Abstract
Accretion of nonessential ions such as sodium and chloride due to the selective ion-uptake process into the root and/or the use of poor-quality irrigation water may adversely affect the production potential of crops such as Chinese kale in closed-loop hydroponic systems. This research identifies the effects of salt on the plant’s growth and the damage avoidance mechanisms in foliage of hydroponically grown Chinese kale. Twenty-one-day-old plants (at the 4-leaf stage) were exposed to salinity (0, 25, 50, 75, and 100 mM NaCl) for 16 days. Results indicated that growth parameters, leaf water status, photosynthetic pigments, and photosystem II efficiency declined as salinity increased in the rhizosphere, whereas proline biosynthesis was stimulated progressively. Salinity induced higher H2O2 endogenesis and lipid peroxidation while triggering antioxidant enzymes activity in the leaf tissues. Activity of ascorbate peroxidase and peroxidase gradually increased with salinity, although peroxidase activity was decreased at high salinity (>75 mM NaCl). Catalase, a crucial component of the free radical neutralization process, was also observed at high salinity (>75 mM NaCl). Chinese kale plants were tolerant to salinity at 25 mM and employed a complex mechanism composed of an excess energy dissipation system along with the enzymatic antioxidant system, thus making it relatively tolerant to salinity beyond 75 mM NaCl.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Salinity is one of the major environmental factors limiting plant productivity worldwide. It affects plant growth and development through osmotic and ionic disruption (Munns and Tester 2008). Accumulation of toxic ions such as sodium and chloride in plant tissues can adversely affect plant biomass production partially due to restriction in the uptake of essential elements (Ghoulam et al. 2002). At higher salinity, ionic cytotoxicity may be the predominant cause of crop susceptibility due to replacement of Na+ instead of K+ and binding Na+ and Cl− ions with amino acids (D’Souza and Devaraj 2010) that can negatively affect major processes including photosynthesis, protein and lipid metabolisms, as well as energy conservation (Parida and Das 2005). Imbalanced metabolism under such condition may result in oxidative stress (D’Souza and Devaraj 2010).
Limited CO2 availability under stress-induced stomatal closure restricts carbon reduction, which implies lower NADP accessibility to act as the specific electron acceptor from photosystem I. This could trigger O2 reduction to superoxide by the Mehler reaction and initiate chain reactions that generate more noxious reactive oxygen species (ROS ). Singlet oxygen as an unusual product might also be generated at photo system II owing to ineffective energy dissipation processes (Sekmen et al. 2007; Gill and Tuteja 2010).
Accelerated ROS formation causes oxidative damage to biomolecules such as membrane lipids, proteins, and nucleic acids and may lead to plant death (D’Souza and Devaraj 2010; Merati et al. 2014). Plants undergo some changes in their normal metabolism to cope with the unbalanced conditions caused by salinity in their environment (Daneshmand et al. 2010). They have evolved specific cellular and subcellular preventative-protective complexes consisting of an energy dissipation mechanism and an antioxidative system to mitigate oxidative damage. For example, superoxide dismutase (SOD) converts superoxide (O2−) to H2O2 in various subcellular compartments. H2O2 is eliminated by ascorbate peroxidase (APX) and catalase (CAT) with a lower affinity than APX (Asada 2006; Sekmen et al. 2007). Different classes of peroxidase (POX) also are associated with scavenging H2O2 generated in chloroplasts and physiological processes such as growth and cell formation (Sekmen et al. 2007). The capability to neutralize ROS and reduce their harmful effects on macromolecules is an important trait of stress tolerance in higher plants (Xiong and Zhu 2002) (Fig. 8.1).
Plants exposed to osmotic stress may accumulate low molecular weight compatible solutes such as proline, which is considered to have multiple roles in osmoregulation, protein and membrane protection, energy conservation , and free radical scavenging, as well as being a component of stress-related signaling processes (Daneshmand et al. 2010; Tayebi-Meigooni et al. 2014).
In closed-loop hydroponic systems, increasing salinity can be caused by differential ion uptake by the crop itself and/or use of low-quality irrigation water containing nonessential ions such as sodium and chloride and may negatively affect salt-sensitive hydroponically grown plants. Chinese kale (Brassica alboglabra L.) can be successfully planted in hydroponic systems and be used as fresh or cooked vegetables in the human diet. Recent research shows a high level of potential anticancer capability of Chinese kale due to high levels of glucosinolates and ascorbic acid (Hecht et al. 2004). Despite the vast literature on salinity, no attention has been particularly devoted to investigate the physio-biochemical mechanisms contributing to salinity tolerance in Chinese kale. Therefore, the aim of this study was to determine the effect of NaCl salinity on subsequent changes on growth, leaf water status, energy harvesting/dissipating complex, and elucidation of antioxidative defense mechanisms. Results obtained in this study may contribute to a better understanding of the responses of Chinese kale plants to salinity and breeding program development .
Materials and Methods
Planting Materials and Growth Conditions
The seeds of Chinese kale (B. alboglabra L.) cv. ‘Standard Kailan’ were sown in trays maintained with frequent water applications until seedling emergence. Young seedlings were then placed in a plastic film pool and fed with basic nutrient solution containing 250, 67, 239, 160, 30, 80, 3, 0.62, 0.44, 0.02, 0.11, and 0.048 mg L−1 of N, P, K, Ca, Mg, S, Fe, Mn, B, Cu, Zn, and Mo, respectively.
Salt Stress Treatment
Fifteen-day-old seedlings of uniform size were installed in a static aerated hydroponic system containing the basic nutrient solution. Six days later, plants at the 4-leaf stage were exposed to salinity by adding 0, 25, 50, 75, and 100 mM NaCl to the basic solution. Osmotic shock was avoided by gradually increasing the NaCl salt (25 mM each day) until reaching the desired concentrations. Salt treatments were continued for 16 days. Plants grown on nutrient solution without NaCl served as the control.
Collecting Samples
Five plants from each experimental unit were used to estimate growth parameters, including leaf surface area as well as the dry mass of leaf, shoot and root tissues. Fresh samples for biochemical assays were taken from new fully expanded leaves and frozen immediately in liquid N on-site and kept at −80 °C. Leaf relative water content (RWC) was also determined using fresh leaf samples.
Determination of Relative Water Content
The relative water content (RWC) was measured according to the following formula described by Smart and Bingham (1974).
Determination of Chlorophyll Fluorescence
Two weeks after initiation of salinity increase , maximum efficiency of photosystem II (Fv/Fm) in dark-adapted leaves (new fully expanded) of five plants for each experimental unit was randomly measured using a portable Plant Efficiency Analyzer (Hansatech, UK). Measurements were done at 10.00am-12.00md with the approximate solar irradiance 1100 to 1300 μmolm−2 s−1. Fv/Fm was calculated according to Schreiber et al. (1995).
Determination of Photosynthetic Pigments
Chlorophylls a and b and carotenoids were extracted in 95% (v/v) acetone and calculated using the following relationships described by Lichtenthaler and Buschmann (2001).
Determination of Proline
Proline content was measured according to the procedure of Bates et al. (1973). Leaf samples (0.2 g) were homogenized in 3 ml sulfosalicylic acid (3% w/v) and then centrifuged. The supernatant was gently mixed with glacial acetic acid and ninhydrin solution, by volume in a ratio of 1:1:1, respectively, and then incubated at 95 °C for 1 h. The cooled mixture was then treated with toluene, and the absorbance of the upper phase (chromophore-containing) was read at 520 nm. Proline content was estimated using a standard curve and was expressed as μmol g−1 fresh weight.
Determination of Hydrogen Peroxide and Lipid Peroxidation
Content of hydrogen peroxide was measured based on Velikova et al. (2000). Fresh leaf samples (0.5 g) were homogenized in 5 ml 0.1% (w:v) trichloroacetic acid (TCA) and centrifuged at 12,000 g for 15 min. 0.5 ml (10 mM) phosphate buffer and 1 ml (1 M) KI were added to 0.5 ml of the supernatants. The absorbance of the final solution was read at 390 nm. The content of H2O2 was estimated using a standard curve.
Lipid peroxidation was estimated by measuring the level of malondialdehyde (MDA) according to the method of Du and Bramlage (1992). Leaf samples (0.25 g) were homogenized in 5 ml of 0.1% (w/v) (TCA) solution and centrifuged at 15000 × g for 15 min. The supernatant (1.0 ml) was added to 4 ml 0.5% (w/v) thiobarbituric acid (TBA) in 20% TCA (w/v). The mixture was then heated at 95 °C for 30 minutes and immediately cooled to stop the reaction. The absorbance of the colored solution was recorded at 532 nm and corrected for nonspecific turbidity by subtracting the absorbance at 600 nm. MDA concentrations were calculated from the extinction coefficient of 155 mM−1 cm−1 using the following formula.
Extraction for Protein and Enzyme Assays
The frozen samples (0.2 g) were homogenized with 3 ml ice-cold extraction buffer (50 mM potassium phosphate buffer, pH 7.8, 0.5% (w/v) PVP, 0.1 mM EDTA, and 0.2 (v/v) triton X-100) and centrifuged at 18,000 × g for 20 min at 4 °C. Extraction media for ascorbate peroxidase (APX) was prepared with inclusion of ascorbate (2 mM) in the main buffer. The supernatants were used as crude extract in the protein and enzymes assays.
Assay of Protein and Antioxidant Enzymes
Total protein content was determined using bovine serum albumin (BSA) as a standard following the method described by Bradford (1976).
Catalase (CAT) activity was determined following the method of Cakmak et al. (1993). The assay mixture contained 25 mM phosphate buffer (pH 7.0), 10 mM H2O2, and enzyme extract (100 μl) in a total volume of 1.0 ml. The reduction of absorbance at 240 nm as a result of H2O2 degradation was recorded every 30 s with spectrophotometer (Model Varian Cary 50 UV-Vis Spectrophotometer, USA). CAT activity was calculated using the extinction coefficient for H2O2 (39.4 mM−1 cm−1).
Ascorbate peroxidase (APX) activity was estimated according to the method of Webb and Allen (1995) with a slight modification. The reaction mixture (1 ml) was made up of enzyme extract (100 μl) along with 50 mM phosphate buffer (pH 7.0), 0.2 mM EDTA, 0.25 mM H2O2, and 0.5 mM sodium ascorbate. The decrease in absorbance at 290 nm was recorded for 1 min. The APX activity was expressed as units mg−1 protein (extinction coefficient 2.8 mM−1 cm−1).
Peroxidase (POX) activity was assayed by the guaiacol oxidation method as described by Ghanati et al. (2002). The reaction mixture contained 100 μl crude extract as well as 50 mM potassium phosphate buffer (pH 7.0), 28 mM guaiacol, and 10 mM H2O2 in the final volume of 3.0 ml. Changes in optical density of the reacting samples were monitored every 30 s by an increase in absorbance at 470 nm. The APX activity was expressed as units per mg soluble protein (extinction coefficient 26.6 mM−1 cm−1).
Experimental Design and Statistical Analysis
A randomized complete block design (RCBD) with five replications was used. Each experimental unit was composed of a hydroponic tray with 12 plants. Data were analyzed using SAS 9.1 software (SAS Institute Inc. 2004). Means comparisons were conducted using least significant differences (LSD) at 5% level of probability.
Results
Increased salt levels in the root zone for 2 weeks resulted in significant changes for all morphological and physio-biochemical traits (Table 8.1).
Plant Growth
Leaf area was significantly reduced by 65% in 100 mM salinity compared to the control. The corresponding reductions were approximately 5.4% for 25, 37.7% for 50, and 52.5% for 75 mM, respectively (Table 8.2). Leaf area IC50 (half the maximum inhibition concentration) occurred at about 75.31 mM NaCl (calculated using linear regression (y = 533.83–3.6566x)).
Reductions were 14.4%, 34.9%, 47.2%, and 58.3% for leaf dry mass (LDM) and 11.7%, 37.2%, 51.7%, and 57.7% for stem dry mass (SDM) as NaCl concentration increased from 0 to 25, 50, 75, and 100 mM, compared to the non-salinized plants, respectively. Root dry mass (RDM) was also significantly reduced by 30.6% for 100 mM salt compared to the control. RDM increased about 2.7% by salt stress at 75 mM (Table 8.2).
Total dry mass (TDM) was significantly reduced by 52.7% at the highest salinity compared to the control. The corresponding reductions were approximately 9.8%, 24.1%, and 38.4% for 25, 50, and 75 mM, respectively (Table 8.2). TDM (IC50) calculated using linear regression (y = 4.1227–0.0245x) was at 84.73 mM NaCl. The negative impact of salinity was greater on SDM than RDM; hence a significant increase in root/shoot ratio was revealed in comparison to the control (Table 8.2).
Relative Water Content
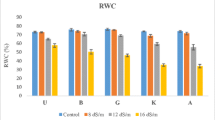
RWC markedly declined in response to increasing salinity. RWC was reduced by 7.92, 11.14, 14.42, and 17.58% as NaCl concentration increased from 0 to 25, 50, 75, and 100 mM, respectively (Fig. 8.2A).
Chlorophyll Fluorescence
Maximum quantum yield (Fv/Fm) was affected by salinity (P < 0.05) and a slight reduction in Fv/Fm (5.2%) observed at 100 mM NaCl compared to the control (Fig. 8.2B).
Photosynthetic Pigments
Chlorophylls a and b and their total were reduced by 41.9, 52.2, and 44.9%, respectively, at 100 mM NaCl (Fig. 8.3A–C). The Chlorophyll a:b ratio increased at higher salinity. At the highest salinity level, Chlorophyll a:b ratio was 1.2-fold greater than the control (Fig. 8.3D). Content of carotenoids decreased about 36.7% at 100 mM NaCl compared to the control (Fig. 8.3E).
Effect of different NaCl concentrations on the contents of chlorophyll a (A), chlorophyll b (B), total chlorophyll (a + b) (C), Chla:b ratio (D), and carotenoid (E) in leaves of Chinese kale (means ± SE, n = 5). Different letters indicate significant differences according to least significant difference (LSD) multiple range test (P < 0.05)
Proline, Hydrogen Peroxide, and Lipid Peroxidation
Changes in proline content were about 2.17-, 4.2-, 5.3-, and 7.6-fold compared to the control as salinity increased to 25, 50, 75, and 100 mM NaCl, respectively (Fig. 8.4A).
Content of H2O2 was boosted from 7.16 (μmol.g−1FW) in control plants to 7.65, 9.96, 11.85, and 13.77 (μmol.g−1FW) in plants subjected to 25, 50, 75, and 100 mM of NaCl, respectively (Fig. 8.4B). MDA level enhancement was about 1.19-, 1.57-, 1.73-, and 1.74-fold as salinity increased from 0 to 25, 50, 75, and 100 mM NaCl, respectively (Fig. 8.4C).
Protein and Antioxidant Enzymes
Protein content decreased with increasing salinity (P ≤ 0.01). These reductions were about 4.1, 6.4, 25.8, and 28.5% for the 25, 50, 75, and 100 mM NaCl, respectively (Fig. 8.5A).
Effect of different NaCl concentrations on the contents of protein (A) and antioxidant enzymes activity of CAT (B), APX (C), and POX (D) in leaves of Chinese kale (means ± SE, n = 5). Different letters indicate significant differences according to least significant difference (LSD) multiple range test (P < 0.05)
CAT activity remained constant in response to salinity up to 50 mM NaCl, although higher salinity (75 and 100 mM NaCl) caused a notable increase (P ≤ 0.01) in CAT activity with as much as 1.65- and 2.35-fold, respectively, compared to the control (Fig. 8.5B).
APX activity showed a significant increase in plants subjected to salinity compared to the control (P ≤ 0.01). APX activity progressively increased from 1.3- to 2.2-fold for 25 to 100 mM NaCl salinity. No significant changes in APX activity between 75 and 100 mM were observed (Fig. 8.5C). A similar increase in POX activity was found as well. POX activity increased (P ≤ 0.01) by 6.6-, 12.1-, 16.3-, and 14.8-fold when salinity increased from 0 to 25, 50, 75, and 100 mM NaCl (Fig. 8.5D).
Discussion
In this study, we investigated the effects of NaCl salinity on growth, water status, content of pigments, proline, hydrogen peroxide, and magnitude of lipid peroxidation , as well as manifestations of adaptive mechanisms in foliage of Chinese kale plant. Increasing level of NaCl in the rhizosphere results in the osmotic stress, ion cytotoxicity, and imbalanced nutrition which can decline plant growth and development (Kholova et al. 2009).
RWC reflects plant water status generally, and it is positively correlated with rates of cell enlargement and leaf elongation (Singh et al. 2007). RWC decreased under salinity and this reduction increased with increasing NaCl concentration in the root zone as reported in previous studies (Singh et al. 2007; Kholova et al. 2009). Lower RWC implies smaller photosynthetic surface under salinity, which can be manifested as lower growth and developments on the whole plant.
Salinity resulted in a significant reduction in growth parameters such as number of leaves, leaf surface area, and plant organ dry masses. Our results (Table 8.2) showed that salinity reduced dry mass of leaf, shoot, and root, which is consistent with earlier reports for various plant species (Ashraf and Orooj 2006; Psarras et al. 2008). Unequal reduction in dry weights of shoot and root under salinity resulted in root/shoot ratio magnification, which is in agreement with the findings of Essa (2002).
Maximum quantum efficiency of PSII photochemistry (Fv/Fm) is usually used as a photoinhibition index (Krause and Weis 1991; Netondo et al. 2004). Several reports have noted that PSII photochemistry is tolerant of moderate salt conditions and few changes in light utilization have been reported (Ma et al. 1997; Lima et al. 2002; Santos 2004). In sorghum, the efficiency of PSII photochemistry was considerably impacted only at high salinity beyond 200 mM (Notondo et al. 2004). In this study, the Fv/Fm ratio was measured to investigate the possible photoinhibition occurrence at different salt levels. Based on the results, downregulation of PSII photochemistry in plants subjected to high salinity implies the existence of a mechanism for keeping excessive energy away from the PSII photo apparatus (Weis and Berry 1987; Krause and Weis 1991). The results show that efficiency of PSII photochemistry changed under moderate and high salt treatments (Fig. 8.2B).
Pigments content was decreased as salinity increased in root media. A positive correlation between chlorophyll degradation and concentration of NaCl has been reported previously (Hernandez et al. 1995, 2000). The rate of chlorophyll degradation under salinity is linked with the size of ROS generation, chlorophyllase activity, and chlorophyll synthesis (Renault 2005). Increase in Chlorophyll a:b ratio (Fig. 8.3A–C) under salt stress implies more vulnerability of Chlorophyll b to salinity than Chlorophyll a (Fig. 8.3D), which is in agreement with result reported by Kholova et al. (2009) and Daneshmand et al. (2010). Decrease in the content of carotenoids under salt stress (Fig. 8.3E) may be attributed to degradation of β-carotene and formation of zeaxanthins (Sultana et al. 1999). The process is thought to provide protection of photosynthetic apparatus by quenching excessive energy of chlorophyll excitation (Daneshmand et al. 2010).
Content of proline was progressively amplified with the increase in salinity (Fig. 8.4A). This phenomenon is considered to facilitate the retention of water and balancing the osmotic potential of the cytoplasm (Hasegawa et al. 2000; Kholova et al. 2009). Osmolytes like proline also are believed to protect the structure and function of various macromolecules related to higher membrane stability and enzymatic antioxidant activity (Kholova et al. 2009).
Salt stress may cause molecular damage to plant cells either directly or indirectly through the formation of ROS . They can react with a target substance, such as lipids, proteins, and/or nucleic acids. H2O2, as well as other ROS , disturbs metabolic functions and causes oxidative damage at sites where it accumulates (Foyer et al. 1997). H2O2 is also responsible for lipid peroxidation (Parida and Das 2005). Estimating malondialdehyde (MDA) is used as an indicator of lipid peroxidation to evaluate the extent of oxidative damage to biomembranes (Lin and Kao 2000). Our results show that content of H2O2 progressively increased with salinity (Fig. 8.4B) while statistically remaining constant at 75–100 mM NaCl salinity. A similar trend for MDA content was also observed (Fig. 8.4C). Changes in H2O2 and MDA contents under salt stress have been reported in several studies (Kholova et al. 2009; Daneshmand et al. 2010; Tayebimeigooni et al. 2012). However, small changes in MDA level and membrane stability index in tolerant plants facing salinity or drought (Egert and Tevini 2002; Sairam et al. 2005; Ashraf and Ali 2008) support the existence of a high potential scavenging system to overcome oxidative stress .
Decrease in the protein content of plants subjected to salinity was observed in the present study (Fig. 8.5A). Reduction of protein content was probably linked with the stimulating effect of ROS on protein degradation (Davies 1987). Faced with salinity, plants employ a number of nonenzymatic and enzymatic antioxidants to prevent and overcome oxidative stress (Sekmen et al. 2007; Manchanda and Garg 2008). The existence of a positive correlation between the activity of the antioxidant enzymes and salinity tolerance has been reported (Sekmen et al. 2007; Garg and Manchanda, 2009; Chawla et al. 2013). SOD dismute superoxide to hydrogen peroxide. H2O2 is later eliminated through conversion to H2O in subsequent reactions by CAT and APX. APX has a higher affinity than CAT for scavenging H2O2 and uses ascorbate as its specific electron donor (Asada 2006; Sekmen et al. 2007). APX association with monodehydroascorbate reductase, dehydroascorbate reductase, and glutathione reductase (GR) appeared to be the most important peroxidase to remove H2O2, through the Foyer-Halliwell-Asada pathway (Hernandez et al. 2010).
Based on our results, CAT and APX activities in leaves of plants subjected to salinity increased (Fig. 8.5B, C). Our results suggest that CAT and APX most likely were significant in the detoxification of hydrogen peroxide in this cultivar. Similar to our results, salt-induced activity of CAT and APX in salt-tolerant plantago and rice have been observed (Turkan et al. 2013; Chawla et al. 2013).
POX involvement in growth and development , the lignification and suberization processes, as well as H2O2 scavenging process has been reported (Dionisio-Sese and Tobita 1998). Under salinity stress, POX activity has been found to be stimulated in salt-tolerant plants (Ashraf and Ali 2008; Turkan et al. 2013; Chawla et al. 2013). In this study, POX activity significantly increased in leaf tissues of plants exposed to NaCl salinity. Induction of POX activity continued to 75 mM NaCl and showed a slight reduction as salinity reached 100 mM NaCl (Fig. 8.5D). Increasing POX activity is in agreement with previous results reported on Indian mustard (Yusuf et al. 2008), plantago (Turkan et al. 2013), and jatropha (Hishida et al. 2014).
Various enzymatic antioxidants participate in ROS detoxification process. SOD counteracts superoxide radical and converts it to H2O2 (Asada 2006). H2O2 is detoxified by scavengers such as APX, CAT, and POX, and in the absence or lack of scavengers, H2O2 accumulates in tissues. Several researches proved that NaCl salinity tolerance is closely correlated to the antioxidant capacity of plants as observed in purslane, canola, Indian mustard, plantago, and rice (Yazici et al. 2007; Ashraf and Ali 2008; Yusuf et al. 2008; Turkan et al. 2013; Chawla et al. 2013)
Conclusions
In this study, changes in pigment content, as well as a downregulation in PSII photochemistry, are manifestations to maintain a balance between harvesting and utilizing energy in order to reduce the risk of salt-induced oxidative stress . Accumulation of osmoregulators such as proline eased water stream continuance throughout the salinized plants, thus facilitating gas exchange and higher PSI performance and less ROS formation. The induction of antioxidant enzyme activity, including APX, CAT, and POX, by NaCl treatment suggests their pivotal role in the detoxification process in leaves of stressed plants. Our result shows that cv. ‘Standard Kailan’ is moderately tolerant to NaCl salinity, whereas higher salinity (>75 mM) suppressed the oxidative defense system. The energy regulation system and enzymatic antioxidant system are prominently contributing to salt tolerance in this study. Chinese kale was less affected by salinity up to 25 mM and employed a complex defense system making it relatively tolerant to high NaCl salinity. Our research potentially provides salinity guidelines for hydroponic production of standard Chinese kale.
References
Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141:391–396. https://doi.org/10.1104/pp.106.082040
Ashraf M, Ali Q (2008) Relative membrane permeability and activities of some antioxidant enzymes as the key determinants of salt tolerance in canola (Brassica napus L.). Environ Exp Bot 63:266–273. https://doi.org/10.1016/j.envexpbot.2007.11.008
Ashraf M, Orooj A (2006) Salt stress effects on growth, ion accumulation and seed oil concentration in an arid zone traditional medicinal plant ajwain (Trachyspermum ammi [L.] Sprague). J Arid Environ 64:209–220. https://doi.org/10.1016/j.jaridenv.2005.04.015
Bates LR, Waldrenand RP, Teare ID (1973) A rapid determination of free proline for water stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Bradford M (1976) A rapid and sensitive method for the quantitation of protein utilizing the principle of protein-dye binding. Annu Rev Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Cakmak I, Strbac D, Marschner H (1993) Activities of hydrogen peroxide scavenging enzymes in germinated wheat seeds. J Exp Bot 44:127–132. https://doi.org/10.1093/jxb/44.1.127
Chawla S, Jain S, Jain V (2013) Salinity induced oxidative stress and antioxidant system in salt-tolerant and salt-sensitive cultivars of rice (Oryza sativa L.). J Plant Biochem Biotechnol 22:27–34. https://doi.org/10.1007/s13562-012-0107-4
D’Souza MR, Devaraj VR (2010) Biochemical responses of Hyacinth bean (Lablab purpureus) to salinity stress. Acta Physiol Plant 32:341–353. https://doi.org/10.1007/s11738-009-0412-2
Daneshmand F, Arvin MJ, Kalantari KM (2010) Physiological responses to NaCl stress in three wild species of potato in vitro. Acta Physiol Plant 32:91–101. https://doi.org/10.1007/s11738-009-0384-2
Davies KJA (1987) Protein damage and degradation by oxygen radicals. I. General aspects. J Biol Chem 262:9895–9901. http://www.jbc.org/content/262/20/9895.full.pdf+html
Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9. https://doi.org/10.1016/S0168-9452(98)00025-9
Du Z, Bramlage WJ (1992) Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J Agric Food Chem 40:1566–1570. https://doi.org/10.1021/jf00021a018
Egert M, Tevini M (2002) Influence of drought on some physiological parameters symptomatic for oxidative stress in leaves of chives (Allium schoenoprasum). Environ Exp Bot 48:43–49. https://doi.org/10.1016/S0098-8472(02)00008-4
Essa TA (2002) Effect of salinity stress on growth and nutrient composition of three soybean (Glycine max L. Merrill) cultivars. J Agron Crop Sci 188:86–93. https://doi.org/10.1046/j.1439-037X.2002.00537.x
Foyer CH, Lopez-Delgado H, Dat JF, Scott IM (1997) Hydrogen peroxide- and glutathione-associated mechanisms of acclimatory stress tolerance and signalling. Physiol Plant 100:241–254. https://doi.org/10.1034/j.1399-3054.1997.1000205.x
Garg N, Manchanda G (2009) ROS generation in plants: boon or bane? Plant Biosyst 143:81–96. https://doi.org/10.1080/11263500802633626
Ghanati F, Morita A, Yokota H (2002) Induction of suberin and increase of lignin content by excess Boron in Tobacco cell. Soil Sci Plant Nutr 48:357–364. https://doi.org/10.1080/00380768.2002.10409212
Ghoulam C, Foursy A, Fares K (2002) Effects of salt stress on growth, inorganic ions and proline accumulation in relation to osmotic adjustment in five sugar beet cultivars. Environ Exp Bot 47:39–50. https://doi.org/10.1016/S0098-8472(01)00109-5
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Hasegawa PM, Bressan R, Zhu JK, Bohnert H (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol 51:463–499. https://doi.org/10.1146/annurev.arplant.51.1.463
Hecht SS, Carmella SG, Kenney PMJ, Low SH, Arakawa K, Yu MC (2004) Effects of cruciferous vegetable consumption on urinary metabolites of the tobacco-specific lung carcinogen 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanone in Singapore Chinese. Cancer Epidemiol Biomark Prev 13:997–1004
Hernandez JA, Jimenez A, Mullineaux P, Sevilla F (2000) Tolerance of pea (Pisum sativum L.) to long-term salt stress is associated with induction of antioxidant defences. Plant Cell Environ 23:853–862. https://doi.org/10.1046/j.1365-3040.2000.00602.x
Hernandez JA, Olmos E, Corpas FJ, Sevilla F, del Río LA (1995) Salt-induced oxidative stress in chloroplasts of pea plants. Plant Sci 105:151–167. https://doi.org/10.1016/0168-9452(94)04047-8
Hernandez M, Fernandez-Garcia N, Diaz-Vivancos P, Olmos E (2010) A different role for hydrogen peroxide and the antioxidative system under short and long salt stress in Brassica oleracea roots. J Exp Bot 61:521–535. https://doi.org/10.1093/jxb/erp321
Hishida M, Ascencio-Valle F, Fujiyama H, Orduño-Cruz A, Endo T, Larrinaga-Mayoral JÁ (2014) Antioxidant enzyme responses to salinity stress of Jatropha curcas and J. cinerea at seedling stage. Russ J Plant Physiol 61:53–62. https://doi.org/10.1134/S1021443714010063
Kholova J, Sairam RK, Meena RC, Srivastava GC (2009) Response of maize genotypes to salinity stress in relation to osmolytes and metal-ions contents, oxidative stress and antioxidant enzymes activity. Biol Plantarum 53:249–256. https://doi.org/10.1007/s10535-009-0047-6
Krause GH, Weis E (1991) Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol 42:313–349. https://doi.org/10.1146/annurev.pp.42.060191.001525
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV–vis spectroscopy. In: Wrolstad RE, Acree TE, An H, Decker EA, Penner MH, Reid DS, Schwartz SJ, Shoemaker CF, Sporns P (eds) Current protocols in food analytical chemistry. Wiley, New York, pp F4.3.1–F4.3.8. https://doi.org/10.1002/0471142913.faf0403s01
Lima ALS, DaMatta FM, Pinheiro HA, Totola MR, Loureiro ME (2002) Photochemical responses and oxidative stress in two clones of Coffea canephora under water deficit conditions. Environ Exp Bot 47:239–247. https://doi.org/10.1016/S0098-8472(01)00130-7
Lin CC, Kao CH (2000) Effect of NaCl stress on H2O2 metabolism in rice leaves. Plant Growth Regul 30:151–155. https://doi.org/10.1023/A:1006345126589
Ma H, Lindsay F, Wang S, Altman A, Hüttermann A (1997) Photosynthetic response of Populus euphratica to salt stress. Forest Ecol Manag 93:55–61. https://doi.org/10.1016/S0378-1127(96)03943-6
Manchanda G, Garg N (2008) Salinity and its effects on the functional biology of legumes. ActaPhysiol Plant 30:595–618. https://doi.org/10.1007/s11738-008-0173-3
Merati MJ, Hassanpour H, Niknam V, Mirmasoumi M (2014) Exogenous application of penconazole regulates plant growth and antioxidative responses in salt-stressed Mentha pulegium L. J Plant Interact 9:791–801. https://doi.org/10.1080/17429145.2014.948084
Munns R, Tester M (2008) Mechanism of salinity tolerance. Annu Rev Plant Biol 59:651–681. https://doi.org/10.1146/annurev.arplant.59.032607.092911
Netondo GW, Onyango JC, Beck E (2004) Sorghum and salinity II: gas exchange and chlorophyll fluorescence of sorghum (Sorghum bicolor L.) under salt stress. Crop Sci 44:806–811. https://doi.org/10.2135/cropsci2004.7970
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotoxicol Environ Saf 60:324–349. https://doi.org/10.1016/j.ecoenv.2004.06.010
Psarras G, Bertak M, Chartzoulakis K (2008) Response of greenhouse tomato to salt stress and K+ supplement. Plant Biosyst 142:149–153. https://doi.org/10.1080/11263500701872903
Renault S (2005) Tamarack response to salinity: effects of sodium chloride on growth and ion, pigment, and soluble carbohydrate levels. Can J For Res 35:2806–2812. https://doi.org/10.1139/x05-194
Sairam RK, Srivastava GC, Agarwal S, Meena RC (2005) Differences in antioxidant activity in response to salinity stress in tolerant and susceptible wheat genotypes. Biol Plant 49:85–91. https://doi.org/10.1007/s10535-005-5091-2
Santos CV (2004) Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves. Sci Hort 103:93–99. https://doi.org/10.1016/j.scienta.2004.04.009
SAS Institute Inc (2004) SAS/STAT user’s guide, version 9.1. Cary, North Carolina
Schreiber U, Bilger W, Neubauer C (1995) Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis. In: Schulze ED, Caldwell MM (eds) Ecophysiology of photosynthesis. Springer, Berlin, pp 49–70. https://doi.org/10.1007/978-3-642-79354-7_3
Sekmen AH, Türkan I, Takio S (2007) Differential responses of antioxidative enzymes and lipid peroxidation to salt stress in salt-tolerant Plantago maritime and salt-sensitive Plantago media. Physiol Plantarum 131:399–411. https://doi.org/10.1111/j.1399-3054.2007.00970.x
Singh MP, Singh DK, Rai M (2007) Assessment of growth, physiological and biochemical parameters and activities of antioxidative enzymes in salinity tolerant and sensitive basmati rice varieties. J Agron Crop Sci 193:398–412. https://doi.org/10.1111/j.1439-037X.2007.00267.x
Smart RE, Bingham GE (1974) Rapid estimates of relative water content. Plant Physiol 53:258–260. https://doi.org/10.1104/pp.53.2.258
Sultana N, Ikeda T, Itoh R (1999) Effect of NaCl salinity on photosynthesis and dry matter accumulation in developing rice grains. Environ Exp Bot 42:211–220. https://doi.org/10.1016/S0098-8472(99)00035-0
Tayebimeigooni A, Awang Y, Mahmood M, Selamat A, Wahab Z (2012) Leaf water status, proline content, lipid peroxidation and accumulation of hydrogen peroxide in salinized Chinese kale (Brassica alboglabra). J Food Agric Environ 10:371–374
Tayebi-Meigooni A, Awang Y, Biggs AR, Rosli M, Madani B, Ghasemzadeh A (2014) Mitigation of salt-induced oxidative damage in Chinese kale (Brassica alboglabra L.) using ascorbic acid. Acta Agric Scand Sect B 64:13–23. https://doi.org/10.1080/09064710.2013.869347
Turkan I, Demiral T, Sekmen AH (2013) The regulation of antioxidant enzymes in two Plantago species differing in salinity tolerance under combination of waterlogging and salinity. Funct Plant Biol 40:484–493. https://doi.org/10.1071/FP12147
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants Protective role of exogenous polyamines. Plant Sci 151:59–66. https://doi.org/10.1016/S0168-9452(99)00197-1
Webb RP, Allen RD (1995) Isolation and characterization of a cDNA for spinach cytosolic ascorbate peroxidase. Plant Physiol 108:13–25. https://doi.org/10.1104/pp.108.3.1325
Weis E, Berry JA (1987) Quantum efficiency of photosystem II in relation to energy-dependent quenching of chlorophyll fluorescence. Biochim Biophys Acta Bioenerg 894:198–208. https://doi.org/10.1016/0005-2728(87)90190-3
Xiong L, Zhu JK (2002) Molecular and genetic aspects of plant responses to osmotic stress. Plant Cell Environ 25:131–139. https://doi.org/10.1046/j.1365-3040.2002.00782.x
Yazici I, Turkan F, Sekmenah DT (2007) Salinity tolerance of purslane (Portulaca oleracea L.) is achieved by enhanced antioxidative system, lower level of lipid peroxidation and proline accumulation. Environ Exp Bot 61:49–57. https://doi.org/10.1016/j.envexpbot.2007.02.010
Yusuf M, Hasan SA, Ali B, Hayat S, Fariduddin Q, Ahmad A (2008) Effect of salicylic acid on salinity-induced changes in Brassica juncea. J Integr Plant Biol 50:1096–1102. https://doi.org/10.1111/j.1744-7909.2008.00697.x
Acknowledgment
The authors gratefully acknowledge the Universiti Putra Malaysia for financial support.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Tayebi-Meigooni, A., Awang, Y., Biggs, A.R., Ghasemzadeh, A. (2019). Salt-Induced Changes in Growth and Damage Avoidance Mechanisms of Hydroponically Grown Chinese Kale (Brassica alboglabra L.). In: Gul, B., Böer, B., Khan, M., Clüsener-Godt, M., Hameed, A. (eds) Sabkha Ecosystems. Tasks for Vegetation Science, vol 49. Springer, Cham. https://doi.org/10.1007/978-3-030-04417-6_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-04417-6_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-04416-9
Online ISBN: 978-3-030-04417-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)