Abstract
Heavy metals (HMs) are one of the major problems worldwide, limiting plant productivity and menacing human health. In this study, we aim to investigate the effects of three HMs (cadmium (Cd), copper (Cu), and lead (Pb)) with three levels (100, 200, and 300 µM) on Origanum majorana L. (Marjoram) and Peganum harmala L. (Harmal) germination and antioxidant enzymatic activities to highlight the possibility of their use in the rehabilitation of HM-polluted soil. Germination test was conducted in sterilized Petri plates using Whatman filter paper and several physiological and biochemical parameters were evaluated: seed germination parameters (such as germination percentage (G%), germination index (GI), Timson’s germination index (TGI), seedling vigor index (SVI), mean germination time (MGT), and metal tolerance (MTI)), shoot and root growth, and biomass, in addition to the antioxidant enzyme activities of both plants. The shoot and root biomass slightly decreased under stress for both plants compared to the control. The results showed that the roots were more affected by stress than shoots. The MTI indicated that Marjoram was more tolerant to Pb and Harmal to Cu exposure at the germination stage. The activity of glutathione peroxidase, glutathione reductase, glutathione S-transferase, and superoxide dismutase increased in both plants. However, nicotinamide adenine dinucleotide phosphate–dependent isocitrate dehydrogenase decreased. The data suggest that Marjoram and Harmal have an efficient antioxidant system protecting them against oxidative stress caused by Cd, Cu, and Pb. Therefore, Marjoram and Harmal might be considered metallotolerants for Pb and Cu respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Heavy metals (HMs) are among the most dangerous pollutants that can be accumulated and persist in the environment for extended periods of time and can be spread to non-contaminated sites through wind and water. They are found in the aquatic environment, the atmosphere, soils, and plants (Farid et al. 2015). Several HMs are essential for living beings (humans, plants, and animals) in adequate quantities, including zinc (Zn), Cu, iron (Fe), manganese (Mn), and nickel (Ni). They have an important role in several biological and metabolic processes (Chaffai and Koyama 2011; Fageria et al. 2009), but they are toxic at higher concentrations (Nedjimi 2020; Zhang et al. 2020; Rajpoot et al. 2020). While non-essential metals such as Cd, mercury (Hg), and Pb have no known vital biological role in living organisms, they are extremely hazardous even at very low doses.
These metals have different effects on natural resources. When they are present in soil, they impact soil quality by affecting physicochemical parameters such as pH, color, and porosity, and may be leached into groundwater. These metals can be harmful to plants while also endangering human and animal health. They can be consumed directly or enter the food chain through eating of these plants by humans or animals (Gupta et al. 2013).
The increasing number of HM-polluted lands worldwide makes their rehabilitation indispensable. Various physical, chemical, electrical, and thermal remediation techniques (or their combination) have emerged to fulfill these needs including containment (surface capping, encapsulation), extraction, solidification, soil washing, soil flushing, immobilization, electrokinetic, vitrification, and thermal desorption, which can be practiced in situ or ex situ (Liu et al. 2018). However, most of them are expensive and difficult to applied, and require a significant amount of energy. Nowadays, the main research interest is to develop alternative solutions to the problem of HM-polluted soil without deteriorating the physical structure of the ecosystem, their biological activities, and chemical properties (Liu et al. 2018). This condition is the main limitation of physical and chemical remediation techniques contrary to biological methods (Kang 2014). Phytoremediation is a sustainable and eco-friendly plant-based strategy to remove, transform, and degrade pollutants present in the air, water, and soil (Nedjimi 2020). Furthermore, economic considerations and technical complexities have made biological techniques the best option for cleaning up the soil, in which bio/phytoremediation is relatively economical compared to physical and chemical remediation techniques.
The proper selection of plant species is very important to develop a phytoremediation process. One of the most important criteria for selecting plants for remediation is their ability to grow in contaminated soil. Despite the rich literature on using many edible crops for this purpose (Chu et al. 2020; Sadia et al. 2016; Angelova et al. 2011; Dimkpa et al. 2009; Marchiol et al. 2004), a little concern is provided to aromatic and medicinal plants (AMPs) as a novel option for phytoremediation use. AMPs may be a potential candidate for cultivation in HM-contaminated soils, as this adverse environment may cause increased production of their secondary metabolite (Ait Elallem et al. 2021). Furthermore, many studies were focused on the effects of HMs on the growing stage of the plant. However, only few studies have been conducted during their early seedling stage, especially for the AMPs. In fact, more comprehensive research on seed germination, which is a key stage in ensuring healthy and tolerant seedlings to cope with the stress caused by pollution, should be conducted.
Plants use different strategies to alleviate absorbed HMs in foliar cells and roots, such as morphological, physiological, biochemical, and genetic mechanisms (Schreck et al. 2012). In fact, the overabundance of HMs leads to an oxidative stress by increasing the reactive oxygen species (ROS) levels (Singh et al. 2006). Thus, these ROS can be eliminated by producing and raising the enzymatic and non-enzymatic antioxidants that assist the alleviation of oxidative stress and enhance environmental stress tolerance (Schutzendubel 2002).
Metallotolerant AMPs were the best candidate for phytoremediation especially phytostabilization of HM-contaminated soils. The studies suggested that the cultivation of these plants could remediate metal-contaminated agricultural soils (Maiti and Kumar 2016; Pandey et al. 2019; Roodi et al. 2012). Several authors investigated the quality of essential oils from AMPs grown on contaminated soils. They have shown that, despite the fact that some AMPs could accumulate substantial amounts of metals when grown on contaminated areas, the essential oils produced by these crops have not been significantly polluted by metals (Asgari Lajayer et al. 2017; Bensabah et al. 2015; Bozhanov et al. 2007; Lal et al. 2013; Lydakis-Simantiris et al. 2016; Zheljazkov et al. 2006). However, it seems important to learn more about the impact of excessive concentration of HMs in aromatic plants commonly used as a source of biologically active compounds.
O. majorana L. and P. harmala L. are two medicinal plants commonly used as culinary herbs; their active ingredients are widely used in cosmetics and pharmaceuticals (Dashti 2020). Researchers reported the existence of many phytochemicals in several parts of the two plants such as phenols, flavonoids, and alkaloids. These two plants are found in polluted soils, which means they have the ability to cope with HMs than other plant species. In this line, O. majorana L. and P. harmala L. were chosen for this study as they are important and popular aromatic and medicinal plants recognized for their essential oil production and therapeutic applications. Though literatures on the effects of HMs on medicinal plants are plenty, conducting germination test under laboratory condition in Petri dishes using these two aromatic plants could be helpful to evaluate their resistance under the stressful conditions of HMs. Therefore, the aim of this study was to evaluate the effect of HMs on seed germination, enzymatic activities, and oxidative state in O. majorana L. and P. harmala L. cultivated in a medium supplemented with Pb, Cu, and Cd.
2 Materials and Methods
2.1 Plant Material
O. majorana L. is a perennial plant from the Lamiaceae family, native to the eastern Mediterranean basin. P. harmala L. is a perennial plant native to a wide area and quite common in Morocco. It is a plant that survives in a stressful environment, and occurs naturally in degraded and contaminated lands due to its tolerance to different environmental stress and ecological conditions (Benidire et al. 2016, 2021; Boularbah et al. 2006). Both plants serve as medicinal and aromatic plants.
Seeds of O. majorana L. were purchased from a commercial store (Batlle—Huerto y Jardín). However, seeds of P. harmala L. were hand-collected in July 2020 from an arid urban zone next to a traffic road in Marrakech region (N 31°64ʹ60ʺ/W 8°02ʹ18ʺ). Seeds were randomly harvested from several individual plants in order to reduce the influence of genetic variation.
2.2 Germination Bioassays
Germination test was conducted in sterilized Petri plates (90 mm of diameter) using Whatman No. 1 filter paper. The papers were soaked with respective concentrations (0, 100, 200, and 300 µM) of metal solutions (supplied as Pb (NO3)2, CdSO4, and CuSO4) as described earlier in Nedjimi (2020). The deionized water (representing the concentration 0) was used as a control. The seeds were placed in a microcentrifuge tube and then, a 2% hypochlorite (NaClO) solution was added. The tube was shaken for 5 min to sterilize the seeds. The seeds were washed several times with sterile distilled water to remove the NaClO traces. Then, they were placed on a sterilized filter paper and left to dry. Twenty-five previously surface-sterilized seeds were put on the Whatman filter paper germination plate, irrigated with 5 mL of the appropriate solution and covered with adhesive tape to prevent moisture loss. The plates were kept in the dark for 2 days to initiate the germination and then placed in a 12/12 h photoperiod growth chamber with an average temperature of 25 ± 2 °C, average humidity of 30 ± 2% RH, and average light intensity of 2600 lx. At the end of 20 days, the seedlings were analyzed for several germination parameters.
2.3 Biomass and Morphological Measurements
The seedlings were first washed thoroughly with distilled water. Then, the height and root length of O. majorana L. and P. harmala L were measured in fresh samples with a metric scale. Humid weight of roots (RHW) and shoots (SHW) was recorded separately (using electronic balance RADWAG, model: AS 220.R2 PLUS, Poland). After taking fresh samples for enzyme extraction, shoots and roots were dried in oven (Memmert oven UF110, Germany) for 48 h at 70 °C and weighed again to establish dry weight (SDW and RDW).
2.4 Germination Parameters
To study the influence of the different metal treatments on initial seedling growth, germination data were recorded every 24 h, and several germination parameters were calculated from the obtained data. Germination index (GI), Timson’s germination index (TGI), mean germination time (MGT), seedling vigor index I (VI), inhibition of germination (Inh G%), and metal tolerance index (MTI) were calculated using the equations described in Table 1.
2.5 Enzymatic Extraction and Essays
A quantity of 0.3 g fresh shoot samples was mixed in an ice bath, each with 3 mL of cold 0.005 M potassium phosphate buffer (pH 7.8), 0.2 mM ethylenediaminetetraacetic acid (EDTA), and 2% (w/v) polyvinylpyrrolidone (PVP). The homogenate was centrifuged at 12,000 rpm for 20 min at 4 °C (centrifuge: 521–1894 VWR Mega Star 600R, Germany). Then, the supernatant was used for glutathione reductase (GR), glutathione S-transferase (GST), glutathione peroxidases (GPx), isocitrate dehydrogenase (ICDH), and superoxide dismutase (SOD) activities assays (Mahdavian et al. 2016).
2.5.1 Glutathione Reductase (GR) Activity
The GR activity was determined by the oxidation of nicotinamide adenine dinucleotide phosphate (NADPH) at 340 nm (extinction coefficient 6.2 mM−1 cm−1) as described by Rao et al. (1996). The reaction mixture contained 100 mM potassium phosphate buffer (pH 7.8), 0.2 mM NADPH, 0.5 mM glutathione disulfide (GSSG), and 10 µL of enzyme extract in a final volume of 1 mL. The reaction was initiated by the addition of NADPH at 30 °C. The activity of GR was expressed as μmol of NADPH oxidized/min/mg of protein.
2.5.2 Glutathione S-Transferase (GST) Activity
The activity of GST was determined by the method of Habig et al. (1974) using 1-chloro-2,4-dinitrobenzene (CDNB) as a substrate. A total of 1 mL of assay mixture contained 5 mM GSH, 2 mM CDNB, 0.1% Triton X100, 0.1 M phosphate buffer (pH 6.5), and 20 µL enzyme extract. The reaction was monitored spectrophotometrically at 340 nm using a UV–Vis spectrophotometer (GENESYS 50). Product concentration was calculated using a molar extinction coefficient of 9.6 mM−1 cm−1. GST activity was expressed as μmol of the GSH-CDNB conjugate formed min−1 mg−1 of protein.
2.5.3 Glutathione Peroxidases (GPx) Activity
GPx activity was measured by the method of Lawrence and Burk (1976) with slight modifications. The reaction mixture contained potassium phosphate (0.05 M, pH 7.4), 1 mM EDTA, 1 mM sodium azide, 1 mM GSH, GR (4 µg/mL), 0.2 mM NADPH, 0.25 mM of H2O2, and 5µL of enzyme extract. The rate of NADPH oxidation was monitored at 340 nm (GENESYS 50 UV–Vis spectrophotometer). GPx activity was calculated and expressed as µmol of NADPH oxidized/min/mg protein using the extinction coefficient of 6.2 mM−1 cm−1.
2.5.4 Isocitrate Dehydrogenase (ICDH) Activity
The NADP-ICDH activity was measured according to Domínguez et al. (2003) with slight modifications. The assay mixture contained 50 mM potassium phosphate buffer (pH 7.5), 1 mM MnCl2, 1 mM NADP+, and 4 mM isocitrate with 10 µL of enzyme extract. ICDH activity was measured by NADPH production at 340 nm (GENESYS 50 UV–Vis spectrophotometer). One unit of ICDHA activity is defined as the amount of enzyme catalyzing the production of 1 µmol of NADPH/min.
2.5.5 The Superoxide Dismutase (SOD) Activity
SOD activity assay was based on the method of Beauchamp and Fridovich (1971). One unit of enzyme activity was defined as the quantity of SOD required to produce a 50% inhibition in the photochemical reduction of nitro blue tetrazolium (NBT) at 560 nm (GENESYS 50 UV–Vis spectrophotometer). The reaction mixture (3 cm3) contained 50 mM Na phosphate buffer (pH 7.8), 0.075 mM NBT, 2 mM l-methionine, 0.001 mM EDTA, 0.002 mM riboflavin, and 5µL of enzyme extract. Reactions were carried out for 10 min at 25 °C, under Photosynthetic Photon Flux Density (PPFD) of about 300 μmol m−2 s−1 (UVP White Light Transilluminator (UVP 95,021,401), 1 × 32 W).
2.6 Statistical Analysis
The software R (version 4.0.2) was used to perform the statistical evaluation. A two-way ANOVA was used to determine the effect of HMs, their concentration, and the interaction of HMs’ concentration on germination parameters and enzymatic activities of both medicinal plants. Tukey’s honest significant difference (HSD) test was applied as a post hoc test at P < 0.05 level to evaluate significant differences between treatments.
A normality test was performed prior analysis; Shapiro–Wilk’s test (P < 0.05) and the analysis of their histograms, normal Q-Q plots, and box plots showed that most variables (germination parameters and enzymatic activities) were approximately normally distributed for each metal. The non-normally distributed variables were transformed (using Box-Cox transformation) to ensure the normal distribution of data. The graphics were generated using Excel.
3 Results
3.1 Heavy Metals’ Effect on Germination Parameters of P. harmala and O. majorana
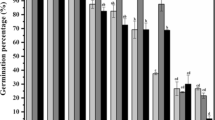
Seeds of O. majorana initiated germination in all media (supplied or not with Cd, Cu, or Pb) after 3 days (Fig. 1a–c). In contrast, the seed germination of P. harmala L. occurred after 2 days (Fig. 1d–f), with a higher number of germinated seeds recorded in the absence of HMs for both plants (Fig. 1). Germination percentage at the end of germination time was 81% and 92.8% for O. majorana L. and P. harmala L., respectively, in the absence of HMs in the medium. The seed germination percentage of the two plants on the medium supplied with Cd, Cu, or Pb was slightly reduced with the increasing concentration compared to the control (Fig. 1). Two-way ANOVA highlights the significant effect of metal type (M) and metal concentration (C) (P ˂ 0.001) on MGT, GI, TGI, and inhG% for both plants (Table 2).
Effect of heavy metal treatments on the cumulative percent germination of O. majorana L. (a–c) and P. harmala L. (d–f) in function of days. Data are mean ± S.D. (n = 6). Significant variation between treatments according to Tukey’s (HSD) test (P < 0.05) indicated with different letters. ns, not significant
Among HMs, Cu had a more inhibitory effect on O. majorana L. seed germination with a maximum decline of germination by 20.94% and 19.51% observed at 300 µM Cd and Cu, respectively. However, the minimum inhibitory effect (3.92%) was recorded under 100 µM Pb. Seeds of O. majorana L. treated with Cu, especially at the highest concentration (300 µM), showed the lowest germination indexes (both GI = 199 and TGI = 0.53) followed by 100 µM and 200 µM Cu and Cd under all concentrations for TGI (0.57–0.58). However, Pb had no significant effect (P ˂ 0.05) on TGI compared to the control (0.63–0.64). The longest main germination time (MGT = 3.808 days) was recorded under 300 µM Cd (Table 2). Compared to the control, it delays germination by 0.43 day (12.72%) (P < 0.05). The level of metal effect on germination parameters of O. majorana L. was in decreasing order: Cu > Cd > Pb.
In contrast, P. harmala L. seeds at 300 µM Cd recorded the highest germination inhibition (23.6%), which showed the longest MGT (3.764 days) as it delays germination by 0.96 days (34.28%) compared to the control. The lowest value of germination indexes (GI = 242.5, TGI = 0.647) was also observed under 300 µM Cd (Table 2). The results indicate that Cd had a significant suppressed effect (P ˂ 0.05) on germination parameters of P. harmala L. seeds. The level of metal effect on germination parameters of P. harmala L. was in decreasing order: Cd > Pb > Cu.
3.2 Effect of Heavy Metals on Biomass and Morphological Parameters of P. harmala L. and O. majorana L.
3.2.1 Shoot and Root Length
The effect of the three metals on the shoot and root length of P. harmala L. and O. majorana L. seeds is presented in Fig. 2. The result showed that the tested HMs affect the shoot and roots length of both plants (P < 0.001). Increasing metal concentrations in the culture media progressively increased the inhibition of root and shoot length in O. majorana L. and P. harmala L. compared to the control (P < 0.001).
Inhibition of shoot and root length of O. majorana L. (a, c) and P. harmala L. (b, d) exposed to increasing metal concentrations (Cd, Cu, and Pb). Data are mean ± S.D. (n = 6). Significant variation between treatments (P < 0.05) indicated with different letters. Uppercase letters indicate the difference between effects of each metal, and lowercase letters indicate the difference between overall treatments effects. A two-way ANOVA indicates a significant effect of metal concentration and their interaction on the shoot length and root length (***P < 0.001)
The shoot length of O. majorana L. was significantly (P < 0.001) inhibited by the Cd concentration (20.7% at 100 µM, 23.4% at 200 µM, and 32.2% at 300 µM) (Fig. 2a). However, Cu had a stronger negative effect on root length (77.27% at 300 µM) when compared with Cd and Pb (Fig. 2c). Cu and Cd had the most significant effect in reducing the shoot and root length in O. majorana L., respectively. The inhibition decreased in the order Cd > Cu > Pb for shoots and Cu > Cd > Pb for roots.
The increasing concentration of Cd had a pronounced effect on decreasing the shoot (32.19% at 300 µM) (Fig. 2b) and root (81.58% at 300 µM) length (Fig. 2d) of P. harmala L. The increasing concentration of Cu influenced especially the root length which decreased significantly and gradually to reach 55.8% at 300 μM Cu. However, Pb did not show any significant effect on the shoot length at 100 µM (0.6% reduction compared to the control) (Fig. 2b) but had a pronounced negative effect on root length starting at the lower concentration (48.5% at 100 µM to 55.35% at 300 µM) (Fig. 2d). Cd was the metal with the most significant effect (P < 0.001) on reducing the shoot and root length in P. harmala L. compared to Cu and Pb. The inhibition decreased in the order Cd > Cu > Pb for shoots and Cd > Pb > Cu for roots.
In terms of elongation, the results showed that the plants’ roots are the most affected organ by the metal exposure. The root elongation inhibition reaches 81.58% under Cd in P. harmala L. and 77.27% under Cu in O. majorana L.
3.2.2 Shoot and Root Dry Weight
The shoot and root dry weights of both species tested in Cd, Cu, and Pb treatments were significantly lower than those in the control. Figure 3 shows that metal exposure had an inhibitory effect on the shoot and root dry weight of O. majorana L. and P. harmala L. starting at 100 µM for the three metals with a clear and pronounced effect on O. majorana L., especially under Pb (81.5% for shoot and 87.6% for root dry weight at 300 µM Pb). However, no significant difference was observed on the inhibition of shoots (Fig. 3a) and root (Fig. 3c) dry weights of O. majorana L. with the rising metal concentration starting from 100 µM. The inhibitory effects of the three metals in the shoot (Fig. 3b) and root (Fig. 3d) dry weight of P. harmala L. were not as pronounced as it was in O. majorana L. The inhibition percentage did not exceed 26.42% in the shoot dry weight (Fig. 3b) and 56.12% in root dry weight (Fig. 3d) under Pb treatment.
Variation in the shoot (SDW) and root (RDW) dry weight of O. majorana L. (a, c) and P. harmala L. (b, d) under increasing Cd, Cu, and Pb concentration. Data are mean ± S.D. (n = 6). Significant variation between treatments (P < 0.05) indicated with different letters. Uppercase letters indicate the difference between effects of each metal, and lowercase letters indicate the difference between metal × concentration effects. A two-way ANOVA indicates a significant effect of metal concentration and their interaction on the SDW and RDW (**P < 0.01; ***P < 0.001)
Metal tolerance index of O. majorana L. (a) and P. harmala L. (b) and their seedling vigor index (c, d) exposed to Cd, Cu, and Pb. Data are mean ± S.D. (n = 6). Significant variation between treatments (P < 0.05) indicated with different letters. Uppercase letters indicate the difference between effects of each metal, and lowercase letters indicate the difference between metal × concentration effects. A two-way ANOVA indicates a significant effect of metal concentration and their interaction on the metal tolerance index and seedling vigor index I (***P < 0.001)
Changes in the enzymatic activities of glutathione peroxidases GPx (a, b), glutathione reductase GR (c, d), glutathione S-transferase GST (e, f), superoxide dismutase SOD (g, h), and isocitrate dehydrogenase ICDH (i, j) in O. majorana L. and P. harmala L. respectively. Shoots after exposure to increasing metal concentrations (Cd, Cu, and Pb). Data are mean ± S.D. (n = 6). Significant variation between treatments according to Tukey’s (HSD) test (P < 0.05) indicated with different letters. Uppercase letters indicate the difference between effects of each metal, and lowercase letters indicate the difference between metal × concentration effects. A two-way ANOVA indicates a significant effect of metal concentration and their interaction on the five enzymatic activities (***P < 0.001) in both plants
3.2.3 Metal Tolerance and Seedling Vigor Index
Metal tolerance index and seedling vigor index I showed similar changes and were significantly impacted by metal type (P < 0.001), the concentration (P < 0.001), and their interaction (P < 0.001). Figure 4 shows MTI (%) and SVI I of O. majorana L. (Fig. 4a, c) and P. harmala L. (Fig. 4b, d).
The increasing concentration of the three studied HMs decreased significantly the SVI and MTI in both plants with a severe reduction at 200 µM Cu in O. majorana L. seedling to reach 122.94 and 22.7% for SVI and MTI respectively at 300 µM. Under Pb and Cd treatment, MTI decreased slowly with the increasing metal concentration to stabilize in 300 µM at 84% and 77.8% respectively. Likewise, SVI decreased under Pb and Cd stress to reach 290 and 278 at 300 µM respectively.
The MTI and SVI under Cu treatment decreased progressively from 100 µM for P. harmala L. to reach 44.2% and 327.6 respectively. Similar value (44.6% and 307.8) was found under 300 µM Pb. SVI and MTI decreased more quickly (100 µM) under Cd and Pb in P. harmala L.; at the highest concentration of Cd, MTI of P. harmala L. decreased to 18.4% and SVI to 185.7.
3.3 Effect of Heavy Metal Treatments on Antioxidant Enzymes of the Plant
The analysis of the main antioxidant enzymatic activities is shown in Fig. 5 for O. majorana L. and P. harmala L. The results demonstrated that HM exposure significantly promotes the antioxidant enzymes over the control.
The GPx activity increased in P. harmala L. (Fig. 5b), and O. majorana L. (Fig. 5a) shoot under different metal exposures. It gradually increases with exposure levels and reaches its highest level at 300 µM Cd (48.66 µmol NADPH min−1 mg−1) increasing by 63% in P. harmala L compared to the control. The GPx activity increased to highest levels in O. majorana L. at 300 µM Pb (35.47 µmol NADPH min−1 mg−1), and it exceeds the control by 80%.
HMs likewise generated GR activity in P. harmala L., particularly under Cd stress, starting at the lowest concentration (29.07 to 35.87 mol NADPH min−1 mg−1 at 100 µM and 300 µM, respectively) (Fig. 5d). It also increased under Cu and Cd in O. majorana L. starting at 200 µM (by 105% compared to the control to reach 12.98 µmol NADPH min−1 mg−1) and 100 µM (exceed by 102.7% to reach 12.83 µmol NADPH min−1 mg−1) respectively and increased with increasing exposure level (Fig. 5c).
The same was observed for GST activity which was higher under Cd stress for both plants. It increased by 41.3% for P. harmala L. and 44% for O. majorana at 300 µM, followed by Cu in P. harmala L. (Fig. 5f) and Pb in O. majorana L. at 100 and 200 µM and reached its higher point at 300 µM Pb (59.63 µmol GSH-CDNB min−1 mg−1) (Fig. 5e). However, the GST activity was not affected under Pb and Cu exposure in P. harmala L. and O. majorana L., respectively.
SOD activity was induced in P. harmala L by Cd (74.64 U min−1 mg−1 at 100 µM) and Cu (65.52 U min−1 mg−1 at 100 µM) and gradually increased to attend its maximum at 300 µM (40% and 33.4% higher than the control for Cd and Cu, respectively) (Fig. 5h). In O. majorana L., SOD activity was also induced by Cu exposure (50.61 U min−1 mg−1 at 100 µM) (Fig. 5g). It increased at first with a peak at 200 µM (26.4% higher than the control) and then decreased at the concentration of 300 µM (to 4.75% higher than the control). At the concentrations 100 µM and 200 µM, no significant effect was observed on O. majorana L. under Cd and Pb. However, when exposed to higher concentrations of those metals, the SOD production has been changed. At 300 µM Cd, the SOD activity was slightly increased while it decreased at 300 µM Pb compared to the control.
Contrary to previous enzymatic activities, the ICDH activity decreased significantly with metal exposure starting at 100 µM. It decreases progressively with the increasing concentration of Pb in P. harmala L. (Fig. 5j) and Cu in O. majorana L. (Fig. 5i). However, a severe decrease in ICDH activity was highlighted under the other metals in both plants.
4 Discussion
The result obtained in the present study revealed that seeds of P. harmala L. and O. majorana L. exhibited different germination responses to Pb, Cu, and Cd treatments with respective concentrations (0, 100, 200, and 300 µM). The inhibition of germination of P. harmala and O. majorana reached its maximum at the highest concentration of Cd. The GI and TGI of P. harmala L. and O. majorana seeds were negatively influenced by the exposure to Cd, Cu, or Pb especially by Cd treatment for P. harmala and Cu for O. majorana. Metals’ effects on the structure and function of plants differ depending on variations in HMs, their concentration, and plant species (Greger 2004). These findings are in concordance with prior research (Nedjimi 2020).
HMs applied in the study caused growth retardation in both plants especially under the highest concentration of Cd. These findings are in concordance with an earlier study, where the germination of O. basilicum seed in the absence of metals began after 4.66 days, and rising Cd and Pb concentrations increased germination time to 7.66 and 10 days, respectively (Fattahi et al. 2019).
In addition, P. harmala plant was the subject of a similar investigation (Nedjimi 2020). Nedjimi (2020) reported the negative influence of metals (Cd, Cr, Pb, and Zn) on plant germination characteristics. In general, plants react differently to different types of metals. Cd and lead treatments significantly reduced seed germination of P. harmala that hardly exceeded 20% in the Nedjimi study (2020). Their results demonstrated that the germination characteristics (G% and TGI) declined as the concentrations of HMs increased. In a recent study, El-Shora et al. (2021) showed that Pb altered the physiological parameters of Salvia officinalis L. The germination percentage of S. officinalis L. seeds was reduced (19.33%) when treated with 400 µM Pb compared to control plants. Reduced germination percentage was also reported for the seeds of Ocimum basilicum L. under Cd and Pb treatment. By raising the Cd concentration to 16 mg L−1, the germination was decreased by 4%. Furthermore, basilic seed germination was significantly lowered to 9.33% when 80 mg L−1 Pb was used (Fattahi et al. 2019). Additionally, due to Cd exposure, the G% of Thymus vulgaris L. seed was significantly reduced (Moori and Ahmadi-Lahijani 2020).
HMs impact seed germination in different ways. Kranner and Colville (2011) suggested that germination is affected either by the overall toxicity of metals or by their ability to limit water intake. Cd, for example, blocked seed imbibition and decreased seedling water content in Dorycnium pentaphyllum (Lefèvre et al. 2009). Likewise, water intake was reduced in Arabidopsis seed exposed to Cu during germination (Li et al. 2005). From the obtained results, in both plants, the harmful effects of Cd and Cu were reversible, and the seeds were able to recover their germination after washing. In fact, the presence of HMs can alter the seed microenvironment and proteins involved in seed germination, which reduces its germination rate (Nanda and Agrawal 2016). Moreover, increasing concentration of HMs reduced seed germination rate, which has been associated with high levels of abscisic acid (ABA) in the seeds of Ocimum basilicum L. (Fattahi et al. 2019). ABA levels of wheat were also increased under Hg, Cd, and Cu stress during the germination stage (Munzuroğlu et al. 2008). Their finding indicates that the inhibition of germination was related with ABA accumulation in the seeds (Munzuroğlu et al. 2008). Since ABA is known to control plant water status, it limits the availability of water, energy, and metabolites that are essential for seed germination and embryo development. As a mechanism of growth inhibition, it might inhibit H+ efflux (H+-ATPase) and cause cytosolic acidification (Planes et al. 2015).
Generally, biomass and elongation reduction are important indicators to evaluate metal toxicity in plants. Previous studies (Fattahi et al. 2019; Ibrahim et al. 2017; Mahdavian et al. 2016; Nanda and Agrawal 2016; Yang and Ye 2015; Zhang et al. 2020) have reported a decline in shoot and root biomass and length due to metal stress. The average shoot length of Cassia angustifolia Vahl was found to be decreased from 5.53 to 1.4 cm, whereas the average root length decreased from 7.12 to 0.3 cm under Cu (Nanda and Agrawal 2016), showing that roots are more sensitive organ to metal exposure than shoots. Similar results were obtained by Zhang et al. (2020) where radicle length was more sensitive to Cu and Zn than other parameters. The present data confirm these findings, as all the examined HMs impact the length of the roots before the shoots. Furthermore, increasing Pb concentrations decreased the dry weights of roots and shoots in P. harmala L. populations (Mahdavian et al. 2016), and Ocimum basilicum L. recorded the lowest SDW under Cd treatment (Fattahi et al. 2019). Yang and Ye (2015) reported that the height and biomass of the 18 wetland species used in their study under Pb treatment were significantly lower than those of the control. In addition, Ibrahim et al. (2017) observed a reduction in Gaultheria procumbens (Lour.) Merr dry weight under Cd and Cu exposure.
Seed vigor index is regarded as a summary of several parameters and criteria developed to answer frequently asked issues concerning seed functioning under unfavorable environmental conditions (Marcos-Filho 2015). In the current study, the effect of HMs on seedling vigor index in O. majorana L. and P. harmala L. seedlings was evaluated. In fact, vigorous seeds are desirable for cultivation in polluted soils to ensure healthy and tolerant seedlings under stressful environmental conditions. The lowest vigor index was recorded in O. majorana L. under Cu exposure. However, P. harmala L. seedling vigor index was strongly affected by Cd at the highest concentration. SVI of T. vulgaris L. was also reported to decrease with increasing Cd concentration (Moori and Ahmadi-Lahijani 2020). Similarly, a gradual decrease was observed on SVI of Thespesia populnea L. under increasing levels of Pb and Cd (Kabir et al. 2008).
In many plants (Astrebla lappacea, Themeda australis, Austrostipa scabra, and Acacia harpophylla), germination percentage, MGT, and the shoot tolerance index (STI) were ineffective indicators of metal toxicity to germination. Root tolerance index (named as the metal tolerance index in our study) was the most effective indicator of species metal tolerance during germination (Guterres et al. 2019). This index was found to be the more sensitive to assess metal tolerance during germination (Zhang et al. 2020). The decrease trend of MTI in O. majorana L. and P. harmala L. was minimal at Pb and Cu exposure, indicating that O. majorana L. may be more tolerant to Pb and P. harmala L. to Cu exposure. Our findings were in the same range of those found by Yang and Ye (2015), where MTI varied from 29 to 82% and from 1 to 80% in the 900 mg Pb kg−1 and 1800 mg Pb kg−1 treatment, respectively, for the 18 wetland plants tested.
In terms of these characteristics, the level of metal toxicity on germination parameters of P. harmala L. was in decreasing order: Cd > Pb > Cu. Similar results have been found for P. harmala L. where Cd was shown to be the most toxic metal, followed by Pb (Nedjimi 2020). This might be due to varying mobility levels of metals, with Cd being more mobile than Pb and Cu (Greger 2004). However, the toxicity of metals on O. majorana L. germination parameters was in decreasing order: Cu ≥ Cd > Pb. A high Cu toxicity on O. majorana L. was emphasized according to our results. Accordingly, other studies reported a toxic effect of Cu on seed germination. Mahmood et al. (2007) highlighted a 35 and 60% decrease in germination of wheat and rice seeds, respectively, after only 10 µM Cu exposure. Germination percentage in rice seeds was also reported to be decreased as Cu level increased from 200 to 1500 µM (Ahsan et al. 2007). Reduced seed germination under Cu exposure may be due to a decrease in alpha-amylase activity (Ahsan et al. 2007). Furthermore, Cu toxicity in the seed germination process has been related to the general alteration of metabolism and water intake (Li et al. 2005; Sethy and Ghosh 2013; Nanda and Agrawal 2016).
Along with the physical parameters, we observed an enhancement in the activity of the antioxidative enzymes (SOD, GPx, GR, and GST) in P. harmala L. and O. majorana L. shoots exposed to the three different HMs.
HMs used in this experiment can be divided into redox-active (Cu) and redox-inactive (Cd, Pb) (Hossain et al. 2012). Plants exposed to Cd or Pb experience oxidative stress via indirect processes, including interaction with the antioxidant defense system, interruption of the electron transport chain, or induction of lipid peroxidation (Hossain et al. 2012). In fact, ROS-induced oxidative stress is one of the major toxic effects during HM exposure of plants, which leads to DNA damage, lipid peroxidation, and inhibition of adenosine triphosphate (ATP) synthesis (Yahaghi et al. 2019).
Glutathione peroxidase is an important antioxidant enzyme involved in plant stress responses and H2O2 detoxification. Its overproduction in several plant species increases resistance to abiotic stress such as HMs and oxidative stresses (Bela et al. 2017). Under different HM stresses, GPx showed a significant rise compared to control. Initially, at lower metal concentrations, the increase of GPx was moderate. Nevertheless, with the increasing concentration of HMs, GPx showed high activity. In fact, the highest significant increase in GPx activity was recorded in Cd-treated P. harmala L. compared to the control. GPx activity was also observed to be higher in Origanum vulgare L. shoots under Cd, Pb, and Zn polluted soil (Stancheva et al. 2014). The study indicated an effective performance of the ascorbate–glutathione cycle in O. vulgare L. Increased GPx activity was observed in Hordeum vulgare L. root tip under Cd exposure and was significantly correlated with root growth inhibition. A similar significant increase in GPx was observed under Cu application. However, a slight increase in GPx was examined following Pb treatment (Halušková et al. 2009).
Glutathione reductase (GR) is an enzyme involved in the AsA-GSH cycle and performs a critical function in scavenging ROS under stress and maintains both AsA and GSH levels in cells (El-Shora and Abd-Elgawad 2014). Increased GR activity in plants leads to increased glutathione (GSH) levels, enhancing plant tolerance. The GR activity in our study was induced by HMs, more strongly by Cd for P. harmala L. It also increased under Cu and Cd in O. majorana L. In fact, Cd increased the mRNA levels of genes involved in GSH production (gsh1 and gsh2) (Semane et al. 2007). These results are confirmed by many authors, who reported a significant increase in GR activity in response to HMs. Similar improvement in GR activity has also been reported in Cu-treated C. angustifolia seedlings (Nanda and Agrawal 2016). The GR activity of Triticum aestivum L. leaves was also increased by 96 and 97% at 1338 mg Cu kg−1 and 24 mg Cd kg−1 respectively over the control (Rizvi and Khan 2017). In the leaves and roots of two Kenaf (Hibiscus cannabinus L.) plants, the highest specific activities of GR were observed at 20 µM Cd and then decreased at higher concentrations (up to 120 µM) (Li et al. 2013).
In addition to GPx and GR, glutathione S-transferase is another family of enzymes involved in the metabolism of glutathione and is known for its ability to assess plants to overcome different abiotic stresses. Several GST enzymes have shown a GPx activity (Halušková et al. 2009). Halušková et al. (2009) have investigated the GST and GPx activities in Hordeum vulgare L. root tip induced by different abiotic stresses, including HMs that caused an increase in GST activity in the root tip. They have also detected a GPx activity in barley root tip despite using an inhibitor of animal GPx, indicating the fact that GST enzymes might have GPx activity. Moreover, an improvement in the three antioxidant enzyme activities (GPx, GST, and GR) was induced by the HM pollution (Cd, Pb, and Zn) in Origanum vulgare L. (Stancheva et al. 2014).
Concerning superoxide dismutase (SOD), the results demonstrated low activity in control plants while it enhanced under treatment with various concentrations. Increased SOD activity in P. harmala L. shoots was shown to be strongly linked with increasing concentrations of the examined HMs, especially under Cd treatment. This increment could be attributed to reducing superoxide radicals (Malecka et al. 2012). Cd causes H2O2 production either directly or indirectly through SOD activity. Comparable findings have been found by Yeboah et al. (2021), where the SOD activity significantly increased (P < 0.001) in Ricinus communis L. under Cd treatment compared to the control. In P. harmala L. shoot under 10 µM Cu and 100 µM Cu, the SOD activity increased at the rate of 126 to 173% compared with the control (Lu et al. 2010). Moreover, SOD activity in leaves of 18 plant species significantly increased under Pb treatments (Yang and Ye 2015). C. angustifolia Vahl. seedlings exposed to Cu had a stronger SOD activity than Zn-treated seedlings (Nanda and Agrawal 2016), indicating that Cu has redox-active characteristics (Hossain et al. 2012). In our study, the specific activity of SOD was also induced in O. majorana L. by HM exposure, especially Cu (maximum at 200 µM). However, the SOD production has been significantly decreased when O. majorana L. seeds were exposed to higher concentrations of Cu and Pb (300 µM). Similar results were revealed by Li et al. (2013). This result might be due to the overproduction of ROS of plants subjected to environmental stresses, which overcome the capacity of the antioxidant system, leading to physiological dysfunction and enzyme damage (Zhang et al. 2007).
NADP-dependent isocitrate dehydrogenase (NADP-ICDH) has been identified as an important component of antioxidative defense systems in animals’ cells and microorganisms. However, few studies were conducted on the antioxidant properties of NADP-ICDH in plant cells. NADPH generated by mitochondrial and chloroplastic ICDH activity may have a critical role in glutathione regeneration in the ascorbate–glutathione cycle and glutathione peroxidase system. Its overproduction resulted in minimized damage induced by ROS generation (Demidchik et al. 2003). Under drought stress, Zahir (2021) found that plant growth was associated with an increase of NADP-ICDH activity. Furthermore, León-Vaz et al. (2021) showed a significant increase in ICDH activity in the microalga Chlorella sorokiniana under Cd and As (III). The content of ICDH in Rosa canina L. collected from HM-polluted soil around a closed mine was also greater than a control soil (Todirascu-Ciornea et al. 2018). Unlike the previously cited studies, our results demonstrated that the increase of metal concentration led to a significant decrease of ICDH in both plants. Rajpoot et al. (2020) also reported that the content of several carbohydrate-metabolizing enzymes, including ICDH, showed a significant reduction in rice plants under excess of Mn. This decline might be attributed to the fact that HM excess affected carbohydrate metabolism, resulting in reduced plant development.
In general, plants have developed a refined mechanism to reinforce the cellular antioxidant system and deal with ROS rise due to HM stress by increasing the production of antioxidant enzymes. Among the HMs tested, Cd has the greater effect on increasing antioxidant enzymes (SOD, GR, GPx, and GST) in P. harmala L. despite its effect on decreasing ICDH activity. However, the antioxidant enzymes of O. majorana L. possessed a different response to each HM. GPx and GST activities were higher under Pb exposure, while GR and SOD were higher under Cu exposure. Moreover, the most suppressive effect of ICDH activity was under Pb.
5 Conclusion
Medicinal and aromatic plants have been proposed as alternative crops in heavy metal–contaminated soils since they can be exposed to different kinds of abiotic stresses. Metal tolerance index is the most effective indicator of species metal tolerance during germination which is a critical phase of the plant in ensuring healthy and tolerant seedlings to mitigate the stress caused by heavy metal. O. majorana L. and P. harmala L have shown the potential to tolerate lead and copper respectively. The reactive oxygen species generated under heavy metal stress were regulated through the different enzymatic pathways activated, suggesting the role of oxidative stress as a component of environmental stress on O. majorana L. and P. harmala L. It appears that the differences in response of enzymatic activities have a direct relation to the sensitivity of the two plants to heavy metals. This study may suggest the possibility of using O. majorana L. for rehabilitation and revegetation of Pb-polluted soils and P. harmala L. for Cu-polluted soils. Thus, further in-depth studies are required to determine the mechanisms of heavy metal uptake and translocation in relation to their impact on medicinal plant growth and development. Furthermore, the roles of enzymatic antioxidants and the activated genes need to be explored for different roots of heavy metal uptake.
References
Abdul-Baki AA, Anderson JD (1973) Vigor determination in soybean seed by multiple criteria1. Crop Sci 13:630–633. https://doi.org/10.2135/CROPSCI1973.0011183X001300060013X
Ahsan N, Lee DG, Lee SH, Kang KY, Lee JJ, Kim PJ, Yoon HS, Kim JS, Lee BH (2007) Excess copper induced physiological and proteomic changes in germinating rice seeds. Chemosphere 67:1182–1193. https://doi.org/10.1016/j.chemosphere.2006.10.075
Ait Elallem K, Sobeh M, Boularbah A, Yasri A (2021) Chemically degraded soil rehabilitation process using medicinal and aromatic plants : review. Environ Sci Pollut Res 28:73–93. https://doi.org/10.1007/s11356-020-10742-y
Al-Mudaris MA (1998) Notes on various parameters recording the speed of seed germination. Der Tropenlandwirt - J Agric Trop Subtrop 99:147–154
Angelova VR, Ivanova RV, Delibaltova VA, Ivanov KI (2011) Use of sorghum crops for in situ phytoremediation of polluted soils. J Agric Sci Technol A 1:693–702
Asgari Lajayer H, Savaghebi G, Hadian J, Hatami M, Pezhmanmehr M (2017) Comparison of copper and zinc effects on growth, micro- and macronutrients status and essential oil constituents in pennyroyal (Mentha pulegium L.). Brazilian J Bot 40:379–388. https://doi.org/10.1007/s40415-016-0353-0
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287. https://doi.org/10.1016/0003-2697(71)90370-8
Bela K, Bangash SAK, Riyazuddin, Csiszár J (2017) Plant glutathione peroxidases: antioxidant enzymes in plant stress responses and tolerance. Glutathione Plant Growth, Dev Stress Toler 113–126https://doi.org/10.1007/978-3-319-66682-2_5
Benidire L, Madline A, Pereira SIA, Castro PML, Boularbah A (2021) Synergistic effect of organo-mineral amendments and plant growth-promoting rhizobacteria (PGPR) on the establishment of vegetation cover and amelioration of mine tailings. Chemosphere 262:127803. https://doi.org/10.1016/j.chemosphere.2020.127803
Benidire L, Pereira SIAA, Castro PMLL, Boularbah A (2016) Assessment of plant growth promoting bacterial populations in the rhizosphere of metallophytes from the Kettara mine, Marrakech. Environ Sci Pollut Res 23:21751–21765. https://doi.org/10.1007/s11356-016-7378-6
Bensabah F, Lamiri A, Naja J (2015) Effect of purified wastewater from the city of Settat (Morocco) on the quality of Lippia citriodora essential oil and infusion. J Saudi Soc Agric Sci 14:101–108. https://doi.org/10.1016/j.jssas.2014.03.001
Boularbah A, Schwartz C, Bitton G, Aboudrar W, Ouhammou A, Morel JL (2006) Heavy metal contamination from mining sites in South Morocco: 2. Assessment of metal accumulation and toxicity in plants. Chemosphere 63:811–817. https://doi.org/10.1016/j.chemosphere.2005.07.076
Bozhanov S, Karadjova I, Alexandrov S (2007) Determination of trace elements in the lavender inflorescence (Lavandula angustifolia Mill.) — lavender oil system. Microchem J 86:119–123. https://doi.org/10.1016/j.microc.2007.01.001
Chaffai R, Koyama H (2011) Heavy metal tolerance in Arabidopsis thaliana. In: Kader J-C, Delseny M (eds) Advances in botanical research. Academic Press, pp 1–49
Chu C, Liu B, Liu J, He J, Lv L, Wang H, Xie X, Tao Q, Chen Q (2020) Phytoremediation of acetochlor residue by transgenic Arabidopsis expressing the acetochlor N-dealkylase from Sphingomonas wittichii DC-6. Sci Total Environ 728:138687. https://doi.org/10.1016/j.scitotenv.2020.138687
Dashti S (2020) Antiviral properties of Peganum Harmala (Espand) as a medicinal plant : a literature review. Herb Med J 5:127–128. https://doi.org/10.22087/herb/20med/20j.v5i3.859
Demidchik V, Shabala SN, Coutts KB, Tester MA, Davies JM (2003) Free oxygen radicals regulate plasma membrane Ca2+- and K+-permeable channels in plant root cells. J Cell Sci 116:81–88. https://doi.org/10.1242/jcs.00201
Dimkpa CO, Merten D, Svatos A, Buchel G, Kothe E (2009) Siderophores mediate reduced and increased uptake of cadmium by Streptomyces tendae F4 and sunflower (Helianthus annuus), respectively. J Appl Microbiol 107:1687–1696. https://doi.org/10.1111/j.1365-2672.2009.04355.x
Domínguez MJ, Gutiérrez F, León R, Vílchez C, Vega JM, Vigara J (2003) Cadmium increases the activity levels of glutamate dehydrogenase and cysteine synthase in Chlamydomonas reinhardtii. Plant Physiol Biochem 41:828–832. https://doi.org/10.1016/S0981-9428(03)00114-1
El-Shora H, Abd-Elgawad AM (2014) Environmental toxicity of arsenic on lupine (Lupinus termis L.) as C3 crop plant and possible alleviation. Int J Agric Crop Sci 7:687–692
El-Shora HM, Massoud GF, El-Sherbeny GA, Alrdahe SS, Darwish DB (2021) Alleviation of lead stress on sage plant by 5-aminolevulinic acid (ALA). Plants 10:1969. https://doi.org/10.3390/PLANTS10091969
Fageria NK, Filho MPB, Moreira A, Guimarães CM (2009) Foliar fertilization of crop plants. J Plant Nutr 32:1044–1064. https://doi.org/10.1080/01904160902872826
Farid G, Sarwar N, Ahmad A, Ghafoor A, Rehman M (2015) Heavy metals (Cd, Ni and Pb) contamination of soils, plants and waters in Madina town of Faisalabad metropolitan and preparation of Gis based maps. 4:1–7. https://doi.org/10.4172/2329-8863.1000199
Fattahi B, Arzani K, Souri MK, Barzegar M (2019) Effects of cadmium and lead on seed germination, morphological traits, and essential oil composition of sweet basil (Ocimum basilicum L). Ind Crops Prod 138:. https://doi.org/10.1016/J.INDCROP.2019.111584
Greger M (2004) Metal availability, uptake, transport and accumulation in plants. In: Prasad MN (ed) Heavy metal stress in plants, 2nd edn. Springer, Berlin Heidelberg, Berlin, Heidelberg, pp 1–27
Gupta AK, Verma SK, Khan K, Verma RK (2013) Phytoremediation using aromatic plants: a sustainable approach for remediation of heavy metals polluted sites. Environ Sci Technol 47:10115–10116. https://doi.org/10.1021/es403469c
Guterres J, Rossato L, Doley D, Pudmenzky A, Bee C, Cobena V (2019) Assessing germination characteristics of Australian native plant species in metal/metalloid solution. J Hazard Mater 364:173–181. https://doi.org/10.1016/j.jhazmat.2018.10.019
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139. https://doi.org/10.1016/S0021-9258(19)42083-8
Halušková L, Valentovičová K, Huttová J, Mistrík I, Tamás L (2009) Effect of abiotic stresses on glutathione peroxidase and glutathione S-transferase activity in barley root tips. Plant Physiol Biochem 47:1069–1074. https://doi.org/10.1016/J.PLAPHY.2009.08.003
Hossain MA, Piyatida P, Da Silva JAT, Fujita M (2012) Molecular mechanism of heavy metal toxicity and tolerance in plants: central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J Bot 2012:37. https://doi.org/10.1155/2012/872875
Ibrahim MH, Kong YC, Zain NAM (2017) Effect of cadmium and copper exposure on growth, secondary metabolites and antioxidant activity in the medicinal plant sambung nyawa (Gynura procumbens (Lour.) Merr). Molecules 22:. https://doi.org/10.3390/molecules22101623
Kabir M, Iqbal MZ, Shafiq M, Farooqi Z (2008) Reduction in germination and seedling growth of Thespesia Populnea L., caused by lead and cadmium treatments. Pakistan J Bot 40:2419–2426
Kang JW (2014) Removing environmental organic pollutants with bioremediation and phytoremediation. Biotechnol Lett 36:1129–1139. https://doi.org/10.1007/s10529-014-1466-9
Khan A, Sharif M, Ali A, Shah SNM, Mian IA, Wahid F, Jan B, Adnan M, Nawaz S, Ali N (2014) Potential of AM fungi in phytoremediation of heavy metals and effect on yield of wheat Crop. Am J Plant Sci 05:1578–1586. https://doi.org/10.4236/AJPS.2014.511171
Khan MA, Gul B (1998) High salt tolerance in germinating dimorphic seeds of Arthrocnemum indicum. Int J Plant Sci 159:826–832. https://doi.org/10.1086/297603
Kranner I, Colville L (2011) Metals and seeds: biochemical and molecular implications and their significance for seed germination. Environ Exp Bot 72:93–105. https://doi.org/10.1016/j.envexpbot.2010.05.005
Lal K, Yadav RK, Kaur R, Bundela DS, Khan MI, Chaudhary M, Meena RL, Dar SR, Singh G (2013) Productivity, essential oil yield, and heavy metal accumulation in lemon grass (Cymbopogon flexuosus) under varied wastewater – groundwater irrigation regimes. Ind Crop Prod 45:270–278. https://doi.org/10.1016/j.indcrop.2013.01.004
Lawrence R, Burk R (1976) Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun 71:952–958. https://doi.org/10.1016/0006-291X(76)90747-6
Lefèvre I, Marchal G, Corréal E, Zanuzzi A (2009) Lutts S (2009) Variation in response to heavy metals during vegetative growth in Dorycnium pentaphyllum Scop. Plant Growth Regul 591(59):1–11. https://doi.org/10.1007/S10725-009-9382-Z
León-Vaz A, León R, Giráldez I, Vega JM, Vigara J (2021) Impact of heavy metals in the microalga Chlorella sorokiniana and assessment of its potential use in cadmium bioremediation. Aquat Toxicol 239:105941. https://doi.org/10.1016/J.AQUATOX.2021.105941
Li FT, Qi JM, Zhang GY, Lin LH, Fang PP, Tao AF, Xu JT (2013) Effect of cadmium stress on the growth, antioxidative enzymes and lipid peroxidation in two Kenaf (Hibiscus cannabinus L.) plant seedlings. J Integr Agric 12:610–620. https://doi.org/10.1016/S2095-3119(13)60279-8
Li W, Khan MA, Yamaguchi S, Kamiya Y (2005) Effects of heavy metals on seed germination and early seedling growth of Arabidopsis thaliana. Plant Growth Regul 46:45–50. https://doi.org/10.1007/S10725-005-6324-2
Liu L, Li W, Song W, Guo M (2018) Remediation techniques for heavy metal-contaminated soils: principles and applicability. Sci Total Environ 633:206–219. https://doi.org/10.1016/j.scitotenv.2018.03.161
Lu Y, Li X, He M, Zhao X, Liu Y, Cui Y, Pan Y, Tan H (2010) Seedlings growth and antioxidative enzymes activities in leaves under heavy metal stress differ between two desert plants: a perennial (Peganum harmala) and an annual (Halogeton glomeratus) grass. Acta Physiol Plant 32:583–590. https://doi.org/10.1007/s11738-009-0436-7
Lydakis-Simantiris N, Fabian M, Skoula M (2016) Cultivation of medicinal and aromatic plants in heavy metal. Glob NEST J 18:630–642
Mahdavian K, Ghaderian SM, Schat H (2016) Pb accumulation, Pb tolerance, antioxidants, thiols, and organic acids in metallicolous and non-metallicolous Peganum harmala L. under Pb exposure. Environ Exp Bot 126:21–31. https://doi.org/10.1016/j.envexpbot.2016.01.010
Mahmood T, Islam KR, Muhammad S (2007) Toxic effects of heavy metals on early growth and tolerance of cereal crops. Pakistan J Bot 39:451–462
Maiti SK, Kumar A (2016) Energy plantations, medicinal and aromatic plants on contaminated soil. In: Prasad, M.N.V (eds) Bioremediation and Bioeconomy. Elsevier Inc., pp 29–47
Malecka A, Piechalak A, Mensinger A, Hanć A, Barałkiewicz D, Tomaszewska B (2012) Antioxidative defense system in Pisum sativum roots exposed to heavy metals (Pb, Cu, Cd, Zn). Polish J Environ Stud 21:1721–1730
Marchiol L, Assolari S, Sacco P, Zerbi G (2004) Phytoextraction of heavy metals by canola (Brassica napus) and radish (Raphanus sativus) grown on multicontaminated soil. Environ Pollut 132:21–27. https://doi.org/10.1016/j.envpol.2004.04.001
Marcos-Filho J (2015) Seed vigor testing: an overview of the past, present and future perspective. Sci Agric 72:363–374. https://doi.org/10.1590/0103-9016-2015-0007
Moori S, Ahmadi-Lahijani MJ (2020) Hormopriming instigates defense mechanisms in thyme (Thymus vulgaris L) seeds under cadmium stress. J Appl Res Med Aromat Plants 19:100268. https://doi.org/10.1016/j.jarmap.2020.100268
Munzuroğlu Ö, Kırbağ Zengin F, Yahyagil Z (2008) The abscisic acid levels of wheat (Triticum aestivum l. cv. Çakmak 79) seeds that were germinated under heavy metal (Hg 2+, Cd 2+, Cu2+) Stress. J Sci 21:1–7
Nanda R, Agrawal V (2016) Elucidation of zinc and copper induced oxidative stress, DNA damage and activation of defence system during seed germination in Cassia angustifolia Vahl. Environ Exp Bot 125:31–41. https://doi.org/10.1016/J.ENVEXPBOT.2016.02.001
Nedjimi B (2020) Germination characteristics of Peganum harmala L. (Nitrariaceae) subjected to heavy metals: implications for the use in polluted dryland restoration. Int J Environ Sci Technol 17:2113–2122. https://doi.org/10.1007/s13762-019-02600-3
Pandey VC, Rai A, Korstad J (2019) Aromatic crops in phytoremediation : from contaminated to waste dumpsites. In: Pandey VC, Bauddh K (eds) Phytomanagement of polluted sites, 1st edn. Elsevier Inc., Amsterdam, The Netherlands, pp 255–275
Planes MD, Niñoles R, Rubio L, Bissoli G, Bueso E, García-Sánchez MJ, Alejandro S, Gonzalez-Guzmán M, Hedrich R, Rodriguez PL, Fernández JA, Serrano R (2015) A mechanism of growth inhibition by abscisic acid in germinating seeds of Arabidopsis thaliana based on inhibition of plasma membrane H+-ATPase and decreased cytosolic pH, K+, and anions. J Exp Bot 66:813–825. https://doi.org/10.1093/jxb/eru442
Rajpoot R, Srivastava RK, Rani A, Pandey P (2020) Dubey RS (2020) Manganese-induced oxidative stress, ultrastructural changes, and proteomics studies in rice plants. Protoplasma 2582(258):319–335. https://doi.org/10.1007/S00709-020-01575-0
Ranal MA, de Santana DG, Ferreira WR, Mendes-Rodrigues C (2009) Calculating germination measurements and organizing spreadsheets. Rev Bras Bot 32:849–855. https://doi.org/10.1590/S0100-84042009000400022
Rao MV, Paliyath G, Ormrod DP (1996) Ultraviolet-B- and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol 110:125. https://doi.org/10.1104/PP.110.1.125
Rizvi A, Khan MS (2017) Biotoxic impact of heavy metals on growth, oxidative stress and morphological changes in root structure of wheat (Triticum aestivum L.) and stress alleviation by Pseudomonas aeruginosa strain CPSB1. Chemosphere 185:942–952. https://doi.org/10.1016/j.chemosphere.2017.07.088
Roodi MM, Bin Md, Said MA, Honari H (2012) Phytoremediation using the influence of aromatic crop on heavy-metal polluted soil, a Review. Adv Environ Biol 6:2663–2668
Sadia K, Asma B, Riffat NM (2016) Role of arbuscular mycorrhizal fungi in phytoremediation of heavy metals and effects on growth and biochemical activities of wheat (Triticum aestivum L.) plants in Zn contaminated soils. African J Biotechnol 15:872–883. https://doi.org/10.5897/AJB2016.15292
Sarma B, Gogoi N, Devi P, Menaka Devi Y (2014) Effects of cobalt induced stress on Triticum aestivum L. Orig Artic Asian J Agri Biol 2:137–147
Schreck E, Foucault Y, Sarret G, Sobanska S, Cécillon L, Castrec-Rouelle M, Uzu G, Dumat C (2012) Metal and metalloid foliar uptake by various plant species exposed to atmospheric industrial fallout: mechanisms involved for lead. Sci Total Environ 427–428:253–262. https://doi.org/10.1016/J.SCITOTENV.2012.03.051
Schutzendubel A (2002) Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot 53:1351–1365. https://doi.org/10.1093/JEXBOT/53.372.1351
Semane B, Cuypers A, Smeets K, Van BF, Horemans N, Schat H, Vangronsveld J (2007) Cadmium responses in Arabidopsis thaliana: glutathione metabolism and antioxidative defence system. Physiol Plant 129:519–528. https://doi.org/10.1111/J.1399-3054.2006.00822.X
Sethy SK, Ghosh S (2013) Effect of heavy metals on germination of seeds. J Nat Sci Biol Med 4:272–275. https://doi.org/10.4103/0976-9668.116964
Singh S, Eapen S, D’Souza SF (2006) Cadmium accumulation and its influence on lipid peroxidation and antioxidative system in an aquatic plant, Bacopa monnieri L. Chemosphere 62:233–246. https://doi.org/10.1016/J.CHEMOSPHERE.2005.05.017
Sovljanski R (Novi SU (Yugoslavia). I for PP, Kisgeci J, Macko V, Obradovic S, Lazic S (1989) The heavy metals contents and quality of hop cones treated by pesticides during the vegetation. Acta Hortic 81–88. https://doi.org/10.17660/ActaHortic.1989.249.9
Stancheva I, Geneva M, Markovska Y, Tzvetkova N, Mitova I, Todorova M, Petrovl P (2014) A comparative study on plant morphology, gas exchange parameters, and antioxidant response of Ocimum basilicum L. and Origanum vulgare L. grown on industrially polluted soil. Turkish J Biol 38:89–102. https://doi.org/10.3906/biy-1304-94
Todirascu-Ciornea E, Dumitru G, Marius Z, Drochioiu G (2018) Heavy metal pollution affects the antioxidant potential of Rosa canina L. species. Rev Chim 69:449–452
Wilkins DA (1978) The measurement of tolerance to edaphic factors by means of root growth. New Phytol 80:623–633. https://doi.org/10.1111/J.1469-8137.1978.TB01595.X
Yahaghi Z, Shirvani M, Nourbakhsh F, Pueyo JJ (2019) Uptake and effects of lead and zinc on alfalfa (Medicago sativa L.) seed germination and seedling growth: role of plant growth promoting bacteria. South African J Bot 124:573–582. https://doi.org/10.1016/J.SAJB.2019.01.006
Yang J, Ye Z (2015) Antioxidant enzymes and proteins of wetland plants: their relation to Pb tolerance and accumulation. Environ Sci Pollut Res 22:1931–1939. https://doi.org/10.1007/s11356-014-3610-4
Yeboah A, Lu J, Gu S, Liu H, Shi Y, Amoanimaa-Dede H, Agyenim-Boateng KG, Payne J, Yin X (2021) Evaluation of two wild castor (Ricinus communis L.) accessions for cadmium tolerance in relation to antioxidant systems and lipid peroxidation. Environ Sci Pollut Res 28:55634–55642. https://doi.org/10.1007/s11356-021-14844-z
Zahir AN (2021) Dynamics of activity of NADP-isocitrate dehydrogenase enzyme in corn seedlings grown under drought stress. International trends in science and technology. Scholarly Publisher, Warsaw, RS Global Sp.z O.O., pp 36–38
Zhang FQ, Wang YS, Lou ZP, De DJ (2007) Effect of heavy metal stress on antioxidative enzymes and lipid peroxidation in leaves and roots of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza). Chemosphere 67:44–50. https://doi.org/10.1016/J.CHEMOSPHERE.2006.10.007
Zhang H, Jiang L, Tanveer M, Ma J, Zhao Z, Wang L (2020) Indexes of radicle are sensitive and effective for assessing copper and zinc tolerance in germinating seeds of Suaeda salsa. Agric 10:1–12. https://doi.org/10.3390/agriculture10100445
Zheljazkov VD, Craker LE, Xing B (2006) Effects of Cd, Pb, and Cu on growth and essential oil contents in dill, peppermint, and basil. Environ Exp Bot 58:9–16. https://doi.org/10.1016/j.envexpbot.2005.06.008
Acknowledgements
The authors acknowledge Mohammed VI Polytechnics University (UM6P) for financial support. The authors would like to thank Professor Ismail RAQI for proofreading the article.
Author information
Authors and Affiliations
Contributions
Ait Elallem Khadija: conceptualization and research design, collection and assembly of data, methodology and laboratory analysis, analysis and interpretation of the data, writing-original draft preparation, critical revision of the article. Ben Bakrim Widad: conceptualization and research design, analysis and interpretation of the data, critical revision of the article for important intellectual content. Ennoury Abdelhamid: collection and assembly of data, methodology, and laboratory analysis. Metougui Mohamed Louay: statistical expertise, critical revision of the article for important intellectual content. Yasri Abdelaziz: critical revision of the article for important intellectual content, final approval of the article, work supervision. Boularbah Ali: conceptualization and research design, critical revision of the article for important intellectual content, final approval of the article, work supervision.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ait Elallem, K., Ben Bakrim, W., Ennoury, A. et al. Germination Parameters and Responses of Antioxidant Enzymatic Activities of Two Medicinal Plants (Peganum harmala L. and Origanum majorana L.) Under Heavy Metal Stress. J Soil Sci Plant Nutr 22, 3942–3957 (2022). https://doi.org/10.1007/s42729-022-00943-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-022-00943-4