Abstract
Borage (Borago officinalis L.) is an oilseed that is being increasingly grown in areas where salinity affects yield. Silicon (Si) supplementation is considered a promising method to improve plant growth in saline conditions, however, there is currently very little information on the effect of Si application on the physiochemical properties of borage under saline conditions. The study aimed to elucidate if the degree of borage resistance to salinity is changed by Si through the physiochemical properties. We conducted a pot experiment to explore photosynthetic pigments, chlorophyll fluorescence parameters, osmolyte content, oxidative stress, and antioxidant response as influenced by increasing salinity levels and Si applications (priming, foliar spray, and priming × foliar spray). Salinity-stressed plants had a noticeable decline in chlorophyll a (56.6%) and b (64.2%), carotenoid content (57.5%), chlorophyll fluorescence parameters (Fv/Fm (7.2%), ETR (23.2%), and ΦPSII (15.2%)), and growth characteristics. Salinity increased oxidative damage through excessive production of malondialdehyde and increased electrolyte leakage in leaves. The salinity-stressed plants also showed high antioxidant activities of catalase and superoxide dismutase and increased accumulation of glycine betaine and soluble sugars compared to non-stressed plants. Si treatments resulted in a significant reduction in oxidative damage and increased antioxidant activity reducing lipid peroxidation and electrolyte leakage in the salinity-stressed leaves. The priming × foliar spray was the most effective at enhancing borage salinity tolerance. We, therefore, assumed that the investigated physicochemical properties could clearly describe the physiological context of salinity tolerance in borage and that Si could be used as a mediator of salinity tolerance and positively affect growth characteristics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Borage (Borago officinalis L.) is an herbaceous annual, belonging to the Boraginaceae family. Borage is grown for its seeds which contain almost 30–40% oil with a high percentage (22– 23%) of gamma-linolenic acid (GLA), an oil used for medicinal purposes (Montaner et al. 2022). Salinity, as one of the pre-eminent stresses facing global agriculture, is a challenge for borage growth and productivity, particularly in arid and semiarid areas (Jaffel et al. 2011), where salinity levels often surpass EC < 4 dS m−1 to reduce borage emergence (Feghhenabi et al. 2021). The response of plants to salinity is multifaceted and involves changes in plant morphology, physiology, and metabolism (Shalaby 2018). Salinity changes the ionic homeostasis and osmotic potential of the cell, which inhibits the ability of the plant to take up water as well as essential minerals and ions, such as K+ and Ca2+, which then reduces photosynthesis and growth (Subramanyam et al. 2019). Cereals exposed to salinity adjust their internal osmotic potential by compartmentalization of either synthesised osmolytes in the cytoplasm or inorganic ions in the vacuole to sustain water uptake (Isayenkov and Maathuis 2019). Compartmentalization of ions in the vacuole is a form of stress avoidance via active exclusion of toxic ions (Isayenkov and Maathuis 2019). When salinity levels exceed the plant’s capacity for compartmentalization, reactive oxygen species (ROS) are produced (Ahanger et al. 2020; Kohil et al. 2019; Mansoor et al. 2022).
Production of ROS adversely affects the cytoplasmic membrane, and cell metabolism, increasing electrolyte leakage (EL) and lipid peroxidation (Alkahtani et al. 2020; Ouzounidou et al. 2016). However, ROS are also considered to be involved in signaling cascades leading to increased antioxidative protection (Alkahtani et al. 2020). In the case of salinity stress, the superoxide radical (O2•−) was found to play a crucial role in signaling the NaCl-induced upregulation of antioxidative enzyme activities and stress tolerance (Kováčik et al. 2009). Among several protective enzymes, superoxide dismutases (SODs) remove superoxide radicals by catalyzing their conversion into hydrogen peroxide (H2O2), which in turn can be broken down by catalase (CAT), ascorbate peroxidase (APX), and guaiacol peroxidase (GPX) to yield oxygen and water (Ahanger et al. 2020; Kohil et al. 2019; Mansoor et al. 2022). Previous reports on quinoa, lettuce, basil, and alfalfa exposed to salinity have indicated increased activities of non-enzymatic and enzymatic antioxidant systems such as SOD and peroxidase catalase (Ouhibi et al. 2014; Panuccio et al. 2014; Tarchoune et al. 2010; Wang et al. 2009). In contrast in turnip, Noreen et al. (2010) reported that salinity substantially decreased the activities of SOD and peroxidase catalase. These contrasting responses demonstrate that plant salinity tolerance is a complex process and not exclusively associated with antioxidant capacity (Noreen et al. 2010). Additionally, addressing changes in biochemical properties and defense mechanisms can partially reflect important changes in plant growth parameters such as plant height, plant dry weight, and leaf area under abiotic stresses (Alkahtani et al. 2020).
The content of photosynthetic pigments is directly proportional to the plant’s photosynthetic rate (Sheteiwy et al. 2021). Salinity has been shown to decrease photosynthetic pigments in common beans (Taïbi et al. 2016). This is more likely due to the increased activity of the chlorophyll-degrading enzyme chlorophyllase which is caused by increased Na+ (Maghsoudi et al. 2016). The deficiency of magnesium caused by salinity is another reason for the reduction of chlorophyll (Łuczak et al. 2021). Reduced photosynthetic pigments cause inhibition of ribulose-1,5-bisphosphate degrading the photosynthetic apparatus (Liu et al. 2019). The destruction of photosynthetic pigments and the inhibition of cellular response and electron transport from PSI to PSII may also reduce Fv/Fm and PSII efficiency (Sheteiwy et al. 2021). Salinity also reduces photosynthetic O2 evolution and reduces fluorescence, which is associated with decreased activity of the PSII complex (Anjum 2011; Hernández 2019). The use of chlorophyll fluorescence analysis is an easy and robust technique used in plant physiology studies to provide valuable insights into the state of the PSII (Acosta-Motos et al. 2017).
Studies have shown that exogenous silicon (Si) applied as a foliar spray on sweet pepper leaves (Abdelaal et al. 2020) or seed priming in maize (Abdel Latef and Tran 2016) could alleviate the adverse effects of salinity through regulating antioxidant activities. Seed priming is thought to enhance salinity tolerance by increasing antioxidant enzyme activity and protecting cell membranes through decreased lipid peroxidation as well as improving the photosynthetic pigments and chlorophyll fluorescence (Abdelhamid et al. 2019; Paul et al. 2017).
To our knowledge, there are no studies investigating the effects of Si seed priming, foliar spray, or combination on antioxidant or chlorophyll dynamics in salinity-stressed borage. The objective of this study was to investigate the efficiency of different Si application techniques in reducing the side effects of salinity concerning the response trend of the physiological, biochemical, and growth traits of borage.
The results of this study will contribute to our understanding of (i) the response trend of physiological and biochemical traits of borage to different levels of salinity, (ii) the effect of different Si application techniques on the response trend of osmolyte content, chlorophyll fluorescence, and photosynthetic pigments,and (iii) the effect of Si application on oxidative stress and response trend of antioxidative enzymes.
2 Materials and Methods
2.1 Plant material and Experimental Design
Healthy and uniform-sized seeds of common borage (Borago officinalis) were prepared by PAKAN BAZR (Isfahan, Iran). The mean 100-seed weight and seed moisture content were 2.03 g and 5–6%, respectively. Prior to the experiments, seeds exhibited a 100% germination rate at 25 °C, as assessed in Petri dishes. For priming, the seeds were soaked for 6 h in a solution containing K2SiO3 (1.5 mM). This agent and its specific concentration were selected following the methods of Feghhenabi et al. (2021). Once primed, the seeds were thoroughly rinsed with tap water and then distilled water, after which they were dried back to their original moisture content under ambient conditions (about 25 °C and 42% relative humidity). The seeds returned to their initial moisture content after 72 h. All seeds were subsequently surface-sterilized in a 1% sodium hypochlorite solution for 10 min to be used in the main experiments.
We conducted two outdoor pot experiments, one in 2017 and 2018. The experiments were factorial with seven salinity levels, three Si treatments, and three replicates arranged in a randomized complete block design. The 2-year average maximum and minimum temperatures were 25.6 and 12.6 °C, the number of sunny hours was 9.4 h, and rainfall throughout the growing season was 36.5 mm. Details of weather data during the whole course of the study are given in Table 1. The growth medium was field soil collected from a 30-cm deep layer at an alfalfa farm. The field soil was classified as loam soil with sand, silt, and clay percentages of 46, 33, and 21%, respectively. The soil was non-saline (the electrical conductivity of the saturation paste was 1.1 dS m−1), containing 18.5% equivalent CaCO3, with a pH value of 7.7. The soil was air-dried, ground, and passed through a 2-mm sieve. In order to maintain the growth of the seedling, a basal dose of urea, di-ammonium phosphate, and potassium sulfate (5, 16, 2 kg h−1, respectively) was added and mixed well into the soil. A small amount of urea was also applied as a top dressing. A total of 16.4 kg of the field soil with a bulk density typical of the plow layer of a cultivated field was placed into each 18.5 L plastic pot. The amount of soil in each pot was measured on a dry weight basis. Pot cultivation was carried out manually at 3 cm depth. Seven seeds were planted per pot. The seedling density per pot was reduced to three after emergence. The addition of saline solution treatments was performed immediately after seed sowing with salinity levels maintained until harvest. The amount of water required per pot to reach field capacity was 2.4 l. Pots were then maintained at 70% field capacity with additional watering. Salinity treatments were 0, 3, 5, 6, 8, 10, and 12 dS m−1, and were obtained by diluting saline water from Lake Urmia, in the northwest of Iran. Rainfall was prevented from diluting saline treatments with the addition of a removable awning during rainfall events.
The application of Si at a concentration of 4 mM has been shown as the most effective in modulating the effects of salinity in wheat (Alzahrani et al. 2018; Bybordi 2015). As such, we used this concentration for the foliar spray treatment. Tween 20 at 0.01% (v/v) was used as a surfactant to increase foliar spray efficiency by increasing solution penetration. Half of the pots were randomly selected and treated with a foliar application of Si. The remaining pots were considered “no-foliar-sprayed” pots. The foliar spray was applied at 5-leaf and 10-leaf stages (32-day-old and 46-day-old plants). The first application was applied before sunset and the second just before dawn. 50 mL per plant of Si solution was applied with a hand-operated small locally made sprayer. Table 2 showed a summary of treatments.
2.2 Plant Sampling
Fresh samples were obtained from the youngest fully expanded leaves of borage at the early flowering stage when 10% of the flowers had appeared. Then, they were frozen in liquid nitrogen and stored at − 80 °C. Liquid nitrogen was also used to powder the frozen samples. Triturating was carried out at 5 °C.
After the borage plants were harvested, shoot length and shoot dry weight were determined.
2.3 Measurement of Photosynthetic Pigments
Chlorophyll a (Chl a) and b (Chl b) as well as carotenoid (CAR) content were determined according to Lichtenthaler and Wellburn (1983), with slight modifications. Samples (0.01 mg) of the youngest fully expanded leaves of borage at the early flowering stage were taken and extracted with 95% methanol. Absorption was read using a spectrophotometer (T60UV-Visible-1100 nm, PG Instruments, UK) at wavelengths 470, 645, and 663 nm. Chlorophyll contents were calculated using the following equations:
where ODx is the absorbance of the extracts at wavelength x.
2.4 Measurement of Chlorophyll Fluorescence Parameters
Chlorophyll fluorescence (CF) was measured on the youngest fully expanded leaves of 50-day-old plants (at 10% of the flowering stage) using a portable fluorometer (PAM-2000, Walz, Germany) following Genty et al.’s (1989) method. After sunset, the fluorometer was connected to a leaf-clip holder with a trifurcated fiberoptic and a computer equipped with data acquisition software. The minimal fluorescence level (Fo) with all PSII reaction centers open, and the maximal fluorescence level (Fm) with all PSII reaction centers closed, were determined on dark-adapted leaves. Then the leaves were continuously illuminated with a white actinic light at an irradiance of 180 µmol m−2 s−1 to measure the steady-state value of fluorescence (Fs), which occurred six minutes after the initiation of white actinic light. The maximal fluorescence level in the light-adapted state (Fm') was recorded after subjecting the leaves to a second saturating pulse at 8000 µmol m−2 s−1. The minimal fluorescence level in the light-adapted state (Fo') was determined by exposing the leaf to far-red light for three seconds. We used the light and dark fluorescence data to calculate the parameters presented in Table 3.
2.5 Measurement of Osmolyte Content
2.5.1 Glycine Betaine
The amount of Glycine betaine (GB) was estimated according to the method of Grieve and Grattan (1983). Frozen samples were mechanically shaken with 20 mL of deionized water for 24 h at 25 °C. The samples were then filtered and filtrates were diluted to 1:1 with 2N H2SO4. Aliquots were kept in centrifuge tubes and cooled in ice water for 1 h. Cold KI–I2 reagent was added and the reactants were gently stirred with a vortex mixer. The tubes were stored at 4 °C for 16 h and then centrifuged at 10,000 rpm for 15 min at 0 °C. The supernatant was carefully aspirated with a fine glass tube. The periodide crystals were dissolved in 9 mL of 1,2-dichloroethane. After 2 h, the absorbance was measured at 365 nm using GB as standard (0–350 mg L−1) and calculated as mg g−1 dry weight.
2.5.2 Soluble Sugars
Soluble sugars were extracted by boiling a known weight of liquid nitrogen-powdered samples in distilled water for 1 h in a water bath. The extract was cooled and centrifuged at 5000 rpm for 10 min, after that the supernatant was made up to a known volume. The total soluble sugars in the extract were determined with an anthrone sulfuric acid reagent. Briefly, 0.5 mL of the extract was mixed with 4.5 mL of anthrone-sulfuric acid reagent and boiled in a water bath for 7 min. After cooling, the developed blue-green color was measured at 620 nm against a water reagent blank. The content of sugars was determined using glucose as a standard and calculated as mg glucose g−1 fresh weight (Yemm and Willis 1954).
2.5.3 Soluble Proteins
Frozen samples were homogenized in ice-cold extraction buffer (50 mM potassium phosphate, pH 7.4, 1 mM EDTA). The extracts were centrifuged at 15,000 rpm for 20 min, and the resulting supernatants were used for the estimation of soluble protein content and protein oxidation damage. Protein content was assayed by following Bradford’s (1976) method with Bovine serum albumin (BSA) as standard.
2.6 Measurement of Oxidative Stress Indicators
2.6.1 Electrolyte Leakage
Electrolyte leakage (EL) was used to assess the membrane permeability. This procedure was based on Lutts et al. (1996). EL was measured using an electrical conductivity meter. Fresh leaf samples were placed in individual stoppered vials containing 10 mL of deionized water after three rounds of washing with distilled water to remove surface contamination. These samples were incubated at room temperature (25 °C) on a shaker (100 rpm) for 24 h. The electrical conductivity (EC) of the bathing solution (EC1) was recorded after incubation. The same samples were then placed in an autoclave at 120 °C for 20 min and the second reading (EC2) was determined after cooling the solution to room temperature. EL was then calculated as:
2.6.2 Malondialdehyde Content
Lipid peroxidation (MDA content) was determined using thiobarbituric acid reactions (Bailly et al. 1996), with slight modifications. The MDA content was calculated using:
where ODx is the absorbance of the extract at a given wavelength x.
2.6.3 Measurement of Antioxidant Enzyme Activities
One gram from the youngest fully expanded leaves was homogenized in 3 mL of 0.05 M sodium phosphate buffer (pH 7.8), including 1 mM EDTA and 2% (w/v) Polyvinylpyrrolidone (PVP). The homogenates were centrifuged at 13,000 × g for 20 min at 4 °C. Supernatants were used for enzyme activity and protein content assays. All steps in the preparation of the enzyme extracts were carried out at 4 °C. The extracts were used for measuring the enzyme activity of CAT, APX, and SOD.
Catalase (CAT) and ascorbate peroxidase (APX), activities were measured according to Cakmak and Marschner (1992), Nakano and Asada (1981), and Egley et al. (1983), respectively. Activities of the extracts were expressed as mM g−1 FWmin−1. One unit of SOD activity was defined as the amount of enzyme which caused 50% inhibition of photochemical reduction of nitro blue tetrazolium (NBT) at 560 nm according to the method of Giannopolitis and Ries (1977). SOD activities of the extracts were expressed as % inhibition g−1 FWmin−1.
2.7 Data Analysis
Data are presented as an average of the 2-year data set, with photosynthetic pigments and osmolyte content, oxidative stress indicators, and antioxidant enzyme activities, reported as means of six replications, while chlorophyll fluorescence parameters were reported as means of three replicates. The data were subjected to analysis of variance (ANOVA) using SAS Statistical software version 9.4. Multiple comparisons were performed using the protected least significant difference (PLSD) test at the p < 0.05 level of significance. Excel 2017 and Sigmaplot 14.0 (Systat software) were used to create the figures and conduct the regression analysis.
3 Results
3.1 The Effect of Salinity and Si Application on Photosynthetic Pigments
The interaction effect of the Si treatments × salinity on chl a, chl b, and CAR was significant (Fig. 1). Salinity caused a significant (p < 0.05) reduction in photosynthetic pigments. The pigments showed the highest decrease at the highest salinity level. Their changes were minor at EC ≤ 6 dS m−1, while beyond EC 6 dS m−1 the rate of reduction increased. Pooled across seasons chl a, chl b, and CAR decreased by 56.6, 64.2, and 57.5% respectively, at EC 12 dS m−1. Si application improved the resilience of chl a, chl b, and CAR and resulted in reductions of 52.2, 59.7, and 53.9%, respectively. Priming was better than foliar spray at maintaining chl a and CAR (Fig. 1a, c). The combination treatment was the most successful strategy to minimize damage across all photosynthetic pigments (Fig. 1a, b, c).
3.2 The Effect Of salinity on Chlorophyll Fluorescence Parameters Under Si Application
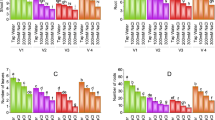
Results showed that salinity affected CF parameters (except ΦPSII and qP) differentially, depending on the Si application method and the salinity level (Figs. 2, 3, and Table 4). Figure 2 shows the effect of Si application on FV/Fm, ΦPSII, qP, and ETR. In Table 4, we present the regression coefficients of qN and NPQ measured at different salinity levels as influenced by Si application. FV/Fm and ETR were altered by Si application and salinity, but there was no interaction. The highest values of FV/Fm and ETR were observed for foliar spray and the combination treatment (Fig. 2a, b). The effect of priming on both parameters was not different from the control. Values of FV/Fm and ETR declined linearly in response to increasing salinity (Fig. 3). At a salinity of EC12 dS m−1, FV/Fm and ETR in leaves decreased by 7.2 and 23.2%, respectively. Like FV/Fm and ETR, priming had no significant effect on ΦPSII and qP. Instead, foliar spray and combination treatment increased ΦPSII and qP by an average of 15.2 and 25.9%, respectively (Fig. 2a). The non-photochemical quenching parameters, qN and NPQ also exhibited a somewhat similar pattern, showing significant differences across all salinity levels as affected by Si application method (Table 4). The qN and NPQ derived from control and priming exhibited uniform changes with increasing salinity so that the slope of their change was between 0.3 and 1.5% per unit increase in salinity. The slope of changes in qN and NPQ as affected by foliar spray and the combination application was negative. Compared to the control and priming, the foliar spray and combination treatment showed the highest values of qN and NPQ at EC ≤ 6 dS m−1 (Table 4). There was no significant difference (p > 0.05) between foliar spray and combination treatment on qN and NPQ. At EC ≤ 6 dS m−1 and compared to the control and priming, qN and NPQ increased by an average of 0.68- and 2.1-fold, respectively, by foliar spray and combination treatment. The difference among the application methods was lessened at EC > 6 dS m−1.
The effect of treatments on chlorophyll fluorescence. Data is the mean (± SD) of three replicates. Different letters above the data columns indicate significant differences between the samples determined by ANOVA, PLSD test (p < 0.05). Detailed information on each CF parameter is presented in Table 2
Changes in Fv/Fm and ETR as affected by different salinity levels. Each plot point represents the mean ± SD. Detailed information on each CF parameter is presented in Table 2
3.3 The Effect of Salinity on Osmolyte Content Under Si application
Osmolyte content was significantly affected by salinity × Si treatment (p < 0.05; Fig. 4). Soluble sugar and GB increased and soluble protein decreased with increasing salinity (Fig. 4a). GB content for the control and Si treatments increased up to EC 6 dS m−1, beyond which it decreased. The positive effect of Si treatments on GB content was observed at all salinity levels. Priming and combination treatment were more efficient than foliar spray across all salinity treatments (P < 0.05). The change in slope of the soluble sugar content of the control was such that it became 2.6-fold higher at EC 12 dS m−1 (Fig. 4b). Compared to the control, the response trend of soluble sugar content to increasing salinity was not changed by priming (p > 0.05). In contrast, foliar spray and the combination treatment increased the soluble sugar content at all salinity levels. The soluble protein content for the control was reduced to less than half at EC 12 dS m−1 (Fig. 4c). Si application increased the soluble protein content at all salinity levels, with foliar spray and the combination treatment having the greatest effects (Fig. 4b, c).
3.4 The Effect of Salinity on Oxidative Stress Indicators Under Si Application
EL% and MDA increased significantly (p < 0.05) with increasing salinity (Fig. 5). The maximum accumulation in EL% (84.3%) and MDA content (7.9 µmol g−1 FW) occurred in the control and was attained at the highest level of salinity (EC 12 dS m−1). The positive effect of Si application on EL% and MDA occurred across all salinity levels (significant interaction effect of the Si treatments × salinity, p < 0.05). For all salinity levels, the combination treatment was the most effective at reducing EL% (Fig. 5a) and MDA content (Fig. 5b). For the combination treatment and at EC 12 dS m−1, the MDA content was reduced by 3 and 31% more than the priming and foliar spray, respectively (Fig. 5b).
3.5 The Effect of Salinity on Antioxidant Enzyme Activities Under Si Application
CAT and SOD activities were significantly affected (p < 0.05) by salinity (Fig. 6), while the interaction effect of the Si treatments × salinity was significant only for APX activity (Fig. 7). Salinity increased CAT and SOD activities with the highest enzyme activities occurring at EC 12 dS m−1 where SOD was increased fourfold and CAT by 2.5-fold (Fig. 6). In the controls APX activity initially increased up to EC5 dS m−1 after which there was a significant (p < 0.05) decrease. All Si treatments caused an increase in APX activity across all salinity levels, with the biggest increases being for the combination treatment (Fig. 7). The Si treatments also caused a significant increase in CAT and SOD activities (excluding priming on SOD, Table 5). The combination treatment caused the greatest increases in CAT and SOD activity. Priming, foliar spray, and the combination treatment increased the CAT activity by 12.3, 10.5, and 21.4%, respectively, and increased SOD activity by 0.5, 8.3, and 10%, respectively (Table 5).
3.6 The Effect of Salinity and Si Application on Growth Characteristics
The interaction effect of the Si treatments × salinity on shoot length and shoot dry weight was significant (p < 0.05; Fig. 8). A significant decrease was observed for both characteristics with increasing salinity. The declining slope was steeper for shoot dry weight. The shoot dry weight and length declined to less than 50% of their initial value at EC > 12 and EC 5 dS m−1, respectively. These reductions were moderated with Si treatments at all salinity levels. The declining slope was reduced for shoot length by Si treatments particularly priming with a 12% reduction (Fig. 8a). Moreover, shoot length driven from Si treatments was more than the control shoot length at all salinity levels, especially at higher salinity. Among Si treatments, the combination treatment was the most effective (Fig. 8a). In spite of been steepened the declining slope of shoot dry weight by Si treatments, its values increased. Furthermore, the effect of Si treatments was greater at low salinities. For instance, at EC 0 dS m−1, the shoot dry weight increased by 4.3, 6, and 11.3 units with priming, foliar spraying, and combination treatment, respectively. While at EC 12 dS m−1, it increased by 0.58, 0.29, and 2.3 units with mentioned treatments, respectively (Fig. 8b).
4 Discussion
Salinity caused a significant reduction in photosynthetic pigments. This reduction has also been reported in Phaseolus vulgaris L. (Taïbi et al. 2016) and on Cucurbita pepo L. (Siddiqui et al. 2014). The reduction in chlorophyll in salinity-stressed plants has been considered a typical symptom of oxidative stress and may be attributed to the inhibition of chlorophyll synthesis, together with the degradation caused by the enzyme chlorophyllase (Hanin et al. 2016). Decreased chl a due to salinity might be the main reason for the decrease of PSII exciting capacity (Oxborough 2004). As such, chlorophyll content can be a useful trait to measure the salinity tolerance of a plant (Saddiq et al. 2019). Reduced chlorophyll, as a result of either slow synthesis or fast breakdown, indicates a possible activation of the photoprotection mechanism through reducing light absorbance (Taïbi et al. 2016). Previously, Muneer et al. (2014) demonstrated that the application of Si as a nutrient solution helped to prevent the degradation of photosynthetic pigments in Solanum lycopersicum L. In rice, Si applied as a nutrient solution alleviated the salinity-induced deficiency of N and increased chlorophyll content (Ávila et al. 2010). At lower N levels, Si treatment tended to increase the transcript levels of certain genes involved in N uptake and assimilation, potentially identifying a feedback response mechanism operating under reduced N availability. Kochanová et al. (2014) concluded that under the presence of Si, maize reacted fast to salt stress and its answer to high salt stress is to supply the plant body with N substances (by increasing the ammonia assimilation), necessary for its survival.
CF parameters can detect small changes in photosynthetic activity and have been broadly used as a tool to study both abiotic and biotic stress responses in rice (Faseela et al. 2020), lettuce (Shin et al. 2020), and sweet pepper (Alkahtani et al. 2020). Fv/Fm reflects the maximum light energy conversion efficiency of PSII after adaptation to darkness, hence photoinhibition reflects the efficiency of light energy conversion in the active center of PSII (Adhikari et al. 2019). In the current study, salinity stress caused a significant decrease in Fv/Fm. This may be due to salinity inhibiting electron transport, damaging the reaction centers at the PSII sites as well as the oxygen-evolving complex. Our results were consistent with a study on sweet pepper that also showed a reduced Fv/Fm in response to salinity (Alkahtani et al. 2020). Our results demonstrated that the application of Si as either foliar or combination treatment caused a significant increase in Fv/Fm in salinity-stressed plants. Li et al. (2018) concluded that the improvement of Fv/Fm by Si was likely via the Si-induced protection of the PSII electron transfer chain as well as the promotion of light-utilization efficiency. The salinity-induced reduction in ETR is thought to be caused by photochemical downregulation (Perez et al. 2014). Our demonstrated foliar and combination Si-mediated increase in ETR suggests an improved capacity to convert photon energy into chemical energy.
ΦPSII and qP are typically known as photochemical quenching parameters. ΦPSII accounts for the efficiency of light use for electron transport by PSII and the principal factor assessing this efficiency is the ability of photosynthetic system to remove electrons from the quinone acceptors of PSII (Faseela et al. 2020). Photochemical quenching of PSII under light conditions indicates the proportion of reaction centers that are open and is used to indicate the photoinhibition and determine the level of photoprotective quenching of fluorescence (Shin et al. 2021). In the current study, ΦPSII and qP had uniform reductions with increased salinity. In contrast, Shin et al. (2020) found that in lettuce ΦPSII and qP had the highest reductions only at the highest NaCl concentration. Our research shows that Si applied as either a foliar spray or a combination treatment increased ΦPSII and qP.
The slope of qN and NPQ in response to increasing salinity was positive for the control and primed plants but negative for plants treated with either foliar spray or combination treatment. qN estimates the rate constant for heat loss from PSII. Increasing qN and NPQ indicates the enhancement of the thermal energy dissipation through the xanthophyll cycle (Shin et al. 2021). The decrease they observed at the highest NaCl concentration (400 mM) implies photo-oxidative damage and that ROS were being generated in the chloroplasts (Shin et al. 2020). Our results suggest that Si application particularly in form of foliar spray and combination treatment could decrease salinity-induced damage to the PSII. Si leads to increased efficiency of PSII by reducing oxidative damage (Dhiman et al. 2021). Si regulates photosynthesis-related proteins during stress and increases antioxidant enzyme activity (Dhiman et al. 2021). Further studies involving chloroplast proteomic expression analysis have shown that light-harvesting complexes (LHCs) are upregulated, cytochrome b6f (Cytb6f), and ATP-synthase complex reduction is mitigated in Si- and salt-treated tomato plants (Dhiman et al. 2021).
Stress-induced accumulation of GB, soluble sugars and proteins act to protect the cell by maintaining the osmotic strength of cytosol with that of the vacuole and the external environment (Soni et al. 2021). This study showed that salinity triggered the accumulation of GB, possibly as an adaptive response. Similar responses have been demonstrated in sunflower exposed to saline conditions (Akram et al. 2012). The increase in soluble sugars that we observed in response to salinity has also been demonstrated in barley, water dropwort, and rice (Hassan et al. 2021; Paul et al. 2017; Soni et al. 2021). Increased concentrations of soluble sugars may be due to the higher enzymatic activities that help in the regulation of cellular structures and functions through the interaction with macromolecules (Ibrahimova et al. 2019). Results of the current study showed that the soluble protein content decreased significantly with increasing salinity. Similar observations have been reported by Migahid et al. (2019) on Silybum marianum (L.) plants and Jaleel et al. (2008) on Catharnathus roseus seedlings. In durum wheat which is considered a more salinity-tolerant crop (Katerji et al. 2005), Soni et al. (2021) showed an increase in the soluble protein content in both roots and shoots in response to salinity. Excess accumulation of Na+ in plants results in the production of ROS which alters plants’ metabolism by causing oxidative stress and protein degradation. Salt stress downregulates proteins due to a decrease in the expression of genes involved in the metabolic pathway, signal transduction, and gene regulation (Dhiman et al. 2021).
It is well documented that increased accumulation of osmolytes plays a significant role in the protection of various vital enzymes, membranes, and proteins in the plant cells or tissues having a key role in scavenging ROS. In line with our results, Gong et al. (2005) showed that the application of Si improved soluble protein content and protein synthesis and metabolism in wheat under drought stress.
We found a general increase of EL% in salt-stressed borage, which suggests the occurrence of cell membrane damage (Chiconato et al. 2021). In salinity stressed barley EL% also increased (Hassan et al. 2021). Salinity causes both cell membrane permeability increases and selectivity decreases, increasing EL% (Hanin et al. 2016; Negrão et al. 2017). The reduction in EL% through the application of Si as either a foliar spray or combination treatment has also been demonstrated in salinity-stressed capsicum (Manivannan et al. 2016). Our results indicated an increase in MDA under saline conditions also indicates oxidative damage to membrane lipids (Rios et al. 2017). In line with our results, Rios et al. (2017) reported that salinity-induced increased MDA was significantly lowered by Si treatment. The borage plants treated with Si showed improved cell membrane stability with decreased formation of MDA in leaves. Dhiman et al. (2021) stated that Si-mediated salinity tolerance was conferred by an increased level of H+-ATPase in roots and SOD in leaves, and a reduced EL% and content of MDA in leaves.
The antioxidative enzymatic system in plants has an efficient ROS scavenging strategy to maintain the steady-state levels of ROS in cells. Higher antioxidant enzyme activity has a role in imparting stress tolerance (Riasat et al. 2018). In this experiment, the activities of CAT, APX, and SOD in borage leaves were significantly upregulated under salinity (except APX at EC > 5 dS m−1). The Si treatments also increased their activity. Increased SOD activity under saline conditions occurs because it converts O2 into H2O2. However, CAT, one of the most potent catalysts known, decomposes H2O2 into water and oxygen (Debona et al. 2017). The combined activity of these two enzymes in borage under salinity can be used as an indicator of tolerance. The beneficial effect of the Si treatments in borage may be partially due to enhanced CAT, APX, and SOD activity. Under salinity, Si significantly increased the activity of antioxidant enzymes GPX and APX, suggesting that Si may also be involved in metabolic or physiological activities (Abdelaal et al. 2020). Our results are in agreement with salinity-stressed bugwang and sweet pepper where Si also prevented oxidative damage by up-regulating the activities of antioxidant enzymes (Abdelaal et al. 2020; Manivannan et al. 2016). Furthermore, Haghighi and Pessarakli (2013) showed that salt-stressed plants treated with Si have shown increased chlorophyll content, leaf photosynthetic activity, decreased permeability of plasma membranes, and maintenance of cell form and structure due to the increased antioxidative enzymes SOD and CAT. Totally, Si supplementation is considered one of the promising methods to improve plant resilience under salt stress. Si supplementation affects the phytohormonal and antioxidant responses under salinity as a key factor defining improved resilience (Dhiman et al. 2021).
It is generally accepted that the first symptom of salt-stressed plants is a reduction in growth (Ouzounidou et al. 2016), as demonstrated here with borage. The salinity leads to hormones-induced sequential reactions, which can diminish the stomatal opening, CO2 assimilation (Sarker and Oba 2020), increase leaf chlorosis, premature senescence (Polash et al. 2019), and ultimately, a declined photosynthetic rate and growth (Kumar et al. 2021; Subramanyam et al. 2019). Another reason for the reduction in growth characteristics might be the diversion of energy from growth and yield to the homeostasis of salinity stress, production of antioxidants, and action of antioxidant enzymes (Kumar et al. 2021; Sarker and Oba 2020). However, when our Si treatments were applied to borage, particularly combination treatment, there was a higher plant shoot dry weight and length. There were some potential mechanisms of Si to increase plant tolerance to stress. It has been proposed that under stress conditions, Si modifies cell wall metabolism to enhance tissue extensibility and remain horizontal delaying the senescence of leaves, increasing leaf content of chlorophyll and activity of ribulose-bisphosphate carboxylase (Ouzounidou et al. 2016). Furthermore, Si decreases the permeability of cell plasma membranes, increasing membranes stabilities and reducing inorganic leakage (i.e., EL) that resulted from increased activities of enzymatic and non-enzymatic antioxidants can become an important assistant mechanism (Merwad et al. 2018).
5 Conclusion
The results of this study confirm that salinity causes a significant reduction in the photosynthetic pigments, chlorophyll fluorescence parameters, and growth characteristics and also increases the ROS production within the cell. Our results also demonstrated oxidative stress with an increase in EL% and MDA content. In response to salinity, borage produced osmolytes and increased the production of antioxidant enzymes, however, these responses were insufficient to prevent damage. The application of Si treatments to salinity-stressed borage reduced damage and improved tolerance, as evidenced by improved concentrations of photosynthetic pigments, chlorophyll fluorescence parameters, osmolyte content, and activities of antioxidative enzymes. Si treatments also caused a decrease in EL% and MDA content and increases in Fv/Fm and ΦPSII. The most effective method of Si application was the priming × foliar spray treatment. The results of this study suggest that Si application may be an option to improve the growth and yield of borage growing under saline conditions with potential applications for other crops grown in saline conditions. To further studies, it would be interesting to have deep look at the role Si plays in cellular defense or cell wall protection.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Abdel Latef AA, Tran L-SP (2016) Impacts of priming with silicon on the growth and tolerance of maize plants to alkaline stress. Front Plant Sci 7:243. https://doi.org/10.3389/fpls.2016.00243
Abdelaal KAA, Mazrou YSA, Hafez YM (2020) Silicon foliar application mitigates salt stress in sweet pepper plants by enhancing water status, photosynthesis, antioxidant enzyme activity, and fruit yield. Plants 9:733. https://doi.org/10.3390/plants9060733
Abdelhamid MT, El-Masry RR, Darwish DS, Abdalla MMF, Oba S, Ragab R, Sabagh AE, Kholy MHE, Omer E (2019) Mechanisms of seed priming involved in salt stress amelioration. In: Hasanuzzaman M, Fotopoulos V (Eds.) Priming and pretreatment of seeds and seedlings. Springer, Nature Singapore Pte Ltd 219–251 https://doi.org/10.1007/978-981-13-8625-1_11
Acosta-motos JR, Ortuño MF, Bernal-Vicente A, Diaz-Vivancos P, Sánchez-Blanco MJ, Hernández JA (2017) Plant responses to salt stress: adaptive mechanisms. Agronomy 7:1–38. https://doi.org/10.3390/agronomy7010018
Adhikari ND, Simko I, Mou B (2019) Phenomic and physiological analysis of salinity effects on lettuce. Sensors 19:1–25. https://doi.org/10.3390/s19214814
Ahanger MA, Aziz U, Alsahli AA, Alyemeni MN, Ahmad P (2020) Influence of exogenous salicylic acid and nitric oxide on growth, photosynthesis, and ascorbate- glutathione cycle in salt stressed Vigna angularis. Biomolecules 10:1–16. https://doi.org/10.3390/biom10010042
Akram NA, Ashraf M, Al-Qurainy F (2012) Aminolevulinic acid-induced changes in some key physiological attributes and activities of antioxidant enzymes in sunflower (Helianthus annuus L.) plants under saline regimes. Sci Hortic 142:143–148. https://doi.org/10.1016/j.scienta.2012.05.007
Alkahtani MDF, Attia KA, Hafez YM, Khan N, Eid AM, Ali MAM, Abdelaal KAA (2020) Chlorophyll fluorescence parameters and antioxidant defense system can display salt tolerance of salt acclimated sweet pepper plants treated with chitosan and plant growth promoting rhizobacteria. Agronomy 10:1–20. https://doi.org/10.3390/agronomy10081180
Alzahrani Y, Kuşvuran A, Alharby HF, Kuşvuran S (2018) The defensive role of silicon in wheat against stress conditions induced by drought, salinity or cadmium. Ecotoxicol Environ Saf 154:187–196. https://doi.org/10.1016/j.ecoenv.2018.02.057
Anjum MA (2011) Effect of exogenously applied spermidine on growth and physiology of citrus rootstock troyer citrange under saline. Turk J Agric For 35:43–53. https://doi.org/10.3906/tar-0912-563
Ávila FW, Baliza DP, Faquin V, Araújo JL, Ramos SJ (2010) Silicon-nitrogen interaction in rice cultivated under nutrient solution. Rev Ciênc Agron 41:184–190. https://doi.org/10.1590/S1806-66902010000200003
Bailly C, Benamar A, Corbineau F, Come D (1996) Changes in malondialdehyde content and in superoxide dismutase, catalase and glutathione reductase activities in sunflower seeds as related to deterioration during accelerated aging. Physiol Plant 97:104–110. https://doi.org/10.1111/j.1399-3054.1996.tb00485.x
Bradford MM (1976) A rapid and sensitive method for the quantitation microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Bybordi A (2015) Influence of exogenous application of silicon and potassium on physiological responses, yield and yield components of salt-stressed wheat. Commun Soil Sci Plant Anal 46:109–122. https://doi.org/10.1080/00103624.2014.956936
Cakmak I, Marschner H (1992) Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol 98:1222–1227. https://doi.org/10.1104/pp.98.4.1222
Chiconato DA, Costa MGS, Balbuena TS, Munns R, Santos DMM (2021) Proteomic analysis of young sugarcane plants with contrasting salt tolerance. Funct Plant Biol 48:588–596. https://doi.org/10.1071/FP20314
Debona D, Rodrigues FA, Datnoff LE (2017) Silicon’s role in abiotic and biotic plant stresses. Annu Rev Phytopathol 55:85–107. https://doi.org/10.1146/annurev-phyto-080516-035312
Dhiman P, Rajora N, Bhardwaj S, Sudhakaran SS, Kumara A, Raturi G, Chakraborty K, Gupta OP, Devanna BN, Tripathi DK, Deshmukh R (2021) Fascinating role of silicon to combat salinity stress in plants: an updated overview. Plant Physiol Biochem 162:110–123. https://doi.org/10.1016/j.plaphy.2021.02.023
Egley GH, Paul RN, Vaughn KC, Duke SO (1983) Role of peroxidase in the development of water impermeable seed coats in Sida spinosa L. Planta 157:224–232. https://doi.org/10.2307/23376182
Faseela P, Sinisha AK, Brestič M, Puthur JT (2020) Chlorophyll a fluorescence parameters as indicators of a particular abiotic stress in rice. Photosynthetic 58:293–300. https://doi.org/10.32615/ps.2019.147
Feghhenabi F, Hadi H, Khodaverdiloo H, van Genuchten MTh (2021) Borage (Borago officinalis L.) response to salinity at early growth stages as influenced by seed pre-treatment. Agric Water Manag 253:1–9. https://doi.org/10.1016/j.agwat.2021.106925
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 99:87–92. https://doi.org/10.1016/S0304-4165(89)80016-9
Giannopolitis CN, Ries SK (1977) Superoxide dismutases. Plant Physiol 59:309–314. https://doi.org/10.1104/pp.59.2.309
Gong H, Zhu X, Chen K, Wang S, Zhang C (2005) Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci 169:313–321. https://doi.org/10.1016/j.plantsci.2005.02.023
Grieve CM, Grattan SR (1983) Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 70:303–307. https://doi.org/10.1007/BF02374789
Haghighi M, Pessarakli M (2013) Influence of silicon and nano-silicon on salinity tolerance of cherry tomatoes (Solanum lycopersicum L.) at early growth stage. Sci Hortic 161:111–117. https://doi.org/10.1016/j.scienta.2013.06.034
Hanin M, Ebel C, Ngom M, Laplaze L, Masmoudi K (2016) New insights on plant salt tolerance mechanisms and their potential use for breeding. Front Plant Sci 7:1–17. https://doi.org/10.3389/fpls.2016.01787
Hassan A, Amjad SF, Saleem MH, Yasmind H, Imran M, Riaz M, Ali Q, Joyia FA, Ahmedi S, Ali S, Alsahli AA, Alyemeni MN (2021) Foliar application of ascorbic acid enhances salinity stress tolerance in barley (Hordeum vulgare L.) through modulation of morpho-physio-biochemical attributes, ions uptake, osmo-protectants and stress response genes expression. Saudi J Biol Sci 28:4276–4290. https://doi.org/10.1016/j.sjbs.2021.03.045
Hernández JA (2019) Salinity tolerance in plants: Trends and perspectives. Int J Mol Sci 20:2408. https://doi.org/10.3390/ijms20102408
Ibrahimova UF, Mammadov AC, Feyziyev YM (2019) The effect of NaCl on some physiological and biochemical parameters in Triticum aestivum L. genotypes. Plant Physiol Rep 24:370–375. https://doi.org/10.1007/s40502-019-00461-z
Isayenkov SV, Maathuis FJM (2019) Plant salinity stress: many unanswered questions remain. Front Plant Sci 10:1–11. https://doi.org/10.3389/fpls.2019.00080
Jaffel K, Sai S, Bouraoui NK, Ammar RB, Legendre L, Lachâal M, Marzouk B (2011) Influence of salt stress on growth, lipid peroxidation and antioxidative enzyme activity in borage (Borago officinalis L.). Plant Biosyst 145:362–369. https://doi.org/10.1080/11263504.2011.558689
Jaleel CA, Gopi R, Kishorekumar A, Manivannan P, Sankar B, Panneerselvam R (2008) Interactive effects of triadimefon and salt stress on antioxidative status and ajmalicine accumulation in Catharanthus roseus. Acta Physiol Plant 30:287–292. https://doi.org/10.1007/s11738-007-0119-1
Katerji N, van Hoorn JW, Hamdy A, Mastrorilli M, Nachit MM, Oweis T (2005) Salt tolerance analysis of chickpea, faba bean and durum wheat varieties: II. Durum Wheat Agric Water Manag 72:195–207. https://doi.org/10.1016/j.agwat.2004.09.014
Kochanová Z, Jašková K, Sedláková B, Luxová M (2014) Silicon improves salinity tolerance and affects ammonia assimilation in maize roots. Biologia 69:1164–1171. https://doi.org/10.2478/s11756-014-0411-7
Kohil SK, Khanna K, Bhardwaj R, Abd-Allah EF, Ahmad P, Corpas FJ (2019) Assessment of subcellular ROS and NO metabolism in higher plants: multifunctional signaling molecules. Antioxidants 8:1–28. https://doi.org/10.3390/antiox8120641
Kováčik J, Klejdus B, Hedbavny J, Bačkor M (2009) Salicylic acid alleviates NaCl-induced changes in the metabolism of matricaria chamomilla plants. Ecotoxicology 18:544–554. https://doi.org/10.1007/s10646-009-0312-7
Kumar S, Li G, Yang J, Huang X, Ji Q, Liu Z, Ke W, Hou H (2021) Effect of salt stress on growth, physiological parameters, and ionic concentration of water dropwort (Oenanthe javanica) cultivars. Front Plant Sci 12:1–26. https://doi.org/10.3389/fpls.2021.660409
Li L, Ai S, Li Y, Wang Y, Tang M (2018) Exogenous silicon mediates alleviation of cadmium stress by promoting photosynthetic activity and activities of antioxidative enzymes in rice. J Plant Growth Regul 37:602–611. https://doi.org/10.1007/s00344-017-9758-7
Lichtenthaler HK, Wellburn AR (1983) Determination of total carotenoids and chlorophyll a and chlorophyll b leaf extracts in different solvents. Biochem Soc Trans 11:591–592. https://doi.org/10.1042/bst0110591
Liu B, Soundararajan P, Manivannan A (2019) Mechanisms of silicon-mediated amelioration of salt salinity in plants. Plants 8:1–13. https://doi.org/10.3390/plants8090307
Łuczak K, Czerniawska-Kusza I, Rosik-Dulewska C, Kusza G (2021) Effect of NaCl road salt on the ionic composition of soils and Aesculus hippocastanum L. foliage and leaf damage intensity. Sci Rep 11:5309. https://doi.org/10.1038/s41598-021-84541-x
Lutts S, Kinet JM, Bouharmont J (1996) NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann Bot 78:389–398. https://doi.org/10.1006/anbo.1996.0134
Maghsoudi K, Emam Y, Pessarakli M (2016) Effect of silicon on photosynthetic gas exchange, photosynthetic pigments, cell membrane stability and relative water content of different wheat cultivars under drought stress conditions. J Plant Nutr 39:1001–1015. https://doi.org/10.1080/01904167.2015.1109108
Manivannan A, Soundararajan P, Muneer S, Ko CH, Jeong BR (2016) Silicon mitigates salinity stress by regulating the physiology, antioxidant enzyme activities, and protein expression in Capsicum annuum ‘Bugwang.’ Biomed Res Int 2016:1–14. https://doi.org/10.1155/2016/3076357
Mansoor S, Wani OA, Lone JK, Manhas S, Kour N, Alam P, Ahmad A, Ahmad P (2022) Reactive oxygen species in plants: from source to sink. Antioxidants 11:1–14. https://doi.org/10.3390/antiox11020225
Merwad A-R, Desoky E-SM, Rady M (2018) Response of water deficit-stressed Vigna unguiculata performances to silicon, proline or methionine foliar application. Sci Hortic 228:132–144. https://doi.org/10.1016/j.scienta.2017.10.008
Migahid MM, Elghobashy RM, Bidak LM, Amin AW (2019) Priming of Silybum marianum L. gaertn seeds with H2O2 and magnetic field ameliorates seawater stress. Heliyon 5:e01886. https://doi.org/10.1016/j.heliyon.2019.e01886
Montaner C, Zufiaurre R, Movila M, Mallor C (2022) Evaluation of borage (Borago officinalis L.) genotypes for nutraceutical value based on leaves fatty acids composition. Foods 11:1–19. https://doi.org/10.3390/foods11010016
Muneer S, Park YG, Manivannan A, Soundararajan P, Jeong BR (2014) Physiological and proteomic analysis in chloroplasts of Solanum lycopersicum L. under silicon efficiency and salinity stress. Int J Mol Sci 15:21803–21824. https://doi.org/10.3390/ijms151221803
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinch chloroplasts. Plant Cell Physiol 22:867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Negrão S, Schmöckel SM, Tester M (2017) Evaluating physiological responses of plants to salinity stress. Ann Bot 119:1–11. https://doi.org/10.1093/aob/mcw191
Noreen Z, Ashraf M, Akram NA (2010) Salt-induced regulation of some key antioxidant enzymes and physio-biochemical phenomena in five diverse cultivars of turnip (Brassica Rapa L.). J Agron Crop Sci 196:273–285. https://doi.org/10.1111/j.1439-037X.2010.00420.x
Ouhibi C, Attia H, Rebah F, Msilini N, Chebbi M, Aarrouf J, Urban L, Lachaal M (2014) Salt stress mitigation by seed priming with UV-C in lettuce plants: growth, antioxidant activity and phenolic compounds. Plant Physiol Biochem 83:126–133. https://doi.org/10.1016/j.plaphy.2014.07.019
Ouzounidou G, Giannakoula A, Ilias I, Zamanidis P (2016) Alleviation of drought and salinity stresses on growth, physiology, biochemistry and quality of two Cucumis sativus L. cultivars by Si application. Braz J Bot 39:531–539. https://doi.org/10.1007/s40415-016-0274-y
Oxborough K (2004) Imaging of chlorophyll a fluorescence: theoretical and practical aspects of an emerging technique for the monitoring of photosynthetic performance. J Exp Bot 55:1195–1205. https://doi.org/10.1093/jxb/erh145
Panuccio MR, Jacobsen SE, Akhtar SS, Muscolo A (2014) Effect of saline water on seed germination and early seedling growth of the halophyte quinoa. AoB PLANTS 6:1–18. https://doi.org/10.1093/aobpla/plu047
Paul S, Roychoudhury A, Banerjee A, Chaudhuri N, Ghosh P (2017) Seed pre-treatment with spermidine alleviates oxidative damages to different extent in the salt (NaCl)-stressed seedlings of three indica rice cultivars with contrasting level of salt tolerance. Plant Gene 11:112–123. https://doi.org/10.1016/j.plgene.2017.04.002
Perez CEA, Rodrigues FÁ, Moreira WR, DaMatta FM (2014) Leaf gas exchange and chlorophyll a fluorescence in wheat plants supplied with silicon and infected with Pyricularia oryzae. Biochem Cell Biol 104:143–149. https://doi.org/10.1094/PHYTO-06-13-0163-R
Polash MAS, Sakil A, Hossain MA (2019) Plants responses and their physiological and biochemical defense mechanisms against salinity: a review. Trop plant res 6:250–274. https://doi.org/10.22271/tpr.2019.v6.i2.035
Riasat M, Pessarakli M, Ahmadi Niaz A, Saed- Moucheshi A (2018) Assessment of different wheat genotypes with altered genetic background in response to different salinity levels. J Plant Nutr 41:1821–1833. https://doi.org/10.1080/01904167.2018.1462383
Rios JJ, Martínez-Ballesta MC, Ruiz JM, Blasco B, Carvajal M (2017) Silicon-mediated improvement in plant salinity tolerance: the role of aquaporins. Front Plant Sci 8:948. https://doi.org/10.3389/fpls.2017.00948
Saddiq MS, Iqbal S, Afzal I, Ibrahim AH, Bakhtavar MA, Hafeez MB, Maqbool M (2019) Mitigation of salinity stress in wheat (Triticum Aestivum L.) seedlings through physiological seed enhancements. J Plant Nutr 42:1192–1204. https://doi.org/10.1080/01904167.2019.1609509
Sarker U, Oba S (2020) The response of salinity stress-induced A. tricolor to growth, anatomy, physiology, non-enzymatic and enzymatic antioxidants. Front Plant Sci 11:559876. https://doi.org/10.3389/fpls.2020.559876
Shalaby OAS (2018) Alleviation of salinity stress in red cabbage plants by urea and sulfur applications. J Plant Nutr 41:1597–1603. https://doi.org/10.1080/01904167.2018.1462387
Sheteiwy MS, Shao H, Qi W, Daly P, Sharma A, Shaghaleh H, Hamoud YA, El-Esawi MA, Pan R, Wan Q, Lu H (2021) Seed priming and foliar application with jasmonic acid enhance salinity stress tolerance of soybean (Glycine Max L.) seedlings. J Sci Food Agric 101:2027–2041. https://doi.org/10.1002/jsfa.10822
Shin YK, Bhandari SR, Jo JS, Song JW, Cho MC, Yang EY, Lee JG (2020) Response to salt stress in lettuce: changes in chlorophyll fluorescence parameters, phytochemical contents, and antioxidant activities. Agronomy 10:1627. https://doi.org/10.3390/agronomy10111627
Shin YK, Bhandari SR, Jo JS, Song JW, Lee JG (2021) Effect of drought stress on chlorophyll fluorescence parameters, phytochemical contents, and antioxidant activities in lettuce seedlings. Horticulturae 7:238. https://doi.org/10.3390/horticulturae7080238
Siddiqui MH, Al-whaibi M, Faisal M, Al Sahli AA (2014) Nano-silicon dioxide mitigates the adverse effects of salt stress on Cucurbita pepo L. Environ Toxicol Chem 33:2429–2437. https://doi.org/10.1002/etc.2697
Soni S, Kumara S, Sehrawat N, Kumara A, Kumara N, Lata C, Mann A (2021) Effect of saline irrigation on plant water traits, photosynthesis and ionic balance in durum wheat genotypes. Saudi J Biol Sci 28:2510–2517. https://doi.org/10.1016/j.sjbs.2021.01.052
Subramanyam K, Laing GD, Van Damme EJM (2019) Sodium selenate treatment using a combination of seed priming and foliar spray alleviates salinity stress in rice. Front Plant Sci 10:116. https://doi.org/10.3389/fpls.2019.00116
Taïbi K, Taïbi F, Abderrahim LA, Ennajah A, Belkhodja M, Mulet JM (2016) Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. S Afr J Bot 105:306–312. https://doi.org/10.1016/j.sajb.2016.03.011
Tarchoune I, Sgherri C, Izzo R, Lachaal M, Ouerghi Z, Navari-Izzo F (2010) Antioxidative responses of Ocimum basilicum to sodium chloride or sodium sulphate salinization. Plant Physiol Biochem 48:772–777. https://doi.org/10.1016/j.plaphy.2010.05.006
Wang WB, Kim YH, Lee HS, Kim KY, Deng XP, Kwak SS (2009) Analysis of antioxidant enzyme activity during germination of alfalfa under salt and drought stresses. Plant Physiol Biochem 47:570–577. https://doi.org/10.1016/j.plaphy.2009.02.009
Yemm EW, Willis AJ (1954) The estimation of carbohydrates in plant extracts by anthrone. Biochem J 57:508–514. https://doi.org/10.1042/bj0570508
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Faride Feghhenabi, Hashem Hadi, and Habib Khodaverdiloo. The first draft of the manuscript was written by Faride Feghhenabi and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Feghhenabi, F., Hadi, H., Khodaverdiloo, H. et al. Demonstration of Borage Salinity Tolerance as affected by Si Application Through Biochemical and Physiological Responses, and Growth Attributes. J Soil Sci Plant Nutr 23, 1678–1693 (2023). https://doi.org/10.1007/s42729-023-01187-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-023-01187-6