contents than BBF2 and BBF4, reaching values close to 112.6 mg (98% of the total P added) up to 264 h. Despite the faster P initial release by BBF2 and BBF4, both BBFs had the lowest contents of P released in CA, up to 264 h.
3.5 Pearson’s Correlation Analysis
The properties and forms of N in the whole soil and soil solution had no correlation with N accumulated in maize shoot (Fig. 5a), and the K accumulated in maize shoot only was correlated with K forms added to the whole soil (fertilizer total K content and fertilizer soluble K pools in water, CA, FA, and NAC+H2O as well). However, the maize dry matter production (SDM, RDM, and TDM) was not correlated with the N (Fig. 5a) or K (Fig. 5c) accumulated in maize shoot (p>0.05). The dry matter production was correlated with P accumulated in maize shoot (p<0.05), with coefficients of correlation of 0.85, 0.73, and 0.85, respectively, for SDM, RDM, and TDM. The accumulation of P in maize shoot was positively correlated with amounts applied of fertilizer-soluble P soluble in CA, available resin P content before or after maize cultivation, as well as with the P content in soil solution at 25 days of cultivation (Fig. 5b). In addition, there was a negative correlation between the accumulation of P in maize shoots and variables that indicate the amounts of P rapidly available, such as the contents of P in solution at 1 and 7 days of maize cultivation, water-soluble P in fertilizers, amounts of P released in water in the kinetics study, as well as the amounts of P released in CA leachates at 0, 4, and 12 h.
Correlation analysis between nitrogen (N) or phosphorus (P) or potassium (K) in maize shoot and nutrients forms applied, as well as with the availability of N, P, and K in the whole soil and in soil solution. T-N-Ap. and N-Min-Ap.: amounts of total and mineral N forms applied during maize cultivation, respectively; NH4+-Sol.1, NH4+-Sol.7, NH4+-Sol.15, and NH4+-Sol.25: ammonium contents in soil solution at 1, 7, 15, and 25 days during maize cultivation, respectively; NO3--Sol.1, NO3--Sol.7, NO3--Sol.15, and NO3--Sol.25: nitrate contents in soil solution at 1, 7, 15, and 25 days during maize cultivation, respectively; N-min-Sol.1, N-min-Sol.7, N-min-Sol.15, and N-min-Sol.25: mineral content of N in soil solution at 1, 7, 15, and 25 days during maize cultivation, respectively; NH4+-Soil Bef. and NH4+-Soil Aft.: ammonium contents in the whole soil before or after maize cultivation, respectively; NO3--Soil Bef. and NO3--Soil Aft.: nitrate contents in the whole soil before or after maize cultivation, respectively; N-min-Soil Bef. and N-min-Soil Aft.: mineral contents of N in the whole soil before or after maize cultivation, respectively; pH-Sol.1, pH-Sol.7, pH-Sol.15, and pH-Sol.25: pH in soil solution at 1, 7, 15, and 25 days during maize cultivation, respectively; pH-Soil Bef. and pH-Soil Aft.: pH in whole soil before and after maize cultivation, respectively; T-P-Ap., P-W-Ap., P-CA-Ap., and P-FA-Ap: amounts of total P and P soluble in water, citric acid, and formic acid applied during maize cultivation, respectively; P-Kin.W-0, P-Kin.W-4, P-Kin.W-12, P-Kin.W-24, P-Kin.W-48, P-Kin.W-72, P-Kin.W-120, P-Kin.W-168, P-Kin.W-216, and P-Kin.W-264: amounts of P released in water in kinetics study at 0, 4, 12, 24, 48, 72, 120, 168, 216, and 264 h, respectively; P-Kin.CA-0, P-Kin.CA-4, P-Kin.CA-12, P-Kin.CA-24, P-Kin.CA-48, P-Kin.CA-72, P-Kin.CA-120, P-Kin.CA-168, P-Kin.CA-216, and P-Kin.CA-264: amounts of P released in citric acid in kinetics study at 0, 4, 12, 24, 48, 72, 120, 168, 216, and 264 h, respectively; P-Sol.1, P-Sol.7, P-Sol.15, and P-Sol.25: content of P in soil solution at 1, 7, 15, and 25 days during maize cultivation, respectively; P-Soil Bef. and P-Soil Aft.: available contents of P in the whole soil before or after maize cultivation, respectively; T-K-Ap., K-W-Ap., K-NAC-Ap., K-CA-Ap., and K-FA-Ap: amounts of total K and K soluble in water, neutral ammonium citrate plus water, citric acid, and formic acid applied during maize cultivation, respectively; K-Sol.1, K-Sol.7, K-Sol.15, and K-Sol.25: contents of K in soil solution at 1, 7, 15, and 25 days during maize cultivation, respectively; K-Soil Bef. and K-Soil Aft.: available contents of K in the whole soil before or after maize cultivation, respectively
4 Discussion
The production of biochar from coffee postharvest residues is a way to generate BBFs with pH values in the alkaline range (Lago et al. 2021). However, due to the apatite acidulation and use of it in the OMFs synthesis, the pH of BBFs ranged from 2.32 to 3.52. The production of BBFs with reduced pH was already verified for the use of phosphoric acid previously in biochar production and BBF synthesis, compared with pyrolysis and BBF production without feedstock acidulation (Lustosa Filho et al. 2017). Fertilizer pH controls the dissolution and reactions of nutrients in the soil-fertilizer interface (Guelfi et al. 2022), and high pH and the presence of Ca in BBFs are conditions that increase the chemical stability of apatite and the formation of Ca-phosphate precipitates in soil, reducing the availability of P in soils to crops (Maluf et al. 2018). However, in our BBFs, controlling the BBF's final pH was a suitable strategy to reduce Ca precipitation with phosphates.
The synthesis of novel BBFs created fertilizers with different proportions of mineral N (47–90%) and organic N (10–53%) forms, depending on the route adopted. Mineral N was the main source of N forms used in BBF synthesis. However, part of the mineral N was converted to organic N linked to the biochar structure, with subsequent formation of N–H from acid amide organic forms, as demonstrated by infrared spectroscopy analysis. Amides from biochar can generate NH4+ from amine and amide hydrolysis as the mineralization process advances. Thus, the N present in BBFs in minerals and organic forms controls the capacity of BBFs to supply and release N to plants (Phillips et al. 2022). In BBFs, N in mineral forms supplies N to growing crops at a fast pace, while organic N pools, when mineralized by microorganisms, supply N to crops throughout the plant cultivation growth stages (Phillips et al. 2022).
Regarding the P pools, in our BBFs, it was possible to reduce the proportion of fertilizer-P soluble in water (11–76%) compared with TSP, whereas the formulated BBFs maintained a high proportion of P-soluble NAC+H2O, CA, and FA in the novel fertilizers. The P solubilized during the acidulation process of APR (apatite) reacted with C compounds from biochars and reduced P solubility in water. In the studies carried out by Lustosa Filho et al. (2017, 2019) and Suwanree et al. (2022), different sources of P, such as TSP or monoammonium phosphate or phosphoric acid, were mixed with different feedstocks previously to pyrolysis, with the subsequent production of BBFs with reduced levels of P soluble in water in fertilizers while maintaining an optimum amount of P released in the long term (P soluble in CA or NAC+H2O or FA). The decrease in fertilizer P soluble in water and the high levels of P soluble in citric acid are effective strategies to improve the agronomic efficiency of biochar and humic substance-based P fertilizers (Maluf et al. 2018). On the contrary, the high levels of water-soluble P-free forms in fully acidulated mineral fertilizer tend to be specifically adsorbed or precipitated in tropical soils (Raniro et al. 2023; Withers et al. 2018).
In addition, the positive interaction of C and P in OMFs, with the subsequent formation of metal-phosphate-humic substance complexes, is a suitable strategy to improve the agronomic efficiency of P fertilizers (Erro et al. 2012). The BBFs formulated in this study mirror the positive properties of the high agronomic value of the P fertilizers synthesized by Erro et al. (2012) when the P solubilized from APR was incorporated into the biochar matrix, the formation of organic P chemical species was verified, i.e., the appearance of peaks related to P–H organophosphorus and ionic bond P+-O− in acid phosphate esters recorded in the FTIR spectra; these peaks were also reported from P-BBFs formulated by Lustosa Filho et al. (2017).
The production of BBFs by mixing NPK mineral sources, coffee organic residues, and acidulated apatite can trigger a more gradual K release, as observed by a reduction in the amounts of K in water leachates, which may increase the use efficiency due to the positive interaction of K with biochar negative charges and the potential diffusion of K into the pores of the biochar matrix (Piash et al. 2022). A more gradual release of K linked to the biochar matrix mimics the process commonly found in soils of retention and gradual release of K to crops. Thus, the development of negative charges on the biochar surface and in the soil itself increases the cation exchange capacity (CEC) and enhances the K retained in the charred matrices by electrostatic bonds.
Considering that N and K supplied by mineral sources were added to the whole soil at the maize sowing (20%) and at 13 days after maize sowing (80%), the N availability was initially higher in BBFs-treated soils compared with N mineral fertilizers; however, the effects of BBFs on N availability in the whole soil and in soil solution did influence the N in maize shoot and N accumulation by plants, which was similar for BBFs and NPK from mineral fertilizers and not explained (low correlation) with amounts of N applied to soils by fertilizers.
In line with Barbosa et al. (2022), a mixture of N-urea with biochar confirmed the interaction of mineral N and biochar due to the interaction and formation of primary amides and amide carbonyls as demonstrated by infrared spectroscopy analysis. Despite the interaction, the N supplied via BBF did not increase N in the shoot in the first common bean cultivation, mirroring the results reported in this study. However, for crops successively grown after maize, the N-BBFs increased the accumulation of N in maize shoot over the exclusive use of urea. The biochar-based N fertilizers formulated with urea show a longer N residual effect than that produced from the exclusive use of urea, which was explained by the gradual N release pattern of BBFs (Barbosa et al. 2022). When N interacts with biochar, N is adsorbed (ammonium) in the negative charges of organic radicals found on biochar surfaces, which can decrease the rate of N release (N kinetic pattern). This specific and gradual release of N by biochar is supposed to increase plant growth and the efficiency of N use by crops over soluble N fertilizers, with an increase in soil residual N (Shi et al. 2020).
In addition to being adsorbed by electrostatic bonds, N is found in biochar in different organic forms, whose mineralization rate is governed by biotic (decomposer activity) and abiotic factors, i.e., soil type (Phillips et al. 2022). Considering the short duration of our experiment (25 days) and the high amounts of N prevailing in BBFs in organic forms over time, BBF1 and BBF2 are more prone to mineralize N (increased residual N effect) for crops successively cultivated after maize. For this reason, further sequential incubation studies should be performed to assess the potential of BBFs to supply and nourish crops with N in the medium and long term.
The K supplied by BBFs was gradually released in the soil, reducing K leaching while improving soil K availability and the use efficiency of K by crops, which decreased the K rate required in topdressing fertilization (Fachini et al. 2021, 2022; Gwenzi et al. 2018; Piash et al. 2022). However, in our study, a more gradual release of K from BBFs on soil K availability and maize nutrition was not verified. The mechanisms involved in the gradual release of K by BBFs are the following: (i) K retention due to electrostatic attraction in negatively charged functional groups of the biochar matrix, such as carboxyl and phenolic groups; (ii) physical protection and diffusion of K inside the biochar micropores; and (iii) the hydrophobic nature of biochar, which decreases water diffusion and, consequently, K dissolution (Fachini et al. 2022; Hossain et al. 2020). For this reason, the use of biochar-based K fertilizers in topdressing fertilization cannot be ruled out. Instead of powder, C-based K fertilizers should be added to soil in granular or, in some cases, pellet form to decrease the rate of K release and improve the uptake of K by crops (Fachini et al. 2022).
Potassium accumulated in maize shoot did not govern the growth of maize plants, considering that K added by fertilizers did explain the K in shoot and maize dry matter production. The lack of a relationship between K in the shoot and maize biomass is partially explained by the relationship between K concentration in the plant tissue and plant growth. If K in the plant leaf is above 50 g kg-1 K (Marschner 2012), increasing the K concentration in the leaf does not translate into higher crop growth. In this study, the K concentration in maize tissue ranged from 88 to 174 g kg-1. Although the availability of K does not explain the accumulation of K in shoot, after maize cultivation, the KCl fertilization splitting practice effectively supplied the K required by maize plants and to the plant and promoted a higher residual effect over the K furnished to plants by BBFs and fully added to soil in a single application at sowing fertilization. Thus, the practice of splitting K improved the efficiency of K use by maize plants. In cropland areas, K is added to soil at sowing and in plant stages in which the root system is abundantly developed, which favors the uptake of K by crops while reducing K leaching in soils (Torabian et al. 2021).
In tropical soils, there is a generalized natural deficiency of NPK and micronutrients that sharply limits crop production in Cerrado soils (Lopes and Guilherme 2016). In our study, no NPK was used, which drastically reduced maize, and the addition of NPK in the maize fertilization improved maize biomass. Soil P availability was the factor governing maize growth, mainly the P contents in the soil solution. Maize SDM, RDM, and TDM were positively correlated with P in the shoot. Use of all BBFs leads to greater maize TDM, RDM and P, N and K in maize shoot than single NPK mineral fertilizers. An improved P acquisition by crops fertilized with BBFs encloses many mechanisms, including (i) the positive effect of BBFs on soil resin P availability; (ii) complexation of P by organic functional groups in biochar; (iii) reduction of P adsorption and phosphate precipitation with cations, notably calcium, in soil solution; (iv) reduction of adsorption in phosphate fixation sites of colloids found in tropical soils; (v) competitive reactions between the P and C organic radical soluble C compounds released by biochar can compete with phosphate for sorption sites in colloids of tropical soils; and (vi) a more gradual release of P from BBFs, which improves the use efficiency of P by crops (An et al. 2021; Bai et al. 2022; Carneiro et al. 2021; Ghodszad et al. 2021; Johan et al. 2021). Concerning soil P-resin content, compared to the NPK mineral sources, the BBFs increased the soil P-resin levels, with greater availability of P in soils treated with BBF1 and BBF3, compared with the other BBFs.
Phosphorus availability is enhanced in biochar-treated soils, and consequently, the uptake and use efficiency of P by crops are improved (Bai et al. 2022). In this study, the increase in P-resin levels in BBF-treated soils positively correlated with P in maize shoot. Additionally, a more gradual release of P by BBFs promoted greater levels of residual P in soils successively cultivated with different crops compared to that of plants nourished with mineral phosphate-soluble fertilizers (Carneiro et al. 2021). In the same direction, the BBFs promote a greater accumulation of P in maize shoot, and a higher residual P (resin P) in BBF1, BBF2, and BBF3 compared with TSP.
A more gradual release of P by BBFs is related to the reduction in the amounts of soluble P in water in fertilizers or the decreased release of P in water leachates, as previously reported (Carneiro et al. 2021; Lustosa Filho et al. 2017; Suwanree et al. 2022). Compared to TSP, our BBFs produced a more gradual release of P, reducing readily available P in water. The reduction in the amounts of readily available and fertilizer-water-soluble P content, the decreased amounts of P released in water throughout the kinetics study (0 to 264 h), and the reduction in the amount of P released in CA leachates explain the positive correlation of fertilizer-P solubility indices, soil-available P content, and P in maize shoot.
Among BBFs, the highest P in maize shoot was verified for plants fertilized with BBFs. A more gradual release of P from BBFs reduced the initial contents of P in soil solution at 1 and 7 days, while TSP rapidly released P into the soil solution, compared with BBFs. Contrary to TSP, BBFs gradually released P into the soil solution in the later stages of maize growth, in which P is more required by roots (25 days after maize sowing). Thus, a higher concentration of P in solution at 25 days after maize cultivation observed in BBF-treated soils explains the maize P nutritional status and growth. High-soluble and fully acidulated P fertilizers promoted a rapid increase in P concentration in soil solution; over time, these high amounts of readily available P to crops precipitate or are specifically adsorbed in soil colloids, decreasing P available levels in whole soil and solution (Johan et al. 2021). Thus, the slow release of P by BBFs is a suitable strategy to reduce P fixation through specific adsorption or precipitation in highly weathered soils, improving soil P available levels and acquisition by crops (Bai et al. 2022; Frazão et al. 2019; Ghodszad et al. 2021).
Phosphorus readily available in water and in CA leachates are the pools of fertilizer-P acquired by crops (Guelfi et al. 2022; Morais et al. 2023). Thus, a suitable strategy to furnish P to crops involves the combined use of water-P fertilizer content, slow release of P in water leachates (P-kinetic study), and high P in CA leachates of BBFs. Phosphorus availability is limited in high P-fixing soils, such as Oxisols, due to the formation of covalent bonds between oxy-hydroxides of iron and aluminum and phosphate in Oxisol. Overall, P rates added to highly weathered soils are based on NAC+H2O for fully soluble P sources, while CA-soluble P content is the main factor governing the definition of low-grade phosphate rock rates for crops.
Some microorganisms and plants exudate organic acids that can sequester Ca from low-grade P chemical species, which can increase the solubilization of phosphate (Johan et al. 2021). Citric acid is able to simulate the capacity of plants to mobilize P from sparing phosphates; thus, the use of CA and water is a suitable protocol to assess both readily available and gradually released P by BBFs. Overall, both CA and water-soluble P in BBFs are key indices to predict the capacity of charred matrices to supply P to crops in the short and medium term. These fertilizer P solubility indices can be employed to predict the agronomic value of BBF, regardless of their capability to nourish crops with P (Morais et al. 2023).
In addition to the fertilizer-P solubility index, the change in the soil solution P levels, which increased in BBF-treated soils over TSP, explained part of the variation in P in maize shoot. The role played by BBFs in increasing soil pH before cultivation was demonstrated by Lustosa Filho et al. (2019), who found that a decreased soil acidity degree did not improve the P nutrition in maize plants, contrary to the results reported in this study. Pyrolysis usually results in the production of biochars with a pH in the alkaline range; thus, depending on the rate and liming value, biochars significantly increase soil pH (Bai et al. 2022; Domingues et al. 2017; Ghodszad et al. 2021; Johan et al. 2021). However, due to the specific synthesis routes and stoichiometric ratio of strong acid:apatite, the BBFs produced and agronomic tested in this work showed pH values close to those adequate for plentiful plant growth in most soils; thus, the effect of BBFs in correcting soil acidity was limited due to the low BBFs pH itself. Biochar acts as a buffer in the growth medium, which is explained due to the presence of organic functional groups and compounds capable of complexing toxic Al, buffering soil acidity, decreasing toxic Al activity, and generating organic metallic complexes in soils (Domingues et al. 2017; Ghodszad et al. 2021; Johan et al. 2021). Regarding the soil solution pH, the BBFs showed a lower variation in the active acidity than that of TSP-treated soils, indicating that the soil acidity was possibly buffered in response to the BBF use in the maize fertilization. As the soil solution pH decreases, soil P availability is reduced due to a greater rate of phosphate specific adsorption and precipitation of P with Fe and Al (hydro)oxid es in tropical soils (Fink et al. 2016).
Thus, synthesized BBFs influenced P nutrition and increased maize biomass production possibly through different mechanisms, such as (i) interaction between P and the biochar organic functional groups, with subsequent formation of organic-metal-P-complexes, in which the presence in BBFs was demonstrated by the FTIR analysis; (ii) increase of readily available P in soil solution and water-soluble P in BBFs was effective in keeping suitable amounts of P throughout the maize cultivation in different plant growth stages; (iii) gradual release of P from BBFs; (iv) reduction in the initial concentration of P in soil solution with subsequent release remaining P in BBFs, notably in maize growth stages of a greater P nutritional requirement; (v) increase in the initial availability of P (resin P) in whole soil; and (vi) higher buffering of soil acidity, i.e., pH control in soils treated with BBFs, which possibly contributed to maintaining P in available forms in different maize growth stages.
Study limitations and prospects BBFs are characterized by the gradual release of NPK, and the proposed novel cultivation method made it possible to sample the soil solution throughout the maize cultivation. Thus, this approach is promising for other experiments because the release of NPK can be simultaneously determined in soil solution as maize growth proceeds in real time. However, the approach was developed for short-term plant growth; thus, some adaptations must be made for long-term plant growth in different soil and cultivation scenarios. The newly synthesized BBFs are promising for reducing N and K topdressing, considering that all N and K were added to the soil in a single fertilization operation at crop sowing fertilization. Regarding agronomic effectiveness, BBFS had the same efficiency in supplying N and K than split N and K added to soil by mineral fertilizer sources.
In addition, the method ensures a higher P use efficiency in crops fertilized with BBFs compared with soluble NPK mineral sources. Therefore, it is necessary to test the agronomic value of BBFs in crop field conditions. Regarding the BBF spectral signature, the presence of C groups in the aliphatic form implies that BBFs contain organic compounds that may act as plant biostimulants. Carbon in aliphatic groups triggers the activity of plasma membrane H+-ATPase in response to the use of humic substance-based fertilizers (Nardi et al. 2021). Plasma membrane H+-ATPase is the initial pump that triggers cell membrane potential in plants; thus, it contributes to improved nutrient acquisition by crops and an improved soil microbiome (Nardi et al. 2021). Therefore, further studies must be carried out to elucidate the role played by BBF bioactive molecules, i.e., water-soluble C content and chemical species, in triggering plant physiological and biochemical processes and the production of exudates by crops and the microbiome in BBF-treated soils as well.
5 Conclusions
In the synthesis of biochar-based fertilizers (BBFs) through nitric acid acidulation of phosphate rock and mixture with biochar, there was an interaction of nitrogen (N) and phosphorus (P) with the biochar matrix. The BBFs had a more gradual release due to a lower proportion of P and potassium (K) soluble in water, but they maintained the high proportion of nutrients soluble over a long period of time. A kinetic study was performed and revealed that triple superphosphate released nearly all the total P, and BBFs released less than 10% of the total P determined after the first 24 h. Biochar-based fertilizers, depending on the synthesis route and the amount of acid used in the apatite solubilization, lead to attend a greater or similar maize biomass, relative agronomic effectiveness, and higher phosphorus acquisition than NPK supplied via soluble mineral fertilizers. When compared with triple superphosphate, BBFs were capable of slowing the release of phosphorus in water leachates while simultaneously releasing high levels of P in citric acid leachates. Phosphorus in the soil solution was quickly released in the mineral fertilizer, while the release of P in the soil solution was low and regular by BBFs. After NPK mineral sources were applied to soils, BBFs effectively increased the levels of resin P in the soil after maize cultivation.
Data Availability
All data generated or analysed during this study are included in this published article [and its supplementary information files]. However, the raw dataset can be requested from the corresponding author on reasonable request.
References
Akaike H (1979) A Bayesian extension of the minimum AIC procedure of autoregressive model fitting. Biometrika 66:237–242. https://doi.org/10.1093/biomet/66.2.237
An X, Wu Z, Liu X, Shi W, Tian F, Yu B (2021) A new class of biochar-based slow-release phosphorus fertilizers with high water retention based on integrated co-pyrolysis and co-polymerization. Chemosphere 285:131481. https://doi.org/10.1016/j.chemosphere.2021.131481
Bai SH, Omidvar N, Gallart M, Kämper W, Tahmasbian I, Farrar MB, Singh K, Zhou G, Muqadass B, Xu C-Y, Koech R, Li Y, Nguyen TTN, van Zwieten L (2022) Combined effects of biochar and fertilizer applications on yield: a review and meta-analysis. Sci Total Environ 808:152073. https://doi.org/10.1016/j.scitotenv.2021.152073
Barbosa CF, Correa DA, da Carneiro JS, Melo LCA (2022) Biochar phosphate fertilizer loaded with urea preserves available nitrogen longer than conventional urea. Sustainability 14:686. https://doi.org/10.3390/su14020686
Baty F, Ritz C, Charles S, Brutsche M, Flandrois J-P, Delignette-Muller M-L (2015) A toolbox for nonlinear regression in R : the Package nlstools. J Stat Softw 66:1–21. https://doi.org/10.18637/jss.v066.i05
Brazil (2017) Manual de métodos analíticos oficiais para fertilizantes e corretivos. MAPA, Brasília
Bremner JM (1965) Total Nitrogen. In: Norman AG (ed) Methods of soil analysis: Part 3 chemical methods. American Society of Agronomy, Madinson, pp 1149–1178
Bremner JM, Keeney DR (1966) Determination and isotope-ratio analysis of different forms of nitrogen in soils: 3. exchangeable ammonium, nitrate, and nitrite by extraction-distillation methods. Soil Sci Soc Am J 30:577–582. https://doi.org/10.2136/sssaj1966.03615995003000050015x
Carneiro JSS, Andrade Ribeiro IC, Nardis BO, Barbosa CF, Lustosa Filho JF, Azevedo Melo LC (2021) Long-term effect of biochar-based fertilizers application in tropical soil: agronomic efficiency and phosphorus availability. Sci Total Environ 760:143955. https://doi.org/10.1016/j.scitotenv.2020.143955
de Morais EG, Silva CA, Jindo K (2023) Biochar-based phosphate fertilizers: synthesis, properties, kinetics of P release and recommendation for crops grown in Oxisols. Agronomy 13(2):326. https://doi.org/10.3390/agronomy13020326
Domingues RR, Trugilho PF, Silva CA, De Melo ICNA, Melo LCA, Magriotis ZM, Sánchez-Monedero MA (2017) Properties of biochar derived from wood and high-nutrient biomasses with the aim of agronomic and environmental benefits. PLoS One 12:e0176884. https://doi.org/10.1371/journal.pone.0176884
Enders A, Lehmann J (2012) Comparison of wet-digestion and dry-ashing methods for total elemental analysis of biochar. Commun Soil Sci Plant Anal 43:1042–1052. https://doi.org/10.1080/00103624.2012.656167
Erro J, Urrutia O, Baigorri R, Aparicio-Tejo P, Irigoyen I, Torino F, Mandado M, Yvin JC, Garcia-Mina JM (2012) Organic complexed superphosphates (CSP): physicochemical characterization and agronomical properties. J Agric Food Chem 60:2008–2017. https://doi.org/10.1021/jf204821j
Fachini J, de Figueiredo CC, Frazão JJ, Rosa SD, da Silva J, do Vale AT (2021) Novel K-enriched organomineral fertilizer from sewage sludge-biochar: chemical, physical and mineralogical characterization. Waste Manag 135:98–108. https://doi.org/10.1016/j.wasman.2021.08.027
Fachini J, de Figueiredo CC, do Vale AT (2022) Assessing potassium release in natural silica sand from novel K-enriched sewage sludge biochar fertilizers. J Environ Manage 314:115080. https://doi.org/10.1016/j.jenvman.2022.115080
FAO (2017) World fertilizer trends and outlook to 2020. In: Summary report. Food Agric Organ United Nations, p 38
Fink JR, Inda AV, Tiecher T, Barrón V (2016) Iron oxides and organic matter on soil phosphorus availability. Ciência e Agrotecnologia 40:369–379. https://doi.org/10.1590/1413-70542016404023016
Fox CL (1951) Stable internal standard flame photometer for potassium and sodium analyses. Anal Chem 23:137–142. https://doi.org/10.1021/ac60049a028
Frazão JJ, de Melo Benites V, Ribeiro JV, Pierobon VM, Lavres J (2019) Agronomic effectiveness of a granular poultry litter-derived organomineral phosphate fertilizer in tropical soils: soil phosphorus fractionation and plant responses. Geoderma 337:582–593. https://doi.org/10.1016/j.geoderma.2018.10.003
Ghodszad L, Reyhanitabar A, Maghsoodi MR, Asgari Lajayer B, Chang SX (2021) Biochar affects the fate of phosphorus in soil and water: a critical review. Chemosphere 283:131176. https://doi.org/10.1016/j.chemosphere.2021.131176
Gmach MR, Cherubin MR, Kaiser K, Cerri CEP (2020) Processes that influence dissolved organic matter in the soil: a review. Sci Agric 77:e20180164. https://doi.org/10.1590/1678-992x-2018-0164
Guelfi D, Nunes APP, Sarkis LF, Oliveira DP (2022) Innovative phosphate fertilizer technologies to improve phosphorus use efficiency in agriculture. Sustainability 14:14266. https://doi.org/10.3390/su142114266
Gwenzi W, Nyambishi TJ, Chaukura N, Mapope N (2018) Synthesis and nutrient release patterns of a biochar-based N–P–K slow-release fertilizer. Int J Environ Sci Technol 15:405–414. https://doi.org/10.1007/s13762-017-1399-7
Hamner B, Frasco M (2018) Metrics: evaluation metrics for machine learning. R package version 0.1, 4, Available via https://www.rdocumentation.org/packages/Metrics/versions/0.1.4
Hoseini M, Cocco S, Casucci C, Cardelli V, Corti G (2021) Coffee by-products derived resources. A review. Biomass Bioenergy 148:106009. https://doi.org/10.1016/j.biombioe.2021.106009
Hossain MZ, Bahar MM, Sarkar B, Donne SW, Ok YS, Palansooriya KN, Kirkham MB, Chowdhury S, Bolan N (2020) Biochar and its importance on nutrient dynamics in soil and plant. Biochar 2:379–420. https://doi.org/10.1007/s42773-020-00065-z
IBGE (2021) Anuário Estatístico do Brasil, 81st edn. Instituto Brasileiro de Geografia e Estatística - IBGE, Rio de Janeiro
Janu R, Mrlik V, Ribitsch D, Hofman J, Sedláček P, Bielská L, Soja G (2021) Biochar surface functional groups as affected by biomass feedstock, biochar composition and pyrolysis temperature. Carbon Resour Convers 4:36–46. https://doi.org/10.1016/j.crcon.2021.01.003
Johan PD, Ahmed OH, Omar L, Hasbullah NA (2021) Phosphorus transformation in soils following co-application of charcoal and wood ash. Agronomy 11:2010. https://doi.org/10.3390/agronomy11102010
Kalra YP (ed) (1998) Handbook of reference methods for plant analysis. CRC Press, New York
Kitson RE, Mellon MG (1944) Colorimetric determination of phosphorus as molybdivanadophosphoric acid. Ind Eng Chem Anal Ed 16:379–383. https://doi.org/10.1021/i560130a017
Lago BC, Silva CA, Melo LCA, de Morais EG (2021) Predicting biochar cation exchange capacity using Fourier transform infrared spectroscopy combined with partial least square regression. Sci Total Environ 794:148762. https://doi.org/10.1016/j.scitotenv.2021.148762
Liang Y, Cao X, Zhao L, Xu X, Harris W (2014) Phosphorus release from dairy manure, the manure-derived biochar, and their amended soil: effects of phosphorus nature and soil property. J Environ Qual 43:1504–1509. https://doi.org/10.2134/jeq2014.01.0021
Lopes AS, Guilherme LRG (2016) A career perspective on soil management in the Cerrado region of Brazil. Adv Agronom 137:1–72. https://doi.org/10.1016/bs.agron.2015.12.004
Lustosa Filho JF, Penido ES, Castro PP, Silva CA, Melo LCA (2017) Co-pyrolysis of poultry litter and phosphate and magnesium generates alternative slow-release fertilizer suitable for tropical soils. ACS Sustain Chem Eng 5:9043–9052. https://doi.org/10.1021/acssuschemeng.7b01935
Lustosa Filho JF, Barbosa CF, Carneiro JSDS, Melo LCA (2019) Diffusion and phosphorus solubility of biochar-based fertilizer: visualization, chemical assessment and availability to plants. Soil Tillage Res 194:104298. https://doi.org/10.1016/j.still.2019.104298
Maluf HJGM, Silva CA, de Morais EG, de Paula LHD (2018) Is composting a route to solubilize low-grade phosphate rocks and improve map-based composts? Rev Bras Ciência do Solo 42:e0170079. https://doi.org/10.1590/18069657rbcs20170079
Marschner P (2012) Marschner’s mineral nutrition of higher plants. Elsevier, Oxford
Mumbach GL, Gatiboni LC, de Bona FD, Schmitt DE, Corrêa JC, Gabriel CA, Dall’Orsoletta DJ, Iochims DA (2020) Agronomic efficiency of organomineral fertilizer in sequential grain crops in southern Brazil. Agron J 112:3037–3049. https://doi.org/10.1002/agj2.20238
Munera-Echeverri JL, Martinsen V, Strand LT, Zivanovic V, Cornelissen G, Mulder J (2018) Cation exchange capacity of biochar: an urgent method modification. Sci Total Environ 642:190–197. https://doi.org/10.1016/j.scitotenv.2018.06.017
Nardi S, Schiavon M, Francioso O (2021) Chemical structure and biological activity of humic substances define their role as plant growth promoters. Molecules 26:2256. https://doi.org/10.3390/molecules26082256
Novais RF, Neves JCL, Barros NF (1991) Ensaio em ambiente controlado. In: Oliveira AJ, Garrido WE, Araújo JD, Lourenço S (eds) Métodos de pesquisa em fertilidade do solo. Embrapa-SEA, Brasília, pp 189–253
Phares CA, Atiah K, Frimpong KA, Danquah A, Asare AT, Aggor-Woananu S (2020) Application of biochar and inorganic phosphorus fertilizer influenced rhizosphere soil characteristics, nodule formation and phytoconstituents of cowpea grown on tropical soil. Heliyon 6:e05255. https://doi.org/10.1016/j.heliyon.2020.e05255
Phillips CL, Meyer KM, Garcia-Jaramillo M, Weidman CS, Stewart CE, Wanzek T, Grusak MA, Watts DW, Novak J, Tripe KM (2022) Towards predicting biochar impacts on plant-available soil nitrogen content. Biochar 4:9. https://doi.org/10.1007/s42773-022-00137-2
Piash MI, Iwabuchi K, Itoh T (2022) Synthesizing biochar-based fertilizer with sustained phosphorus and potassium release: co-pyrolysis of nutrient-rich chicken manure and Ca-bentonite. Sci Total Environ 822:153509. https://doi.org/10.1016/j.scitotenv.2022.153509
R Core Team (2020) R: A language and environment for statistical computing. Available via https://cran.r-project.org/bin/windows/base/
Raij BV, Quaggio JA, da Silva NM (1986) Extraction of phosphorus, potassium, calcium, and magnesium from soils by an ion-exchange resin procedure. Commun Soil Sci Plant Anal 17:547–566. https://doi.org/10.1080/00103628609367733
Raniro HR, Papera J, José LU, Valença RM, Pavinato PS, Hermann L, Santner J (2023) New investments in phosphorus research and training are paramount for Brazilian long-term environmental and food security. Environ Syst Decis 43:504–508. https://doi.org/10.1007/s10669-023-09903-4
Russel VL (2021) Emmeans: estimated marginal means, aka least-squares means. Available via https://cran.r-project.org/web/packages/emmeans/index.html
Shi W, Ju Y, Bian R, Li L, Joseph S, Mitchell DRG, Munroe P, Taherymoosavi S, Pan G (2020) Biochar bound urea boosts plant growth and reduces nitrogen leaching. Sci Total Environ 701:134424. https://doi.org/10.1016/j.scitotenv.2019.134424
Silva CA, Morais EG (2022) Fertilizante organomineral NPK de liberação controlada produzido a partir de compósito de rocha fosfatada-biocarvão. Brazil Patent No BR1020220127840. Brazilian National Institute of Industrial Property - INPI, Rio de Janeiro, RJ
Singh B, Camps-Arbestain M, Lehmann J (2017) Biochar: a guide to analytical methods. CRC Press, New York
Sparks DL, Page AL, Helmke PA, Loeppert RH (eds) (2020) Methods of soil analysis, Part 3: Chemical Methods, 14rd edn. New York, John Wiley & Sons
Suwanree S, Knijnenburg JTN, Kasemsiri P, Kraithong W, Chindaprasirt P, Jetsrisuparb K (2022) Engineered biochar from sugarcane leaves with slow phosphorus release kinetics. Biomass Bioenergy 156:106304. https://doi.org/10.1016/j.biombioe.2021.106304
Torabian S, Farhangi-Abriz S, Qin R, Noulas C, Sathuvalli V, Charlton B, Loka DA (2021) Potassium: a vital macronutrient in potato production-a review. Agronomy 11:543. https://doi.org/10.3390/agronomy11030543
Tumbure A, Bishop P, Bretherton M, Hedley M (2020) Co-pyrolysis of maize stover and igneous phosphate rock to produce potential biochar-based phosphate fertilizer with improved carbon retention and liming value. ACS Sustain Chem Eng 8:4178–4184. https://doi.org/10.1021/acssuschemeng.9b06958
United Nations Industrial Development Organization Staff (1998) Fertilizer manual. Kluwer Academic Publishers, Dordrecht
Wei T, Simko V (2017) R package “corrplot”: visualization of a correlation matrix. Available via https://cran.r-project.org/web/packages/corrplot/index.html
Wickham H, Averick M, Bryan J et al (2019) Welcome to the Tidyverse. J Open Source Softw 4:1686. https://doi.org/10.21105/joss.01686
Withers PJ, Rodrigues M, Soltangheisi A, De Carvalho TS, Guilherme LR, Benites VD, Gatiboni LC, De Sousa DM, Nunes RD, Rosolem CA, Andreote FD, de Oliveira A, ELM C, Pavinato PS (2018) Transitions to sustainable management of phosphorus in Brazilian agriculture. Sci Rep 8:2537. https://doi.org/10.1038/s41598-018-20887-z
Funding
This study received grants from the Coordination for the Improvement of Higher Education Personnel (CAPES) (CAPES-PROEX/AUXPE 593/2018), National Council for Scientific and Technological Development (CNPq) (303899/2015-8 and 307447/2019-7 grants), and the Foundation for Research of the State of Minas Gerais (FAPEMIG).
Author information
Authors and Affiliations
Contributions
Conceptualization: EGM and CAS; methodology: EGM and CAS; software: EGM; validation: EGM and CAS; formal analysis: EGM; investigation: EGM; resources: CAS; data curation: EGM; writing—original draft: EGM and CAS; writing—review and editing: EGM and CAS; visualization: EGM; supervision: CAS; project administration: CAS; funding acquisition: CAS.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
We understand that the corresponding author is the sole contact for the editorial process. He is responsible for communicating with the other authors about progress, submissions of revisions, and final approval of proofs.
We, the undersigned, declare that this manuscript is original, has not been published before, and is not currently being considered for publication elsewhere.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 472 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Morais, E.G., Silva, C.A. Novel Slow-Release NPK Biochar-Based Fertilizers with Acidulated Apatite: Evaluation of the Fertilization Value in a Short-Term Experiment. J Soil Sci Plant Nutr 23, 4937–4954 (2023). https://doi.org/10.1007/s42729-023-01494-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-023-01494-y