Abstract
Developing salt-tolerant plants has emerged as a highly efficient approach to cope with salinity damage on crop growth and productivity. This study aimed to elucidate the mechanisms of salt acclimation in cowpea plants [Vigna unguiculata (L.) Walp] and screen salt-tolerant potential genotypes during the early vegetative stage. Seven cowpea genotypes (Epace, Juruá, Maratauã, Milagroso, Pitiúba, Sempre verde, and TVU) were irrigated for 24 days with saline solutions of electrical conductivity 0.8 (control), 4.0 (moderate stress), and 8.0 dS m−1 (severe stress). Growth, water status, membrane damage, and variables related to photosynthetic machinery efficiency were evaluated. Biomass accumulation dramatically decreased with salinity, and the reductions were intensified by increasing the salt level. Nevertheless, under moderate salinity, Pitiúba plants showed less reductions in growth than other genotypes. Under moderate stress, Pitiúba plants exhibited maintenance of osmotic potential and photosynthetic pigments, which was consistent with unaltered membrane and elevated leaf succulence, resulting in improved photochemical performance. Conversely, although TVU, Juruá, and Milagroso plants had activated responses against moderate salinity, including reduced leaf osmotic potential and improved stomatal conductance, these responses were not sufficient to mitigate salt injury. The findings clearly show that the Pitiúba genotype activates coordinated responses to mitigate moderate salt damage, constituting an alternative for cultivating cowpea plants in saline environments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Soil salinity is a major limiting environmental factor for crop growth and productivity, especially in arid and semiarid regions. Globally, it is estimated that 45 million ha (20%) of irrigated areas are salt-affected soils, disturbing crop production, the economy, ecosystems, the quality of natural resources, and the living conditions of farmers (FAO 2020). Salt harmful effects in plants arise from an ionic and osmotic imbalance that impairs structural functions and physiological and biochemical processes (Miranda et al. 2014, 2017; Farooq et al. 2017; Van Nguyen et al. 2020).
Salt-tolerant plants usually display mechanisms to optimize water absorption, extrude toxic ions, and avoid oxidative damage (Miranda et al. 2017; Freitas et al. 2018; Causin et al. 2020; Coelho et al. 2020). In addition, success in acclimating species to saline environments depends on the capability to rapidly perceive salinity and signalize defense mechanisms, a complex genetically determined trait (Bao et al. 2014; Freitas et al. 2018).
Over the last decades, numerous efforts have been made to genetically engineer plants for the overproduction of sensors and signaling elements and specific genes responsive to salinity. Target species are glycophytic plants, which correspond to the most food-agricultural crops and display elevated sensitivity to salt stress (Bargaz et al. 2016; Ismail and Horie 2017). Nevertheless, engineered plants have shown improved salt tolerance, mainly at the laboratory level (Huertas et al. 2012; Mishra et al. 2014; Huo et al. 2021; Li et al. 2021), but little success has been reached for commercially producing crops (Agarwal et al. 2013; Cabello et al. 2014). Otherwise, although the exogenous application of organic/inorganic compounds has also improved tolerance to salt stress for numerous plant species, this approach has not received adequate consideration in the field (Hu et al. 2012; Fan et al. 2013; Gondim et al. 2013; Freitas et al. 2019). Hence, developing salt-tolerant genotypes/varieties constitutes a highly efficient approach to cope with the damaging effects of soil salinity in crop production.
Cowpea [Vigna unguiculata (L.) Walp] is a crop widely cultivated in arid and semiarid regions, constituting one of the most important leguminous crops worldwide. Studies focusing on cowpea cultivars with contrasting responses to saline stress were previously conducted (Costa et al. 2003; Murillo-Amador et al. 2006; Praxedes et al. 2014). Nonetheless, little information has been described into the genetic variability of the response to salinity and how intricate mechanism networks related to the photosynthetic machinery of cowpea plants are determinants for salt tolerance.
Our working hypothesis was that cowpea genotypes vary in their responses to salinity for several physiological processes and oxidative damage to plant tissues. This hypothesis was tested by investigating the impact of three salinity levels in water irrigation on different indicative tolerance parameters (growth, leaf gas exchange, photosynthetic pigments, photosystem II efficiency, oxidative damage, and water status) in seven cowpea genotypes.
2 Material and Methods
2.1 Plant Material and Growth Conditions
To select salt-tolerant cowpea genotypes, experiments were carried out in a greenhouse from November 2017 to July 2018 at the Technological Development Center (CDT) of Brazilian Airport Infrastructure Company (Infraero), Imperatriz, Maranhão, Brazil, with geographic coordinates of 5°31′32″ S and 47°26′35″ W at approximately 92 m altitude. The experiments were conducted in 8 dm3 plastic pots containing 7.5 kg of soils collected at different sites from agricultural areas of Imperatriz city. Compound soil samples were analyzed for characterization of the chemical attributes, and the soil was corrected as necessary, according to Souza and Lobato (2014) (Table 1).
The experiment was conducted in a randomized completely design in a 7 × 3 factorial scheme composed of seven cowpea genotypes (Epace, Juruá, Maratauã, Milagroso, Pitiúba, Sempre verde, and TVU 2331) and three salinity levels [electrical conductivity (EC) 0.8 (control), 4.0 (moderate stress), and 8.0 dS m−1 (severe stress)]. Four replications per treatment (one plant per pot) were employed, totaling 84 experimental units.
V. unguiculata genotypes were selected based on productivity and cultivation in agricultural areas from Brazilian semiarid. Seeds were obtained from Banco Ativo de Germoplasma (BAG) at Federal University of Ceará (UFC, Fortaleza, Ceará, Brazil), sterilized with 2% sodium hypochlorite, and subsequently sown in soils previously irrigated to field capacity. Twelve days after sowing, salt stress was started by irrigation with saline water prepared by dissolving NaCl, CaCl2·2H2O, and MgCl2 (7:2:1 w/w) in tap water (Rhoades et al. 2000), adjusting the values of EC to 0.8, 4.0, and 8.0 dS m−1 (Silva et al. 2018). The plants were regularly watered to field capacity using the principle of lysimeter drainage (Bernardo et al. 2008) and adding a leaching fraction of 15% to prevent excessive salt accumulation.
Environmental conditions in the greenhouse during the experiments were as follows: an average air relative humidity of 60.6%; an average day/night temperature of 32.7/28.1 °C; an average midday photosynthetic photon flux density (PPFD) of approximately 1,200 µmol m−2 s−1; and a 12/12 h photoperiod. The harvests were performed 24 days after beginning the salinity treatments.
2.2 Gas Exchange and Chlorophyll Fluorescence Assays
Leaf gas exchange was measured using a portable photosynthesis system (IRGA, Licor-6400-XT, USA) equipped with a fluorometer (LI-6400–40, LI-COR Inc., Lincoln, USA) from 09:00 to 11:30 a.m. on days with full sun. The values of net photosynthesis (A, μmol CO2 m−2 s−1), transpiration (E, mol H2O m−2 s−1), stomatal conductance (gs, mol H2O m−2 s−1), and internal CO2 concentration (Ci, ppm) were measured under constant CO2 concentration (390 μmol CO2 mol−1 air) and PPFD (1,000 μmol photons m−2 s−1).
Simultaneously, chlorophyll fluorescence parameters were measured in light-adapted leaves (Fm’, maximum fluorescence; Fo’, minimal fluorescence; Fv’, variable fluorescence) and dark-adapted leaves (Fm, maximum fluorescence; Fo, minimal fluorescence; Fv, variable fluorescence; Fs, fluorescence emission at steady state) for 40 min. The data were used to calculate the maximum quantum efficiency of PSII photochemistry [Fv/Fm = (Fm − Fo)/Fm]; PSII operating efficiency [ΦPSII = (Fm’ − Fs)/Fm’]; relative energy excess at the PSII level [EXC = (Fv/Fm) − (ΦPSII)/(Fv/Fm)]; and nonphotochemical [NPQ = (Fm − Fm’)/Fm’] and photochemical [qP = (Fm’ − Fs)/(Fm’ − Fo’)] quenching. The electron transport rate (ETR) was calculated using the formula ETR = ΦPSII × PPFD × 0.84 × 0.5, where 0.84 is the assumed light absorbance by the leaves, 0.5 is the fraction of excitation energy diverted to PSII, and PPFD of 1,000 μmol m−2 s−1. The alternative sink of photosynthetic electrons (ETR/A ratio) was also evaluated.
2.3 Plant Growth
During the harvest, four plants from each treatment were individually collected and divided into leaves, stems, and roots. The plant tissues were dried in a forced-air oven at 80 °C for 3 days prior to dry mass measurement. The relative salt tolerance was measured as the total dry mass of plants grown in saline conditions (moderate or severe stress) relative to the total dry mass of plants in the control treatment (Miranda et al. 2017).
2.4 Photosynthetic Pigments
Chlorophylls (a and b) and carotenoids were extracted after incubating disks from leaves in CaCO3 saturated in dimethyl sulfoxide (DMSO) at room temperature in the dark for 72 h. Photosynthetic pigment contents were measured by spectrophotometric readings at 480, 649, and 665 nm, according to equations defined by Wellburn (1994).
2.5 Membrane Damage
Cell membrane damage was measured as electrolyte leakage using a conductivity meter. Leaf disks were placed in closed vials containing deionized water and incubated at room temperature on a rotary shaker for 12 h. Then, the EC of the solution was determined (S1). Subsequently, the homogenate was incubated at 100 °C for 15 min, and the conductivity of the solution was measured again (S2). Membrane damage (%) was calculated as S1/S2 × 100.
2.6 Relative Water Content, Leaf Succulence, and Osmotic Potential
Relative water content (RWC) was employed to estimate plant hydric status. Leaf disks were first weighed to obtain fresh weight (FW). Thereafter, the samples were immersed in deionized water to full turgidity for 6 h at room temperature to obtain turgid weight (TW). Samples were then oven-dried at 70 °C for 24 h and weighed to measure dry weight (DW). RWC was calculated as RWC (%) = [(FW − DW)/(TW − DW)] × 100. Leaf succulence was measured through the difference between the fresh and dry mass of leaves (adapted from Mantovani 1999).
Leaf osmotic potential (Ψs) was assayed through a vapor pressure osmometer (model 5600, Vapro®) in the first fully expanded leaves. The data were expressed as MPa according to the Van’t Hoff equation: Π = − R × T × Ci, where R, T, and Ci correspond to the universal gas constant (0.00831 MPa kg mol−1 K−1), absolute temperature (298 K), and molar concentration of solute (mol kg−1), respectively, as described by Bao et al. (2014).
2.7 Statistical Analysis
All data were subjected to analysis of variance (ANOVA) employing the F test at 95%, and average values were grouped using the Scott-Knott test (p < 0.05) (statistical details in Tables S1-S6). Pearson correlation analysis was carried out between all evaluated analyses to obtain an integrated view of plant performance under salinity and establish a correlation between physiological, biochemical, and phenotypic datasets. Statistical assays were carried out using Sisvar (version 5.10) and JASP (version 0.12.2) programs, and the graphs were plotted using SigmaPlot (version 11.0) software.
3 Results
3.1 Water Status and Membrane Damage
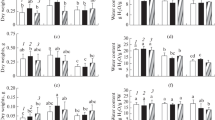
Salt stress significantly decreased the relative water content (RWC) of leaves in the Milagroso, Pitiúba, and Sempre verde genotypes, irrespective of stress level. Under severe salinity (8.0 dS m−1), the RWC values from Milagroso, Pitiúba, and Sempre verde plants were found to be 18.1, 26.6, and 13.3% lower than those of the respective controls (Fig. 1a).
Relative water content (a), leaf succulence (b), and osmotic potential (c) from leaves of cowpea genotypes after 24 days of salinity stress imposed by irrigation with salt solutions containing electric conductivities (ECs) of 0.8 (control—white bar), 4.0 (moderate salinity—gray bar), and 8.0 dS m−1 (severe salinity—black bar). Data are expressed as average of four repetitions. Within the same genotype (same bar), significant differences due to salt stress are represented by the presence of asterisks, while significant differences due to the cowpea genotype within the salt treatment (white, gray, or black filled bar portions) are denoted by different lowercase letters, according to the Scott-Knott test (p < 0.05). For absolute values, see Table S1
Leaf succulence was decreased by salt stress in all cowpea plants, irrespective of genotype and stress level (Fig. 1b); however, the most conspicuous reductions by moderate stress were registered in TVU (41.8%) and Milagroso (48.4%) plants compared to the control treatment. Under moderate stress, Pitiúba, Epace, and Sempre verde plants showed leaf succulence greater than that of other studied genotypes. On the other hand, succulence was dramatically decreased in the leaves of severely stressed plants, with an average reduction of 45.3% from all cowpea genotypes compared to controls (Fig. 1b).
Interestingly, the cowpea plants displayed differential modulation of osmotic potential under salinity (Fig. 1c). For all cases, Pitiúba and Sempre verde genotypes showed no or slight alteration in leaf osmotic potential by salt imposition, while TVU, Epace, Juruá, Maratauã, and Milagroso plants displayed a substantial decrease in osmotic potential in response to salt imposition, especially under severe stress (except for TVU) (Fig. 1c).
Salt-stressed plants from the Epace, Juruá, Sempre verde, Maratauã, and Milagroso genotypes showed a marked increase in leaf membrane damage, whereas no significant alteration by salinity was registered in Pitiúba and TVU plants compared to the respective controls (Fig. 2). At both salt levels, although the highest values of membrane damage were registered in the salt-stressed Pitiúba genotype, these plants showed minor variations in comparison to the control condition (Fig. 2).
Membrane damage from leaves of cowpea genotypes after 24 days of salinity imposition. Irrigation salt solutions contained electric conductivities (ECs) of 0.8 (control), 4.0 (moderate salinity), and 8.0 dS m−1 (severe salinity). Additional details same as in Fig. 1
3.2 Plant Growth and Salt Tolerance
In general, salt-stressed cowpea plants showed a significant decrease in leaf, stem, root, and total dry mass, regardless of salinity level and cowpea genotype, except for root of Pitiúba, Juruá, and Sempre verde plants subjected to moderate stress (Figs. 3 and 4). In the majority of cowpea genotypes, the dry mass decrease was intensified by increasing the salinity level under water irrigation (from 4.0 to 8.0 dS m−1) (Fig. 4). For all cases, the average decrease in dry mass of leaf, stem, and roots was found to be 28.4, 36.6, and 25.0% under moderate stress, and 53.0, 56.7, and 48.8% under severe salinity, respectively, compared to control plants (Fig. 3a, b, c) However, at 4.0 dS m−1 salinity, the highest total dry mass accumulation was registered in Pitiúba plants, followed by Epace, Maratauã, and Sempre verde, whereas the lowest dry mass production was observed in Milagroso, Juruá, and TVU plants (Fig. 3d). Yet, at 8.0 dS m−1 (severe stress), the total dry mass accumulation was similar in all salt-stressed cowpea genotypes (Fig. 3d).
Leaf (a), stem (b), root (c), and total dry mass (d) of cowpea genotypes after 24 days of salinity stress imposed by irrigation with salt solutions containing electric conductivities (ECs) of 0.8 (control), 4.0 (moderate salinity), and 8.0 dS m−1 (severe salinity). More details same as in Fig. 1 and Table S2
By analyzing the growth performance, the Pitiúba genotype was found to be the most tolerant to moderate salinity, while TVU, Juruá, and Milagroso displayed the most inexpressive responses to mild stress (Fig. 5). On the other hand, all cowpea genotypes showed strong reductions in biomass production and elevated sensitivity to salinity at 8.0 dS m−1, which resulted in similar values of relative tolerance to severe stress (Fig. 5).
Relative tolerance to moderate (CE of 4.0 dS m−1) and severe salinity (CE of 8.0 dS m−1) of cowpea genotypes after 24 days of stress imposition. Within the same genotype (same bar), significant differences due to intensity of salt stress are represented by presence of asterisks, while significant differences due to cowpea genotype within the same salt treatment (gray or black filled bar portions) are denoted by different lowercase letters, according to Scott-Knott test (p < 0.05). For absolute values, see Table S2
3.3 Accumulation of Photosynthetic Pigments
In general, under control conditions and severe salinity, the Juruá genotype showed the highest accumulation of all photosynthetic pigments at the evaluated time point (Fig. 6). Salinity-induced alterations were predominantly observed in Chl b, Chl total, and carotenoids, depending on plant genotype and stress level. The Chl b content was enormously increased by severe salinity in the Pitiúba and TVU genotypes, whereas under mild stress, a similar response was registered only for Epace plants compared to the respective controls (Fig. 6b). Additionally, under severe salinity, the lowest Chl b accumulation was observed in the Maratauã genotype.
Contents of chlorophyll a (Chl a) (A), b (Chl b) (B), and total (Chl total) (C) and carotenoids (D) of cowpea genotypes after 24 days of salinity imposition. Irrigation salt solutions contained electric conductivities (ECs) of 0.8 (control), 4.0 (moderate salinity), and 8.0 dS m−1 (severe salinity). More details same as in Fig. 1 and Table S3
The genotypes TVU, Epace, Juruá, and Sempre verde displayed an increase in Chl total by salinity imposition in comparison to the control, depending on salt level (moderate or severe) (Fig. 6c). On the other hand, the carotenoids content was exclusively altered by moderate salinity, with an increase in Epace plants and a decrease in Juruá and Sempre verde genotypes (Fig. 6d). Unexpectedly, the Maratauã and Milagroso genotypes displayed no significant regulation in any photosynthetic pigment by salt stress at either moderate or severe levels.
3.4 Photosynthetic Performance
Salinity promoted a drastic decrease in net photosynthesis and stomatal conductance from all cowpea genotypes, except for net photosynthesis of moderate salt-stressed Sempre verde plants (Fig. 7a, b). The most significant decreases by moderate salinity were registered in the Pitiúba, Epace, and Juruá genotypes, which showed net photosynthesis rates 47.6, 43.2, and 47.6% lower than those of the control treatments (Fig. 7a). This response was intensified by salt level, and the most considerable reductions under severe stress were found to be 65.7, 66.9, and 69.3% in Pitiúba, Epace, and Maratauã plants, respectively.
Net photosynthesis (a), stomatal conductance (b), and transpiration rates (c) and internal CO2 concentrations (d) of cowpea genotypes after 24 days of salinity imposition. Irrigation salt solutions contained electric conductivities (ECs) of 0.8 (control), 4.0 (moderate salinity), and 8.0 dS m−1 (severe salinity). More details same as in Fig. 1 and Table S4
The transpiration rate was significantly decreased by moderate saline stress only in Pitiúba (53.6%), Epace (51.4%), and Juruá (36.3%) plants, but it was strongly reduced by severe salinity in all evaluated genotypes (Fig. 7c). Under mild salinity, the lowest values of stomatal conductance and transpiration rate were registered in Pitiúba, Epace, and Juruá plants.
Regulation of internal CO2 concentrations was not closely associated with stomatal conductance modulation (Fig. 7d). In this study, the internal CO2 was slightly decreased by salt treatment solely in plants from genotypes Pitiúba, Sempre verde, and Maratauã (only at mild stress).
3.5 Photosystem II Efficiency
Salinity did not promote photoinhibition of PSII photochemistry in cowpea plants, as provided by unaltered Fv/Fm ratios (Fig. 8a). Nonetheless, under moderate salinity, plants from the Epace, Juruá, and Maratauã genotypes displayed a significant decrease in the effective quantum yield of PSII (ΦPSII), electron transport rate (ETR), and photochemical quenching (qP) compared to the respective controls (Fig. 8b, c, e). Surprisingly, Pitiúba and Sempre verde plants exhibited a significant increase in photochemical quenching (qP) under moderate and severe salinity. Consequently, under severe salinity, the highest qP values were registered in Pitiúba and TVU genotypes, followed by Sempre verde and Epace plants (Fig. 8e).
Photosystem II maximum efficiency (Fv/Fm) (a), effective quantum yield of PSII (ΦPSII) (b), electron transport rate (ETR) (c), energy excess at the PSII level (EXC) (d), photochemical (qP) (e), and nonphotochemical quenching (NPQ) (f) of cowpea genotypes after 24 days of salinity imposition. Irrigation salt solutions contained electric conductivities (ECs) of 0.8 (control), 4.0 (moderate salinity), and 8.0 dS m−1 (severe salinity). More details same as in Fig. 1 and Table S5
In general, nonphotochemical quenching (NPQ) was higher in salt-stressed plants than in control plants, irrespective of cowpea genotype (Fig. 8f). On the other hand, only salt-stressed plants from the TVU, Epace, and Maratauã genotypes showed an increase in energy excess at the PSII level (EXC) in comparison to the control (Fig. 8d).
Under salinity, all cowpea genotypes exhibited an increase in the ETR/A ratio, indicating activation of an alternative sink of photosynthetic electrons (Fig. 9). For all cases, the highest percentage increases were noticed in Pitiúba plants, with ETR/A ratio values under moderate and severe salinity found to be 90.6 and 190.2% higher than those of control plants, respectively.
Alternative sink of photosynthetic electrons (ETR/A ratio) of cowpea genotypes after 24 days of salinity imposition. Irrigation salt solutions contained electric conductivities (ECs) of 0.8 (control), 4.0 (moderate salinity), and 8.0 dS m−1 (severe salinity). More details same as in Fig. 1 and Table S5
4 Discussion
4.1 Cowpea Genotypes Display Contrasting Responses to Salinity Stress
In the current study, seven cowpea genotypes (Pitiúba, TVU, Epace, Juruá, Sempre verde, Maratauã, and Milagroso) were challenged to different salinity levels to select the most salt-contrasting plants and elucidate salt tolerance mechanisms. The salt concentrations were defined to promote a remarkable inhibition of growth without constituting a lethal level for the plants.
Salt tolerance has been cited to include metabolically and genetically determined mechanisms that might vary among plant species and genotypes, depending on the salt level in the growth medium (Miranda et al. 2014, 2016, 2017; Bulgari et al. 2019; Mann et al. 2019). Our findings showed that cowpea genotypes displayed a substantial decrease in dry mass accumulation under salt stress, but differential tolerance to salinity was noticeable in moderate salt-stressed plants, while all studied genotypes showed similar performance under severe stress (Figs. 3, 4 and 5). The Pitiúba genotype was more tolerant to salt-moderate stress (4.0 dS m−1) than the other cowpea genotypes (Fig. 5). Tolerance to NaCl was associated with a lesser reduction in biomass accumulation that enabled plants to survive and maintain growth under such conditions. In parallel to the greater biomass production, efficient control of osmotic potential and leaf succulence was observed in salt-stressed Pitiúba plants, as well as maintenance of membrane damage that culminated in the preservation of photosynthetic pigments and improved qP (Figs. 1, 2, 6, 8 and 9). In contrast, the Milagroso, Juruá, and TVU genotypes displayed ineffective responses to moderate salinity, which resulted in drastic reductions in biomass accumulation, characterizing them as the most sensitive genotypes to salt stress. The studied V. unguiculata genotypes seemed to be similarly sensitive to salinities above 8.0 dS m−1, constituting a critical level for their cultivation in salinity-prone regions. The data reinforce that the performance of cowpea under salinity depends on the stress level and plant genotype.
4.2 Water Status Does Not Seem to Be Fundamental for Cowpea Salt Tolerance
Osmotic adjustment traits are frequently cited as crucial indicators to improve salt tolerance in some plant species. Plants able to decrease osmotic potential frequently restore osmotic homeostasis to maintain cell turgor and typically do not suffer oxidative damage; consequently, they are commonly more salt tolerant than osmotically unadjusted plants (Cabello et al. 2014; Chang et al. 2014; Miranda et al. 2016). Under moderate salinity, a possible osmotic adjustment attempt was elicited by the most salt-sensitive genotypes, as demonstrated by the conspicuous decrease in leaf osmotic potential of TVU, Juruá, and Milagroso plants (Figs. 1c, 5 and 10). Curiously, the substantial reduction in osmotic potential of the Milagroso genotype was not enough to maintain water uptake and cell turgor, as evidenced by low RWC and leaf succulence in salt-stressed plants (Fig. 1a, b). In a similar way, osmotic adjustment seemed not to be a determinant for water relations in stressed TVU plants, as the reduced osmotic potential under mild stress was associated with elevated transpiration rates (compared to mild salt-stressed Pitiúba), but the plants no longer controlled the water status once the leaf succulence was dramatically decreased (Figs. 1b and 7c).
Pearson correlation coefficient between membrane damage (MD); relative water content (RWC); chlorophyll a (Chl a), b (Chl b), and total (Chl total); carotenoids; leaf (LDM), stem (SDM), root (RDM), and total dry mass (TDM); leaf succulence; salt tolerance; osmotic potential; net photosynthesis; stomatal conductance (gs); transpiration; internal CO2; photosystem II maximum efficiency (Fv/Fm); effective quantum yield of PSII (ΦPSII); electron transport rate (ETR); photochemical (qP) and nonphotochemical quenching (NPQ); energy excess at the PSII level (EXC); and alternative sink of photosynthetic electrons (ETR/A ratio) in cowpea genotypes under salinity stress. Statistical details in Table S6
Previous reports have shown that tolerance to salt stress in cowpea is largely unrelated to carbohydrate accumulation and water relations (Praxedes et al. 2010, 2011). In agreement, the salt-tolerant cowpea genotypes did not exhibit any key regulation for variables related to water status. The Pitiúba plants maintained the osmotic potential (Fig. 1c) and showed a slight leaf RWC decline (Fig. 1a) after salt imposition, a response mostly activated to maintain water balance as affected by salt-induced stomatal closure (Fig. 7b). Thus, under moderate salinity, the Pitiúba genotype was capable of overcoming the slight RWC decrease, exhibiting elevated leaf succulence and unchanged membrane damage, fundamental traits for plant metabolism (Figs. 1a, b, 2 and 10), suggesting that the salt tolerance of cowpea plants is not closely related to water status adjustment.
4.3 Salinity Tolerance of the Pitiúba Genotype Is Associated with Improved Photochemical Performance and Structural Compounds Rather than Photosynthetic Capacity
Numerous efforts have been made to figure out the photosynthetic plant defense against salt stress (Chaves et al. 2009; Doubnerová and Ryšlavá 2011; Kumar et al. 2016; Wang et al. 2019); however, the mechanisms underlying plant responses are complex and remain to be completely elucidated. Here, the functioning of photosynthetic machinery in plants subjected to salt stress was differentially regulated in the salt-contrasting cowpea genotypes. For salt-tolerant Pitiúba plants, a decrease in stomatal conductance promoted a reduction in CO2 availability as a consequence of diffusion restrictions through stomata and mesophyll (Flexas et al. 2007; Poor et al. 2011; Batista et al. 2019) and was directly reflected in impaired net photosynthesis (Figs. 1a, b, d and 10). These data reinforce the idea that low net photosynthesis mainly results from stomatal limitations rather than nonstomatal limitations. Nonetheless, although the CO2 assimilation rate was reduced under moderate stress, it was enough to match the demand of energy compounds by the plant defense against harmful salinity effects, resulting in high growth under stressful conditions (Figs. 3 and 4).
The superior performance of Pitiúba plants under moderate stress was associated with unaltered membrane damage and photosynthetic pigments and high photochemical quenching (Figs. 2, 6 and 8e). Therefore, the maintenance of membrane composition and photosynthetic pigments were fundamental to elevated electron transport and photochemical quenching in the thylakoid membrane of moderate salt-stressed plants, resulting in an unaltered effective quantum yield of PSII. This idea was corroborated by greater ΦPSII and ETR rates under mild salinity and, consequently, improved qP without being accompanied by an increase in net photosynthesis. These data clearly show that nonphotochemical pathways efficiently dissipated the excess energy in Pitiúba plants, as evidenced by improved NPQ and ETR/A (Figs. 8f and 9). In concordance, recent findings showed that chlorophyll and carotenoids can act as salinity markers, and plants able to maintain elevated levels of photosynthetic pigments usually display improved growth under salt stress (Mittal et al. 2012; Araújo et al. 2018).
The photosynthetic performance of salt-sensitive cowpea genotypes was seriously injured by salt-induced oxidative damage in structural compounds and photosystem II operation (Yan et al. 2012; Zahra et al. 2014; Araújo et al. 2018). This argument was corroborated by the low ΦPSII and ETR values in the severe salt-stressed Epace and Juruá genotypes, indicating that a considerable amount of absorbed energy was not transferred to reaction centers. As a consequence, nonphotochemical processes should be activated to dissipate the excess energy (EXC) in stressed plants, such as heat dissipation or the xanthophyll cycle, as demonstrated by increased NPQ and ETR/A (Figs. 8f and 9) (Juneau et al. 2005; Yin et al. 2010). Nonetheless, although Juruá plants activated mechanisms against damage to the photosynthetic apparatus, including stability in the Fv/Fm ratio and increased NPQ, these pathways seem not to be enough to avoid injury in PSII, since ΦPSII and ETR and, consequently, CO2 assimilation were negatively affected by salinity (Fig. 7a). To conclude, the sensitivity to moderate salinity of the Juruá genotype was attributed to oxidative damage to leaf cells (Figs. 2 and 5), which culminated in the degradation of all photosynthetic pigments (Fig. 6), and damage to ΦPSII and ETR (Fig. 8b, c), thus decreasing carbon assimilation by the Benson-Calvin cycle (net photosynthesis) (Fig. 7a) (Elkelish et al. 2019; Causin et al. 2020).
5 Conclusion
Our investigative study reveals the existence of genotype-specific responses to salinity in the cowpea, thus describing an order of tolerance to moderate salt stress: Pitiúba > Maratauã = Epace = Sempre verde > Juruá = TVU = Milagroso. The data showed significant correlations between physiological and biochemical indices and morphological parameters (Fig. 10). The correlations suggested that salt tolerance was positively correlated with the effective quantum yield of PSII (ΦPSII), electron transport rate (ETR), leaf succulence, relative water content (RWC), gas exchange, and dry mass accumulation but negatively correlated with osmotic potential, chlorophyll b (Chl b), nonphotochemical quenching (NPQ), and energy excess at the PSII level (EXC). Taken together, the findings indicate that the superior performance of the Pitiúba genotype is attributed to an efficient mechanism to counteract salt damage on photochemical performance and structural compounds of the photosynthetic machinery. The Pitiúba genotype emerges as a potential alternative for cultivating cowpea plants in saline- or salinity-prone environments.
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Agarwal PK, Sheel P, Gupta K, Jha B (2013) Bioengineering for salinity tolerance in plants: state of the art. Mol Biotechnol 54:102–123. https://doi.org/10.1007/s12033-012-9538-3
Araújo GS, Miranda RS, Mesquita RO, Paula SO, Prisco JT, Gomes-Filho E (2018) Nitrogen assimilation pathways and ionic homeostasis are crucial for photosynthetic apparatus efficiency in salt-tolerant sunflower genotypes. Plant Growth Regul 86(3):375–388. https://doi.org/10.1007/s10725-018-0436-y
Bao AK, Wang YW, Xi JJ, Liu C, Zhang JL, Wang SM (2014) Co-expression of xerophyte Zygophyllum xanthoxylum ZxNHX and ZxVP1-1 enhances salt and drought tolerance in transgenic Lotus corniculatus by increasing cations accumulation. Funct Plant Biol 41(2):203–214. https://doi.org/10.1071/fp13106
Bargaz A, Nassar RMA, Rady MM, Gaballah MS, Thompson SM, Brestic M, Schmidhalter U, Abdelhamid MT (2016) Improved salinity tolerance by phosphorus fertilizer in two Phaseolus vulgaris recombinant inbred lines contrasting in their P-efficiency. J Agron Crop Sci 202(6):497–507. https://doi.org/10.1111/jac.12181
Batista VCV, Pereira IMC, Paula-Marinho SO, Canuto KM, Pereira RCA, Rodrigues THS, Daloso DM, Gomes-Filho E, Carvalho HH (2019) Salicylic acid modulates primary and volatile metabolites to alleviate salt stress-induced photosynthesis impairment on medicinal plant Egletes viscosa. Environ Exp Bot 167:103870. https://doi.org/10.1016/j.envexpbot.2019.103870
Bernardo S, Soares AA, Mantovani EC (2008) Manual de irrigação, 8th edn. Viçosa, UFV
Bulgari R, Trivellini A, Ferrante A (2019) Effects of two doses of organic extract-based biostimulant on greenhouse lettuce grown under increasing NaCl concentrations. Front Plant Sci 9:1870. https://doi.org/10.3389/fpls.2018.01870
Cabello JV, Lodeyro AF, Zurbriggen MD (2014) Novel perspectives for the engineering of abiotic stress tolerance in plants. Curr Opin Biotech 26:62–70. https://doi.org/10.1016/j.copbio.2013.09.011
Causin HF, Bordón DAE, Burrieza H (2020) Salinity tolerance mechanisms during germination and early seedling growth in Chenopodium quinoa Wild. genotypes with different sensitivity to saline stress. Environ Exp Bot 172:103995. https://doi.org/10.1016/j.envexpbot.2020.103995
Chang B, Yang L, Cong W, Zu Y, Tang Z (2014) The improved resistance to high salinity induced by trehalose is associated with ionic regulation and osmotic adjustment in Catharanthus roseus. Plant Physiol Bioch 77:140–148. https://doi.org/10.1016/j.plaphy.2014.02.001
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103(4):551–560. https://doi.org/10.1093/aob/mcn125
Coelho DG, Miranda RS, Paula-Marinho SO, Carvalho HH, Prisco JT, Gomes-Filho E (2020) Ammonium nutrition modulates K and N uptake, transport and accumulation during salt stress acclimation of sorghum plants. Arch Agron Soil Sci 66(14):1991–2004. https://doi.org/10.1080/03650340.2019.1704736
Costa PHA, Silva JV, Bezerra MA, Enéas-Filho J, Prisco JT, Gomes-Filho E (2003) Crescimento e níveis de solutos orgânicos e inorgânicos em cultivares de Vigna unguiculata submetidos à salinidade. Rev Bras Bot 26(3):289–297. https://doi.org/10.1590/S0100-84042003000300002
Doubnerová V, Ryšlavá H (2011) What can enzymes of C4 photosynthesis do for C3 plants under stress? Plant Sci 180(4):575–583. https://doi.org/10.1016/j.plantsci.2010.12.005
Elkelish AA, Soliman MH, Alhaithloul HA, El-Esawi MA (2019) Selenium protects wheat seedlings against salt stress-mediated oxidative damage by up-regulating antioxidants and osmolytes metabolism. Plant Physiol Bioch 137:144–153. https://doi.org/10.1016/j.plaphy.2019.02.004
Fan H-F, Du C-X, Guo S-R (2013) Nitric oxide enhances salt tolerance in cucumber seedlings by regulating free polyamine content. Environ Exp Bot 86:52–59. https://doi.org/10.1016/j.envexpbot.2010.09.007
FAO (2020) Salt-affected soils. http://www.fao.org/soils-portal/soil-management/management-of-some-problem-soils/salt-affected-soils/more-information-on-salt-affected-soils/en/. Accessed 20 May 2020
Farooq M, Gogoi N, Hussain M, Barthakur S, Paul S, Bharadwaj N, Migdadi HM, Alghamdi SS, Siddique KHM (2017) Effects, tolerance mechanisms and management of salt stress in grain legumes. Plant Physiol Biochem 118:199–217. https://doi.org/10.1016/j.plaphy.2017.06.020
Flexas JA, Diaz-Espejo A, Galmés J, Kaldenhoff R, Medrano H, Ribas-Carbo M (2007) Rapid variations of mesophyll conductance in response to changes in CO2 concentration around leaves. Plant Cell Environ 30(10):1284–1298. https://doi.org/10.1111/j.1365-3040.2007.01700.x
Freitas VS, Miranda RS, Costa JH, Oliveira DF, Paula SO, Miguel EC, Freire RS, Prisco JT, Gomes-Filho E (2018) Ethylene triggers salt tolerance in maize genotypes by modulating polyamine catabolism enzymes associated with H2O2 production. Environ Exp Bot 145:75–86. https://doi.org/10.1016/j.envexpbot.2017.10.022
Freitas PAF, Carvalho HH, Costa JH, Miranda RS, Saraiva KDC, Oliveira FDB, Coelho DG, Prisco JT, Gomes-Filho E (2019) Salt acclimation in sorghum plants by exogenous proline: physiological and biochemical changes and regulation of proline metabolism. Plant Cell Rep 38(3):403–416. https://doi.org/10.1007/s00299-019-02382-5
Gondim FA, Miranda RS, Gomes-Filho E, Prisco JT (2013) Enhanced salt tolerance in maize plants induced by H2O2 leaf spraying is associated with improved gas exchange rather than with non-enzymatic antioxidant system. Theor Exp Plant Phys 25(4):251–260. https://doi.org/10.1590/S2197-00252013000400003
Hu M, Shi Z, Zhang Z, Zhang Y, Li H (2012) Effects of exogenous glucose on seed germination and antioxidant capacity in wheat seedlings under salt stress. Plant Growth Regul 68(2):177–188. https://doi.org/10.1007/s10725-012-9705-3
Huertas R, Olías R, Eljakaoui Z, Gálvez FJ, Li J, Morales AP, Belver A, Rodríguez-Rosales MP (2012) Overexpression of SlSOS2 (SlCIPK24) confers salt tolerance to transgenic tomato. Plant Cell Environ 2:1–16. https://doi.org/10.1111/j.1365-3040.2012.02504.x
Huo T, Wang CT, Yu TF, Wang DM, Li M, Zhao D, Li XT, Fu JD, Xu ZS, Song XY (2021) Overexpression of ZmWRKY65 transcription factor from maize confers stress resistances in transgenic Arabidopsis. Sci Rep-UK 11(1):1–15. https://doi.org/10.1038/s41598-021-83440-5
Ismail AM, Horie T (2017) Genomics, physiology, and molecular breeding approaches for improving salt tolerance. Annu Rev Plant Biol 68:405–434. https://doi.org/10.1146/annurev-arplant-042916-040936
Juneau P, Green BR, Harrison PJ (2005) Simulation of pulse amplitude-modulated (PAM) fluorescence: limitations of some PAM-parameters in studying environmental stress effects. Photosynthetica 43(1):75–83. https://doi.org/10.1007/s11099-005-5083-7
Kumar A, Lata C, Kumar P, Devi R, Singh K, Krishnamurthy SL, Kulshreshtha N, Yadav RK, Sharma SK (2016) Salinity and drought induced changes in gas exchange attributes and chlorophyll fluorescence characteristics of rice (Oryza sativa) varieties. Indian J Agr Sci 86(6):718–726
Li M, Chen R, Jiang Q, Sun X, Zhang H, Hu Z (2021) GmNAC06, a NAC domain transcription factor enhances salt stress tolerance in soybean. Plant Mol Biol 105(3):333–345. https://doi.org/10.1007/s11103-020-01091-y
Mann A, Kaur G, Kumar A, Sanwal SK, Singh J, Sharma PC (2019) Physiological response of chickpea (Cicer arietinum L.) at early seedling stage under salt stress conditions. Legume Res 42(5):625–632. https://doi.org/10.18805/LR-4059
Mantovani A (1999) A method to improve leaf succulence quantification. Braz Arch Biol Technol 42(1):9–14. https://doi.org/10.1590/S1516-89131999000100002
Miranda RS, Mesquita RO, Freitas NS, Prisco JT, Gomes-Filho E (2014) Nitrate:ammonium nutrition alleviates detrimental effects of salinity by enhancing photosystem II efficiency in sorghum plants. Rev Bras Eng Agr Amb 18(Suplement):8–12
Miranda RS, Gomes-Filho E, Prisco JT, Alvarez-Pizarro JC (2016) Ammonium improves tolerance to salinity stress in Sorghum bicolor plants. Plant Growth Regul 78(1):121–131. https://doi.org/10.1007/s10725-015-0079-1
Miranda RS, Mesquita RO, Costa JH, Alvarez-Pizarro JC, Prisco JT, Gomes-Filho E (2017) Integrative control between proton pumps and SOS1 antiporters in roots is crucial for maintaining low Na+ accumulation and salt tolerance in ammonium-supplied Sorghum bicolor. Plant Cell Physiol 58(3):522–536. https://doi.org/10.1093/pcp/pcw231
Mishra S, Behura R, Awasthi JP, Dey M, Sahoo D, Bhowmik SS, Panda SK, Sahoo L (2014) Ectopic overexpression of a mungbean vacuolar Na+/H+ antiporter gene (VrNHX1) leads to increased salinity stress tolerance in transgenic Vigna unguiculata L. Walp Mol Breeding 34(3):1345–1359. https://doi.org/10.1007/s11032-014-0120-5
Mittal S, Kumari N, Sharma V (2012) Differential response of salt stress on Brassica juncea: photosynthetic performance, pigment, proline, D1 and antioxidant enzymes. Plant Physiol Biochem 54:17–26. https://doi.org/10.1016/j.plaphy.2012.02.003
Murillo-Amador B, Troyo-Diéguez E, García-Hernández JL, López-Aguilar R, Ávila-Serrano NY, Salgado SZ, Rueda-Puente EO, Kaya C (2006) Effect of NaCl salinity in the genotypic variation of cowpea (Vigna unguiculata) during early vegetative growth. Sci Hortic 108(4):423–431. https://doi.org/10.1016/j.scienta.2006.02.010
Poor P, Gemes K, Horvath F, Szepesi A, Simon ML, Tari I (2011) Salicylic acid treatment via the rooting medium interferes with stomatal response, CO2 fixation rate and carbohydrate metabolism in tomato, and decreases harmful effects of subsequent salt stress. Plant Biol 13(1):105–114. https://doi.org/10.1111/j.1438-8677.2010.00344.x
Praxedes SC, Lacerda CF, DaMatta FM, Prisco JT, Gomes-Filho E (2010) Salt tolerance is associated with differences in ion accumulation, biomass allocation and photosynthesis in cowpea cultivars. J Agron Crop Sci 196:193–204. https://doi.org/10.1111/j.1439-037X.2009.00412.x
Praxedes SC, Lacerda CF, Ferreira TM, Prisco JT, DaMatta FM, Gomes-Filho E (2011) Salt tolerance is unrelated to carbohydrate metabolism in cowpea cultivars. Acta Physiol Plant 33:887–896. https://doi.org/10.1007/s11738-010-0615-6
Praxedes SC, Gomes-Filho E, Damatta FM, Lacerda CF, Prisco JT (2014) Salt stress tolerance in cowpea is poorly related to the ability to cope with oxidative stress. Acta Bot Croat 73(1):51–62. https://doi.org/10.2478/botcro-2013-0010
Rhoades JD, Kandiah A, Mashali AM (2000) Uso de águas salinas para produção agrícola. UFPB, Campina Grande
Silva ARA, Bezerra FML, Lacerda CF, Miranda RS, Marques EC (2018) Ion accumulation in young plants of the “green dwarf” coconut under water and salt stress. Rev Cienc Agron 49(2):249–258. https://doi.org/10.5935/1806-6690.20180028
Souza DMG, Lobato E (2014) Cerrado: correção do solo e adubação. Embrapa Informação Tecnológica, Brasília, p 416
Van Nguyen L, Bertero D, Nguyen LV (2020) Genetic variation in root development responses to salt stresses of quinoa. J Agron Crop Sci 206:538–547. https://doi.org/10.1111/jac.12411
Wang Q, Kang L, Lin C, Song Z, Tao C, Liu W, Sang T, Yan J (2019) Transcriptomic evaluation of Miscanthus photosynthetic traits to salinity stress. Biomass Bioenerg 125:123–130. https://doi.org/10.1016/j.biombioe.2019.03.005
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144(3):307–314. https://doi.org/10.1016/S0176-1617(11)81192-2
Yan K, Chen P, Shao H, Zhao S, Zhang L, Xu G, Sun J (2012) Responses of photosynthesis and photosystem II to higher temperature and salt stress in sorghum. J Agron Crop Sci 198(3):218–226. https://doi.org/10.1111/j.1439-037X.2011.00498.x
Yin Y, Li S, Liao W, Lu Q, Wen X, Lu C (2010) Photosystem II photochemistry, photoinhibition, and the xanthophyll cycle in heat-stressed rice leaves. J Plant Physiol 167(12):959–966. https://doi.org/10.1016/j.jplph.2009.12.021
Zahra J, Nazim H, Cai S, Han Y, Wu D, Zhang B, Haider SI, Zhang G (2014) The influence of salinity on cell ultrastructures and photosynthetic apparatus of barley genotypes differing in salt stress tolerance. Acta Physiol Plant 36:1261–1269. https://doi.org/10.1007/s11738-014-1506-z
Acknowledgements
The Fundação de Amparo à Pesquisa e ao Desenvolvimento Científico e Tecnológico do Maranhão (FAPEMA), Fundação de Amparo à Pesquisa do Estado do Piauí (FAPEPI), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ) for financial support.
Funding
This research was supported by the Fundação de Amparo à Pesquisa e ao Desenvolvimento Científico e Tecnológico do Maranhão (FAPEMA) under grant number PVIG-02334/17; Fundação de Amparo à Pesquisa do Estado do Piauí (FAPEPI) under grant Edital PPP FAPEPI/MCT/CNPq/CT-INFRA n° 007/2018; and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) under grant number 427219/2018–3.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Souza Miranda, R., de Souza, F.I.L., Alves, A.F. et al. Salt-Acclimation Physiological Mechanisms at the Vegetative Stage of Cowpea Genotypes in Soils from a Semiarid Region. J Soil Sci Plant Nutr 21, 3530–3543 (2021). https://doi.org/10.1007/s42729-021-00625-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-021-00625-7