Abstract
Arbuscular mycorrhizal fungi (AMF) establish symbiotic interactions that improve productivity of agricultural crops. This study aims to evaluate the effect of different doses of the fungus Glomus intraradices on ex vitro development of sugarcane plantlets in the acclimatization stage. In vitro sugarcane (Saccharum spp. cv Mex 69–290) plantlets were inoculated with 0, 50, 100, 200, and 400 spores per plant of G. intraradices. After 60 days of acclimatization in a greenhouse, survival rate, colonization percentage, plant growth, dry matter, total chlorophyll content, and macronutrient and micronutrient contents were evaluated. Mycorrhizae were characterized by bright field and multiphoton microscopy. Effects of mycorrhizae on the different variables evaluated were observed. The doses of 50 and 100 spores per plant, with 30 and 58% colonization, respectively, achieved a symbiotic interaction, while doses of 200 and 400 spores per plant, with the highest colonization percentages (80 and 86%), had negative effects on survival, development, chlorophyll content, and nutritional status. Microscopy demonstrates the symbiotic association between G. intraradices and Saccharum spp. Early application of adequate mycorrhizal doses in plantlets during acclimatization provides a conditioning advantage prior to transplanting for the establishment of basic sugarcane seedbeds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Ecological interactions between different species can have a practical application in plant biotechnology (Sales et al. 2021). Arbuscular mycorrhizal fungi (AMF) are obligate biotrophs that establish endosymbiotic associations with the roots of 85% of vascular plants (Choi et al. 2018; Di Martino et al. 2019). AMF enhance mineral nutrient uptake in the host plant (Di Martino et al. 2019; Ortas 2019; Juntahum et al. 2020), have positive effects on photosynthetic activity (Al-Karaki and Williams 2021), induce sclerification of leaf tissue, indirectly contribute to reducing herbivorous insect attack (Damin et al. 2020), decrease disease invasion (Wu et al. 2021a, 2021b), and confer tolerance to different types of abiotic stresses in plants such as drought, salinity, sudden temperature changes (Chandrasekaran et al. 2021), and heavy metals (Gupta et al. 2021). Positive effects of AMF have been demonstrated in agri-food crops such as tomato (Lycopersicon esculentum) (Di Martino et al. 2019), sugarcane (Saccharum spp.) (Da Silva Barros et al. 2019; Fors et al. 2020; Sales et al. 2021), rice (Oryza sativa) (Campo et al. 2020), melon (Cucumis melo) (Meddich et al. 2021), and the woody plant Cinnamomum migao (Xiao et al. 2022).
Sugarcane (Saccharum spp.), a member of the grass family (Poaceae), is grown mainly in tropical and subtropical areas of the world (Bigott et al. 2019; Tripathi et al. 2022). It is one of the main agroindustrial and economically important crops from which various products and by-products used in the food, energy, chemical, pharmaceutical, and other industries are obtained (Rivero et al. 2020; Sentíes-Herrera et al. 2017; Shabbir et al. 2021). However, sugarcane cultivation faces great challenges in terms of productivity and competitiveness, since the conventional method of propagation by buds does not guarantee the sanitation and rejuvenation of commercial plantations (Bello-Bello et al. 2018). Recently, plant biotechnology has offered alternatives to address these limitations, one of which is through micropropagation using plant tissue culture techniques, which guarantee obtaining rejuvenated plants with high genetic and phytosanitary quality. Acclimatization is the final stage of micropropagation that consists of transferring plantlets in vitro to external conditions and initiating their ex vitro growth (Gómez-Kosky et al. 2020). In addition, acclimatization is a process that allows early inoculation of mycorrhizae prior to transplanting in the field for the establishment of basic sugarcane seedbeds (Vergara et al. 2019). Several studies using AMF during acclimatization stage have been reported in different species such as lily (Gloriosa superba L.) (Yadav et al. 2013), giant reed (Arundo donax) (Tauler and Baraza 2015), banana (Dwarf Cavendish) (Ortas et al. 2017), and salvia (Salvia miltiorrhiza B.) (Wu et al. 2021a, 2021b). This study aims to evaluate the effect of different doses of AMF of the species Glomus intraradices on the ex vitro development of sugarcane plantlets in the acclimatization stage.
2 Materials and Methods
2.1 Plant Material and Micropropagation
For in vitro establishment of sugarcane (Saccharum spp.), 25-cm apices of the Mex 69–290 variety were collected at eight months of age. The apices were washed with water and Axion® commercial soap (Mission Hills, S.A. de C.V., San José de Iturbide, Guanajuato, MX) wrapped in paper bags, and kept at 4 °C for 24 h. The apices were cut to a length of 15 cm and subjected to hydrothermotherapy in a circulating thermostatic bath (Ecoshel, SC-15, McAllen, TX) at 50 °C for 20 min. Apices were reduced to 2 cm, rinsed for five min in a 10% (v/v) commercial sodium hydrochloride solution with three drops of Tween 20® (Sigma-Aldrich® Chemical Company, Saint Louis, MO) per 100 mL of water. The explants were individually placed in test tubes containing 10 mL of MS (Murashige and Skoog 1962) medium supplemented with 30 g L−1 sucrose, without growth regulators. The medium pH was adjusted to 5.8 and 2.5 g L−1 Phytagel™ (Sigma-Aldrich®) was added as a gelling agent. It was sterilized in an autoclave for 15 min at 120 °C and 115 kPa. The explants were incubated at 24 ± 2 °C, under 40 ± 5 μmol m−2 s−1 irradiance and a 16-h photoperiod. After 1 week, the apices were transferred for multiplication to MS medium supplemented with 1 mg L−1 kinetin (KIN, Sigma-Aldrich®), 1 mg L−1 indoleacetic acid (IAA, Sigma-Aldrich®), and 2 mg L−1 6-benzylaminopurine (BAP, Sigma-Aldrich®). After four subcultures (45 d each), the shoots were individualized and rooted in semi-solid MS medium supplemented with 2 mg L−1 activated charcoal.
2.2 Mycorrhizal Fungi Inoculation and Culture Conditions
Inoculation with mycorrhizal fungi was carried out ex vitro under greenhouse conditions using plantlets with a length of 5 cm and Glomus intraradices (Biofertilizante INIFAP®, Chiapas, MX). Plant-mycorrhizal inoculation was performed in 32-cavity polypropylene trays with a substrate made of compost, peat moss and agrolite (2:1:1 v/v) (Table S1). The substrate was autoclaved for 30 min at 120 °C and 115 kPa. Subsequently, G. intraradices was added to the substrate at 0, 50, 100, 200, and 400 spores per plant. The sugarcane plantlets were covered with a translucent dome to control humidity conditions and in a greenhouse with 60% shade at 30 ± 2 °C, relative humidity of 60 ± 10%, and natural light at an irradiance of 80 ± 10 μmol m−2 s−1 for 1 month. In a second phase, the dome was removed and plantlets were kept for 1 month under greenhouse conditions at 35 ± 2 °C, relative humidity of 30%, and natural light at an irradiance of 150 ± 10 μmol m−2 s−1. Throughout the experiment, irrigation with osmosis water was applied twice a week. After 60 days, the survival rate, colonization percentage, number of shoots, plant length, number and length of roots, and dry matter were evaluated. Total chlorophyll content and macronutrient and micronutrient contents (N, P, K, Ca, Mg, Fe, Cu, Zn, Mn, and B) were also recorded. To assess the effect of survival rate on different treatments during acclimatization, 30 plantlets were evaluated in triplet for each dose of G. intraradices. Dry weight was determined after placing the shoots and roots in a drying oven (Felisa, FE292, JAL, MX) at 75 °C for 72 h. Dry matter content was calculated using dry weight/fresh weight × 100.
2.3 Total Chlorophyll Content
Total chlorophyll content was determined according to the methodology proposed by Harborne (1973). For each sample, 1 g of fresh material was macerated with 80% acetone and allowed to stand at − 4 °C for 24 h in 80% acetone to a final volume of 10 mL. Subsequently, the mixture was sieved through filter paper, then adjusted to a volume of 25 mL with 80% acetone. Two milliliters per sample were used and read at an absorbance of 663 and 645 nm for chlorophyll a and b, respectively. Readings were performed using a spectrophotometer (Genesys 10S, Thermo Scientific; MA).
2.4 Mycorrhizal Colonization and Characterization
2.4.1 Mycorrhizal Colonization
From the microscopic observations, the percentages of mycorrhizal colonization were determined using the following formula: Percentage of root colonization (%) = No. of infected segments/No. of examined root segments × 100.
2.4.2 Bright-Field Microscopy
To visualize the effect of the different doses of arbuscular mycorrhizal fungi (AMF) on sugarcane roots, segments of the roots were obtained and fixed in 4% paraformaldehyde, and then incubated for 48 h at room temperature. Root segments were washed three times with distilled water and then incubated in 10% KOH for 15 min at 120 °C. The supernatant was removed and washed three times with distilled water. An alkaline hydrogen peroxide solution was added and incubated for 20 min at room temperature, after which the samples were washed with tap water and incubated in 10% HCl (Sigma-Aldrich®) for 5 min, after which they were decanted and 0.05% trypan blue (Sigma-Aldrich®) was added and incubated for 24 h at room temperature. Finally, the trypan blue was removed and an acetoglycerol solution was added. The samples were observed under a compound microscope (BX50, Olympus, Tokyo, JP) using 20X/0.50, UPlan-FL (α − 0.17) and 40 × /1.00 (U-Plan-Apochromat, Olympus, Tokyo, JP) objectives. Image acquisition was performed with an Infinity3 high-sensitivity fluorescence camera (Lumenera, Montreal, CA) synchronized through Image Pro Premier 9.1 software (Media Cybernetics, Rockville, MD).
2.4.3 Multiphoton Microscopy
Cleared roots were washed three times with deionized water and suspended in a solution of 500 µL of deionized water with 200 µL of 0.002% propidium iodide (Sigma Aldrich®). Samples were incubated for 15 min in darkness at 4 °C. Then, 10 µL of solophenylflavine 7GFE (Sigma-Aldrich®) at 0.1% were added in 500 µL of deionized water and were incubated in darkness for 10 min at 4 °C. The samples were mounted and covered with a high-performance cover glass slide (D = 0.17 mm ± 0.005 mm refractive index = 1.5255 ± 0.0015, Abbe number = 56 ± 2) and observed in the multiphoton microscope system (LSM 880-NLO, Zeiss, Walthman, MA) coupled to infrared laser Ti: Sapphire (Chameleon vision II, COHERENT, SCT). The operating conditions in all experiments were Chameleon laser at 750 nm with 1.0% of power and 514 nm laser with 2.2% of power, for solophenylflavine 7GFE and propidium iodide, respectively. Observations were performed with a 20 × /0.5 objective (NA ∞ − 0.17, Zeiss Plan NEOFLUAR). All images were acquired by separation of the emission into two channels, green region for solophenyl flavine stain (496–580 nm) and red region for propidium iodide stain (607–700 nm). All micrographs were captured in CZI format at 1024 × 1024 pixels and RGB.
2.5 Macronutrient and Micronutrient Contents
To determine macronutrient and micronutrient contents, the samples were dried at 70 °C in a drying oven for 72 h and pulverized in a blender (Oster 6832, Milwaukee, WI). Samples were subjected to wet digestion in a mixture of perchloric and nitric acids at a 2:1 (v/v) ratio, according to the protocol described by Alcántar and Sandoval (1999). To determine the concentrations of macronutrients (P, K, Ca, and Mg) and micronutrients (Fe, Cu, Zn, Mn, and B), the extracts were analyzed using a coupled plasma induction optical emission spectrometer (ICP- OES, Varian 725-ES, Agilent; Mulgrave, VIC, AUS). The N concentration was determined by the semi-microkjeldahl method according to the protocol described by Bremner (1965).
2.6 Experimental Design and Statistical Analysis
All experiments were performed using a completely randomized design and replicated three times. An analysis of variance (ANOVA) and then Tukey’s test (p ≤ 0.05) were performed using SPSS statistical software (Windows version 22). Percentage data were transformed with the formula Y = arcsine (√ (× /100)), where × is the percentage value.
3 Results
3.1 Mycorrhizal Effect on Ex Vitro Development
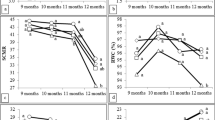
When evaluating the effect of different AMF concentrations on the development of sugarcane plantlets, significant differences were observed for the number of shoots, plant length, number of roots and length, percentage of dry matter, and chlorophyll content during acclimatization (Fig. 1). The highest number of shoots per plant was found in the treatments with 50, 100, and 200 spores per plant, respectively, whereas the lowest number of shoots per plant was found in the control and 400 treatments, respectively (Fig. 1a). For the plant length variable, the tallest plantlets were observed in the treatment with 100 spores per plant, while in the rest of the treatments, the plant height was between 33 and 36 cm per plantlet (Fig. 1b). Regarding the number of roots per plant, the highest number of roots was obtained in the treatments with 50 and 100 spores per plant, respectively, whereas the lowest number of roots was obtained in the control and the 200 and 400 spores per plant treatments, respectively (Fig. 1c). For root length, the largest ones were observed in the treatments with 100 and 200 spores per plant, respectively, whereas the smallest roots were found in the control and 50 spores per plant, respectively (Fig. 1d). As for the percentage of dry matter (DM), roots, shoots, and complete plantlets treated with mycorrhizae showed the highest DM percentages, whereas the lowest amount of dry matter was observed in the control treatment (Fig. 1e). In relation to total chlorophyll, the highest chlorophyll content was observed in the treatments with 50, 100, 20, and 400 spores per plant, whereas the lowest content was found in the control treatment (Fig. 1f).
Effect of arbuscular mycorrhizal fungi (Glomus intraradices) on development, dry matter, and chlorophyll. a Number of shoots, b plant length, c number of roots, d root length, e % dry matter, and f total chlorophyll, evaluated at 60 days of ex vitro culture of sugarcane (Saccharum spp. cv Mex 69˗290). Results are shown as mean ± SE (standard error). Means with a different letter are significantly different (Tukey, p ≤ 0.05)
3.2 Survival Percentage, Mycorrhizal Colonization, and Characterization
The results obtained in this study demonstrate an effect of the mycorrhizae on plantlet survival percentage and mycorrhizal colonization percentage (Fig. 2). Inoculation with AMF at doses between 0 and 100 spores per plant had no effect on survival i.e., more than 95% survival was obtained, whereas the 200 and 400 doses had the greatest effect on plantlet mortality, respectively (Fig. 2a). Regarding colonization, the higher the spore content per plant, the higher the colonization percentage observed (Fig. 2b).
Effect of arbuscular mycorrhizal fungi (Glomus intraradices) on a survival and b colonization evaluated at 60 days of ex vitro culture of sugarcane (Saccharum spp. cv Mex 69˗290). Results are shown as mean ± SE (standard error). Means with a different letter are significantly different (Tukey, p ≤ 0.05)
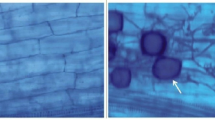
According to the fungus-plant characterization carried out by microscopy, a symbiotic interaction between plant and fungus was observed, with the presence of spores, hyphae, and chlamydospores (Fig. 3). The structures observed in the mycorrhizal treatments were spores and hyphae. Spores were most frequently located in the periphery of the root, between the epidermis and the covering mucilage, whereas hyphae were observed internalized between the central cylinder and the parenchyma. Arbuscular structures were observed inside the cells, forming extensions of the hyphae to the outside of the root.
Colonization of Glomus intraradices on ex vitro sugarcane (Saccharum spp. cv Mex 69˗290) plantlets at 60 days after spore inoculation. Typical fungal structures, such as intracellular hyphae (ih), spores (sp), and chlamydospores (cl), were observed. Left: bright-field microscopy and right: fluorescence by a multiphoton microscope a–b control treatment, c-d presence of spores, e–f hyphae, and g-h chlamydospores. Bar = 50 μm
3.3 Macronutrient and Micronutrient Contents
When applying different doses of spores per plant, differences were observed in the contents of P, K, Ca, Mg, Fe, Cu, Zn, and Mn, whereas no significant differences were observed for the nutrients N and B (Table 1). For the macronutrients P, K, and Mg, the highest contents (g kg−1 dry weight) were obtained at the doses of 0, 50, and 100 spores per plant, whereas the lowest contents of these nutrients were observed at the doses of 200 and 400 spores per plant. For the Ca macronutrient, the highest contents were obtained at the doses of 100 and 200 spores per plant, whereas the lowest contents of this element were obtained at the doses of 0, 50 and 400 spores per plant. Regarding micronutrients, the highest contents of Fe and Cu (mg kg−1 dry weight) were observed in the doses of 0, 50, and 100 spores per plant, whereas the lowest contents of these micronutrients were obtained at the doses of 200 and 400 spores per plant. For the weight of Zn and Mn, the highest contents were obtained at the doses of 0 and 50 spores per plant, whereas the lowest contents of these micronutrients were obtained at the doses of 100, 200 and 400 spores per plant.
4 Discussion
The results demonstrate the effect of different mycorrhizal inoculant doses on the development variables evaluated, and the recommended dose per plant is between 50 and 100 spores. The positive effect of these doses on development could be due to the fact that 30 and 58% colonization promotes an adequate symbiotic interaction which could favor some physiological processes for the plant such as photosynthetic efficiency and nutrient and water absorption, which allows adequate development of the plantlet for its subsequent transfer to the field. In crops such as apple (An et al. 1993), olives (Porras-Soriano et al. 2009), grapevine (Trouvelot et al. 2015), and red tangerine (Citrus tangerine Hort. ex Tanaka) (Wu et al. 2011), increases in shoot number were observed when inoculated with AMF. However, the effects of mycorrhizae on development depend on the type and dose of AMF in relation to the host plant species (Aslani et al. 2019; Ren et al. 2019). According to studies by Xiao et al. (2022) on Cinnamomum migao plants, greater plant and root length and increased shoot diameter were obtained when inoculating with 1400 spores per plant with the fungus Glomus etunicatum compared to Funneliformis mosseae and non-inoculated plants. Meddich et al. (2021) observed that when using a mycorrhizal consortium with Glomus sp. in melon (Cucumis melo) at a dose of 44 spores per plant, shoot and root biomass increased significantly. Wu et al. (2021a, 2021b) observed that colonization with mycorrhizae for vegetable at a dose of 300 spores per plant promoted greater biomass accumulation in watermelon (Citrullus lanatus L. cv. Qilin) seedlings. Sales et al. (2021) in sugarcane (Saccharum spp.) hybrids obtained an increase in yield when inoculating with native AMF at a dose of 260 spores per plant. In this regard, Wu et al. (2022) note that AMF are well recognized for improving plant growth and biomass accumulation.
In this study, the negative effect on different development variables of the highest mycorrhizal doses could be due to a disruption of the mycorrhizal symbiosis caused by excessive root colonization. Lopes et al. (2021) state that under favorable conditions, plants can obtain nutrients and water by their own means, the role of mycorrhiza being less important to the plant. Thus, plants have an autoregulation mechanism to control excessive root colonization, which consists of suppressing the colonization once an efficient mycorrhization is reached (López-Ráez and Pozo 2013). In addition, AMF are obligate biotrophs that inhabit the root system and obtain carbon provided by the plant. The AMF may receive between 10 and 30% of the plant photosynthates (Lopes et al. 2021). Thus, high colonization rates could cause competition for photosynthates, which leads to lower plant development. In this study, the increase in dry matter by the AMF is probably because G. intraradices is an endomycorrhizal fungus. This type of fungus lives between the cells and tissues of the plant and could cause a relative increase in root dry matter compared to the control treatment, without the presence of colonies in the roots. In addition, G. intraradices hyphae could act as root hairs which form networks between plant roots and soil and are effective in improving plant nutritional requirements and availability of water (Kheyri et al. 2022; El-Sawah et al. 2021).
An increase in chlorophyll content was observed in all mycorrhizal treatments. The increase in total chlorophyll content at doses of 50 and 100 spores per plant could be related to increased photosynthetic activity as a consequence of efficient colonization. Studies by Chen et al. (2017) and Di Martino et al. (2019) found that mycorrhizal colonization increases the concentration of photosynthetic pigments. On the other hand, Campo et al. (2020) and Gupta et al. (2021) report that AMF colonization promotes photosynthesis by increasing Rubisco carboxylation and RuBP (ribulose-1,5-bis-phosphate) regeneration. Meddich et al. (2021) found in melon (Cucumis melo) seedlings that administering doses of 44 spores increased chlorophyll content. Wu et al. (2021a, 2021b) found an increase in the photosynthetic rate in watermelon (Citrullus lanatus L. cv. Qilin) at a dose of 300 spores per plant. According to Aseel et al. (2019), adequate nutritional status promotes an increase in chlorophyll content and photosynthetic efficiency.
The results obtained in this study demonstrate the effect of different doses of mycorrhizae on the survival and colonization percentages of sugarcane plantlets. In general, the survival percentage tends to decrease as the mycorrhizal colonization percentage increases. Lotfi et al. (2019) in in vitro pear (Pyrus communis) shoots inoculated with Rhizophagus irregularis at a dose of 50 spores per plant obtained 95% survival compared to the control with 70% survival. Similarly, de Souza-Ferrari et al. (2020) inoculated turmeric (Curcuma longa L.) with a dose of 150 spores per plant and obtained a survival rate of 100% compared to the control, with 90% survival. The negative effect on survival at high mycorrhizal doses has been reported in other studies. Gomes et al. (2021) in strawberry tree (Arbutus unedo L.) obtained 65.9% survival when inoculated at a dose of 3,200 spores per plant of arbutoid mycorrhizae. These results agree with those reported by Wu et al. (2021a, 2021b), who determined that high doses of mycorrhizal inoculant are not appropriate for the establishment of a symbiotic interaction between AMF and plant roots due to a toxic effect of the fungus on the plant that could affect plant survival and development. On the other hand, according to Lerat et al. (2003), mycorrhizae have the ability to store nutrients in their tissues while consuming the photosynthetic products of plants, which could lead to nutritional competition. In addition, excessive AMF colonization can cause changes in plant metabolism (Hodge et al. 2010; Shtark et al. 2021).
The characterization of the mycorrhizal-sugarcane interaction showed a symbiotic association between the two species at doses of 50 and 100 spores per plant. This fact gives an advantage to the symbiotic interaction; however, an excess of hyphae as a result of increased colonization could cause competition for the elements that showed deficiencies. The extension of AMF hyphae allows them to obtain more soil volume compared to non-mycorrhizal plants. The deficiency of some elements in plants can be explained by a competition in the symbiotic interaction because it is an obligate association for the fungus, but not for the plant, which could cause an antagonistic interaction.
The AMF inoculation had an effect on the content of some macronutrients and micronutrients in sugarcane plantlets grown ex vitro. However, although AMF hyphae have the ability to absorb and transfer N from the soil to the roots, there were no significant differences in N accumulation. The low content of the macronutrients P, K, and Mg at doses of 200 and 400 spores per plant may have occurred due to competition for these nutrients as a result of excess AMF colonization. The high magnesium content at doses of 50 and 100 spores per plant may also be related to the high chlorophyll content in this treatment. Magnesium plays an important role in the chlorophyll molecule because it participates in light absorption and CO2 assimilation reactions in the chloroplast (Chaudhry et al. 2021). Regarding Ca, the higher content of this element at doses of 50 and 100 spores could be due to an efficient colonization without affecting the symbiotic interaction, whereas the decrease in Ca at doses of 400 spores per plant could also be due to an excess in colonization, causing competition for this element. The micronutrients Fe and Cu were also affected at doses of 200 and 400 spores per plant, whereas Zn and Mn were only affected at doses higher than 100 spores per plant. Bouskout et al. (2022), when inoculating caper (Capparis spinosa) with 30 spores per plant of a consortium of native AMF, observed that plantlet growth improved considerably due to a higher accumulation of P, K, Mg, Fe, and Zn. Al-Karaki and Williams (2021) reported that there was a higher concentration of macro- (N, P, and K) and micronutrients (Fe and Cu) in leaves of plants inoculated with the mycorrhizal consortium (Rhizophagus clarus, Rhizophagus intraradices, Septoglomus desertícola and Funneliformis mosseae) at a dose of 750 spores per plant compared to the control. AMF have been shown to positively influence the accumulation of some mineral nutrients, especially those with low mobility such as P and Zn (Lotfi et al. 2019; Ortas 2019).
The results obtained in this study have a practical implication for the acclimatization phase during micropropagation due to the importance of early inoculation with mycorrhizae in plantlets obtained in vitro. The application of mycorrhizae is expected to result in vigorous plantlets with an adequate root system, a better use of fertilization in the field and better survival in the field.
5 Conclusions
Inoculation of Glomus intraradices at different doses has effects on the survival and colonization percentages and physiology of ex vitro plantlets of Saccharum spp. cv Mex 69–290 during the acclimatization stage. Arbuscular mycorrhizal fungi, at doses of 50 and 100 spores per plant, with 30 and 58% colonization, respectively, improved plantlet development and promoted an increase in chlorophyll content without affecting survival during acclimatization. Different doses of arbuscular mycorrhizal fungi spores per plant were shown to have an effect on the uptake of some nutrients. Doses of 200 and 400 spores per plant, which represent the highest colonization percentage, had negative effects on survival, development, chlorophyll content, and nutritional status of some elements. The early application of mycorrhizae to sugarcane plantlets during acclimatization could represent a preconditioning advantage before transplanting for the establishment of basic seedbeds.
Data Availability
The datasets generated during and, or, analyzed during the current study are available from the corresponding author on reasonable request.
References
Alcántar GG, Sandoval VM (1999) Manual de análisis químico de tejido vegetal. In: Sociedad Mexicana de la Ciencia del Suelo AC (ed) Publicación especial 10 Chapingo, México, p 156
Al-Karaki GN, Williams M (2021) Mycorrhizal mixtures affect the growth, nutrition, and physiological responses of soybean to water deficit. Acta Physiol Plant 43:1–9. https://doi.org/10.1007/s11738-021-03250-0
An ZQ, Shen T, Wang HG (1993) Mycorrhizal fungi in relation to growth and mineral nutrition of apple seedlings. Sci Hortic 54:275–285. https://doi.org/10.1016/0304-4238(93)90106-Z
Aseel DG, Rashad YM, Hammad SM (2019) Arbuscular mycorrhizal fungi trigger transcriptional expression of flavonoid and chlorogenic acid biosynthetic pathway genes in tomato against Tomato Mosaic Virus. Sci Rep 9:9692. https://doi.org/10.1038/s41598-019-46281-x
Aslani F, Juraimi AS, Ahmad-Hamdani MS, Alam MA, Hasan MM, Hashemi FSG, Bahram M (2019) The role of arbuscular mycorrhizal fungi in plant invasion trajectory. Plant Soil 441:1–14
Bello-Bello JJ, Mendoza-Mexicano M, Pérez-Sato JA (2018) In vitro propagation of sugarcane for certified seed production. In Sugar Tech and Research. Intech. https://doi.org/10.5772/intechopen.74037
Bigott AF, Hoy JW, Fultz LM (2019) Soil properties, microbial communities, and sugarcane yield in paired fields with short-or long-term sugarcane cultivation histories. Appl Soil Ecol 142:166–176. https://doi.org/10.1016/j.apsoil.2019.04.027
Bouskout M, Bourhia M, Al Feddy MN, Dounas H, Salamatullah AM, Soufan W, Nafidi H-A, Ouahmane L (2022) Mycorrhizal fungi inoculation improves Capparis spinosa’s yield, nutrient uptake and photosynthetic efficiency under water deficit. Agronomy 12:149. https://doi.org/10.3390/agronomy12010149
Bremner JT (1965) Inorganic forms of nitrogen. In Methods of soil analysis: part 2. Chemical and microbiological properties; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 9; 1179–1237
Campo S, Martín-Cardoso H, Olivé M, Pla E, Catala-Forner M, Martínez-Eixarch M, San Segundo B (2020) Effect of root colonization by arbuscular mycorrhizal fungi on growth, productivity and blast resistance in rice. Rice 13:1–14. https://doi.org/10.1186/s12284-020-00402-7
Chandrasekaran M, Boopathi T, Manivannan P (2021) Comprehensive assessment of ameliorative effects of AMF in alleviating abiotic stress in tomato plants. J Fungi 7:303. https://doi.org/10.3390/jof7040303
Chaudhry AH, Nayab S, Hussain SB, Ali M, Pan Z (2021) Current understandings on magnesium deficiency and future outlooks for sustainable agriculture. J Mol Sci 22:1819. https://doi.org/10.3390/ijms22041819
Chen S, Zhao H, Zou C, Li Y, Chen Y, Wang Z, Jiang Y, Liu A, Zhao P, Wang M, Ahammed GJ (2017) Combined inoculation with multiple arbuscular mycorrhizal fungi improves growth, nutrient uptake and photosynthesis in cucumber seedlings. Front Microbiol 8:2516. https://doi.org/10.3389/fmicb.2017.02516
Choi J, Summers W, Paszkowski U (2018) Mechanisms underlying establishment of arbuscular mycorrhizal symbioses. Annu Rev Phytopathol 56:135–160. https://doi.org/10.1146/annurev-phyto-080516-035521
Da Silva Barros TH, de Araujo Pereira AP, de Souza AJ, Ribeiro NL, Cardoso EJBN, Coelho RD (2019) Influence of sugarcane genotype and soil moisture level on the arbuscular mycorrhizal fungi community. Sugar Tech 21:505–513. https://doi.org/10.1007/s12355-018-0640-0
Damin S, Carrenho R, Martins S (2020) The influence of mycorrhization on the growth of Zea mays L. and the sclerification of foliar tissues susceptible to chewing insect attacks. Braz J Bot 43:493–502. https://doi.org/10.1007/s40415-020-00621-8
De Souza Ferrari MP, Da Cruz RMS, Dos Santos QM et al (2020) Efficient ex vitro rooting, acclimatization, and cultivation of Curcuma longa L. from mycorrhizal fungi. J Crop Sci Biotechnol 23:469–482. https://doi.org/10.1007/s12892-020-00057-2
Di Martino C, Fioretto A, Palmieri D, Torino V, Palumbo G (2019) Influence of tomato plant mycorrhization on nitrogen metabolism, growth and fructification on P-limited soil. J Plant Growth Regul 38:1183–1195. https://doi.org/10.1007/s00344-019-09923-y
El-Sawah AM, El-Keblawy A, Ali DFI, Ibrahim HM, El-Sheikh MA, Sharma A, Alhaj Hamoud Y, Shaghaleh H, Brestic M, Skalicky M, Xiong Y-C, Sheteiwy MS (2021) Arbuscular Mycorrhizal Fungi and Plant Growth-Promoting Rhizobacteria Enhance Soil Key Enzymes, Plant Growth, Seed Yield, and Qualitative Attributes of Guar. Agriculture 11:194. https://doi.org/10.3390/agriculture11030194
Fors RO, Saggin OJ, Carneiro MAC, Berbara RLL (2020) Selection of arbuscular mycorrhizal fungi for sugarcane in four soils with the presence of dark septate endophytes. Acta Sci Agron 42. https://doi.org/10.4025/actasciagron.v42i1.42477
Gomes B, Castro F, Santos R, Figueiredo P, Silva M, Vidal M, Gomes F (2021) Effect of Quercetin on mycorrhizal synthesis between Tuberborchii and Arbutusunedo L In Vitro Plants. Microbiol Res 12:69–81. https://doi.org/10.3390/microbiolres12010007
Gómez-Kosky R, Jaramillo DN, Esquiro CR, Villegas AB, Calimano MB, Armas PM, Daniels DD (2020) Effect of VIUSID Agro® and FitoMas-E® on the ex vitro acclimatization of sugarcane plants (Saccharum spp.) Cultivar C90–469. Sugar Tech 22:42–51. https://doi.org/10.1007/s12355-019-00752-7
Gupta S, Thokchom SD, Kapoor R (2021) Arbuscular mycorrhiza improves photosynthesis and restores alteration in sugar metabolism in Triticum aestivum L. grown in arsenic contaminated soil. Front Plant Sci 12:334. https://doi.org/10.3389/fpls.2021.640379
Harborne JB (1973) Compuestos fenólicos en Métodos fitoquímicos. Springer, Dordrecht, Holanda, pp 33–88
Hodge A, Fitter AH, Díaz SM (2010) Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. Proc Natl Acad Sci USA 107:13754–13759. http://www.jstor.org/stable/25708797. Accessed 7 Jan 2022
Juntahum S, Jongrungklang N, Kaewpradit W et al (2020) Impact of arbuscular mycorrhizal fungi on growth and productivity of sugarcane under field conditions. Sugar Tech 22:451–459. https://doi.org/10.1007/s12355-019-00784-z
Kheyri Z, Moghaddam M, Farhadi N (2022) Inoculation efficiency of different mycorrhizal species on growth, nutrient uptake, and antioxidant capacity of Calendula officinalis L.: a comparative study. J Soil Sci Plant Nutr 22:1160–1172. https://doi.org/10.1007/s42729-021-00721-8
Lerat S, Lapointe L, Piché Y, Vierheilig H (2003) Variable carbon-sink strength of different Glomus mosseae strains colonizing barley roots. Rev Can Bot 81:886–889. https://doi.org/10.1139/b03-070
Lopes JI, Correia CM, Gonçalves A, Silva E, Martins S, Arrobas M, Rodrigues MÂ (2021) Arbuscular mycorrhizal fungi inoculation reduced the growth of pre-rooted olive cuttings in a greenhouse. Soil Systems 5:30. https://doi.org/10.3390/soilsystems5020030
López-Ráez JA, Pozo MJ (2013) Chemical signaling in the arbuscular mycorrhizal symbiosis: biotechnological applications. In Symbiotic endophytes (pp. 215–232). Springer, Berlin, Heidelberg.
Lotfi M, Fernandez K, Vermeir P et al (2019) In vitro mycorrhization of pear (Pyrus communis). Mycorrhiza 29:607–614. https://doi.org/10.1007/s00572-019-00919-w
Meddich A, Ait Rahou Y, Boutasknit A, Ait-El-Mokhtar M, Fakhech A, Lahbouki S, Wahbi S (2021) Role of mycorrhizal fungi in improving the tolerance of melon (Cucumis melo) under two water deficit partial root drying and regulated deficit irrigation. Plant Biosyst 1-11. https://doi.org/10.1080/11263504.2021.1881644
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Ortas I (2019) Under filed conditions, mycorrhizal inoculum effectiveness depends on plant species and phosphorus nutrition. J Plant Nutr. https://doi.org/10.1080/01904167.2019.1659336
Ortas I, Rafique M, Akpinar C, Kacar YA (2017) Growth media and mycorrhizal species effect on acclimatization and nutrient uptake of banana plantlets. Sci Hortic 217:55–60. https://doi.org/10.1016/j.scienta.2017.01.025
Porras-Soriano A, Soriano-Martın ML, Porras-Piedra A, Azcon R (2009) Arbuscular mycorrhizal fungi increased growth, nutrient uptake and tolerance to salinity in olive trees under nursery conditions. J Plant Physiol 166:1350–1359. https://doi.org/10.1016/j.jplph.2009.02.010
Ren L, Wang B, Yue C, Zhou S, Zhang S, Huo H, Xu G (2019) Mechanism of application nursery cultivation arbuscular mycorrhizal seedling in watermelon in the field. Ann Appl Biol 174:51–60. https://doi.org/10.1111/aab.12469
Rivero AM, Ramírez-Mosqueda MA, Frómeta OM, Morgado MME, Paneca MR, Escriba RCR, Bello-Bello JJ (2020) Influence of Vitrofural® on sugarcane micropropagation using temporary immersion system. Plant Cell Tiss Organ Cult 1-7. https://doi.org/10.1007/s11240-020-01800-x
Sales FR, Silva AO, Sales LR, Rodrigues TL, de Souza Moreira FM, Carneiro MAC (2021) Native arbuscular mycorrhizal fungi exhibit biotechnological potential in improvement of soil biochemical quality and in increasing yield in sugarcane cultivars. Sugar Tech 1-12. https://doi.org/10.1007/s12355-021-01016-z
Sentíes-Herrera HE, Trejo-Téllez LI, Gómez-Merino FC (2017) The Mexican sugarcane production system: History, current status, and new trends. Sugarcane: Production Systems, Uses and Economic Importance; Murphy, R., Ed, 39–71
Shabbir R, Javed T, Afzal I, Sabagh AE, Ali A, Vicente O, Chen P (2021) Modern biotechnologies: innovative and sustainable approaches for the improvement of sugarcane tolerance to environmental stresses. Agronomy 11:1042. https://doi.org/10.3390/agronomy11061042
Shtark O, Puzanskiy R, Avdeeva G, Yemelyanov V, Shavarda A, Romanyuk D, Kliukova M, Kirpichnikova A, Tikhonovich I, Zhukov V, Shishova M (2021) Metabolic alterations in Pisum sativum roots during plant growth and arbuscular mycorrhiza development. Plants 10:1033. https://doi.org/10.3390/plants10061033
Tauler M, Baraza E (2015) Improving the acclimatization and establishment of Arundo donax L. plantlets, a promising energy crop, using a mycorrhiza-based biofertilizer. Ind Crops Prod 66:299–304. https://doi.org/10.1016/j.indcrop.2014.12.039
Tripathi P, Chandra A, Prakash J (2022) Changes in physio-biochemical attributes and dry matter accumulation vis a vis analysis of genes during drought and stress recovery at tillering stage of sugarcane. Acta Physiol Plant 44:1–10. https://doi.org/10.1007/s11738-021-03336-9
Trouvelot S, Trouvelot S, Bonneau L, Van Tuinen D, Adrian M, Wipf D (2015) Arbuscular mycorrhiza symbiosis in viticulture: a review. Agron Sustain Dev 35:1449–1467. https://doi.org/10.1007/s13593-015-0329-7
Vergara C, Araujo K, de Souza SR, Schultz N, Saggin-Júnior OJ, Sperandio M, Zilli JE (2019) Plant-mycorrhizal fungi interaction and response to inoculation with different growth-promoting fungi. Pesqui Agropecu Bras 54:e25140. https://doi.org/10.1590/S1678-3921.pab2019.v54.25140
Wu QS, Zou YN, Wang GY (2011) Arbuscular mycorrhizal fungi and acclimatization of micropropagated citrus. Commun Soil Sci Plant Anal 42:1825–1832. https://doi.org/10.1080/00103624.2011.587570
Wu M, Yan Y, Wang Y, Mao Q, Fu Y, Peng X, Yang Z, Ren J, Liu A, Chen S, Ahammed GJ (2021a) Arbuscular mycorrhizal fungi for vegetable (VT) enhance resistance to Rhizoctonia solani in watermelon by alleviating oxidative stress. Biol Control 152:104433. https://doi.org/10.1016/j.biocontrol.2020.104433
Wu YH, Wang H, Liu M, Li B, Chen X, Ma YT, Yan ZY (2021b) Effects of native arbuscular mycorrhizae isolated on root biomass and secondary metabolites of Salvia miltiorrhiza Bge. Front Plant Sci 12:66. https://doi.org/10.3389/fpls.2021.61789
Wu S, Shi Z, Chen X, Gao J, Wang X (2022) Arbuscular mycorrhizal fungi increase crop yields by improving biomass under rainfed condition: a meta-analysis. Peer J 10:e12861. https://doi.org/10.7717/peerj.12861
Xiao X, Chen J, Liao X, Yan Q, Liang G, Liu J, Wang D, Guan R (2022) Different arbuscular mycorrhizal fungi established by two inoculation methods improve growth and drought resistance of Cinnamomum Migao seedlings differently. Biology 11:220. https://doi.org/10.3390/biology11020220
Yadav K, Aggarwal A, Singh N (2013) Arbuscular mycorrhizal fungi (AMF) induced acclimatization, growth enhancement and colchicine content of micropropagated Gloriosa superba L. plantlets. Ind Crops Prod 45:88–93. https://doi.org/10.1016/j.indcrop.2012.12.001
Author information
Authors and Affiliations
Contributions
Conceptualization, R. S. P., L. S. S., and J. L. S. C.; data curation, M. R. M. H.; formal analysis, M. R. M. H.; methodology, J. J. B. B.; project administration, J. J. B. B.; writing—original draft, M. R. M. H.; L. S. S., provided supervision and guidance about all the microscopic assays; writing—review and editing, R. S. P., L. S. S. and J. L. S. C. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
del Rosario, MH.M., Luis, SC.J., Lino, SS. et al. Arbuscular Mycorrhizal Fungi: Inoculum Dose Affects Plant Development and Performance of Sugarcane (Saccharum spp.) Plantlets During Acclimatization Stage. J Soil Sci Plant Nutr 22, 4847–4856 (2022). https://doi.org/10.1007/s42729-022-00964-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-022-00964-z