Abstract
Arbuscular mycorrhizal fungi (AMF) have been reported to improve the resistance to drought stress in some plant species. The aim of this study was to evaluate the effects of Rhizophagus irregularis inoculation on the growth, photosynthetic capacity, nutrient uptake, and nutrient distribution of poplar cuttings under drought stress. The experiment was performed with a randomized block design with two factors: (i) AMF treatment, inoculated with R. irregularis (AM) or not (NM); (ii) drought treatment, well-watered (WW, 70–75% of field capacity), mild stressed (MS, 50–55% of field capacity), or severe stressed (SS, 30–35% of field capacity). The results showed that R. irregularis colonized more than 70% of the roots of poplar cuttings. Drought stress limited the plant growth and photosynthetic capacity of poplar, while inoculation increased the plant height, stem diameter, stem dry weight, root dry weight, net photosynthetic rate (PN), stomatal conductance (gs), and intrinsic water use efficiency (WUEi) regardless of the drought stress treatment. Drought stress decreased the absorption of nutrients and affected their distribution in plant tissues. Regardless of drought stress treatment, inoculation increased the concentrations of Ca and Mn in leaves and the concentration of Cu in roots. Under mild drought stress conditions, the contents of P, Ca, Cu, Fe, and Zn increased significantly in the leaves of inoculated plants, while the contents of P, Ca, Fe, and Mn increased significantly in the roots. Under severe drought stress, inoculation decreased the distribution of N, P, K, and Mg in the leaves; the distribution of K, Ca, Mn, and Zn in roots; and the distribution of Cu in roots. Moreover, a principal components analysis showed that under well-watered and severe drought stress conditions, the inoculation of poplars with R. irregularis could significantly increase the absorption of nutrients. The results of a correlation analysis indicated that the growth parameters and gas exchange parameters positively correlated with the concentrations of leaf P, K, Ca, Fe, Mn, Cu, and Zn. Photosynthetic capacity, nutrient absorption, and a change in nutrient distribution were enhanced in the mycorrhizal poplar cuttings, which resulted in enhanced growth and a limited loss of biomass during drought stress compared with the non-mycorrhizal cuttings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Populus species and their hybrids have high economic value and are cultivated worldwide (Feodorova and Alexandrov 2020). Populus × canadensis ‘Neva’, a hybrid of P. nigra × P. deltoides, is widely planted in China as a woody species under intensive management (Li et al. 2021). The fast-growing poplar species/genotypes are generally vulnerable to drought stress owing to their high requirement for water (Yi et al. 2020).
Drought is considered a severe abiotic stress that causes plant growth inhibition. In general, drought stress can reduce the availability of nutrients in the soil, the absorption of nutrients by roots, the transport from roots to aboveground parts, and the distribution among plants (Hussain et al. 2019). Under drought stress, phosphorus (P) and potassium (K) contents usually decrease (Püschel et al. 2021; Qi et al. 2019). The nutritional disturbance occurs in poplars when they are under water deficit (Tripathi et al. 2018).
The fossil records suggest that arbuscular mycorrhiza (AM), which appeared 400 million years ago (Walker et al. 2018), are the most common and widely distributed type of plant symbiosis (Brundrett and Tedersoo 2018). AM are important mutualistic symbioses formed between fungi from the phylum Glomeromycota and more than 72% of terrestrial plants (Brundrett and Tedersoo 2018). The spread of arbuscular mycorrhizal fungal (AMF) mycelia can increase the absorption area of the host plant’s root in the soil. Moreover, AMF rely on photosynthates from the host to complete their life span and supply water and mineral nutrients to the host (Ortuño et al. 2018; Wu et al. 2017a). AMF can enhance plant drought resistance by improving plant nutrition. On the one hand, AMF can alleviate the decrease in soil available nutrients caused by drought by improving the absorption of slowly diffusing mineral ions, such as PO42− and Zn2+ (Hu et al. 2017; Watts-Williams et al. 2019). A previous study identified a positive effect of the AMF Rhizophagus irregularis and R. arabicus on drought resistance of Sorghum bicolor by increasing nutrient uptake (Symanczik et al. 2018). Alternatively, AMF regulates the ion and osmotic balances in plants under drought stress (Liu et al. 2020). Potassium accumulation in tobacco seedlings was regulated by AMF, and mycorrhizal tobacco seedlings had a better osmotic balance than the uninoculated control under drought stress (Liu et al. 2020). Alterations in the uptake, distribution, and composition of nutrients were observed in apple, palm, and Ailanthus altissima, inoculated with AMF under different soil conditions (Costa et al. 2021; Nejad et al. 2021; Zai et al. 2021). Exogenous inoculation with R. irregularis can increase the distribution of calcium (Ca), magnesium (Mg), iron (Fe), manganese (Mn), and zinc (Zn) to the leaves of poplar (Wu et al. 2018). Arbuscular mycorrhizal fungus modulated Cd/Zn accumulation and distribution in poplar (De Oliveira et al. 2019). Alterations in the concentrations and distribution of nutrients may lead to changes in the photosynthetic capacity (Wu et al. 2018; Zai et al. 2021).
Previous research had confirmed that the shoot biomass and transpiration rates of poplars could be increased by Rhizophagus irregularis under cadmium (Cd) stress. (De Oliveira et al. 2019). Inoculation with AMF increased the area of absorption of the poplar roots and enhanced the absorption capacity of the roots for nitrate (NO3−) (Wu et al. 2020). An improvement in photosynthetic capacity and drought tolerance of P. × canadensis ‘Neva’ by AMF was found in a previous study (Liu et al. 2015), but the influence of AMF on nutrient uptake and distribution of P. × canadensis ‘Neva’ remains elusive. This study was conducted to determine the effects of AMF on the nutrient contents and their distribution in different organs of P. × canadensis ‘Neva’ under different drought stress levels.
2 Material and Methods
2.1 Plant Material and AM Colonization
The cuttings (15 cm in length) of P. × canadensis ‘Neva’ used in this study were obtained from a nursery of poplar cuttings that were produced vegetatively in Rougu county, Yangling district, Shaanxi Province, China. The surface disinfection process of cuttings consisted of soaking them in 75% (v/v) ethanol for 15 s and then rinsing them three times with sterile distilled water for 10 s at a time. The surface-disinfected cuttings were planted with 2 cm exposed outside of the pots (19.5 × 21.5 cm), which contained 5 kg of soil substrate.
The substrate was a mixture of soil and sand (1:1, v/v) that was autoclaved at 121 °C for 2 h under pressure (0.11 MPa), and then placed in a storage room for 3 days before use. Soil (0–20 cm) was obtained from a nursery on the campus of Northwest A&F University (Yangling, China) and sieved with a 2-mm mesh. The soil contained 16.21 g kg−1 soil organic matter, 12.78 mg kg−1 available P, 33.89 mg kg−1 available nitrogen (N), and 132.54 mg kg−1 available K. The soil pH was 7.6 (soil: water, 1:5). Thoroughly washed river sand was mixed with soil.
The inoculum of Rhizophagus irregularis (Blaszk, Wubet, Renker & Buscot) Walker & Schüßler (BGC B109) was supplied by the Institute of Plant Nutrition and Resources, Beijing Academy of Agriculture and Forestry Sciences (Beijing, China). The AMF inoculum consisted of soil, infected root fragments, spores, and hyphae. The inoculum (20 g) was placed next to the cutting in the pot, while the non-inoculated treatments were provided with 20 g of autoclaved inoculum, to ensure consistent with the nutritional environment of the inoculation treatment.
2.2 Experimental Design and Growth Conditions
The experiment was performed using a randomized block design with two factors: (i) AM treatment, inoculated with R. irregularis (AM) or not (NM); (ii) drought treatment, well-watered (WW, 70–75% of field capacity), mildly stressed (MS, 50–55% of field capacity), or severely stressed (SS, 30–35% of field capacity). Field capacity was calculated as follows: X = (saturated soil weight − dry soil weight)/dry soil weight × 100%.
The pots were weighed every day to control the soil water content. The experiment included six treatments. One cutting was planted per pot, and three pots were merged into one replicate. Each treatment contained four replicates, and there were 72 cuttings in this experiment.
The experiment was carried out in a greenhouse at Northwest A&F University, Shaanxi Province, China, with 12–14 h light per day, a relative humidity of 55–78%, and a temperature of 25–35 °C.
During the experiment, cuttings were fertilized with 100 mL Hoagland’s solution every 2 weeks and grown under well-watered conditions. Thirty days after planting, the drought treatment started and continued for 60 days. The positions of pots were changed every other week.
2.3 Plant Growth Parameters and Mycorrhizal Colonization
Cuttings were harvested 90 days after planting. The plant height was determined by a tape (Swordfish, China) and the stem diameter with Vernier calipers (ECV150C, China). The roots from each treatment were collected, washed with tap water to remove the soil particles, and dried with paper towels. The fresh weights of leaves, stems, and roots were recorded. The dry weight of leaves, stems, and roots was recorded after oven drying to a constant weight at 80 °C. Parts of the roots were cut into 1-cm-long fragments and stained with trypan blue (Phillips and Hayman 1970). A total of 200 root segments were collected for each treatment. The rates of AM colonization were examined using the gridline intercept method (Giovannetti and Mosse 1980).
2.4 Gas Exchange Parameters
The leaf without visible injury was selected for the gas exchange measurements between 08:00 and 13:30 before harvest. The net photosynthetic rate (PN), stomatal conductance (gs), intercellular CO2 concentration (Ci), and transpiration rate (E) were measured with a Li-Cor 6400 portable photosynthesis system (Li-Cor Inc., Lincoln, NE, USA) and red/blue LED. All of the measurements were conducted with the following parameters: photosynthetically active irradiation of 1000 µmol m−2 s−1, a CO2 concentration of 400 cm3 m−3, and a leaf temperature of 50 ℃. The intrinsic water use efficiency (WUEi) was calculated as follows: WUEi = PN/gs.
2.5 Nutrient Analysis
The nutrient analysis was performed using ground and homogenized dry samples of roots, stems, and leaves. The N concentration was determined as described by Kong et al. (2015) using 0.2 g of dried leaves, stems, and roots, and the P concentration was determined using 1 g of dried leaves, stems, and roots that were digested with HNO3-HClO4 using the vanadomolybdate method (Wu et al. 2018). The K, Mg, Ca, Fe, Zn, copper (Cu), and Mn concentrations were determined as described by Nisha and Rao (2017) using an atomic absorption spectrophotometer (Hitachi Z-2000, Tokyo, Japan). The nutrient contents equal the nutrient concentrations multiplied by the dry weight. The distribution of elements in the leaf, stem, and root equal nutrient contents divided by the total element contents.
2.6 Statistical Analysis
The data were examined for normality and homogeneity. The differences in associated measured parameters according to AM status, water status, and their interaction were analyzed using SPSS v. 17.0 for Windows (SPSS, Inc., Chicago, IL, USA). A two-way analysis of variance (ANOVA) was used to test the effects of drought, inoculation, and their interactions at P ≤ 0.05. Multiple comparisons were performed using a Duncan’s post-hoc test. The principal component analysis (PCA) of the nutrient parameters was analyzed using MetaboAnalyst 4.0 (Chong et al. 2018). Correlations between gas exchange parameters and leaf nutrient concentrations were analyzed by Pearson’s correlation coefficients.
3 Results
3.1 Plant Growth and AM Colonization
The AM colonization is significantly influenced by drought treatment, AM treatment, and their interaction (Table 1). The colonization rate of R. irregularis is higher than 70% in all the inoculated treatments; no mycorrhizal colonization is observed in non-inoculated plant roots (Fig. 1).
Plant height and stem diameter are significantly influenced by drought treatment and AM treatments (Table 1). Compared with non-inoculated treatments, the inoculation increases plant height and stem diameter under the different water regimes, while drought stress decreases the plant height and stem diameter for both non-inoculated and inoculated treatments (Fig. 2).
Drought treatment and AM treatments had significant effects on the dry weights of leaves, stems, and roots (Table 1). Compared with non-inoculated treatments, inoculation increases the stem and root dry weights under the different water regimes, while drought stress decreases the root dry weight for both inoculated and non-inoculated treatments (Fig. 2).
3.2 Gas Exchange Parameters
Drought treatment and AM treatments had significant effects on the leaf gas exchange parameters (Fig. 3). Drought stress decreases PN, gs, Ci, E, and WUEi, while inoculation increases Ci and E under well-watered and severe drought stress and increases PN, gs, and WUEi regardless of drought stress treatment (Fig. 3).
Effect of AM fungus and drought conditions on gas exchange. PN, net photosynthesis; Ci, intercellular CO2 concentration; gs, stomatal conductance, E, transpiration rate, WUEi, intrinsic water use efficiency; WW, well-watered; MS, mildly stressed; SS, severely stressed; AM, inoculated with Rhizophagus irregularis; NM, non-mycorrhizal; AMF, AM treatments; *P ≤ 0.05; NS, not significant. Values with different letters indicate significant differences (Duncan’s test P = 0.05, n = 4)
3.3 Macronutrient Concentrations
The concentration of P in all plant tissues, Ca in leaves and roots, and Mg in roots is significantly affected by drought stress, inoculation, and their interaction (Table 1). Compared with non-inoculated treatments, inoculation increases the concentration of leaf and root P under different soil water contents, while drought stress decreases the concentration of stem P for both inoculated and non-inoculated treatments (Fig. 4). Compared with non-inoculated treatments, inoculation increases the concentrations of leaf Ca and root Mg under drought stress, while drought stress decreases the concentration of leaf Ca and increases that of root Mg for both non-inoculated and inoculated treatments (Fig. 4).
Effect of AM fungus and drought condition on the concentration of macronutrient (N, P, K, Ca, Mg). WW, well-watered; MS, mildly stressed; SS, severely stressed; AM, inoculated with Rhizophagus irregularis; NM, non-mycorrhizal. Values with different letters indicate significant differences (Duncan’s test P = 0.05, n = 4)
3.4 Micronutrient Concentrations
The concentration of Zn in all tissues, leaf Mn, and root Cu is significantly affected by drought stress, inoculation, and their interaction (Table 1). Drought stress decreases the concentrations of leaf Mn and Zn and root Cu, while inoculation increases the concentrations of leaf Mn and root Cu regardless of whether the plants were subjected to drought stress treatment (Fig. 5). Drought stress increases the concentration of stem Zn, while inoculation decreases the concentration of stem Zn regardless of drought stress treatment (Fig. 5).
Effect of AM fungus and drought conditions on the concentrations of micronutrients (Fe, Mn, Cu, Zn). WW, well-water; MS, mildly stressed; SS, severely stressed; AM, inoculated with Rhizophagus irregularis; NM, non-mycorrhizal. Values with different letters indicate significant differences (Duncan’s test P = 0.05, n = 4)
3.5 Macronutrient Contents
The contents of P and Ca in leaves and roots and Mg in roots are significantly affected by drought stress, inoculation, and their interaction (Table 2). Drought stress decreases the contents of P and Ca in leaves and roots, while inoculation increases the contents of P and Ca in leaves and roots under drought stress treatment (Table 3).
3.6 Micronutrient Contents
The contents of Cu, Fe, and Mn in roots and Mn in leaves are significantly affected by drought stress, inoculation, and their interaction (Table 2). Drought treatment and AM treatments had significant effects on the contents of Zn, Cu, and Fe in leaves. Drought stress decreased the contents of Cu, Fe, and Mn in leaves and roots, and the content of leaf Zn, while inoculation increased the contents of leaf Cu, Fe, and Zn and the root Fe and Mn contents under well-watered and mild drought stress conditions. Inoculation significantly increases the contents of leaf Mn and root Cu regardless of drought stress treatment (Table 4).
3.7 Nutrient Distribution
The distribution of K, Ca, and Cu occurred mostly in stems, whereas N, P, Mg, and Zn were distributed more in the leaves. Fe is primarily distributed in the roots (Fig. 6).
Effect of AM fungus and drought conditions on N, P, K, Ca, Mg, Fe, Mn, Cu, and Zn distribution percentage in different plant parts of poplar seedlings. WW, well-watered; MS, mildly stressed; SS, severely stressed; AM, inoculated with Rhizophagus irregularis; NM, non-mycorrhizal. Values with different letters indicate significant differences (Duncan’s test P = 0.05, n = 4)
Under severe drought stress, inoculation decreased the distribution of N, P, K, and Mg in leaves and the distribution of K, Ca, Mn, and Zn in roots, and increased the distribution of N, P, K, Mg, Ca, Zn, and Mn in stems. Under well-watered and mild drought stress conditions, inoculation significantly increases the distribution of Mn and Zn in leaves and decreases the distribution of Cu and Mn in stems (Fig. 6). Drought stress significantly decreases the distribution of Cu in roots for both inoculated and non-inoculated treatments, while inoculation increases the distribution of Cu in roots under the different water regimes compared with non-inoculated treatments (Fig. 6).
3.8 Principal Components Analysis and Correlation Coefficients of Nutrients
Principal components PC1 and PC2 together explain 91.6% of the variance (Fig. 7). The principal components analysis indicates that under well-watered and severely stressed conditions, inoculation significantly affected the absorption of nutrients, more under well-watered than under severely stressed (Fig. 7). Drought stress had a significant impact on the absorption of nutrients for both inoculated and non-inoculated treatments, and it had a greater effect on the absorption of nutrients in inoculated than non-inoculated plants (Fig. 7).
The results of a correlation analysis indicated that the growth parameters and gas exchange parameters are positively correlated with the concentrations of leaf P, K, Ca, Fe, Mn, Cu, and Zn and negatively correlate with the concentration of leaf N (P < 0.05, Table 5).
4 Discussion
Poplars are widely planted to meet various demands (Chen et al. 2017) and require a large supply of water and nutrients (Goehing et al. 2019). AMF rely on the lipid from host plants to complete their life span, and they supply mineral nutrients and water for exchange (Jiang et al. 2017). In this symbiotic relationship, both partners control the trade (Hu et al. 2017). In this study, the degree of AM colonization of the roots of plants inoculated with R. irregularis exceeded 70%. This was observed in previous studies where P. × canadensis ‘Neva’ could form symbiosis with AMF (Wu et al. 2017b). To control the loss of photosynthates that AM fungi demand, it has been suggested to decrease the colonization of plants under drought stress (Wang et al. 2017). The symbiosis established with the AMF increased plant height, stem diameter, and biomass, and the interaction between P. × canadensis ‘Neva’ and R. irregularis has been documented in experiments (Wu et al. 2017b). Mycorrhizal P. trichocarpa showed improved growth (De Oliveira et al. 2019). Consistent with previous studies on poplar (Wu et al. 2017b), inoculation increased the parameters of gas exchange and photosynthesis, suggesting that mycorrhizal plants had a higher photosynthetic capacity.
Under drought stress, the concentrations of leaf Ca, Mn, and Zn and stem P diminished, whereas there were no changes in nutrient concentration in the roots, suggesting a notable decline in nutrient transportation from the belowground to aboveground plant tissues under drought stress. Moreover, the percentage of distribution of P in the plant suggested a larger accumulation in roots but a decline of their transportation to leaves under drought stress.
The nutrient ions available to plants are dissolved in the soil solution. Thus, the absorption of nutrients by plants relies on water flow in the soil-root-shoot continuum, so that water and different nutrients always coexist in plant tissues (Keller 2020). The roots absorb soil solution that contains essential nutrients aided by leaf transpiration, which provides the necessary tension (Keller 2020).
Drought stress exerts adverse effects on plant nutrition. First, it causes a decrease in the growth rate of trees, particularly in the expansion rate of leaves (Zhang et al. 2018a, b). The reason for this is thought to be owing to an increase in the down-regulation effect on nutrient uptake. Secondly, the water deficit has a negative impact on the availability of nutrients around the root. Third, it decreases stomatal conductance, which results in a lower leaf internal CO2 concentration and photosynthetic rate, transpiration rate, and mass flow of nutrients among others.
Under well-watered and severe drought stress conditions, the inoculation of poplars with R. irregularis could have significantly affected the absorption of nutrients other than P, while the AMF apparently increased P concentration and contents in the leaves and roots, suggesting that R. irregularis can promote the absorption of P (Hu et al. 2017). Phosphorus is vital for plants because of its key role in signal transduction pathways and the structural composition of nucleic acids and phospholipids, and because it is a significant factor in energy transfer (Ramos-Artuso et al. 2019). Both P mineralization and mobility are generally constrained by many environmental conditions, such as drought (Goll et al. 2018). In addition, P uptake is affected by the water deficit, which can cause an inhibition of root growth (Zhang et al. 2018a, b). In fact, there are several adaptive strategies for plants to respond to low availability of P in soil. For instance, in order to increase the acquisition of P, the plant allocates more carbon to the root (Hu et al. 2017) and establishes mycorrhizal symbiosis, whose extended extraradical mycelia supplement the function of plant roots (Brundrett and Tedersoo 2018).
Under the drought stress treatments, inoculation with R. irregularis increased Ca concentration and contents in leaves. Thus, we considered that the diffusible molecules released by AMF can cause changes in the Ca contents in plant cells (Sujkowska-Rybkowska and Znojek 2018), transmitting drought signals to plants and causing a series of physiological changes to further resist the arid environment (Mbengue et al. 2020). Drought activates calcium ion channels located on the plasma membrane of the cell to generate specific calcium signals in the cytoplasm. Signals mediated by Ca2+ could act as core sensors and regulators in multiple adaptive and developmental aspects of plant metabolism (Bredow and Monaghan 2019). Calcium has been shown to promote the ability of leaves and their cellular membranes to preserve water, so as to effectively alleviate plant hydropenia under drought stress (Khan et al. 2017).
In this experiment, mycorrhization increased the concentration and contents of Mn in leaves, and the proportion of Mn transferred to the leaves under well-watered and mild drought stress. This was associated with an increase in the photosynthetic capacity in the inoculated plants, which is to be expected, because Mn is a component element in the water-splitting system of photosystem II and supplies essential electrons for photosynthesis in the thylakoid membranes (Cao et al. 2018). Recently, increasing numbers of studies have reported that Mn takes part in redox processes and could be an activator or cofactor of a variety of enzymes, including those needed for light-induced water oxidation in photosystem II (Lubitz et al. 2019).
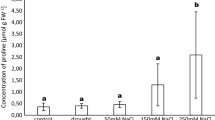
The inoculation of poplars with R. irregularis significantly increased the concentration and contents of root Cu and the distribution of Cu in roots. The results might suggest that the biosorption of plant root cell wall and extracellular secretions produced by AMF could change the availability of Cu in the rhizosphere, thereby reducing the transmission to the shoot, as previously shown by Sun et al. (2019). Copper is also necessary for redox systems and serves as an activator of many enzymes, primarily those required for superoxide radical detoxification and lignin synthesis (Ishka and Vatamaniuk 2020).
In this study, the contents and distribution of Mg were higher in leaves, supporting the concept that Mg is easily transferred to leaves and engages in photosynthesis (Geng et al. 2021). In most cases, Mg plays an important role in metabolic processes by activating numerous enzymes. For instance, RUBISCO, a Mg-activated enzyme, could participate in photosynthesis and other biological processes (Rodrigues et al. 2021). Under drought stress, inoculated plants accumulated less Mg in leaves and more in the roots. These results showed that the Mg in leaves was sufficient for photosynthesis, and AMF maintained needless Mg in the roots, reducing the energy required for mineral nutrient transport (Lopes et al. 2020).
The distribution of iron was higher in the roots, and our results support the concept that Fe may be tightly bound to root cells, as previously indicated (Sterckeman et al. 2021). In this study, inoculation with R. irregularis increased the contents and distribution of Fe in roots under mild drought stress. These results are consistent with a previous study in which Funneliformis mosseae enhanced the uptake of Fe to maize plants that were grown under drought stress (Bahraminia et al. 2020).
Drought stress had a significant influence on the absorption of Zn, decreasing its contents and distribution in the leaves, while inoculation increased them. Zinc is essential to the membrane integrity, detoxification of superoxide radicals, and the synthesis of the phytohormone IAA and proteins (Nakandalage and Seneweera 2018). It has been demonstrated that Zn can be transferred by AMF external mycelia (Upadhayay et al. 2019). Our results showed that AMF could absorb and deliver Zn to the host, thereby improving plant Zn nutrition (Ruytinx et al. 2019).
Nutrient transport in plants is a very complex process, which involves many transporters. Nutrient transporters play an important role in nutrient absorption and signal transduction (Sun et al. 2020). Studies showed that one transporter can simultaneously transport multiple nutrient elements. For example, PtrZIP gene is expressed not only under Zn, Fe, Cu, and Mn deficiency or excess stress, but also under cadmium (Cd) and lead (Pb) excess stress (Zhang et al. 2017). Moreover, the genes involved in the absorption, transport, and distribution of nutrient are also different. Therefore, it is difficult to select the appropriate transporter gene for research. However, research on nutrient transporters is very meaningful and can be focused on in the future.
5 Conclusions
In summary, this indicated that arbuscular mycorrhizal fungi promote the growth and gas exchange parameters of poplar under drought stress. Moreover, the gas exchange parameters positively correlated with the concentrations of leaf P, K, Ca, Fe, Mn, Cu, and Zn. Under severe drought stress, inoculation decreased the distribution of N, P, K, and Mg in leaves and the distribution of K, Ca, Mn, and Zn in the roots and increased the distribution of N, P, K, Mg, Ca, Zn, and Mn in stems. The results demonstrated that nutrient absorption and changes in the distribution of nutrients were enhanced in the mycorrhizal poplar cuttings, which resulted in the enhanced photosynthetic capacity of poplar, plant growth, and limited biomass loss during drought stress compared with the non-mycorrhizal cuttings, leading to an improvement in the drought resistance of poplar.
References
Bahraminia M, Zarei M, Ronaghi A, Sepehri M, Etesami H (2020) Ionomic and biochemical responses of maize plant (Zea mays L.) inoculated with Funneliformis mosseae to water-deficit stress. Rhizosphere 16:100269. https://doi.org/10.1016/j.rhisph.2020.100269
Bredow M, Monaghan J (2019) Regulation of plant immune signaling by calcium-dependent protein kinases. Mol Plant Microbe in 32:6–19. https://doi.org/10.1094/MPMI-09-18-0267-FI
Brundrett MC, Tedersoo L (2018) Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol 220:1108–1115. https://doi.org/10.1111/nph.14976
Cao P, Su XD, Pan XW, Liu ZF, Chang WR, Li M (2018) Structure, assembly and energy transfer of plant photosystem II supercomplex. BBA-Biomembranes 1859:633–644. https://doi.org/10.1016/j.bbabio.2018.03.007
Chen H, Dong Y, Xu T, Wang YP, Wang HT, Duan BL (2017) Root order-dependent seasonal dynamics in the carbon and nitrogen chemistry of poplar fine roots. New for 48:587–607. https://doi.org/10.1007/s11056-017-9587-3
Chong J, Soufan O, Li C, Caraus I, Li SZ, Bourque G, Wishart DS, Xia JG (2018) MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res 46:486–494. https://doi.org/10.1093/nar/gky310
Costa DM, Rech TD, Primieri S, Pigozzi BG, Werner SS, Sturmer SL (2021) Inoculation with isolates of arbuscular mycorrhizal fungi influences growth, nutrient use efficiency and gas exchange traits in micropropagated apple rootstock ‘Marubakaido.’ Plant Cell Tiss Org 145:1–11. https://doi.org/10.1007/s11240-020-01994-0
De Oliveira VH, Ullah I, Dunwell JM, Tibbett M (2019) Mycorrhizal symbiosis induces divergent patterns of transport and partitioning of Cd and Zn in Populus trichocarpa. Environ Exp Bot 171:103925. https://doi.org/10.1016/j.envexpbot.2019.103925
Feodorova TA, Alexandrov OS (2020) Comparative studying of leaf trichomes, teeth and glands in Populus nigra L., Populus deltoides W. Bartram ex Marshall and their hybrids. Forests 11:1267. https://doi.org/10.3390/f11121267
Geng GT, Cakmak I, Ren T, Lu ZF, Lu JW (2021) Effect of magnesium fertilization on seed yield, seed quality, carbon assimilation and nutrient uptake of rapeseed plants. Field Crop Res 264:108082. https://doi.org/10.1016/j.fcr.2021.108082
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84:489–500. https://doi.org/10.1111/j.1469-8137.1980.tb04556.x
Goehing J, Henkel-Johnson D, Macdonald SE, Bork EW, Thomas BR (2019) Spatial partitioning of competitive effects from neighbouring herbaceous vegetation on establishing hybrid poplars in plantations. Can J Forest Res 49:595–605. https://doi.org/10.1139/cjfr-2018-0410
Goll DS, Joetzjer E, Huang M, Ciais P (2018) Low phosphorus availability decreases susceptibility of tropical primary productivity to droughts. Geophys Res Lett 45:8231–8240. https://doi.org/10.1029/2018GL077736
Hu WT, Zhang HQ, Zhang XY, Chen H, Tang M (2017) Characterization of six PHT1 members in Lycium barbarum and their response to arbuscular mycorrhiza and water stress. Tree Physiol 37:351–366. https://doi.org/10.1093/treephys/tpw125
Hussain HA, Men S, Hussain S, Chen Y, Ali S, Zhang S, Zhang KP, Li Y, Xu QW, Liao CQ, Wang LC (2019) Interactive effects of drought and heat stresses on morpho-physiological attributes, yield, nutrient uptake and oxidative status in maize hybrids. Sci Rep 9:3890. https://doi.org/10.1038/s41598-019-40362-7
Ishka MR, Vatamaniuk OK (2020) Copper deficiency alters shoot architecture and reduces fertility of both gynoecium and androecium in Arabidopsis thaliana. Plant Direct 4:e00288. https://doi.org/10.1002/pld3.288
Jiang Y, Wang W, Xie Q, Liu N, Liu L, Wang D, Zhang X, Yang C, Chen X, Tang D, Wang E (2017) Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science 356:1172–1175. https://doi.org/10.1126/science.aam9970
Keller M (2020) Water relations and nutrient uptake. In: Keller M (ed) The science of grapevines, 3rd edn. Academic Press, New York, pp 105–127. https://doi.org/10.1016/B978-0-12-816365-8.00003-8
Khan A, Anwar Y, Hasan M, Iqbal A, Ali M, Alharby H, Hakeem KR, Hasanuzzaman M (2017) Attenuation of drought stress in Brassica seedlings with exogenous application of Ca2+ and H2O2. Plants 6:20. https://doi.org/10.3390/plants6020020
Kong Z, Glick BR, Duan J, Ding S, Tian J, McConkey BJ, Wei G (2015) Effects of 1-aminocyclopropane-1-carboxylate (ACC) deaminase-overproducing Sinorhizobium meliloti on plant growth and copper tolerance of Medicago lupulina. Plant Soil 39:383–398. https://doi.org/10.1007/s11104-015-2434-4
Li X, Mao XH, Xu YJ, Li Y, Zhao N, Yao JX, Dong YF, Tigabu M, Zhao XY, Li SW (2021) Comparative transcriptomic analysis reveals the coordinated mechanisms of Populus × canadensis ‘Neva’ leaves in response to cadmium stress. Ecotox Environ Safe 216:112179. https://doi.org/10.1016/j.ecoenv.2021.112179
Liu L, Li D, Ma YL, Shen HT, Zhao SM, Wang YF (2020) Combined application of arbuscular mycorrhizal fungi and exogenous melatonin alleviates drought stress and improves plant growth in tobacco seedlings. J Plant Growth Regul. https://doi.org/10.1007/s00344-020-10165-6
Liu T, Sheng M, Wang CY, Chen H, Li Z, Tang M (2015) Impact of arbuscular mycorrhizal fungi on the growth, water status and photosynthesis of hybrid poplar under drought stress and recovery. Photosynthetica 5:250–258. https://doi.org/10.1007/s11099-015-0100-y
Lopes JI, Arrobas M, Brito C, Goncalves A, Silva E, Martins S, Raimundo S, Rodrigues MA, Correia CM (2020) Mycorrhizal fungi were more effective than zeolites in increasing the growth of non-irrigated young olive trees. Sustainability 12:10630. https://doi.org/10.3390/su122410630
Lubitz W, Chrysina M, Cox N (2019) Water oxidation in photosystem II. Photosynth Res 142:105–125. https://doi.org/10.1007/s11120-019-00648-3
Mbengue MD, Hervé C, Debellé F (2020) Nod factor signaling in symbiotic nodulation. Adv Bot Res 94:1–39. https://doi.org/10.1016/bs.abr.2019.10.002
Nakandalage N, Seneweera S (2018) Micronutrients use efficiency of crop-plants under changing climate. In: Hossain MA, Kamiya T, Burritt DJ, Tran L-S P, Fujiwara T (Eds) Plant micronutrient use efficiency. Academic Press, New York, pp 209–214. https://doi.org/10.1016/B978-0-12-812104-7.00015-0
Nejad RAH, Kafi M, Jari SK, Mozafari H, Motesharezadeh B (2021) Arbuscular mycorrhizal fungi improve growth, physiological status and nutrients accumulation of Ailanthus altissima seedlings under cadmium pollution and salinity. Russ J Plant Physi 68:266–273. https://doi.org/10.1134/S102144372102014X
Nisha N, Rao PB (2017) Atomic absorption spectrophotometer (AAS) analysis for evaluation of variation in mineral content in different varieties of Trigonella foenumgraecum L. Legume Res 41:132–134. https://doi.org/10.18805/lr.v0i0.8396
Ortuño MF, Lorente B, Hernández JA, Sánchez-Blanco MJ (2018) Mycorrhizal inoculation on compost substrate affects nutritional balance, water uptake and photosynthetic efficiency in Cistus albidus plants submitted to water stress. Braz J Bot 41:299–310. https://doi.org/10.1007/s40415-018-0457-9
Phillips J, Hayman D (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161. https://doi.org/10.1016/S0007-1536(70)80110-3
Püschel D, Bitterlich M, Rydlová J, Jansa J (2021) Drought accentuates the role of mycorrhiza in phosphorus uptake. Soil Biol Biochem 157:108243. https://doi.org/10.1016/j.soilbio.2021.108243
Qi JG, Sun SM, Yang L, Li MJ, Ma FW, Zou YJ (2019) Potassium uptake and transport in apple roots under drought stress. Hortic Plant J 5:10–16. CNKI:SUN:YYZW.0.2019–01–002
Ramos-Artuso F, Galatro A, Lima A, Batthyány C, Simontacchi M (2019) Early events following phosphorus restriction involve changes in proteome and affects nitric oxide metabolism in soybean leaves. Environ Exp Bot 161:203–217. https://doi.org/10.1016/j.envexpbot.2019.01.002
Rodrigues VA, Crusciol CAC, Bossolani JW, Moretti LG, Portugal JR, Mundt TT, De Oliveira SL, Garcia A, Calonego JC, Lollato RP (2021) Magnesium foliar supplementation increases grain yield of soybean and maize by improving photosynthetic carbon metabolism and antioxidant metabolism. Plants, 10:797. https://doi.org/10.3390/plants10040797
Ruytinx J, Kafle A, Usman M, Coninx L, Zimmermann S, Garcia K (2019) Micronutrient transport in mycorrhizal symbiosis; zinc steals the show. Fungal Biol Rev 34:1–9. https://doi.org/10.1016/j.fbr.2019.09.001
Sterckeman T, Moyne C, Le TD (2021) A modelling study to evaluate the mechanisms of root iron uptake by Noccaea caerulescens. Plant Soil. https://doi.org/10.1007/s11104-021-04873-5
Sujkowska-Rybkowska M, Znojek E (2018) Localization of calreticulin and calcium ions in mycorrhizal roots of Medicago truncatula in response to aluminum stress. J Plant Physiol 229:22–31. https://doi.org/10.1016/j.jplph.2018.05.014
Sun H, Zheng YL, Xie YX, Lin YL, Yang FY (2019) Varied responses of growth and mineral elements concentrations in Pennisetum ericanum and Festuca arundinacea under Cd/Cu addition. Pol J Environ Stud 28:1385–1396. https://doi.org/10.15244/pjoes/81562
Sun YM, Wang M, Mur LAJ, Shen QR, Guo SW (2020) The cros-kingdom roles of mineral nutrient transporters in plant-microbe relations. Physiol Plantarum 171:771–784. https://doi.org/10.1111/ppl.13318
Symanczik S, Lehmann MF, Wiemken A, Boller T, Courty PE (2018) Effects of two contrasted arbuscular mycorrhizal fungal isolates on nutrient uptake by Sorghum bicolor under drought. Mycorrhiza 28:779–785. https://doi.org/10.1007/s00572-018-0853-9
Tripathi AM, Klem K, Fischer M, Orság M, Trnka M, Marek MV (2018) Water availability influences accumulation and allocation of nutrients and metals in short-rotation poplar plantation. Biomass Bioenerg 116:151–160. https://doi.org/10.1016/j.biombioe.2018.06.010
Upadhayay VK, Singh J, Khan A, Lohani S, Singh AV (2019) Mycorrhizal mediated micronutrients transportation in food based plants: a biofortification strategy. In: Varma A, Choudhary D (eds) Mycorrhizosphere and pedogenesis. Springer, Singapore, pp 1–24. https://doi.org/10.1007/978-981-13-6480-8-1
Walker C, Harper CJ, Brundrett MC, Krings M (2018) Looking for arbuscular mycorrhizal fungi in the fossil record: an illustrated guide. In: Krings M, Harper CJ, Cuneo NR, Rothwell GW (eds) Transformative paleobotany: papers to commemorate the life and legacy of Thomas N. Taylor. Academic Press, London, pp 481–517. https://doi.org/10.1016/B978-0-12-813012-4.00020-6
Wang WX, Zhang F, Chen ZL, Liu J, Guo C, He JD, Zou YN, Wu QS (2017) Responses of phytohormones and gas exchange to mycorrhizal colonization in trifoliate orange subjected to drought stress. Arch Agron Soil Sci 63:14–23. https://doi.org/10.1080/03650340.2016.1175556
Watts-Williams SJ, Nguyen TD, Kabiri S, Losic D, McLaughlin MJ (2019) Potential of zinc-loaded graphene oxide and arbuscular mycorrhizal fungi to improve the growth and zinc nutrition of Hordeum vulgare and Medicago truncatula. Appl Soil Ecol 150:103464. https://doi.org/10.1016/j.apsoil.2019.103464
Wu F, Zhang HQ, Fang FR, Liu HG, Tang M (2017a) Arbuscular mycorrhizal fungi alter nitrogen allocation in the leaves of Populus × canadensis ‘Neva.’ Plant Soil 421:477–491. https://doi.org/10.1007/s11104-017-3461-0
Wu F, Zhang HQ, Fang FR, Wu N, Zhang YX, Tang M (2017b) Effects of nitrogen and exogenous Rhizophagus irregularis on the nutrient status, photosynthesis and leaf anatomy of Populus × canadensis ‘Neva.’ J Plant Growth Regul 36:824–835. https://doi.org/10.1007/s00344-017-9686-6
Wu F, Zhang HQ, Fang FR, Tang M (2018) Nutrient allocation and photochemical responses of Populus × canadensis ‘Neva’ to nitrogen fertilization and exogenous Rhizophagus irregularis inoculation. Acta Physiol Plant 40:152. https://doi.org/10.1007/s11738-018-2728-2
Wu F, Fang FR, Wu N, Li L, Tang M (2020) Nitrate transporter gene expression and kinetics of nitrate uptake by Populus × canadensis ‘Neva’ in relation to arbuscular mycorrhizal fungi and nitrogen availability. Front Microbiol 11:176. https://doi.org/10.3389/fmicb.2020.00176
Yi L, Li B, Korpelainen H, Yu F, Wu L, Tong L, Liu M (2020) Mechanisms of drought response in Populus. South Forests 82:359–366. https://doi.org/10.2989/20702620.2020.1733755
Zai XM, Fan JJ, Hao ZP, Li XM, Zhang WX (2021) Effect of co-inoculation with arbuscular mycorrhizal fungi and phosphate solubilizing fungi on nutrient uptake and photosynthesis of beach palm under salt stress environment. Sci Rep 11:5761. https://doi.org/10.1038/s41598-021-84284-9
Zhang HZ, Zhao SH, Li DD, Xu XM, Li CH (2017) Genome-wide analysis of the ZRT, IRT-Like protein (ZIP) family and their responses to metal stress in Populus trichocarpa. Plant Mol Biol Rep 35:534–549. https://doi.org/10.1007/s11105-017-1042-2
Zhang BB, Zhang H, Wang H, Wang P, Wu YX, Wang MM (2018a) Effect of phosphorus additions and arbuscular mycorrhizal fungal inoculation on the growth, physiology, and phosphorus uptake of wheat under two water regimes. Commun Soil Sci Plan 49:862–874. https://doi.org/10.1080/00103624.2018.1435798
Zhang JS, Zhang H, Srivastava AK, Pan YJ, Bai JJ, Fang JJ, Shi HZ, Zhu JK (2018b) Knockdown of rice microRNA166 confers drought resistance by causing leaf rolling and altering stem xylem development. Plant Physiol 176:2082–2094. https://doi.org/10.1104/pp.17.01432
Funding
This research was funded by the National Natural Science Foundation of China (32071639 and 31700530), the National Key Research and Development Program of China (2018YFD0600203-3), the China Postdoctoral Science Foundation (2016M592849), and the Postdoctoral Foundation of Shaanxi Province (2016BSHYDZZ19). The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, L., Zhang, H., Tang, M. et al. Nutrient Uptake and Distribution in Mycorrhizal Cuttings of Populus × canadensis ‘Neva’ Under Drought Stress. J Soil Sci Plant Nutr 21, 2310–2324 (2021). https://doi.org/10.1007/s42729-021-00523-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-021-00523-y