Abstract
Apple rootstocks establish symbiosis with arbuscular mycorrhizal fungi (AMF), however the influence of fungal isolates on nutritional and physiological parameters are limited. The aim of this work was to evaluate the growth, nutrient uptake and use efficiency, and gas exchange of apple micropropagated rootstock ‘Marubakaido’ inoculated with four isolates of AMF with differing levels of phosphorus (P). We grew plantlets in a non-sterilized soil inoculated with AMF isolates Acaulospora colombiana SCT115A, A. morrowiae SCT400B, Claroideoglomus etunicatum SCT101A, and Gigaspora albida SCT200A, plus a non-inoculated treatment at three levels of P (0%, 50% e 100%). After 90 days of AMF inoculation, internal CO2 concentration (ci), transpiration rate (E), stomatal conductance (gs) and photosynthetic rate (A) were evaluated and after 315 days, total dry biomass, macro and micronutrient contents and mycorrhizal colonization were determined. AMF inoculation, regardless of P levels, decreased ci, E and gs, and increased the intrinsic water use efficiency (A/gs) and water use efficiency (A/E). The total biomass results differed among the AMF isolates, where G. albida stood out increasing apple rootstock growth in all levels of P. Gigaspora albida also increased the relative accumulation of N, K, Ca, Mg, Cu and B and had lower mycorrhizal colonization rates. Nutrient use efficiency was higher in plants inoculated with G. albida compared to control plants. In conclusion, although the AMF isolates demonstrated positive results depending on the soil P concentration, we found evidence that G. albida has the potential to be used as inoculant on apple rootstock ‘Marubakaido’ production in nurseries to enhance tree performance.
Key message
Apple micropropagated rootostocks inoculated with Gigaspora albida increase growth, nutrients content and use efficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Inoculation of apple plantlets with different isolates of arbuscular mycorrhizal fungi (AMF) increases plant growth and nutrition (Cavallazzi et al. 2007; Gastol et al. 2016), however the influence of these fungi on physiological parameters and nutrient use efficiency of micropropagated apple plantlets is not well understood (McDonald et al. 2013; Huang et al. 2020; Choudhary et al. 2019). AMF establish the arbuscular mycorrhizal (AM) symbiosis with ca. 72% of land plants (Brundrett and Tedersoo 2018) including wild plants and most of crop and fruit trees. Several ecosystem services have been attributed to the AM as improvement of soil structure, plant growth promotion associated with reduced fertilizer input, increased plant tolerance/resistance to abiotic and biotic stress, and increase of plant quality attributes that impact human health (Gianinazzi et al. 2010). These attributes evidence that AMF and the AM symbiosis are important components in agricultural systems that aim sustainability and decreasing of fertilizer input (Hart and Trevors 2005). An example of this system is the production of micropropagated fruit trees, in which the inoculation of AMF in the acclimatization promotes beneficial effects to the growth of plants by increasing photosynthetic rate, root growth, and water and nutrient inputs. This reduces the adaptation period to the ex vitro environment, which is reflected in time and cost savings (Lovato et al. 1996; Kapoor et al. 2008).
Inoculation of AMF influences physiological parameters as respiration and photosynthesis and regulate the water use efficiency in the host plant (Jayne and Quigley 2014; Augé et al. 2016). A detailed meta-analysis of 400 studies detected increments of up to 20% for stomatal conductance in mycorrhizal plants compared to non-mycorrhizal ones (Augé et al. 2016). Transpiration rate is also higher in AM plants under water (Zhu et al. 2012) and saline (Wu et al. 2010) stresses. Considering that 10–23% of photosynthetic C is allocated to support mycorrhizal structures in the roots, mycorrhizal plants have higher photosynthetic rates than non-mycorrhizal (Valentine et al. 2013). In M. hupehensis, inoculation with AMF increased net photosynthetic and transpiration rate but not stomatal conductance in drought-stressed plants (Huang et al. 2020) and AMF did not influence chlorophyll content of three-year old apple M. pumila under field conditions (Berdeni et al. 2018). These studies however did not address the influence of AMF inoculation on physiological parameters on early stages of growth for micropropagated apple plants, which is important to overcome some problems during the switch from in vitro to ex vitro conditions.
Previous papers evidenced that mycorrhizal inoculation confers several benefits to the apple plants like improving plant dry biomass and nutrition (Cavallazzi et al. 2007; Gastol et al. 2016), protecting against root lesion nematode Pratylenchus penetrans (Forge et al. 2001; Ceustermans et al. 2018) and Botryosphaeria canker disease (Krishna et al. 2010), inducing faster root growth (Resendes et al. 2008), and increasing fruit production in replant-disease soils (Utkhede and Smith 2000). However, these benefits depend on several factors like soil conditions, water availability, temperature, soil P levels and the appropriate combination between the AMF type and the plant host (Rutto and Mizutani 2006; Camprubí et al. 2008; Ortas 2012). The importance of local adaptation to establish worthwhile relationships between AMF isolates and plants was highlighted by a meta-analysis study that included 1170 studies whose results recommend to consider the origin of the plant, soil, and fungal components for mycorrhizal relationships (Rúa et al. 2016). Thus, the use of native AMF isolates may be more efficient than using fungal inoculum from geographically distant location.
Screening of native AMF isolates should be performed preferentially over a range of soil phosphorus (P) levels and considering the recommended P dose for the targeted host. Phosphorus availability in the soil is very low when compared to the plant demand, especially in tropical acidic soils (Osorio et al. 2017) making phosphorus one of the main mineral nutrients provided to crop plants (Gianinazzi et al. 2010). The fungal mycelium network differentiated by AMF when colonizing host roots increases the soil volume explored for P uptake and translocate it back to the host (Smith and Read 2008). This function is highly important in agricultural systems when considering that 80% of the recommended P supply could be reduced by AMF inoculation (Jakobsen 1995). Indeed, mycorrhizal biofertilizer effect was equivalent to adding 254 kg ha−1 P2O5 in coffee plants (Siqueira et al. 1998); these values could be significant and supplies the apple trees demands considering the amounts of up to 130 kg ha−1 P2O5 that is applied to maintain orchards and guarantee fruit production (CQFS-RS/SC 2004). Consequently, crop systems that maximize the benefits of mycorrhizal association and AMF inoculation can increase the use efficiency of P and other nutrients (Koide 1991; Verzeaux et al. 2017; Schütz et al. 2018), decreasing the need for fertilizer input and increasing crop production sustainability. The relative efficiency of inoculation of AMF can be estimated by measuring the output (shoot and / or roots plant biomass or crop yield) as the numerator, and the input (nutrient applied or absorbed by plants) as the denominator (McDonald et al. 2013; Choudhary et al. 2019). Plants with higher nutrient use efficiency have higher growth or yield with relatively lower levels of applied or absorbed nutrients, reducing the cost–benefit of agricultural systems (Siddiqi and Glass 1981; McDonald et al. 2013; Choudhary et al. 2019).
Apple is one of the most worldwide cultivated fruit crops that contributes significantly to global agricultural production (FAO 2018). The state of Santa Catarina, in the Southern region of Brazil, is the largest apple producer in the country with an estimated 489,000 tons of apples in 2020 (Goulart Jr 2020). As part of a large project to propagate apple rootstocks adapted to soil conditions in Santa Catarina, previous studies developed protocols for acclimatization and inoculation with AMF (Locatelli and Lovato 2002), investigated apple root architecture in the presence or absence of AMF (Locatelli et al. 2002), and screened AMF isolates over distinct pH level for inoculation during post vitro phase (Cavallazzi et al. 2007). Herein, we investigate the effect of AMF inoculation on nutritional and physiological measurements of apple plantlets in different soil P levels. Our goal was to evaluate growth, nutrient content, nutrient use efficiency (NUE), and gas exchange of micropropagated apple ‘Marubakaido’, the main rootstock used in Santa Catarina state, inoculated with four AMF isolates under different levels of soil phosphorus. We hypothesize that AMF inoculation increase leaf physiological traits of micropropagated apple plantlets on early stages of post vitro growth. The hypothesis is that physiological and nutritional responses of micropropagated plantlets of the Marubakaido apple rootstock are variable between AMF rates anda MF species and are affected by phosphate fertilization levels.
Material and methods
Biological material
In vitro shoots of Marubakaido apple rootstock (Malus prunifolia (Willd.) Borkh.) were micropropagated on culture media consisting of MS salts and vitamins (Murashige and Skoog 1962) with NH4NO3 and KNO3 reduced to ¾ of the original concentration and supplemented with 0.8 mg L−1 6-benzylaminopurine (BAP), 30 g L−1 sucrose and 7 g L−1 agar–agar (Sigma Aldrich); subculture was done every 7–8 weeks. After this proliferation phase, plantlets were acclimatized ex vitro for 65 days; 2 cm minicuttings basal ends were dipped in 1000 mg L−1 of indol butiric acid (IBA) solution for 10 s to induce rooting. Plantlets were then transferred to Styrofoam trays containing a mix of sterilized sand and commercial substrate Tecnomax® (3:7, v/v) until the onset of the experiment.
AMF isolates used in the experiments were Acaulospora colombiana (Spain & N.C. Schenck) Kaonongbua, J.B. Morton & Bever (isolate SCT115A), A. morrowiae Spain & N.C Schenck (isolate SCT400B), Claroideoglomus etunicatum (W.N. Becker & Gerd.) C. Walker & A. Schüssler (isolate SCT101A), and Gigaspora albida N.C. Schenck & G.S. Sm. (isolate SCT200A). Mycorrhizal inoculum of these isolates were obtained from the International Culture Collection of Glomeromycota (CICG at FURB, Blumenau, SC, Brazil—http://www.furb.br/cicg) and consisted of spores, hyphal fragments and colonized root pieces in a soil:sand mix medium. Whole soil inoculum of each AMF isolate was produced by diluting (10%) inoculum from a stock culture with a sterile substrate consisting of a 1:1 mixture (vol/vol) of a silt loam soil and quartzite sand and conditioned in 1.5 kg plastic pots. Seeds of Urochloa brizantha Moench were added to each pot and plants grown in a greenhouse. After four months, watering was ceased, plant tops removed and discarded, and the substrate with roots balls chopped, homogenized, and stored at 4° C until the onset of the experiment.
Experimental design
A factorial, randomized complete block design was adopted with six replications consisting of 15 treatments. Treatments consisted of five inoculation treatments (A. colombiana, A. morrowiae, C.etunicatum, G. albida, and non-inoculated control) and three levels of soil P (0%, 50% and 100%). Levels of P were established by adding Na2HPO4 and consisted of the rate recommended for apple culture (130 kg ha−1 P2O5), half the rate recommended (65 kg ha−1 P2O5), and no P added (0 kg ha−1 P2O5). Levels of P were added according to the Soil Chemistry and Fertility Commission of Rio Grande do Sul and Santa Catarina (CQFS-RS/SC 2004). Block treatment was established to account for variability on plantlets height at the beginning of the ex vitro phase.
Plant growth experiment was established in 3.6 L plastic pots containing a natural, non-sterile Oxisol with the following properties: pH of 5.1; 1.37 mg dm−3 P; 60 mg dm−3 K; 0.4% of organic matter; 1.4 cmolc dm−3 Al; 0.53 cmolc dm−3 Ca; 0.24 cmolc dm−3 Mg; 11.8 cmolc dm−3 H + Al; 2.8 cmolc dm−3 CEC. The soil pH and levels of N, K, and B were adjusted as recommended for apple culture (CQFS-RS/SC 2004). Mycorrhizal inoculation was done for each experimental unit and consisted of adding 5 g of inoculum of each fungal isolate 1 cm below the surface of substrate and then transplanting a plantlet per pot. Five grams of sterile AMF inoculum (sterilized twice, for 30 min at 121 °C) were added to non-mycorrhizal treatment plus an inoculum filtered suspension (10 g L−1 Whatmann # 1) to standardize the non-mycorrhizal microbiota.
Plants were grown in a partially climate-controlled greenhouse, with day temperature of 26 ± 2 °C. Pots were watered daily in order maintain the field capacity. Ninety days after AMF inoculation, the following traits were evaluated: internal CO2 concentration (ci), transpiration rate (E), stomatal conductance (gs) photosynthetic rate (A), intrinsic water use efficiency (A/gs) and water use efficiency (A/E). Measurements were obtained using a LCpro-SD Portable Photosynthesis System with artificial light of 1,680 μmols s−1 of photosynthetic photon flux, temperature of 25 °C and constant levels of CO2.
Experiments were carried out for 315 days, going through one period of dormancy, when shoots were cut at the soil line, stored in paper bags, dried for 72 h in a forced-air oven at 60 °C to a constant mass and weighed to obtain shoot dry biomass. Shoots and roots were ground before being analyzed for macro and micronutrients (Schveitzer and Suzuki 2013). The N concentration was determined by titration with 0,05 N H2SO4 after Kjeldahl digestion. The nitro-perchloric digestion method was used to solubilization of P, Mg, Ca, K, Zn e Cu in plant tissue. The P concentration was determined by vanadium molybdate yellow colorimetric method at 420 nm. The Cu, Zn, Ca, K e Mg concentration was determined by atomic absorption spectrophotometry (Perkin Elmer model PE-2400 elemental analyzer). After dry digestion, B concentration was determined by the azomethine H method. NUE for each treatment was calculated by squared plant biomass:nutrient concentration according to Siddiqi and Glass (1981).
Fresh root (ca. 1 g) were sampled after 90 and 315 days of AMF inoculation, washed and stored in 50% alcohol until staining. To tissue clearing, root fragments (1–2 cm) were washed under tap water, soaked in 10% KOH for 16 h at 28° C and then for 1 h at 121° C. To increased the bleaching, the roots were washed under tap water and placed in 10% H2O2 for 30 min (Koske and Gemma 1989). After this step, cleared roots were soaked in 5% acetic acid for 30–60 min and stained with 5% black ink (Shaeffer) diluted in 5% acetic acid for 10 min at 90° C. Roots were destaining in tap water (Vierheilig et al. 1998). Roots were stored in distilled water at 4 °C. Stained root fragments were placed in water on a microscopic slide and covered with a coverslip, The mycorrhizal colonization intensity (%) were analyzed in 20 root fragments per replicate, under an optical microscopic, and estimated the area of root cortex colonized by AMF relative to the total area of the root fragment (Trouvelot et al. 1986).
The effect of AMF inoculation was analyzed by calculating the mycorrhizal growth response (MGR) considering shoot dry biomass and nutrient content results, according to formulas described by Veiga et al. (2011) and Köhl et al. (2016):
Results of N and P contents (mg plant−1) were used to estimate the N:P ratio for each combination of AMF and P treatments and correlated with total dry biomass data.
Statistical analyses
Data were checked for normality and homogeneity of variances and Box-Cox transformation was used when necessary. A fully factorial analyses of variance (ANOVA) was used to detect the effect of each mycorrhizal inoculation, P level and interaction of AMF × P on gas exchange parameters, mycorrhizal colonization and nutrient use efficiency. When the F ratio was significant, Tukey was used as a post hoc test (p ≤ 0.05). Statistical analyses were performed with R software (R CORE TEAM 2018). MGR results were evaluated using Student’s t test to detect differences between the inoculated and non-inoculated treatments. Positive MGR indicates that the plant parameter analyzed have benefited from AMF inoculation and negative MGR indicates that AMF inoculation negatively affect the plant parameter analyzed (Köhl et al. 2016).
Results
Leaf physiological traits were significantly affected only by AMF inoculation treatment (Table S1 in online resource). Sub stomatic CO2 (Ci) ranged from 266 to 289 vpm in plants inoculated with A. colombiana, A. morrowiae and Gi. albida and these values were significantly lower than in control plant (323 vpm). Ci of plants associated with C. etunicatum were not different from the control plants (Table 1). Transpiration rate (E) increased 10–20% in control plants compared to mycorrhizal treatments. Stomatal conductance was 0.26 mol m−2 s−1 in control plants and differed significantly (p < 0.0001) from all mycorrhizal treatments. Overall, photosynthetic rates were not affected by mycorrhizal treatments, but water use efficiency was 4.09 and 4.02 for A. colombiana and A. morrowiae, respectively, and these values differed from control treatment. Mycorrhizal treatment increased water use efficiency by 50–83% compared to control plants (Table 1).
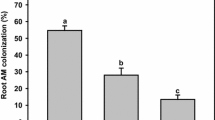
Overall, increasing P levels in the soils did not influence significantly mycorrhizal colonization (Table 2). Plants inoculated with C. etunicatum, A. morrowiae and A. colombiana had the same levels of root colonization intensity (34.7 to 52.5) but these values were not significantly different from control plants (Table 2). Root colonization intensity in plants inoculated with G. albida were 45.5% significantly lower than control plants (p < 0.05).
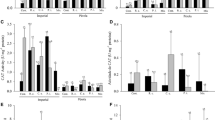
Mycorrhizal growth responses (MGR) related to total dry biomass and nutrient contents were positively affected by mycorrhizal inoculation, mainly at a 50% P level (Fig. 1). Considering total dry biomass (DB), MGR was positive and significantly higher in plants inoculated with G. albida in all P levels while all AMF isolates increased DB by 20–30% at intermediate P level. At 50% P treatment, inoculation with A.colombiana, A. morrowiae, and G. albida increased significantly N content in shoots and roots of apple plants relative to control plants. At 0% and 100% P levels, G. albida increased by 11 to 48% apple contents of K, Ca, Mg, Cu, and B, while others AMF isolates did not influence the contents of these nutrients or significantly decreased them (e.g. Ca and Zn for A. colombiana at 100% P) (Fig. 1). At 50% P level, A. colombiana increased contents of P, Zn, Cu, and B relative to control treatment, while C. etunicatum increased only Mg and Cu (p < 0.05).
Mycorrhizal growth responses (MGR) of dry biomass (DB) and nutrients accumulation of apple rootstock ‘Marubakaido’ inoculated with Acaulospora colombiana, A. morrowiae, Claroideoglomus etunicatum or Gigaspora albida under three levels of P (0, 50 and 100%). Bars represent means and error bars the standard deviation. Asterisks represent that the effect size is significantly different from zero (p < 0.05*, p < 0.01**, p < 0.001***) according to a one-sample t-test
Nutrient use efficiency (NUE) for N, P, K, Ca, Cu, and B were influenced by mycorrhizal, phosphorus levels, and the interaction among the treatments (see the online resource Table S2 for details). Plants inoculated with G. albida at 50% P had significantly higher NUE for all nutrients except for Cu when compared to control plants. At 0% P level, NUE for N and B in plants inoculated with G. albida were significantly higher than in control plants (Table 3). Overall, NUE was significantly higher with 100% P level, except for NUE of P that was 43–53% higher at 0% P level compared to 50% and 100% P level (Table 3).
Most of the plants (76%) have grown in N limitation condition and plants grown under P limitation were associated with G. albida, C. etunicatum at 0% P level (Fig. 2), considering the correlation between N:P ratio and total dry biomass associated with the N and P limitation zones for plant growth established by Koerselman and Meuleman (1996).
Correlation between the N:P ratio and total dry biomass of apple rootstock ‘Marubakaido’ inoculated with AMF Acaulospora colombiana (Ac), A. morrowiae (Am), Claroideoglomus etunicatum (Ce), Gigaspora albida (Ga) or non-inoculated (nom-myc), after 315 days of AMF inoculation, under three levels of P (0, 50 and 100%). Nitrogen and phosphorus limitation zones to plant growth (KOERSELMAN; MEULEMAN 1996)
Discussion
We found that AMF inoculation influenced positively micropropagated apple rootstock growth, nutrient content and use efficiency, and leaf physiological traits in ten-month old plants growing on non-sterilized soil. The effects of AMF inoculation were clearly observed in plantlets grown at 50% of the recommended P level for apple culture where most of AMF isolates increased growth and nutrient contents. This results have practical implication for micropropagated apple production because improvement of growth and nutrient content can be achieved with half of the phosphorus being added to the soil, and plants reached earlier the proper plant size to be transferred to the field which reduces rootstock production costs.
Results obtained provided some support for our working hypothesis that AMF inoculation affects leaf physiological parameters in the early stages of apple growth. Mycorrhizal treatments decreased significantly sub-stomatic CO2 (ci), transpiration rate (E) and stomatal conductance (gs) compared to control plants colonized by indigenous AMF, once control plants were cultivated in a non-sterile soil (Table S3 in online resource). Conversely, these parameters were found to be positively affected by AMF inoculation in meta-analysis studies (Augé et al. 2014, 2016). The lower gs results in apple trees were also identified in plants under water stress (Sun et al. 2013) where the cultivar and the water status played an important role in this parameter (Sun et al. 2013). The water use efficiency (A/E) and intrinsic water use efficiency (A/gs) were increased by AMF inoculation in our experiment (Table 1). Plants with high A/E have better water conservation strategies allowing greater capacity to tolerate water stress, one of the benefits resulting from functional mycorrhizae (Miransari 2010) and important when plantlets are moved from the in vitro to ex vitro condition. We noticed that there are few studies relating AMF colonization with water use efficiency in other fruit trees, which have been focused on the interaction between plants and ectomycorrhizal fungi (Canton et al. 2016).
Investigation on inoculation of AMF isolates in apple and others fruit trees also found positive results using sterilized soil as substrate (Cavallazzi et al. 2007; Camprubí et al. 2008; Ozdemir et al. 2010; Kapoor et al. 2008). MGR is more commonly estimated in herbaceous and pasture crops and it increases in woody species with AMF inoculation (Corrêa et al. 2015). Conversely, our study showed that AMF inoculation in a non-sterilized soil also improved growth, nutrition, and physiological parameters. Screening of AMF using non-sterilized soil simulates the environment found at field conditions and allows to select more competitive and efficient isolates to be applied in the apple production system. Further benefits of AMF inoculation in the field include tree growth and fruit yield as demonstrated by Utkhede and Smith (2000) on apple replant disease soil or rejuvenation of older apple trees (Zhang et al. 2019). AMF isolates used herein pertain to species that are naturally found in apple orchards in Santa Catarina state cultivated under organic and conventional management (Purin et al. 2006). Gigaspora albida and Acaulospora morrowiae used in this work have been demonstrated to be tolerant to acid soils with high levels of Al (Aguilera et al. 2015; Seguel et al. 2013, 2015). Despite the AMF isolates used herein are different from those used by Aguilera and coworkers and Seguel and coworkers, this tolerance to acidity and Al might be a characteristic of isolates of these two species that could explain partially our results, since both species were among the most efficient in improving apple parameters measured.
Gigaspora albida was the isolate that influenced apple growth and nutrient contents in all P levels despite the root colonization intensity lower than control plants (Table 2). G. albida belongs to the family Gigasporaceae and members of this family are characterized for producing more mycelium in soil compared to roots (Hart and Reader 2002) and having a more localized colonization within the root cortex (Antunes et al. 2011). Investing primarily on external mycelium, members of Gigasporaceae are hypothesized to be more “mutualistic” (Hart and Reader 2002) and results of our study provide some support for this hypothesis. Similar results were found by Cavallazzi et al. (2007) who showed that Cetraspora pellucida and Dentiscutata heterogama (Gigasporaceae) and an isolate of Claroideoglomus etunicatum (Claroideoglomeraceae) improved apple growth and nutrition under sterilized soil in a soil pH gradient. Maherali and Klironomos (2007) proposed that higher investment on internal mycelium, as observed in Glomeraceae, provides greater resistance to pathogens while higher investment on external mycelium, as observed in Gigasporaceae, improves nutrient uptake and consequently the plant host growth.
Plant responses to AMF inoculation are influenced by several factors, especially by soil phosphorus availability and AMF species or isolate (Novais et al. 2014; Mensah et al. 2015; Holland et al. 2018). In this study, most isolates influenced plant growth and nutrient content when apple plants were grown at half of the soil P dose recommended for the culture. For instance, in this P level apple growth increased by 30% when inoculated with G. albida and A. morrowiae. Similar results were found by Hoeksema et al. (2010) in a meta-analysis, where plants in the functional group of apple trees showed a 45% increase on growth when inoculated with AMF, indicating that mycorrhizal colonization can decrease the needs of P fertilization without influencing plant growth.
Significant effects of nutrients uptake have also been observed in apple rootstock when inoculated with all isolated at 50% P-dose. Mycorrhizal plants are able to absorb soil nutrients more efficiently in soils with low nutrient availability (Pérez-Tienda et al. 2012) due to the higher affinity of external mycelium to some nutrients. For example, nitrogen has five times more affinity by mycorrhizal mycelium than plant roots (Bücking and Kafle 2015) that can be absorbed by extraradical fungal and incorporated into amino acids (Wang et al. 2017). In contrast, Reynolds et al. (2005) found that the increase in nutrient uptake of mycorrhizal plants must be an indirect effect of plant growth improved by phosphate acquisition. Although the aim here was not to distinguish whether or not AMF influence directly or indirectly nutrient contents on apple plants, the results evidenced that apple nutrient contents were increased by AMF inoculation when plants were grown at half of the soil P dose recommended. Increasing shoot contents of several plant nutrients due to AMF inoculation have been observed for apple (An et al. 1993), grapevine (Trouvelot et al. 2015), citrus (Wu et al. 2011), and olives (Porras-Soriano et al. 2009). Despite the fact that increased K, Ca, and Mg contents due to mycorrhiza may be an indirect effect of improved growth (Wang et al. 2017), higher nutrient contents in apple mycorrhizal plants at the onset of plantlet production might be important for the survival and establishment of apple rootstock when transplanted to the field.
Data from this study demonstrated that inoculation with AMF improved considerably nutrient use efficiency (NUE) of micropropagated apple. Previous results indicate that colonization of Malphigia emarginata (acerola cherry) with G. albida increased NUE of P (Balota et al. 2011) while colonization of Funneliformis mosseae in olive trees decreased NUE of P, N, and K (Porras-Soriano et al. 2009). Therefore, NUE of woody plant species is also affected by the AMF isolate used in the experimental condition. In our study, increase of NUE in mycorrhizal plants for N, P and Ca were observed with Acaulospora morrowiae and Gigaspora albida in plants growing at 50% P level. Indeed, NUE of P for plants colonized by G. albida at 50% P level was three times higher than that of control plants, but no improvement of nutrient contents was observed. Transfer of P and other nutrients mediated by AMF for plant metabolism is regulated by C flow from the host to fungus metabolism. P accumulation on AMF hyphae can be nine times higher compared to roots under C-limiting conditions (Hammer et al. 2011). Pulses of P into the soil increased three times P shoot concentration in non-mycorrhizal plants compared to mycorrhizal plants, indicating that AMF increase nutrient uptake under limiting P soil condition and modulate within narrow boundaries the supply of P to plant metabolism (Nazeri et al. 2014). Allocation in external hypha by G. albida and regulation of P supply to the host could explain that this isolate had no influence on P content but increased NUE of P. Increasing of NUE by AMF inoculation reduces the need for fertilization which impact on production costs and environmental contamination.
Depicting the relationship of plant biomass and N:P ratio showed that most of our experimental units were standing below the N:P ratio of 14 (Fig. 2) which is considered as a limiting condition in N for plant metabolism (Koerselman and Meuleman 1996). It is not clear how N availability affects the contribution of mycorrhiza to nitrogen nutrition, especially considering that plant growth responses and nitrogen nutrition responses may not be related (Corrêa et al. 2015). The results found in this study indicate that for apple trees inoculated with G. albida there is an interaction between increased N content and the plant biomass, especially in soils with P limitation. The P accumulation in plant tissues is one of the main effects provided by mycorrhizal fungi (Wang et al. 2017) but it has been observed in our work only when plants were inoculated with A. colombiana at 50% P level.
In conclusion, this work demonstrated that inoculation with AMF isolates increases apple plant growth, nutrient accumulation and use efficiency, and influence leaf physiological traits associated with photosynthesis, in non-sterilized soil with half of the recommended P dose applied. Among the AMF isolates used herein, we detected that G. albida SCT200A was the one with the best performance especially when plant growth and contents of different nutrients are considered. This support early studies (Cavallazzi et al. 2007) with micropropagated apple plants and the condition of member of Gigasporaceae being better ‘mutualistic’ (Hart and Reader 2002). Inoculation with selected AMF isolates over distinct soil pH range (Cavallazzi et al. 2007) and different P doses (this work) provide further evidence that the AM symbiosis is crucial to be used in the apple plantlets production system of ‘Marubakaido’ rootstock.
Data availability
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
References
Aguilera P, Cumming J, Oehl F, Cornejo P (2015) Diversity of arbuscular mycorrhizal fungi in acidic soils and their contribution to aluminum phytotoxicity alleviation. In: Panda SK, Baluska F (eds) Aluminum stress adaptation in plants. Springer, New York, pp 203–228
An ZQ, Shen T, Wang HG (1993) Mycorrhizal fungi in relation to growth and mineral nutrition of apple seedlings. Sci Hortic 54:275–285. https://doi.org/10.1016/0304-4238(93)90106-Z
Antunes PM, Koch AM, Morton JB, Rillig MC, Klironomos JN (2011) Evidence for functional divergence in arbuscular mycorrhizal fungi from contrasting climatic origins. New Phytol. https://doi.org/10.1111/j.1469-8137.2010.03480.x
Augé RM, Toler HD, Saxton AM (2014) Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: a meta-analysis. Mycorrhiza 25:13–24. https://doi.org/10.1007/s00572-014-0585-4
Augé RM, Toler HD, Saxton AM (2016) Mycorrhizal stimulation of leaf gas exchange in relation to root colonization, shoot size, leaf phosphorus and nitrogen : a quantitative analysis of the literature using meta-regression. Front Plant Sci 7:1–16. https://doi.org/10.3389/fpls.2016.01084
Balota EL, Machineski O, Stenzel NMC (2011) Resposta da acerola à inoculação de fungos micorrízicos arbusculares em solo com diferentes níveis de fósforo. Bragantia 70:166–175. https://doi.org/10.1590/S0006-87052011000100023
Berdeni D, Cotton TEA, Daniell TJ, Bidartondo MI, Cameron DD, Evans KL (2018) The effects of arbuscular mycorrhizal fungal colonisation on nutrient status, growth, productivity, and canker resistance of apple (Malus pumila). Front Microbiol 9:1461. https://doi.org/10.3389/fmicb.2018.01461
Brundrett MC, Tedersoo L (2018) Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol 220:1108–1115. https://doi.org/10.1111/nph.14976
Bücking H, Kafle A (2015) Role of arbuscular mycorrhizal fungi in the nitrogen uptake of plants: current knowledge and research gaps. Agronomy 5:587–612. https://doi.org/10.3390/agronomy5040587
Camprubí A, Estaún V, Nogales A, García-Figueres F, Pitet M, Calvet C (2008) Response of the grapevine rootstock Richter 110 to inoculation with native and selected arbuscular mycorrhizal fungi and growth performance in a replant vineyard. Mycorrhiza 18:211–216. https://doi.org/10.1007/s00572-008-0168-3
Canton GC, Bertolazi AA, Cogo AJD et al (2016) Biochemical and ecophysiological responses to manganese stress by ectomycorrhizal fungus Pisolithus tinctorius and in association with Eucalyptus grandis. Mycorrhiza 26:475–487. https://doi.org/10.1007/s00572-016-0686-3
Cavallazzi JRP, Klauberg-Filho O, Sturmer SL, Rygiewicz PT, de Mendonça MM (2007) Screening and selecting arbuscular mycorrhizal fungi for inoculating micropropagated apple rootstocks in acid soils. Plant Cell Tissue Organ Cult 90:117–129. https://doi.org/10.1007/s11240-006-9163-6
Ceustermans A, Van Hemelrijck W, Van Campenhout J, Bylemans D (2018) Effect of arbuscular mycorrhizal fungi on pratylenchus penetrans infestation in apple seedlings under greenhouse conditions. Pathogens 7:76. https://doi.org/10.3390/pathogens7040076
Choudhary M, Meena VS, Yadav RP et al (2019) Does PGPR and mycorrhizae enhance nutrient use efficiency and efficacy in relation to crop productivity? In: Maheshwari DK, Dheeman S (eds) Field crops: sustainable management by PGPR. Springer, Cham, pp 45–68
Corrêa A, Cruz C, Ferrol N (2015) Nitrogen and carbon/nitrogen dynamics in arbuscular mycorrhiza: the great unknown. Mycorrhiza 25:499–515. https://doi.org/10.1007/s00572-015-0627-6
CQFS-RS/SC – Chemistry Commission and Soil Fertility in the states of Rio Grande do Sul and Santa Catarina (2004) Manual fertilization and liming to the states of Rio Grande do Sul and Santa Catarina, Brazil. 10. ed. Porto Alegre
de Novais CB, Borges WL, da Jesus E, C, Júnior OJS, Siqueira JO, (2014) Inter- and intraspecific functional variability of tropical arbuscular mycorrhizal fungi isolates colonizing corn plants. Appl Soil Ecol 76:78–86. https://doi.org/10.1016/j.apsoil.2013.12.010
FAO (2018) FAOSTAT Database Collections. Food and Agriculture Organization of the United Nations. http://www.fao.org/faostat/en/#data/QC. Accessed 10 May 2020
Forge T, Muehlchen A, Hackenberg C et al (2001) Effects of preplant inoculation of apple (Malus domestica Borkh.) with arbuscular mycorrhizal fungi on population growth of the root-lesion nematode, Pratylenchus penetrans. Plant Soil 236:185–196. https://doi.org/10.1023/A:1012743028974
Gastol M, Domagała-Swiatkiewicz I, Bijak M (2016) The effect of mycorrhizal inoculation and phosphorus application on the growth and mineral nutrient status of apple seedlings. J Plant Nutr 39:288–299. https://doi.org/10.1080/01904167.2015.1109114
Gianinazzi S, Gollotte A, Binet MN, van Tuinen D, Redecker D, Wipf D (2010) Agroecology: the key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 20:519–530. https://doi.org/10.1007/s00572-010-0333-3
Goulart Jr. R (2020) Maçã. In: Epagri/Cepa. Boletim Agropecuário Novembro/2020. Epagri, Florianópolis, pp 7–9. http://docweb.epagri.sc.gov.br/website_cepa/Boletim_agropecuario/boletim_agropecuario_n90.pdf
Hammer EC, Pallon J, Wallander H, Olsson PA (2011) Tit for tat? A mycorrhizal fungus accumulates phosphorus under low plant carbon availability. FEMS Microbiol Ecol 76:236–244. https://doi.org/10.1111/j.1574-6941.2011.01043.x
Hart MM, Reader RJ (2002) Host plant benefit from association with arbuscular mycorrhizal fungi: variation due to differences in size of mycelium. Biol Fertil Soils 36:357–366. https://doi.org/10.1007/s00374-002-0539-4
Hart MM, Trevors JT (2005) Microbe management: application of mycorrhyzal fungi in sustainable agriculture. Front Ecol Environ 3:533–539. https://doi.org/10.1017/CBO9781107415324.004
Hoeksema JD, Chaudhary VB, Gehring CA et al (2010) A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol Lett 13:394–407. https://doi.org/10.1111/j.1461-0248.2009.01430.x
Holland TC, Hart MM, Bogdanoff C, Bowen P (2018) Response of grapevine rootstocks to soil inocula from different sources. Am J Enol Vitic 1:94–100. https://doi.org/10.5344/ajev.2017.17090
Huang D, Ma M, Wang Q, Zhang M, Jing G, Li C, Ma F (2020) Arbuscular mycorrhizal fungi enhanced drought resistance in apple by regulating genes in the MAPK pathway. Plant Physiol Biochem 149:245–255. https://doi.org/10.1016/j.plaphy.2020.02.020
Jakobsen I (1995) Transport of phosphorus and carbon in VA mycorrhizas. In: Varma A, Hock B (eds) Mycorrhiza. Springer-Verlag, Berlin, pp 297–324
Jayne B, Quigley M (2014) Influence of arbuscular mycorrhiza on growth and reproductive response of plants under water deficit: a meta-analysis. Mycorrhiza 24:109–119. https://doi.org/10.1007/s00572-013-0515-x
Kapoor R, Sharma D, Bhatnagar AK (2008) Arbuscular mycorrhizae in micropropagation systems and their potential applications. Sci Hortic 116:227–239. https://doi.org/10.1016/j.scienta.2008.02.002
Koerselman W, Meuleman AFM (1996) The vegetation N:P ratio: a new tool to detect the nature of nutrient limitation. J Appl Ecol 33:1441–1450. https://doi.org/10.2307/2404783
Köhl L, Lukasiewicz CE, Van Der HMGA (2016) Establishment and effectiveness of inoculated arbuscular mycorrhizal fungi in agricultural soils. Plant Cell Environ 39:136–146. https://doi.org/10.1111/pce.12600
Koide RT (1991) Nutrient supply, nutrient demand and plant response to mycorrhizal infection. New Phytol 117:365–386. https://doi.org/10.1111/j.1469-8137.1991.tb00001.x
Koske RE, Gemma JN (1989) A modified procedure for staining roots to detect VA mycorrhizas. Mycol Res 92:486–488. https://doi.org/10.1016/S0953-7562(89)80195-9
Krishna H, Das B, Attri BL et al (2010) Suppression of Botryosphaeria canker of apple by arbuscular mycorrhizal fungi. Crop Prot 29:1049–1054. https://doi.org/10.1016/j.cropro.2010.05.005
Locatelli LM, Lovato PE (2002) Inoculação micorrízica e aclimatização de dois porta-enxertos de macieira micropropagados. Pesqui Agropecu Bras 37:177–184. https://doi.org/10.1590/s0100-204x2002000200009
Locatelli LM, Vitovski CA, Lovato PE (2002) Sistema radicular de porta-enxertos micropropagados de macieira colonizados com fungos micorrízicos arbusculares. Pesqui Agropecu Bras 37:1239–1245. https://doi.org/10.1590/s0100-204x2002000900006
Lovato PE, Gianinazzi-Pearson V, Trouvelot A, Gianinazzi S (1996) The state of art of mycorrhizas and micropropagation. Adv Hortic Sci 10:46–52. https://doi.org/10.1400/75291
Maherali H, Klironomos JN (2007) Influence of phylogeny on fungal community assembly and ecosystem functioning. Science 316:1746–1748. https://doi.org/10.1126/science.1143082
McDonald G, Bovill W, Huang C, Lightfoot D (2013) Nutrient use efficiency. In: Kole C (ed) Genomics and breeding for climate-resilient crops, vol 2. Springer-Verlag, Berlin, pp 333–393
Mensah JA, Koch AM, Antunes PM, Kiers ET, Hart M, Bücking H (2015) High functional diversity within species of arbuscular mycorrhizal fungi is associated with differences in phosphate and nitrogen uptake and fungal phosphate metabolism. Mycorrhiza 25:533–546. https://doi.org/10.1007/s00572-015-0631-x
Miransari M (2010) Contribution of arbuscular mycorrhizal symbiosis to plant growth under different types of soil stress. Plant Biol 12:563–569. https://doi.org/10.1111/j.1438-8677.2009.00308.x
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1016/S0031-9422(01)00179-0
Nazeri NK, Lambers H, Tibbett M, Ryan MH (2014) Moderating mycorrhizas: arbuscular mycorrhizas modify rhizosphere chemistry and maintain plant phosphorus status within narrow boundaries. Plant Cell Environ 37:911–921. https://doi.org/10.1111/pce.12207
Ortas I (2012) The effect of mycorrhizal fungal inoculation on plant yield, nutrient uptake and inoculation effectiveness under long-term field conditions. For Crop Res 125:35–48. https://doi.org/10.1016/j.fcr.2011.08.005
Osorio NW, Osorno L, Leon JD, Álvarez C (2017) Plant-microbe interactions for phosphate management in tropical soils. In: Naeem M, Ansari AA, Gill SS (eds) Essential plant nutrients: uptake, use efficiency, and management. Springer, New York, pp 491–512
Ozdemir G, Akpinar C, Sabir A et al (2010) Effect of inoculation with mycorrhizal fungi on growth and nutrient uptake of grapevine genotypes (Vitis spp.). Eur J Hortic Sci 75:103–110
Pérez-Tienda J, Valderas A, Camañes G, García-Agustín P, Ferrol N (2012) Kinetics of NH4+ uptake by the arbuscular mycorrhizal fungus Rhizophagus irregularis. Mycorrhiza 22:485–491. https://doi.org/10.1007/s00572-012-0452-0
Porras-Soriano A, Soriano-Martın ML, Porras-Piedra A, Azcon R (2009) Arbuscular mycorrhizal fungi increased growth, nutrient uptake and tolerance to salinity in olive trees under nursery conditions. J Plant Physiol 166:1350–1359. https://doi.org/10.1016/j.jplph.2009.02.010
Purin S, Klauberg O, Stürmer SL (2006) Mycorrhizae activity and diversity in conventional and organic apple orchards from Brazil. Soil Biol Biochem 38:1831–1839. https://doi.org/10.1016/j.soilbio.2005.12.008
R Core Team (2018) R: a language andenvironmentfor statisticalcomputing. Version 3.3.1. Vienna, Austria: R Foundation for Statistical Computing. https://www.R-project.org/
Resendes ML, Bryla DR, Eissenstat DM (2008) Early events in the life of apple roots: variation in root growth rate is linked to mycorrhizal and nonmycorrhizal fungal colonization. Plant Soil 313:175–186. https://doi.org/10.1007/s11104-008-9690-5
Reynolds HL, Hartley AE, Vogelsang KM, Bever JD, Schultz PA (2005) Arbuscular mycorrhizal fungi do not enhance nitrogen acquisition and growth of old-field perennials under low nitrogen supply in glasshouse culture. New Phytol 167:869–880. https://doi.org/10.1111/j.1469-8137.2005.01455.x
Rúa MA, Antoninka A, Antunes PM et al (2016) Home-field advantage ? Evidence of local adaptation among plants, soil, and arbuscular mycorrhizal fungi through meta-analysis. BMC Evol Biol 16:1–15. https://doi.org/10.1186/s12862-016-0698-9
Rutto KL, Mizutani F (2006) Peach seedling growth in replant and non-replant soils after inoculation with arbuscular mycorrhizal fungi. Soil Biol Biochem 38:2536–2542. https://doi.org/10.1016/j.soilbio.2006.03.012
Schütz L, Gattinger A, Meier M, Müller A, Boller T, Mäder P, Mathimaran N (2018) Improving crop yield and nutrient use efficiency via biofertilization - a global meta-analysis. Front Plant Sci 8:2204. https://doi.org/10.3389/fpls.2017.02204
Schweitzer B, Suzuki Z (2013) Métodos de análise foliar utilizados no Laboratório de Ensaio Químico da Epagri/ EECd. Epagri, Florianópolis
Seguel A, Cumming JR, Klugh-Stewart K (2013) The role of arbuscular mycorrhizas in decreasing aluminium phytotoxicity in acidic soils : a review. Mycorrhiza 23:167–183. https://doi.org/10.1007/s00572-013-0479-x
Seguel A, Barea JM, Cornejo P, Borie F (2015) Role of arbuscular mycorrhizal symbiosis in phosphorus-uptake efficiency and aluminium tolerance in barley growing in acid soils. Crop Pasture Sci 66:696–705. https://doi.org/10.1071/CP14305
Siddiqi MY, Glass ADM (1981) Utilization index: a modified approach to the estimation and comparison of nutrient utilization efficiency in plants. J Plant Nutr 4:289–302. https://doi.org/10.1080/01904168109362919
Siqueira JO, Saggin-Júnior OJ, Flores-Aylas WW, Guimarães PTG (1998) Arbuscular mycorrhizal inoculation and superphosphate application influence plant development and yield of coffee in Brazil. Mycorrhiza 7:293–300. https://doi.org/10.1007/s005720050195
Smith S, Read D (2008) Mycorrhizal symbiosis. Elsevier Ltd, Amsterdam
Sun X, Yan HL, Kang XY, Ma FW (2013) Growth, gas exchange, and water-use efficiency response of two young apple cultivars to drought stress in two scion-one rootstock grafting system. Photosynthetica 51:404–410. https://doi.org/10.1007/s11099-013-0040-3
Trouvelot A, Kough J, Gianinazzi-Pearson V (1986) Mesure du taux de mycorhization VA d’un système radiculaire. Recherche de méthodes d’estimation ayant une signification fonctionnelle. In: Gianinazzi-Pearson V, Gianinazzi S (eds) Physiological and genetical aspects of Mycorrhizae. INRA, Paris, pp 217–221
Trouvelot S, Trouvelot S, Bonneau L, Van Tuinen D, Adrian M, Wipf D (2015) Arbuscular mycorrhiza symbiosis in viticulture: a review. Agron Sustain Dev 35:1449–1467. https://doi.org/10.1007/s13593-015-0329-7
Utkhede RS, Smith EM (2000) Impact of chemical, biological and cultural treatments on the growth and yield of apple in replant-disease soil. Australas Plant Pathol 29:129–136. https://doi.org/10.1071/AP00021
Valentine AJ, Mortimer PE, Kleinert A, Kang Y, Benedito VA (2013) Carbon metabolism and costs of arbuscular mycorrhizal associations to host roots. In: Aroca R (ed) Symbiotic endophytes. Springer-Verlag, Berlin, pp 233–252
Veiga RSL, Jansa J, Frossard E, Van Der HMGA (2011) Can arbuscular mycorrhizal fungi reduce the growth of agricultural weeds ? PLoS ONE 6:1–10. https://doi.org/10.1371/journal.pone.0027825
Verzeaux J, Hirel B, Dubois F, Lea PJ, Tétu T (2017) Agricultural practices to improve nitrogen use efficiency through the use of arbuscular mycorrhizae : basic and agronomic aspects. Plant Sci 264:48–56. https://doi.org/10.1016/j.plantsci.2017.08.004
Vierheilig H, Coughlan AP, Wyss URS, Piché Y (1998) Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl Environ Microbiol 64:5004–5007. https://doi.org/10.1128/AEM.64.12.5004-5007.1998
Wang W, Shi J, Xie Q, Jiang Y, Yu N, Wang E (2017) Nutrient exchange and regulation in arbuscular mycorrhizal symbiosis. Mol Plant 10:1147–1158. https://doi.org/10.1016/j.molp.2017.07.012
Wu Q-S, Zou Y-N, He X-H (2010) Contributions of arbuscular mycorrhizal fungi to growth, photosynthesis, root morphology and ionic balance of citrus seedlings under salt stress. Acta Physiol Plant 32:297–304. https://doi.org/10.1007/s11738-009-0407-z
Wu Q, Zou Y, Wang G (2011) Arbuscular mycorrhizal fungi and acclimatization of micropropagated citrus. Commun Soil Sci Plant Anal 42:1825–1832. https://doi.org/10.1080/00103624.2011.587570
Zhang S, Lehmann A, Zheng W, You Z, Rillig MC (2019) Arbuscular mycorrhizal fungi increase grain yields : a meta-analysis. New Phytol 222:543–555. https://doi.org/10.1111/nph.15570
Zhu XC, Song FB, Liu SQ, Liu TD, Zhou X (2012) Arbuscular mycorrhizae improves photosynthesis and water status of Zea mays L. under drought stress. Plant soil Environ 58:186–191
Acknowledgements
We thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq – Brazil) for a Research Assistantship to Sidney Luiz Stürmer (Process 307995/2019-4) and Bruna Greicy Pigozzi (Process 562862/2010-2).
Funding
This study was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq – Brazil) for a Research Assistantship, Process 307995/2019-4 and 562862/2010-2.
Author information
Authors and Affiliations
Contributions
MDC conceived of the presented hypothesis and experimental design, carried out the experiment, interpretation of the results, and wrote the manuscript with support from other authors. TDR carried out the experiment and wrote the manuscript with support from other authors. SP carried out the experiment and wrote the manuscript with support from other authors. BGP carried out gas exchange analysis. SSW performed experimental design and statistical analysis, contributed to the interpretation of the results and wrote the manuscript with support from other authors. SLS provided fungal inoculum for experiment, provided suggestion for the manuscript, and edited the manuscript. All authors discussed the results, provided critical feedback and contributed to the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Maurizio Lambardi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dalla Costa, M., Rech, T.D., Primieri, S. et al. Inoculation with isolates of arbuscular mycorrhizal fungi influences growth, nutrient use efficiency and gas exchange traits in micropropagated apple rootstock ‘Marubakaido’. Plant Cell Tiss Organ Cult 145, 89–99 (2021). https://doi.org/10.1007/s11240-020-01994-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-020-01994-0