Abstract
The present work evaluates the role of silicon (Si) in barley (Hordeum vulgare L. var. Rihane) plants subjected to potassium (K+) deficiency. Plants were grown hydroponically either under K+-sufficient (+ K, 3 mM) or K+-deficient (-K, 10 µM) conditions supplied or not with 1 mM Si. Several parameters were determined in both shoots and roots [growth, macronutrients (K and Ca) and micronutrients (Fe and Zn) dynamics, protein and malondialdehyde (MDA) contents, and antioxidative enzymes activities (superoxide dismutase (SOD), catalase (CAT), and guaiacol peroxidase (GPX)]. Results showed that K+ deficiency resulted in a reduction of growth and K+ concentration in both shoots and roots compared to control plants. The exogenous application of Si alleviated the reduction of plant growth induced by K+ deficiency but did not influence K+ nutrition. Concerning Ca, Fe, and Fe nutrients, the addition of Si adjusts their uptake, translocation, and use efficiencies. Leaf MDA concentration was not affected by K+ deficiency, while an increase was noted in roots. The increase of root MDA concentration was mitigated by the addition of Si. Modulation of antioxidant enzyme activities was also observed. As a whole, the data indicate the beneficial effects of Si on barley plants grown under a K+-deficient medium by modulating mineral nutrition and antioxidative response. This suggests the possible use of Si as an effective, cheap, and environmentally safe fertilizer to improve the plant’s response to K+ deficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In their natural habitats, plants are frequently exposed to a shortage supply of several nutrients. Among them, K+ deficiency constitutes a serious environmental stress restricting plant growth as well as crop yield and quality (Hafsi et al. 2014; Wang and Wu 2015). Despite its abundance in the upper soil layer of the majority of agricultural soils, around 10–20 g kg−1 (Sparks 1987), K+ deficiency affects the majority of arable fields worldwide because of its very low availability (2–10%) to plants (Römheld and Kirkby 2010). K+ is implicated in several physiological processes including photosynthesis, enzyme activation, osmoregulation, protein synthesis, and ion homeostasis (Blaha et al. 2000; Hafsi et al. 2016; Zhu et al. 2019). Upon K+ deficiency, plant growth and productivity were generally restricted as a consequence of reduced photosynthetic activity (Lu et al. 2016; Hafsi et al. 2016). In addition, K+ deficiency may lead to an overproduction of reactive oxygen species (ROS) such as superoxide anion (O2.−), hydroxyl radical (.OH), hydrogen peroxide (H2O2), and singlet oxygen (1O2) produced in different cellular sites including plasma membranes, apoplast, mitochondria, chloroplasts, mitochondria, and peroxisomes (Singh et al. 2019; Hasanuzzaman et al. 2020). Owing to their high reactivity, ROS can provoke oxidative damage to lipids, proteins, pigments, and nucleic acids (Hafsi et al. 2011b; Choudhary et al. 2020). These negative effects may be alleviated by antioxidative mechanisms including non-enzymatic and enzymatic defense systems. The non-enzymatic defense system comprises numerous molecules such as ascorbate, glutathione, and polyphenols (Hafsi et al. 2011b, 2021). The enzymatic system includes several enzymes implicated in the detoxification of ROS such as superoxide dismutase, catalase, peroxidases, ascorbate peroxidase, glutathione reductase, monodehydroascorbate reductase, and dehydroascorbate reductase (Hafsi et al. 2011b; Hernandez et al. 2012; Tang et al. 2015).
To remedy K+ deficiency, a conventional approach consists to apply potassium fertilization. Nevertheless, especially in developing countries, a limiting factor restricting the use of fertilizers is related to their economic burdens (Miao et al. 2010).
Over the last decade, several studies were conducted to investigate the mechanisms by which silicon (Si) can improve plant growth and development (da Silva et al. 2021; Hussain et al. 2021). In this context, the role of Si in mitigating the deleterious effects of abiotic constraints was explored in several research works (Wu et al. 2019; Laifa et al. 2021; Das et al. 2021). In rice plants grown under manganese toxicity, Che et al. (2016) demonstrated that Si is important in alleviating the toxicity of this microelement by decreasing its uptake and translocation from roots to shoots. Khan et al. (2020) demonstrated that the exogenous application of Si to date palm subjected to the combined effects of salinity and cadmium resulted in a reduction of Cd uptake and an improvement of macronutrient uptake. The beneficial effect of Si on the plant growth of sorghum and sunflower plants grown under salinity stress was associated with a decrease of Na+ uptake and an increase in macronutrients and micronutrients uptake and use efficiencies (Hurtado et al. 2019). Laifa et al. (2021) showed that silicon was of great efficiency enabling sea barley plants to successfully recover from salt stress effects. An increase in Fe use and translocation efficiencies was registered in sorghum (Teixeira et al. 2020) and sea barley (Ksiaa et al. 2021) plants subjected to iron deficiency. The ameliorative role of this element on plants subjected to K+ deficiency may be attributed to several ways including (i) increasing plant growth, leaf area, root length, root density, and root area (dos Santos Sarah et al. 2021), (ii) improving water status by increasing K+ accumulation in the xylem (Chen et al. 2016a), (iii) decreasing oxidative stress to membrane lipids and H2O2 accumulation and modulating the activities of antioxidant enzymes, alleviating leaf chlorosis by decreasing the accumulation of the polyamine putrescine, increasing pigment and protein contents, and photosynthetic activity (Miao et al. 2010; Chen et al. 2016b; dos Santos Sarah et al. 2022), and (iv) stimulating K+ absorption and distribution efficiencies (Miao et al. 2010). It was demonstrated that Si modifies the expression of numerous genes implicated in the uptake and translocation of several mineral nutrients such as P (Hu et al. 2018), N (Haddad et al. 2018), and K (Chen et al. 2016a). Interactions between K uptake and utilization with the other nutrients have been observed in previous research works (Pujos and Morard 1997; Hafsi et al. 2011a) which can either increase or decrease their uptake and use efficiencies. However, the effect of Si on nutrient dynamics in plants grown under K+-deficient conditions is not well documented. So, the present work was conducted to investigate the effect of exogenous Si addition in barley plants subjected to K+ deficiency by analyzing plant growth, nutrient uptake, use, and translocation efficiencies, and antioxidant enzymes response.
2 Materials and methods
2.1 Plant material and culture conditions
Seeds of Hordeum vulgare (var. Rihane) were kindly provided by Dr. Abderrazak SMAOUI (LPE—CBBC, Tunisia). After surface sterilization with 1% NaClO and washed several times with distilled water, they were germinated in Petri dishes on filter paper moistened with distilled water for five days. Thereafter, seedlings were transferred into plastic pots and irrigated with 5 L of modified Hewitt’s (1966) nutrient solution (Hafsi et al. 2017). After a pretreatment period (14 days), plants were divided into four lots corresponding to the following treatments: + K-Si = complete medium containing 3 mM K+ without Si, + K + Si = complete medium containing 3 mM K+ with 1 mM Si, -K-Si = complete medium containing 10 µM K+ and without Si, and -K + Si = complete medium containing 10 µM K+ with 1 mM Si. Potassium deficiency was created by replacing KCl by an equivalent amount of NaCl. For control plants (without Si), 2 mM NaCl was added to the nutrient solution to maintain the same concentration of Na+. The low K+ concentration was chosen based on previous research works conducted in barley (Zeng et al. 2018; Ye et al. 2021). Silicon was applied as Na2SiO3. The aerated hydroponic culture was maintained in a greenhouse under sunlight conditions in the Centre of Biotechnology of Borj-Cedria (North-East of Tunisia, 36°42 ′32.9″ N, 10°25 ′40.9″ E) with day/night temperatures of 25 °C/18 °C and relative humidity of 70–75%. Nutrient solutions were renewed twice a week to prevent nutrient depletion. After 17 days of treatment, plants were harvested. To remove extracellular nutrients, roots were carefully washed with cold HCl solution (0.01 M), then rinsed three times with distilled water, and finally dried with filter paper as described by Laifa et al. (2021). The fresh weights (FW) were immediately weighted. Samples were then oven-dried for 48 h at 60 °C for dry weight (DW) determination. Fresh leaves and roots were collected for the determination of malondialdehyde contents (MDA) and the antioxidant enzyme activities (superoxide dismutase (SOD), catalase (CAT), and guaiacol peroxidase (GPX)).
2.2 Nutrient extraction and analysis
For nutrients extraction, 100 mg of shoot and root dried samples were digested with 1 N H2SO4 (20 ml) at 80 °C for 1 h according to Zorrig et al. (2019). K was determined by a flame photometer (BWB). Ca, Fe, and Zn concentrations were measured by atomic absorption spectrophotometer (SpectrAA 220; Varian, Australia).
Nutrient uptake efficiency = Total nutrient accumulation in plant/Root dry weight (Rabhi et al. 2007).
Translocation factor = Nutrient contents in shoots/Nutrient contents in roots (Malik et al. 2010).
Nutrient utilization efficiency = Shoot or root dry weight/Nutrient content of each element (Hafsi et al. 2011a).
2.3 Relative growth rate (RGR) determination
The mean relative growth rate (RGR) was calculated according to the following equation (Hunt 1990):
RGR = (lnDW2 − lnDW1)/(t2 − t1), with DW, leaf, root or whole plant dry weight (g), t = time (d), and the subscripts 1 and 2 = initial and final harvest.
2.4 Estimation of lipid peroxidation
Lipid peroxidation was estimated by measuring malondialdehyde (MDA) formation using the thiobarbituric acid method as described by Buege and Aust (1978). Briefly, leaf and root samples were ground in a cold mortar using 50 mM Tris–HCl (pH 7.5) as a buffer containing 0.2 mM EDTA and 0.2% (v/v) Triton X-100. The obtained homogenate was centrifuged at 16,700 g for 30 min. To 200 µl of supernatant was added 1 ml of 0.375% (w/v) thiobarbituric acid containing 15% (w/v) trichloroacetic acid and 0.01% butylated hydroxytoluene. The mixture was heated at 100 °C for 15 min and then quickly cooled in an ice bath. After centrifugation at 5,500 g for 5 min, the absorbance of the supernatant was read at 535 and 600 nm. The MDA content was calculated by using a molar extinction coefficient of 155 mM−1 cm−1.
2.5 Enzyme extraction and activity assays
Enzyme extractions were carried out at 4 °C according to Pereira et al. (2002). Fresh plant leaves and roots were frozen in liquid nitrogen and ground with an ice-cold pestle and mortar and then extracted in 100 mM potassium phosphate buffer (pH 7.5) containing 1 mM EDTA, 3 mM DTT, and 5% (w/v) insoluble PVP in the ratio of 1:3 (w/v). The resulting homogenate was centrifuged at 14 000 g for 30 min. After that, the supernatant was collected and stored at -80 °C for SOD, CAT, and GPX analysis. Protein was determined by the method of Bradford (1976) using bovine serum albumin (BSA) as a standard.
SOD (EC 1.15.1.1) activity was measured according to the method described by Giannopolitis and Ries (1977). The assay is based on the inhibition of the photochemical reduction of nitroblue tetrazolium (NBT). One unit of SOD activity was defined as the amount of protein inhibiting 50% of the initial reduction of NBT under illumination, expressed as U mg−1 protein.
CAT (EC 1.11.1.6) activity was by the UV method previously described by Aebi (1984). The activity was assayed by monitoring the disappearance of H2O2 at 240 nm and was expressed as U mg−1 protein min−1.
GPX (EC 1.11.1.7) activity, expressed as U mg−1 protein min−1, was determined by monitoring the increase in absorbance after H2O2-induced guaiacol oxidation at 470 nm according to the method of Chance and Maehly (1955).
2.6 Statistical analysis
Data were subjected to a one-way analysis using the XLSTAT software, version 2014 (Addinsoft, Paris, France). Means were compared using one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test at p ≤ 5% level.
3 Results
3.1 Plant growth
K+ deficiency resulted in a reduction of root length, shoot DW, root DW, and whole plant DW by 15.5%, 31.3%, 37.5%, and 32.1%, respectively (Table 1). The exogenous application of Si restores these parameters to a level comparable to control plants (+ K-Si treatment). K+ deficiency had no significant effect on shoot height and root/shoot DW ratio compared to K+-sufficient plants. An increase of shoot and root length was observed in K+-sufficient plants supplied with Si. Concerning RGR, a significant reduction was registered in shoots, roots, and whole plants by K+ deficiency. Si had a beneficial effect on the root and whole plant’s RGR (Table 1).
3.2 Mineral concentrations
3.2.1 Potassium and calcium concentrations
K+ deficiency reduced K+ concentration in both shoots and roots compared to control plants (+ K-Si-treated plants) (reductions of 79.4% and 76%, respectively) (Fig. 1A,B). Concerning Ca2+ concentration, a reduction of 30.8% was observed in shoots, while no significant effect was registered in roots (Fig. 1C,D). The addition of Si in the culture medium had no significant effect on the concentrations of these two macronutrients under K+-deficient conditions (Fig. 1C,D).
Shoot and root K+, Ca2+, Fe2+, and Zn2+ concentrations in H. vulgare (var. Rihane) grown hydroponically during 17 days either under K+-sufficient (+ K, 3 mM) or K+-deficient (-K, 10 µM) conditions supplied or not with 1 mM Si. Data are the mean of six replicates ± SE. Means followed by the same letters are not significantly different at 5% according to Duncan’s multiple range test
3.2.2 Iron and zinc concentrations
Contrarily to shoots where K+ deficiency had no significant effect on Fe concentration, a reduction of 62.2% was registered in roots compared to control plants (+ K-Si treatment). This reduction was more pronounced following Si addition (a reduction of 82.4%). Si had no significant effect in K+ sufficient plants (Fig. 1E,F). In shoots, K+ deficiency had no significant effect on Zn concentration while a reduction was observed in roots (-64%). Si application to K+-deficient plants increased Zn concentration in shoots (+ 127.4%) but had no significant effect on roots (Fig. 1G,H).
3.3 Mineral absorption and translocation efficiencies
3.3.1 Potassium and calcium absorption and translocation efficiencies
K+ deficiency caused a decrease in KAE (78.6%) and CaAE (23.7%) compared to control plants. The exogenous supply of Si to plants grown under K+-deficient conditions did not change KAE and CaAE (Fig. 2A,C).
Potassium absorption efficiency (KAE), K translocation efficiency, calcium absorption efficiency (CaAE), Ca translocation efficiency, iron absorption efficiency (FeAE), Fe translocation efficiency, Zinc absorption efficiency (ZnAE), and Zn translocation efficiency in H. vulgare (var. Rihane) grown hydroponically during 17 days either under K+-sufficient (+ K, 3 mM) or K+-deficient (-K, 10 µM) conditions supplied or not with 1 mM Si. Data are the mean of six replicates ± SE. Means followed by the same letters are not significantly different at 5% according to Duncan’s multiple range test
Concerning K+ translocation efficiency, no significant effect was observed between all applied treatments (Fig. 2B). K+ deficiency provokes a decrease of Ca2+ translocation efficiency by 53.2% in comparison to control plants (Fig. 2D). The addition of Si resulted in a higher reduction of Ca translocation both in plants grown under sufficient and K+-deficient conditions (Fig. 2D).
3.3.2 Iron and zinc absorption and translocation efficiencies
FeAE and ZnAE were reduced by K+ deficiency (reductions of 34.7% and 42.5%, respectively) compared to control plants (+ K-Si treatment). The addition of Si in the culture medium caused a more pronounced reduction of FeAE (-59.2%), while an increase of ZnAE to a value similar to control plants was observed (increase by 63.6% compared to K+-deficient plants (-K-Si plants). Si had no significant effect in control plants (Fig. 2E,G).
K+ deficiency increased Fe translocation efficiency (3.8-fold) compared to control plants (+ K-Si-treated plants). This increase was more important following Si application (5.9-fold) in comparison to control plants (Fig. 2F). Concerning Zn, an increase was observed only in plants subjected simultaneously to K+ deficiency and exogenous Si supply compared to control plants. Si had no significant effect on Fe and Zn translocation efficiencies in K+ sufficient plants (Fig. 2H).
3.4 Mineral contents and partitioning
3.4.1 Potassium and calcium contents and partitioning
The relative partitioning of K+ among shoots and roots was 91% and 9%, 92% and 8%, 90%, and 10%, and 90% and 10% in plants subjected to + K-Si, + K + Si, -K-Si, and -K + Si-treated plants, respectively (Fig. 3A). The Ca2+ partitioning among shoots and roots was 96% and 4% under control conditions (+ K-Si treatment), 75% and 25% in plants grown under + K + Si-treated plants, 92% and 8% in plants subjected to K+ deficiency without Si (-K-Si treatment), and 87% and 13% in plants grown under -K + Si treatment (Fig. 3B).
Potassium (A), calcium (B), iron (C), and zinc (D) partitioning between shoots (the upper portions of the histograms) and roots (the lower portions of the histograms) and quantities (numbers in brackets) in shoots and roots of H. vulgare (var. Rihane) grown hydroponically during 17 days either under K+-sufficient (+ K, 3 mM) or K+-deficient (-K, 10 µM) conditions supplied or not with 1 mM Si. Data are the mean of six replicates ± SE. Means followed by the same letters are not significantly different at 5% according to Duncan’s multiple range test
Except for root Ca2+ content which remained constant, shoot and root K+ and shoot Ca2+ contents were reduced by K+ deficiency. The exogenous application of Si to plants grown under K+-deficient conditions had no significant effect (Fig. 3A,B).
3.4.2 Iron and zinc contents and partitioning
The Fe partitioning among shoots and roots was 31% and 69% under control conditions (C-Si treatment), 36% and 64% in plants grown under + K + Si-treated plants, 59% and 41% in plants subjected to K+ deficiency without Si (-K-Si treatment), and 70% and 30% in plants grown under -K + Si treatment (Fig. 3C). The relative partitioning of Zn among shoots and roots was 64% and 36%, 63% and 37%, 77% and 23%, and 85% and 15% in plants subjected to + K-Si, + K + Si, -K-Si, and -K + Si-treated plants, respectively (Fig. 3D).
K+ deficiency caused significant reductions in the root Fe, shoot Zn, and root Zn contents. The addition of Si to K+-deficient plants increased shoot Zn contents, whereas no significant effects were observed for shoot and root Fe contents and root Zn contents (Fig. 3C,D).
3.5 Mineral use efficiencies
3.5.1 Potassium and calcium use efficiencies
K+ deficiency increased KUE 5 and 4.2-fold in both shoots and roots, respectively compared to control plants. Si application did not influence KUE in both vegetative organs (Fig. 4A,B). K+ deficiency alone had no significant effect on CaUE in both shoots and roots. The addition of Si to K+ deficient plants increased the root CaUE (increase by 1.9-fold), while no significant effect was registered in shoots. Under control conditions, the exogenous supply increased CaUE in both shoots and roots (Fig. 4C,D).
Potassium use efficiency (KUE), calcium use efficiency (CaUE), iron use efficiency (FeUE), and zinc use efficiency (ZnUE) in shoots and roots of H. vulgare (var. Rihane) grown hydroponically during 17 days either under K+-sufficient (+ K, 3 mM) or K+-deficient (-K, 10 µM) conditions supplied or not with 1 mM Si. Data are the mean of six replicates ± SE. Means followed by the same letters are not significantly different at 5% according to Duncan’s multiple range test
3.5.2 Iron and zinc use efficiencies
Shoot FeUE was decreased by about 32% following K+ deficiency compared to control plants (+ K-Si treatment) (Fig. 4E). An increase by 2.4-fold was registered in roots (Fig. 4F). The addition of Si to K+-deficient plants caused an increase in FeUE in both shoots and roots (1.3- and 2.4-fold, respectively). Si had no significant effect on FeUE in K+-sufficient plants (Fig. 4E,F).
K+ deficiency increased ZnUE by 52.8% and 81.8% in shoots and roots, respectively in comparison to K+-sufficient plants. The addition of Si to K+-deficient plants decreased ZnUE in shoots to a value similar to control plants, while an increase by about 109% was observed in roots (Fig. 4G,H).
3.6 Protein and malondialdehyde concentrations
K+ deficiency reduced leaf protein content by about 58% in comparison to control plants. The addition of Si to K+-deficient plants did not change protein content (Fig. 5A). In roots, K+ deficiency caused an increase in protein content compared to K+-sufficient plants (about 159%). The exogenous application of Si had no significant effect on protein content in K+-sufficient and deficient plants compared to their respective control plants (Fig. 5B).
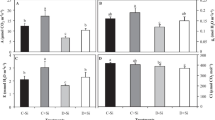
Soluble protein and malondialdehyde (MDA) concentrations in shoots and roots of H. vulgare (var. Rihane) grown hydroponically during 17 days either under K+-sufficient (+ K, 3 mM) or K+-deficient (-K, 10 µM) conditions supplied or not with 1 mM Si. Data are the mean of three replicates ± SE. Means followed by the same letters are not significantly different at 5% according to Duncan’s multiple range test
Leaf malondialdehyde (MDA) concentration, an estimation of lipid peroxidation, was not affected by K+ deficiency either without or with Si compared to control plants. A decrease was registered in K+-sufficient plants supplied with Si (Fig. 5C). In roots, K+ deficiency increases MDA concentration (+ 75%). The addition of Si to K+-deficient plants decreased MDA concentration to a level similar to K+-sufficient plants (Fig. 5D).
3.7 Antioxidative enzymes
In leaves, K+ deficiency increased SOD activity (+ 59%) compared to K+-sufficient plants. This increase was more pronounced following the Si application (+ 117.4%). The addition of Si to K+-sufficient plants had no significant effect on SOD activity (Fig. 6A). In roots, a decrease of SOD activity (-67.7%) was noted in comparison with the control. This decrease was comparable following Si application to K+-deficient plants. A reduction of SOD activity was also observed in K+-sufficient plants receiving Si (Fig. 6B).
Superoxide dismutase (SOD), catalase (CAT), and guaiacol peroxidase (GPX) activities in shoots and roots of H. vulgare (var. Rihane) grown hydroponically during 17 days either under K+-sufficient (+ K, 3 mM) or K+-deficient (-K, 10 µM) conditions supplied or not with 1 mM Si. Data are the mean of three replicates ± SE. Means followed by the same letters are not significantly different at 5% according to Duncan’s multiple range test
Leaf CAT activity was not significantly affected by all applied treatments (Fig. 6C). In roots, CAT activity was reduced by K+ deficiency (-73.3% compared to control). The addition of Si to plants subjected to K+ deficiency resulted in an increase of CAT activity in both leaves and roots, while no significant effect was observed in K+-sufficient plants (Fig. 6D).
Leaf GPX activity was significantly increased by K+ deficiency (4.6-fold) in comparison with control plants (+ K-Si treatment). The addition of Si did not change GPX activity either for K+-sufficient or deficient plants (Fig. 6E). In roots, GPX activity was significantly decreased by K+ deficiency (-64.5% compared with the K+-sufficient plants). In contrast to K+-sufficient plants where a reduction of root GPX activity was observed, the addition of Si to K+-deficient plants had no significant effect on the activity of this enzyme (Fig. 6F).
4 Discussion
The present work studies the effect of K+ deficiency–silicon interaction on growth, nutrient dynamics, and antioxidant enzymes activity in the cultivated barley H. vulgare.
Results showed that K+ deficiency results in a decrease in dry biomass production of vegetative organs (shoots and roots). This reduction may be explained partially by a decrease in root length and leaf number (Table 1). Similar results were observed in several previous reports (Hafsi et al. 2011a, 2016; Wang et al. 2015). The addition of Si to the growing medium improved the growth of shoots and roots of barley plants grown under K+-deficient conditions. However, the addition of Si to the medium containing adequate K+ concentration (3 mM: control) did not influence dry biomass production. These results are in agreement with those obtained by Miao et al. (2010) on soybean, Chen et al. (2016a) on sorghum, and Buchelt et al. (2020) on two forage plants subjected to K+ deficiency. The present data suggest that Si may play an important role in alleviating the negative impact of K+ deficiency on plant growth.
It has been suggested that K+ deficiency may affect biomass distribution between shoots and roots as a consequence of a difference in photoassimilate distribution between the two organs (Gerardeaux et al. 2010). Our results showed that the root/shoot dry mass ratio was affected neither by K+ deficiency nor Si supply (Table 1). Similar results were observed by Chen et al. (2016a). Contrarily, Miao et al. (2010) observed an increase in this ratio following Si application under K+-deficient conditions. An increase of root length was registered following exposure of Sulla carnosa (Hafsi et al. 2016) to K+ deficiency, while a decrease was observed in Triticum aestivum (Ruan et al. 2018), and banana (He et al. 2020). Our results showed a negative impact of low K+ on root length (Table 1). The addition of Si significantly increased the length of these organs in concordance with the results obtained by Miao et al. (2010).
The effects of Si on the uptake, translocation, and distribution of several nutrients are not well documented (Greger et al. 2018) particularly under K+-deficient conditions. In this context, we analyzed the dynamics of two macronutrients (K and Ca) and two micronutrients (Fe and Zn).
K+ deficiency resulted in a reduction of shoot and root K+ concentrations and contents (Fig. 1A,B3A). This is verified by a reduction in KAE (Fig. 2A). The translocation factor, an important parameter used to assess K+ efficiency (Liu et al. 2017), was not affected by K+ deficiency (Fig. 2B). It is important to note the preferential allocation of K+ toward shoots. Expressed as percent of the total K+ uptake, shoot quantities represented 91% in control plants (+ K-Si treatment) and 90% in K+-deficient plants (-K-Si treatment) (Fig. 3A). This suggests that roots accumulate sufficient K+ and hence regulate its unloading to the xylem sap probably via activation of outward-rectifying K+ channel (SKOR, Stellar K+ outward rectifier) expressed in stellar parenchyma (Gaymard et al. 1998; Liu et al. 2006). Miao et al. (2010) observed that Si increased the K+ contents of vegetative organs of soybean plants grown under low-K+ conditions. In wheat plants grown in a nutrient solution, Mali and Aery (2008) demonstrated that Si improved K+ uptake through the activation of H+-ATPase. Chen et al. (2016a) showed that K+ deficiency results in a decrease of K+ concentrations in shoots and roots and that addition of Si to the growing medium had no ameliorative effect suggesting that the beneficial effect of Si on growth is not due to enhanced K+ uptake and translocation efficiencies. Our results are in agreement with the latter suggestion. Indeed, silicon did not influence K+ concentrations and contents under control and K+-deficient conditions (Fig. 1A,B;3A). As a response to K+ limitation, H. vulgare increased KUE for biomass production.
A fivefold increase in shoot KUE was registered in K+ deficient plants compared to the K+-sufficient plants (Fig. 4A). In roots, KUE was increased by 4.2-fold in plants subjected to -K-Si treatment (Fig. 4B). Similar results were observed by Degl'Innocenti et al. (2009) and Hafsi et al. (2011b). Increased KUE reflects (i) the ability of H. vulgare to economize this macronutrient (Hafsi et al. 2007), (ii) its capacity to substitute the nonspecific functions of K+ with other ions like Na+ and Mg2+ (Hafsi et al. 2011a) and molecules such as carbohydrates and amino acids (Hermans et al. 2006; Zhu et al. 2019), and (iii) the capacity to translocate and redistribute the absorbed K+ within the plant (White 2013). The addition of Si did not improve KUE in both vegetative organs. (Fig. 4A,B) because no effect on K+ concentration was observed (Fig. 1A,B).
A modulation of Ca2+ concentrations in response to K+ deficiency has been reported in several research works (Pujos and Morard 1997; Jordan-Meille and Pellerin 2008; Hafsi et al. 2011a). In our study, K+ deficiency caused a reduction in shoot Ca2+ concentrations (Fig. 1C) and contents (Fig. 3B) but not in roots (Fig. 1D) suggesting the presence of an antagonism K+-Ca2+ in the aerial parts. Similar results were observed in Hordeum maritimum (Hafsi et al. 2011a). Like K+, the highest Ca2+ quantities were observed in shoots representing between 75 and 96% depending on the treatments (Fig. 3B). Si application reduced the translocation of Ca2+ in plants grown under sufficient and K+-deficient conditions (Fig. 2D). CaAE was reduced by K+ deficiency; Si had no significant effect under these conditions. The application of Si reduced significantly CaAE in plants grown under sufficient K+-conditions (Fig. 2C). Despite these reductions, H. vulgare plants were able to save Ca for biomass production. In fact, an increase in CaUE was registered generally following Si application, except for shoots from plants subjected to K+ deficiency (Fig. 4C,D). This may be due to a replacement of the role of Ca by Si in the formation of cell wall components. Hence, it was observed that Si forms complexes with several structural cell polymers, like pectin and callose (Boylston et al. 1990).
In Arabidopsis thaliana, Ye et al. (2019) demonstrated that K+ nutrition plays an important role in mitigating Fe deficiency through amelioration of Fe reutilization from cell walls and vacuoles as well as an increase of Fe translocation from roots to leaves. A synergistic relationship between K and Fe was noted by Tewari et al. (2013). It was observed that Si application ameliorates Fe uptake in barley by overexpression of strategy II genes (Nikolic et al. 2019) and improved Fe translocation and use efficiencies (Teixeira et al. 2020). In the present study, shoot Fe2+ concentration was not affected by K+ deficiency, while a decrease was observed in roots. Si application further decreases the Fe2+ concentration in roots (Fig. 1E,F). Fe quantities were also slightly decreased following Si application (Fig. 3C). This is proven by a decrease in FeAE (Fig. 2E). It is important to note that K+ deficiency caused an increase in Fe2+ translocation factor being the most pronounced following Si supply in K+-deficient plants (Fig. 2F). This might be explained by a beneficial effect of Si on Fe2+ translocation to shoots. In this context, an opposite behavior was registered between FeAE and Fe translocation factor in plants grown under K+-deficiency conditions (Fig. 2E,F). The increased Fe translocation could be attributed to the beneficial effect of Si on (i) the mobility of Fe and its translocation from roots to shoots in the xylem and (ii) the induced-biosynthesis of some Fe-chelation compounds like citrate in the xylem and leaf tissues as suggested by Pavlovic et al. (2013) and Bityutskii et al. (2014). Consequently, in the current work, the primary nutritional role of Si is Fe translocation rather than its uptake. An opposite response was observed by Greger et al. (2018). FeAE was significantly reduced by K+ deficiency alone or in combination with Si and consequently plants improved their FeUE for the shoot and root biomass production (Fig. 4E,F). Under K+ sufficient conditions, Si had no significant effect on FeAE and Fe translocation (Fig. 2E,F).
Concerning Zn2+ concentrations, a reduction was observed only in roots following K+ deficiency compared to K+-sufficient plants. The exogenous application of Si provokes an increase of shoot Zn2+ concentrations, no beneficial effect of Si was noted in roots (Fig. 1G,H). Under these conditions, an amelioration of ZnAE and Zn2+ translocation factor by Si was observed (Fig. 2G,H) which led to more allocation of Zn2+ to shoots (85% vs 77% in -K + Si and -K-Si-treated plants, respectively) (Fig. 3D). In contrast, a reduction of root-to-shoot translocation of Zn was registered in Cardaminopsis likely by the formation of Zn-Si complex in the roots which reduced Zn toxicity (Neumann and Zurnieden, 2001). K+ deficiency increased shoot and root ZnUE. The addition of Si decreased shoot ZnUE, while no change was found in roots (Fig. 4G,H).
K+ deficiency led to a reduction of leaf soluble protein contents (Fig. 5A). These results are in concordance with the crucial role played by K+ for protein synthesis owing particularly to its implication in the synthesis of ribosomes and aminoacyl-tRNA, the transport and movement of ribosomes, and the binding of tRNA to ribosomes (Evans and Wildes 1971; Blaha et al. 2000; Austin and First 2002). The decrease in protein contents may reflect an accumulation of amino acids which may replace K+ for osmotic adjustment as suggested by Zhu et al. (2019). In contrast, Ashraf and Zafar (1997) observed no effect of K+ deficiency on soluble proteins accumulation in lentil plants. A surprising effect was registered in roots where an increase in soluble proteins was noted (Fig. 5B) which may be an adaptative response controlling several processes including morphological, physiological, metabolism, and stress and antioxidative defense traits. Application of Si had no significant effect on soluble protein contents either under K+-sufficient and deficient conditions (Fig. 5A,B). In contrast, an improvement of soluble protein accumulation following Si application was observed by Chen et al. (2016b).
Malondialdehyde (MDA), a product of lipid peroxidation, is often used as a tool to assess the severity of the oxidative stress and the degree of cell membranes damage in plants subjected to environmental constraints (Miao et al. 2010; Ma et al. 2015). In the present work, K+ deficiency affects differentially MDA content in both roots and shoots. While no significant effect was registered in shoots, an increase in MDA content was observed in roots (Fig. 5C,D) suggesting the induction of oxidative stress in these organs. An increase in MDA contents was previously observed in several works (Tewari et al. 2007; Hafsi et al. 2011b; Hernandez et al. 2012; Zhao et al. 2021). Miao et al. (2010) suggested that exogenous application of Si could alleviate the oxidative damage caused by K+ deficiency. Our results are in agreement with these suggestions. Indeed, a decrease in MDA levels is recorded in the roots of plants subjected to the -K + Si treatment compared to those of the -K-Si treatment (Fig. 5D).
A modulation of antioxidant activities by K+ deficiency is observed in several works (Hafsi et al. 2011b; Hernandez et al. 2012; Tang et al. 2015). The beneficial effects of Si on plants growing under environmental stresses may be in part due to a regulation of the antioxidant enzyme activities (Liang et al. 2007; Miao et al. 2010; Chen et al. 2016b). Because of this, a determination of the activity of some enzymes (SOD, CAT, and GPX) is determined in leaves and roots (Fig. 6).
SODs, a family of metalloenzymes containing Mn, Fe or Cu, and Zn as prosthetic groups in their active sites, represent the first line of defense against the toxic effects of superoxide radicals produced in various cellular compartments (Wang et al. 2016). This enzyme catalyzes the dismutation of superoxide ions (O2.−) to hydrogen peroxide (H2O2) and oxygen (O2). In the present study, an increase in SOD activity following K+ deficiency is observed in leaves (Fig. 6A) in contrast to roots (Fig. 6B) where a reduction is noted. These results suggest an increase in the production of superoxide ions in leaves under the culture conditions and thus indicating their important role in the conversion of O2.− to H2O2. An increase in the activity of this enzyme was observed in several works (Tewari et al. 2007; Hafsi et al. 2011b; Qi et al. 2019). The addition of Si to K+-deficient plants further increased leaf SOD activity. On the contrary, Miao et al. (2010) and Chen et al. (2016b) observed that the addition of Si to the growing medium resulted in a decrease in SOD activity.
The detoxification of H2O2 is performed by several enzymes such as ascorbate peroxidase, catalase, and guaiacol peroxidase (Azevedo Neto et al. 2006). Catalase, an enzyme exclusively localized in peroxisomes, ensures the conversion of H2O2 to H2O and O2 (Corpas et al. 1999). An increase in catalase activity was observed by Tewari et al. (2004) in maize. No effect of K+ deficiency on the activity of this enzyme is observed in Morus alba (Tewari et al. 2007) and H. maritimum (Hafsi et al. 2011b). On the contrary, a reduction is noted in H. vulgare (Hafsi et al. 2010). Our results showed that K+ deficiency did not alter catalase activity in leaves (Fig. 6C), whereas a reduction was recorded in roots (Fig. 6D) indicating a limited role of catalase in detoxifying H2O2 under our conditions. It is important to note that the addition of Si to K+-deficient plants enhanced the activity of this enzyme in both leaves and roots. A reduction of this enzyme activity by Si was observed in previous research works (Miao et al. 2010; Chen et al. 2016b). In plants grown under K+ deficiency and Si supply (-K + Si treatment), root catalase may play a protective role in preventing oxidative stress as evidenced by the observed increase of catalase activity (Fig. 6D) and a reduction of MDA concentration (Fig. 5D). These results indicate that Si mitigates oxidative damage by enhancing antioxidant system. In addition to catalase, H2O2 detoxification could also be carried out by peroxidases including guaiacol peroxidase that uses guaiacol as an electron donor (Azevedo Neto et al. 2006). The strong increase in the activity of this enzyme in leaves (Fig. 6E) indicates its involvement in H2O2 detoxification in these organs in contrast to roots where a significant reduction was observed (Fig. 6F). Under K+-deficient conditions, the application of Si did not influence GPX activity in contrast to the results registered by Miao et al. (2010) where a reduction was noted. It is noteworthy that the increase in SOD activity in leaves and its reduction in roots is in agreement with the activities of enzymes involved in H2O2 removal.
5 Conclusion
K+ deficiency reduced plant growth and K+ concentrations in shoots and roots. A beneficial effect of Si was recorded for plant growth but not K+ nutrition indicating that the beneficial effect of Si on growth is not due to enhanced K+ uptake and translocation efficiencies. In addition, silicon differentially influences K+, Ca2+, Fe2+, and Zn2+ uptake, translocation, distribution, and use efficiencies suggesting an individual regulation of each nutrient. A differential regulation of antioxidant enzymes was also registered. These physiological and biochemical changes mediated by Si may contribute to the improvement of barley growth under K+-deficient conditions.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Ashraf M, Zafar ZU (1997) Effect of potassium deficiency on growth and some biochemical characteristics in two lines of lentil (Lens culinaris Medic.). Acta Physiol Plant 19:9–15
Austin J, First EA (2002) Potassium functionally replaces the second lysine of the KMSKS signature sequence in human tyrosyl-tRNA synthetase. J Biol Chem 277:20243–20248. https://doi.org/10.1074/jbc.M201923200
Azevedo Neto AD, Prisco JT, Enéas-Filho J, Braga de Abreu CE, Gomes-Filho E (2006) Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ Exp Bot 56:87–94. https://doi.org/10.1016/j.envexpbot.2005.01.008
Bityutskii N, Pavlovic J, Yakkonen K, Maksimović V, Nikolic M (2014) Contrasting effect of silicon on iron, zinc, and manganese status and accumulation of metal-mobilizing compounds in micronutrient-deficient cucumber. Plant Physiol Biochem 74:205–211. https://doi.org/10.1016/j.plaphy.2013.11.015
Blaha G, Stelzl U, Spahn CMT, Agrawal RK, Frank J, Nierhaus KH (2000) Preparation of functional ribosomal complexes and effect of buffer conditions on tRNA positions observed by cryoelectron microscopy. Methods Enzymol 317:292–309. https://doi.org/10.1016/s0076-6879(00)17021-1
Boylston EK, Hebert JJ, Hensarling TP, Bradow JM, Thibodeaux DP (1990) Role of silicon in developing cotton fibers. J Plant Nutr 13:131–148. https://doi.org/10.1080/01904169009364063
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilising the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Buchelt AC, Teixeira GCM, Oliveira KS, Rocha AMS, de Mello PR, Caione G (2020) Silicon contribution via nutrient solution in forage plants to mitigate nitrogen, potassium, calcium, magnesium, and sulfur deficiency. J Soil Sci Plant Nutr 20:1532–1548. https://doi.org/10.1007/s42729-020-00245-7
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310. https://doi.org/10.1016/S0076-6879(78)52032-6
Chance B, Maehly A (1955) Assay of catalases and peroxidases. Methods Enzymol 2:764–775. https://doi.org/10.1016/S0076-6879(55)02300-8
Che J, Yamaji N, Ji Shao F, Ma JF, Shen RF (2016) Silicon decreases both uptake and root-to-shoot translocation of manganese in rice. J Exp Bot 67:1535–1544. https://doi.org/10.1093/jxb/erv545
Chen D, Cao B, Qi L, Yin L, Wang S, Deng X (2016a) Silicon moderated K-deficiency-induced leaf chlorosis by decreasing putrescine accumulation in sorghum. Ann Bot 118:305–315. https://doi.org/10.1093/aob/mcw111
Chen D, Cao B, Wang S, Liu P, Deng X, Yin L, Zhang S (2016b) Silicon moderated the K deficiency by improving the plant-water status in sorghum. Sci Rep 6:22882. https://doi.org/10.1038/srep22882
Choudhary A, Kumar A, Kaur N (2020) ROS and oxidative burst: Roots in plant development. Plant Divers 42:33–43. https://doi.org/10.1016/j.pld.2019.10.002
Corpas F, Palma JM, Sandalio LM, Lopez-Huertas E, Romero-Puertas MC, Barroso JB, del Río LA (1999) Purification of catalase from pea leaf peroxisomes: identification of five different isoforms. Free Rad Res 31:235–241. https://doi.org/10.1080/10715769900301561
da Silva DL, Prado RM, Tenesaca LFL, da Silva JLF, Mattiuz BH (2021) Silicon attenuates calcium defciency by increasing ascorbic acid content, growth and quality of cabbage leaves. Sci Rep 11:1770. https://doi.org/10.1038/s41598-020-80934-6
Das P, Manna I, Sil P, Bandyopadhyay M, Biswas AK (2021) Silicon augments salt tolerance through modulation of polyamine and GABA metabolism in two indica rice (Oryza sativa L.) cultivars. Plant Physiol Biochem 166:41–52. https://doi.org/10.1016/j.plaphy.2021.05.030
Degl’Innocenti E, Hafsi C, Guidi L, Navari-Izzo F (2009) The effect of salinity on photosynthetic activity in potassium-deficient barley species. J Plant Physiol 166:1968–1981. https://doi.org/10.1016/j.jplph.2009.06.013
dos Santos Sarah MM, de Mello PR, de Souza Júnior JP, Teixeira GCM, dos Santos Duarte JC, de Medeiros RLS (2021) Silicon supplied via foliar application and root to attenuate potassium defciency in common bean plants. Sci Rep 11:19690. https://doi.org/10.1038/s41598-021-99194-z
dos Santos Sarah MM, de Mello PR, Teixeira GCM, de Souza Júnior JP, de Medeiros RLS, Barreto RF (2022) Silicon supplied via roots or leaves relieves potassium deficiency in maize plants. SILICON 14:773–782. https://doi.org/10.1007/s12633-020-00908-1
Evans HJ, Wildes RA (1971) Potassium and its role in enzyme activation. Potassium in biochemistry and physiology. International Potash Institute, Berne 13–39
Gaymard F, Pilot G, Lacombe B, Bouchez D, Bruneau D, Boucherez J, Michaux-Ferriere N, Thibaud JB, Sentenac H (1998) Identification and disruption of a plant Shaker-like outward channel involved in K+ release into the xylem sap. Cell 94:647–655. https://doi.org/10.1016/s0092-8674(00)81606-2
Gerardeaux E, Jordan-Meille L, Constantin J, Pellerin S, Dingkuhn M (2010) Changes in plant morphology and dry matter partitioning caused by potassium deficiency in Gossypium hirsutum (L.). Environ Exp Bot 67:451–459. https://doi.org/10.1016/j.envexpbot.2009.09.008
Giannopolitis CN, Ries SK (1977) Superoxide dismutases I. Occurrence in Higher Plants Plant Physiol 59:309–314. https://doi.org/10.1104/pp.59.2.309
Greger M, Landberg T, Vaculík M (2018) Silicon influences soil availability and accumulation of mineral nutrients in various plant species. Plants 7:41. https://doi.org/10.3390/plants7020041
Haddad C, Arkoun M, Jamois F, Schwarzenberg A, Yvin JC, Etienne P, Laîné P (2018) Silicon promotes growth of Brassica napus L. and delays leaf senescence induced by nitrogen starvation. Front Plant Sci 9:516. https://doi.org/10.3389/fpls.2018.00516
Hafsi C, Atia A, Lakhdar A, Debez A, Abdelly C (2011a) Differential responses in potassium absorption and use efficiencies in the halophytes Catapodium rigidum and Hordeum maritimum to various potassium concentrations in the medium. Plant Prod Sci 14:135–140. https://doi.org/10.1626/pps.14.135
Hafsi C, Bettaib J, Falleh H, Zorrig W, Ksouri R, Abdelly C, Debez A (2021) Phenolic accumulation and related antioxidant capacity in stems and roots of the Tunisian extremophile Sulla carnosa as influenced by potassium application under salinity stress. Arab J Geosci 14:34. https://doi.org/10.1007/s12517-020-06334-2
Hafsi C, Debez A, Abdelly C (2014) Potassium deficiency in plants: effects and signaling cascades. Acta Physiol Plant 36:1055–1070. https://doi.org/10.1007/s11738-014-1491-2
Hafsi C, Falleh H, Saada M, Ksouri R, Abdelly C (2017) Potassium deficiency alters growth, photosynthetic performance, secondary metabolites content, and related antioxidant capacity in Sulla carnosa grown under moderate salinity. Plant Physiol Biochem 118:609–617. https://doi.org/10.1016/j.plaphy.2017.08.002
Hafsi C, Falleh H, Saada M, Rabhi M, Mkadmini K, Ksouri R, Abdelly C, Smaoui A (2016) Effects of potassium supply on growth, gas exchange, phenolic composition, and related antioxidant properties in the forage legume Sulla carnosa. Flora 223:38–45. https://doi.org/10.1016/j.flora.2016.04.012
Hafsi C, Lakhdhar A, Rabhi M, Debez A, Abdelly C, Ouerghi Z (2007) Interactive effects of salinity and potassium availability on growth, water status, and ionic composition of Hordeum maritimum. J Plant Nutr Soil Sci 170:469–473. https://doi.org/10.1002/jpln.200625203
Hafsi C, Romero-Puertas MC, del Río LA, Abdelly C, Sandalio LM (2011b) Antioxidative response of Hordeum maritimum L. to potassium deficiency. Acta Physiol Plant 33:193–202. https://doi.org/10.1007/s11738-010-0537-3
Hafsi C, Romero-Puertas MC, del Río LA, Sandalio LM, Abdelly C (2010) Differential antioxidative response in barley leaves subjected to the interactive effects of salinity and potassium deprivation. Plant Soil 334:449–460. https://doi.org/10.1007/s11104-010-0395-1
Hasanuzzaman M, Borhannuddin Bhuyan MHM, Zulfiqar F, Raza A, Mohsin SM, Al Mahmud J, Fujita M, Fotopoulos V (2020) Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator. Antioxidants 9:681. https://doi.org/10.3390/antiox9080681
He Y, Li R, Lin F, Xiong Y, Wang L, Wang B, Guo J, Hu C (2020) Transcriptome changes induced by different potassium levels in banana roots. Plants 9:11. https://doi.org/10.3390/plants9010011
Hermans C, Hammond JP, White PJ, Verbruggen N (2006) How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci 11:610–617. https://doi.org/10.1016/j.tplants.2006.10.007
Hernandez M, Fernandez-Garciaa N, Garcia-Garmaa J, Rubio-Asensioa JS, Rubio F, Olmos E (2012) Potassium starvation induces oxidative stress in Solanum lycopersicum L. roots. J Plant Physiol 169:1366–1374. https://doi.org/10.1016/j.jplph.2012.05.015
Hewitt EJ (1966) Sand and water culture methods used in the study of plant nutrition. Commonwealth Bureau of Horticultural Plantation Crops Tech. Commun N 22. https://doi.org/10.2134/agronj1952.0002196200440012001
Hu AY, Che J, Shao JF, Yokosho K, Zhao XQ, Shen RF, Ma JF (2018) Silicon accumulated in the shoots results in down-regulation of phosphorus transporter gene expression and decrease of phosphorus uptake in rice. Plant Soil 423:317–325. https://doi.org/10.1007/s11104-017-3512-6
Hunt R (1990) Basic Growth Analysis Plant Growth Analysis for Beginners. Unwin Hyman, London
Hurtado AC, Chiconato DA, Prado RM, Junior GSS, Felisberto G (2019) Silicon attenuates sodium toxicity by improving nutritional efficiency in sorghum and sunflower plants. Plant Physiol Biochem 142:224–233. https://doi.org/10.1016/j.plaphy.2019.07.010
Hussain S, Shuxian L, Mumtaz M, Shafiq I, Iqbal N, Brestic M, Shoaib M, Sisi Q, Li W, Mei X, Bing C, Zivcak M, Rastogi A, Skalicky M, Hejnak V, Weiguo L, Wenyu Y (2021) Foliar application of silicon improves stem strength under low light stress by regulating lignin biosynthesis genes in soybean (Glycine max (L) Merr). J Hazard Materials 401:123256. https://doi.org/10.1016/j.jhazmat.2020.123256
Jordan-Meille L, Pellerin S (2008) Shoot and root growth of hydroponic maize (Zea mays L.) as influence by K deficiency. Plant Soil 304:157–168. https://doi.org/10.1007/s11104-007-9534-8
Khan A, Bilalb S, Khan AL, Imran M, Al-Harrasi A, Al-Rawahi A, Lee IJ (2020) Silicon-mediated alleviation of combined salinity and cadmium stress in date palm (Phoenix dactylifera L.) by regulating physio-hormonal alteration. Ecotox Environ Safe 188:109885. https://doi.org/10.1016/j.ecoenv.2019.109885
Ksiaa M, Farhat N, Rabhi M, Elkhouni A, Smaoui A, Debez A, Cabassa-Hourton C, Savouré A, Abdelly C, Zorrig W (2021). Silicon (Si) alleviates iron deficiency effects in sea barley (Hordeum marinum) by enhancing iron accumulation and photosystem activities. Silicon 1-16https://doi.org/10.1007/s12633-021-01376-x
Laifa I, Hajji M, Farhat N, Elkhouni A, Smaoui A, M’nif A, Hamzaoui AH, Savouré A, Abdelly C, Zorrig W (2021) Beneficial effects of silicon (Si) on sea barley (Hordeum marinum Huds.) under salt stress. Silicon 13:4501–4517. https://doi.org/10.1007/s12633-020-00770-1
Liang YC, Sun WC, Zhu YG, Christie P (2007) Mechanisms of silicon mediated alleviation of abiotic stresses in higher plants: a review. Environ Pollut 147:422–428. https://doi.org/10.1016/j.envpol.2006.06.008
Liu C, Tu B, Li Y, Tian B, Zhang Q, Liu X, Herbert SJ (2017) Potassium application affects key enzyme activities of sucrose metabolism during seed filling in vegetable soybean. Crop Sci 57:2707–2717. https://doi.org/10.2135/cropsci2016.08.0648
Liu K, Li L, Luan S (2006) Intracellular K+ sensing of SKOR, a Shaker-type K+ channel from Arabidopsis. Plant J 46:260–268. https://doi.org/10.1111/j.1365-313X.2006.02689.x
Lu Z, Ren T, Pan Y, Li X, Cong R, Lu J (2016) Differences on photosynthetic limitations between leaf margins and leaf centers under potassium deficiency for Brassica napus L. Sci Rep 6:21725. https://doi.org/10.1038/srep21725
Ma J, Du G, Li X, Zhang C, Guo JA (2015) Major locus controlling malondialdehyde content under water stress is associated with Fusarium crown rot resistance in wheat. Mol Genet Genom 290:1955–1962. https://doi.org/10.1007/s00438-015-1053-3
Mali M, Aery NC (2008) Influence of silicon on growth, relative water contents and uptake of silicon, calcium and potassium in wheat grown in nutrient solution. J Plant Nutr 31:1867–1876. https://doi.org/10.1080/01904160802402666
Malik RN, Husain SZ, Nazir I (2010) Heavy metal contamination and accumulation in soil and wild plant species from industrial area of Islamabad, Pakistan. Pak J Bot 42:291–301
Miao BH, Han XG, Zhang WH (2010) The ameliorative effect of silicon on soybean seedlings grown in potassium-deficient medium. Ann Bot 105:967–973. https://doi.org/10.1093/aob/mcq063
Neumann D, Zurnieden U (2001) Silicon and heavy metal tolerance of higher plants. Phytochemistry 56:685–692. https://doi.org/10.1016/S0031-9422(00)00472-6
Nikolic DB, Nesic S, Bosnic D, Kostic L, Nikolic M, Samardzic JT (2019) Siliconalleviates iron deficiency in barley by enhancing expression of strategy II genes and metal redistribution. Front Plant Sci 10:416. https://doi.org/10.3389/fpls.2019.00416
Pavlovic J, Samardzic J, Maksimović V, Timotijevic G, Stevic N, Laursen KH, Hansen TH, Husted S, Schjoerring JK, Liang Y, Nikolic M (2013) Silicon alleviates iron deficiency in cucumber by promoting mobilization of iron in the root apoplast. New Phytol 198:1096–1107. https://doi.org/10.1111/nph.12213
Pereira GJG, Molina SMG, Lea PJ, Azevedo RA (2002) Activity of antioxidant enzymes in response to cadmium in Crotalaria juncea. Plant Soil 239:123–132. https://doi.org/10.1023/A:1014951524286
Pujos A, Morard P (1997) Effects of potassium deficiency on tomato growth and mineral nutrition at the early production stage. Plant Soil 189:189–196. https://doi.org/10.1023/A:1004263304657
Qi D, Xin-hua Z, Le X, Chun-ji J, Xiao-guang W, Yi H, Jing W, Hai-qiu Y (2019) Effects of potassium deficiency on photosynthesis, chloroplast ultrastructure, ROS, and antioxidant activities in maize (Zea mays L.). J Integr Agr 18:395–406. https://doi.org/10.1016/S2095-3119(18)61953-7
Rabhi M, Barhoumi Z, Ksouri R, Abdelly C, Gharsalli M (2007) Interactive effects of salinity and iron deficiency in Medicago ciliaris. CR Biologies 330:779–788. https://doi.org/10.1016/j.crvi.2007.08.007
Römheld V, Kirkby EA (2010) Research on potassium in agriculture: needs and prospects. Plant Soil 335:155–180. https://doi.org/10.1007/s11104-010-0520-1
Ruan L, Xin X, Zhang J, Zhao B, Cheng H, Zhang C, Ma D, Chen L (2018) Potential root foraging strategy of wheat (Triticum aestivum L.) for potassium heterogeneity. Front Plant Sci 9:1755. https://doi.org/10.3389/fpls.2018.01755
Singh A, Kumar A, Yadav S, Singh IK (2019) Reactive oxygen species-mediated signaling during abiotic stress. Plant Gene 18:100173. https://doi.org/10.1016/j.plgene.2019.100173
Tang ZH, Zhang AJ, Wei M, Chen XG, Liu ZH, Li HM, Ding YF (2015) Physiological response to potassium deficiency in three sweet potato (Ipomoea batatas [L.] Lam.) genotypes differing in potassium utilization efficiency. Acta Physiol Plant 37:184. https://doi.org/10.1007/s11738-015-1901-0
Teixeira GCM, Prado RM, Oliveira KS, D’Amico-Damião V, Junior GSS (2020) Silicon increases leaf chlorophyll content and iron nutritional efficiency and reduces iron deficiency in sorghum plants. J Soil Sci Plant Nutr 20:1311–1320. https://doi.org/10.1007/s42729-020-00214-0
Tewari RK, Hadacek F, Sassmann S, Lang I (2013) Iron deprivation-induced reactive oxygen species generation leads to non-autolytic PCD in Brassica napus leaves. Environ Exp Bot 91:74–83. https://doi.org/10.1016/j.envexpbot.2013.03.006
Tewari RK, Kumar P, Sharma PN (2007) Oxidative stress and antioxidant responses in young leaves of mulberry plants under nitrogen, phosphorus or potassium deficiency. J Integr Plant Biol 49(3):313–322. https://doi.org/10.1111/j.1744-7909.2007.00358.x
Tewari RK, Kumar P, Tewari N, Srivastava S, Sharma PN (2004) Macronutrient deficiencies and differential antioxidant responses influence on the activity and expression of superoxide dismutase in maize. Plant Sci 166:687–694. https://doi.org/10.1016/j.plantsci.2003.11.004
Wang W, Xia MX, Chen J, Yuan R, Deng FN, Shen FF (2016) Gene expression characteristics and regulation mechanisms of superoxide dismutase and its physiological roles in plants under stress. Biochem Mosc 81:465–480. https://doi.org/10.1134/S0006297916050047
Wang XG, Zhao XH, Jiang CJ, Li CH, Cong S, Wu D, Chen YQ, Wang CY (2015) Effects of potassium deficiency on photosynthesis and photoprotection mechanisms in soybean (Glycine max (L.) Merr.). J Integr Agric 14:856–863. https://doi.org/10.1016/S2095-3119(14)60848-0
Wang Y, Wu WH (2015) Genetic approaches for improvement of the crop potassium acquisition and utilization efficiency. Curr Opin Plant Biol 25:46–52. https://doi.org/10.1016/j.pbi.2015.04.007
White PJ (2013) Improving potassium acquisition and utilisation by crop plants. J Plant Nutr Soil Sci 176:305–316. https://doi.org/10.1002/jpln.201200121
Wu J, Mock HP, Giehl RF, Pitann B, Mühling KH (2019) Silicon decreases cadmium concentrations by modulating root endodermal suberin development in wheat plants. J Hazard Materials 364:581–590. https://doi.org/10.1016/j.jhazmat.2018.10.052
Ye YQ, Luo HY, Li M, Zhang JJ, Cao GQ, Lin KM, Lin SZ, Xu SS (2019) Potassium ameliorates iron deficiency by facilitating the remobilization of iron from root cell walls and promoting its translocation from roots to shoots. Plant Soil 440:507–521. https://doi.org/10.1007/s11104-019-04111-z
Ye Z, Zeng J, Ma X, Long L, Zhang G (2021) Transcriptome profiling analysis reveals involvement of SAM cycle and methionine pathway in low potassium tolerance in barley. Curr Plant Biol 25:100190. https://doi.org/10.1016/j.cpb.2020.100190
Zeng J, Quan X, He X, Cai S, Ye Z, Chen G, Zhang G (2018) Root and leaf metabolite profiles analysis reveals the adaptive strategies to low potassium stress in barley. BMC Plant Biol 18:187. https://doi.org/10.1186/s12870-018-1404-4
Zhao X, Zhang N, Liu X, Jiang J (2021) The effects of K+-deficiency on H2O2 dynamics and sucrose in tomato. Hort Sci (Prague) 48:90–97. https://doi.org/10.17221/103/2020-HORTSCI
Zhu B, Xu Q, Zou Y, Ma S, Zhang X, Xie X, Wang L (2019) Effect of potassium deficiency on growth, antioxidants, ionome and metabolism in rapeseed under drought stress. Plant Growth Regul 90:455–466. https://doi.org/10.1007/s10725-019-00545-8
Zorrig W, Cornu JY, Maisonneuve B, Rouached A, Sarrobert C, Shahzad Z, Abdelly C, Davidian JC, Berthomieu P (2019) Genetic analysis of cadmium accumulation in lettuce (Lactuca sativa). Plant Physiol Biochem 136:67–75. https://doi.org/10.1016/j.plaphy.2019.01.011
Acknowledgements
This work was supported by the Tunisian Ministry of Higher Education and Scientific Research (LR19CBBC02). A part of the work was also supported by the PHC Maghreb 2019 network (Partenariat Hubert CURIEN Maghreb 2019 no. 41482RL—19MAG41). We thank the staff of the Centre of Biotechnology of Borj-Cedria (CBBC) for technical and administrative supports.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Benslima, W., Ellouzi, H., Zorrig, W. et al. Beneficial effects of silicon on growth, nutrient dynamics, and antioxidative response in barley (Hordeum vulgare L.) plants under potassium deficiency. J Soil Sci Plant Nutr 22, 2633–2646 (2022). https://doi.org/10.1007/s42729-022-00832-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-022-00832-w