Abstract
This work was aimed at characterizing the effects of foliarly applied rutile (TiO2) nanoparticles (NPs) on Ti translocation as well as biomass production and antioxidant system in tomato (Solanum lycopersicum L.). The seeds were germinated and grown on a substrate in individual pots in a growth chamber. The TiO2 NPs were characterized using transmission electron microscopy (TEM), Raman spectroscopy, dynamic light scattering (DLS), and laser doppler velocimetry (LDV). Titanium dioxide NPs had a rod-shaped form and were moderately prone to agglomeration. The TiO2 NPs treatments were applied at 0, 5, 10, 20, 40, 80, and 160 mg L−1 by foliar spraying on 20-day-old S. lycopersicum plants. After 7 days of treatment exposure, tissue Ti concentration was determined by inductively coupled plasma-mass spectrometry (ICP-MS). Nanoparticle treatments increased tissue Ti concentration; Ti was translocated from leaves to stem, triggering a significant decrease in biomass production. With respect to the control, an increase in total reducing capacity (1.7-fold), antioxidant activity (1.5-fold), and superoxide dismutase activity (2-fold) were observed in the treatments with intermediate to high doses. The TiO2 NPs triggered an increase in tissue Ti concentration, increasing the antioxidant system activity and lipid peroxidation at low to intermediate doses, and decreasing biomass production at intermediate to high doses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The nanoparticles (NPs) may have natural or anthropogenic origin, ranging in size between 1 and 100 nm, and can be dispersed as individual particles in liquid, solid, or gaseous media (Schmid 1994; Ghosh and Pal 2007). In plants, metallic NPs are associated with either negative (inducing structural and/or functional damages), neutral, or positive responses (Hong et al. 2005; Da Costa and Sharma 2016; Sarmast and Salehi 2016; Tripathi et al. 2017; Tighe-Neira et al. 2018). Some metallic NPs causing negative responses in plants are Ag NPs in Triticum aestivum L. (Da Costa and Sharma 2016) and Cu NPs in Oryza sativa L. (Karimi and Mohsenzadeh 2017). In both species, a decrease in growth and biomass and an increase in lipid peroxidation was observed. In particular, Cu NPs had a strong negative impact on photosynthetic performance (Da Costa and Sharma 2016). In contrast, positive responses to TiO2 were reported in Ocimum basilicum L. regarding photosynthetic parameters and biomass production depending on TiO2 NPs rate and treatment duration (Tan et al. 2018). Similarly, TiO2 NPs had a positive effect in Brassica napus L. (photosynthetic parameters, antioxidant system, and biomass production) (Li et al. 2015). Hence, there are contradictory reports on the TiO2 NPs impacts on plants (Gogos et al. 2012; Zahra et al. 2017; Manesh et al. 2018). Indeed, the effects of NPs in plants may be influenced by multiple and complex interactions dependent on four factors: (i) the NPs properties (e.g., shape, size, surface, coating, crystal chemistry); (ii) plant species characteristics (genotype, phenological state, etc.); (iii) environmental conditions of plant growth (mainly soil/substrate properties and light intensity); and (iv) NPs application and absorption by roots (soil-grown, seed imbibition, and/or hydroponic systems) or foliage (Moaveni et al. 2011; Conway et al. 2015; Cox et al. 2017; Tan et al. 2017; Tripathi et al. 2017).
The exposure of plants to NPs via roots or leaves influences internal translocation and concentration of the relevant element in various plant tissues (e.g., Ti for TiO2) (Silva et al. 2017). Upon TiO2 application to the root medium, the Ti concentration in roots and shoots of T. aestivum was found to vary in a dose-dependent manner (Jiang et al. 2017). In contrast, there was no change in Ti concentration in Hordeum vulgare L. tissues using the same exposure pathway (Marchiol et al. 2016). In addition, Larue et al. (2012) reported difference in Ti concentration in plant tissues of T. aestivum and rapeseed at the same dose of TiO2 applied to the root medium. In Solanum lycopersicum L., grown in sludge-amended soil containing TiO2 NPs, the Ti concentration in stem, leaves, and fruit was similar and lower than the control in case of leaves (Bakshi et al. 2019). Regarding the impact of TiO2 on growth, a decrease in root biomass of T. aestivum was found in the treatments with high doses of TiO2 NPs, whereas shoot biomass was unchanged (Jiang et al. 2017). In contrast, Tan et al. (2017) observed a reduction (more than 30%) in shoot biomass of O. basilicum treated with TiO2 NPs compared to the control. Others studies reported no change in biomass production in species such as Phaseolus vulgaris L. and T. aestivum (Jacob et al. 2013; Larue et al. 2012).
The antioxidant system is used by plants to counteract possible toxic effects associated with application of TiO2 NPs. Ultraviolet light exacerbates generation of ROS under TiO2 NPs exposure (Kőrösi et al. 2019), differentially activating the enzymatic and non-enzymatic antioxidative mechanisms in plants (Silva et al. 2019). In general, the antioxidant system has been activated by TiO2 in several crops, such as O. sativa (Wu et al. 2017), T. aestivum (Silva et al. 2017), S. lycopersicum (Tiwari et al. 2017), and Vicia narbonensis L. (Castiglione et al. 2014).
Solanum lycopersicum L. is an important edible crop grown worldwide (Tiwari et al. 2017). This species has been recognized as a terrestrial plant model for toxicity studies testing chemicals in the stages of seedling emergence and seedling growth (OECD 2003). In this context, S. lycopersicum has been used in the evaluation of toxicity, absorption, transport, and accumulation of TiO2 NPs applied in doses ranging from 0 to 5000 mg L−1. In addition, the TiO2 effects have been evaluated on seed germination and on seedlings, including growth, biomass production, photosynthesis, water conductance, transpiration, and antioxidant system (Qi et al. 2013; Song et al. 2013; Raliya et al. 2015; Singh et al. 2016; Tiwari et al. 2017). Nevertheless, there is a paucity of information on Ti translocation and concentration in S. lycopersicum plants exposed to TiO2 NPs (Raliya et al. 2015). In addition, no study could be found about foliar TiO2 exposure and the interaction between Ti concentration in tissues with biomass production and antioxidant system activity in S. lycopersicum as the model species.
The foliar application of NPs is an interesting mode of exposure because of multiple potential entry pathways, e.g., through stomata, trichomes, cuticle, and hydathodes (Khan et al. 2019; Rodríguez-González et al. 2019). The working hypothesis was that the foliar-applied NPs would induce an increment in Ti accumulation and its translocation within the seedlings, as well as activate the antioxidant system and decrease biomass production. Therefore, this work was aimed at characterizing the effects of foliar-applied TiO2 NPs on Ti absorption and translocation, biomass production, and the antioxidant system in S. lycopersicum L.
2 Materials and Methods
2.1 Physical Characterization of Nanoparticles
The TiO2 NPs used in this work (catalog #637262, Sigma Aldrich Co., St. Louis, USA) had <100 nm, 99.5% purity, specific surface area 50 m2 g−1, and 4.17 g mL−1 density at 25 °C. The characterization of these NPs was performed according to Nanogenotox (2011). In brief, TiO2 NPs were dispersed in 0.5% v/v ethanol and subsequently suspended in 0.05% w/v filtered bovine serum albumin and dissolved in autoclaved Milli-Q® water. Then, they were sonicated at 10% of amplitude for 16 min. To corroborate TiO2 nanoparticles were rutile, Raman spectroscopy was carried out on a WITec alpha 300 R Confocal Raman Microscope (WITec GmbH, Germany) using a laser at 785 nm. Transmission electron microscopy (TEM) (JEOL JEM-1400, Jeol LTD, Tokyo, Japan) was used to determine size and morphology of dried nanoparticles. The hydrodynamic size and Z-potential were determined by dynamic light scattering (DLS) and laser Doppler velocimetry (LDV) in a Malvern Zetasizer Nano-ZS ZEN3600 device (Malvern, UK) at 0, 24, and 48 h after sonication.
2.2 Plant Material and Growth Conditions
The assay was performed in the plant tissue culture laboratory at Universidad Católica de Temuco (38° 42′ 08.4″ S 72° 32′ 53.5″ W, and 149 m altitude).
Six S. lycopersicum seeds were germinated and then grown on a substrate (peat + perlite, volumetric ratio 2:1) in individual pots (500 mL) placed in a large box (3 L) for irrigation with distilled water by capillarity. S. lycopersicum was grown in a control-environment chamber under 200-μmol photons m−2 s−1, 23 ± 1 °C, 50% relative humidity, and 16/8 h photoperiod for 20 days followed by treatment application and additional 7 days of growth.
2.3 Treatments
Titanium dioxide NPs used were rutile in the crystalline form; these NPs are considered thermodynamically more stable than anatase and with higher hardness (Muscat et al. 2002; Pacheco et al. 2014). These characteristics give some advantages in terms of less agglomeration, and a lower photocatalytic activity to protect the plant system from photodegradation (Gogos et al. 2012). In addition, there is a precedent of using rutile in S. lycopersicum research (Tiwari et al. 2017).
The NPs were suspended in ultrapure water and stirred for 30 min, followed by breaking up possible aggregates for further 30 min in a sonicator (Elmasonic VC300) at room temperature, just before application. The treatments were applied 20 days after sowing, and lasted for 7 days. The doses of TiO2 NPs applied were 0, 5, 10, 20, 40, 80, and 160 mg L−1 (with ultrapure water serving as the control) by spraying 2.5 mL per plant foliarly to ensure the complete wetting. All the treatments were applied in the morning from 09:00 to 11:00 h, and the substrate was covered with aluminum foil to avoid contact with NPs.
2.4 Biomass Production
For the biomass measurement, after 7 days of exposure, plants were separated into leaves, stems, and roots, rinsed with abundant deionized water, blotted gently with absorbent paper, and weighed. Subsequently, they were dried in a forced-air oven to constant weight. Dry matter (DM) was calculated according to Balestri et al. (2014).
2.5 Samples for Analysis
The plants were harvested in the morning (from 09:00 to 11:00 h), pooled for each experimental unit, and rinsed three times with deionized water; subsequently, subsamples created for different assays were stored at −20 or − 80 °C according to the established protocols for lipid peroxidation and other biochemical assays, respectively.
2.6 Titanium Concentration in Plant Tissues
Titanium concentration was determined according to Gao et al. (2013); to remove TiO2 NPs from the leaf surface, the leaves were washed with tap water and distilled water. Subsequently, samples were dried at 100 °C for 72 h and digested in concentrated HNO3 at 115 °C for 1 h. The measurement of Ti concentration was performed using ICP-MS (Thermo iCAP RQ model) at the Geosciences Institute of the Universidad Austral de Chile.
2.7 Lipid Peroxidation
Oxidative stress was determined according to the protocol described by Heath and Packer (1968), based on the reaction with thiobarbituric acid. For extraction, the samples stored at −20 °C for 1 week were used, and the absorbance was measured at 532, 600, and 440 nm in a UV-Vis spectrophotometer to correct for the interference generated by TBARS-sugar complexes. The peroxidation was expressed in nanomoles of malondialdehyde per gram of fresh weight (nmol MDA g−1 FW).
2.8 Antioxidant Activity
The radical scavenging was determined using methanolic extracts and the free 2.2-diphenyl-1-picrylhydrazyl (DPPH) method according to Chinnici et al. (2004). The samples frozen at −80 °C for 2 weeks were ground and homogenized in 80% v/v methanol solution, centrifuged at 13,000g at 4 °C for 5 min, and 200 μL of the supernatant was used for reaction with the DPPH reagent. The absorbance was measured at 515 nm using a spectrophotometer (Thermo Scientific Spectronic Genesys 10 UV-Vis Scanning, Madison, WI, USA) using Trolox as standard.
2.9 Total Reducing Capacity
The total reducing capacity was measured by the Folin-Ciocalteu method as described by Slinkard and Singleton (1977), with chlorogenic acid used as a standard. The extraction was performed as described above, and 10 μL of the supernatant was used for mixing with the reagent, followed by measurements at 765 nm using the UV-Vis spectrophotometer. The total reducing capacity was expressed in milligrams of chlorogenic acid equivalents per g of fresh weight (mg CAE g−1 FW).
2.10 Superoxide Dismutase Activity
The superoxide dismutase (SOD, EC 1.15.1.1) activity was measured in fresh leaves stored at −80 °C for 2 weeks. The SOD activity was determined according to Giannopolitis and Ries (1977) with minor modifications (Mora et al. 2009), based on the photochemical inhibition of nitroblue tetrazolium (NBT) reduction. The SOD values were standardized by the total protein content, which was determined according to Bradford (1976). One unit of SOD activity (U g−1) was defined as the amount of enzyme required to cause 50% inhibition of the reduction of NBT measured at 560 nm by the UV-Vis spectrophotometer.
2.11 Experimental Design and Statistical Analysis
The experiment was set up in a randomized complete block design with three replicates composed of three plants each. The data were tested using one-way ANOVA and Tukey test for the multiple comparisons. Correlation analysis was used to establish relationships between the variables. All statistical analyses were done using JMP Software 5.01®.
3 Results
3.1 Nanoparticle Characterization
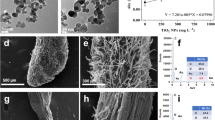
In the Raman spectrum of TiO2 NPs, the two characteristic peaks of rutile were observed at 446 and 608 Raman shift (cm−1) (Fig. 1a). The TiO2 NPs had a rod form, ranging in size from 30 to 60 (width) to 60–90 nm (length). The average width and length of TiO2 rod-shaped nanoparticles were 52 nm and 77 nm, respectively (Fig. 1b and c).
The main hydrodynamic parameters of TiO2 rod nanoparticles are summarized in Table 1. In general, TiO2 nanoparticles showed a moderate stability (with some trend to agglomeration), given a hydrodynamic diameter of 236 nm and a Z-potential of −13 mV in an aqueous medium. In addition, PDI index was relatively low, indicating that particles showed homogeneous distribution in aqueous media.
3.2 TiO2 NPs and Ti Translocation
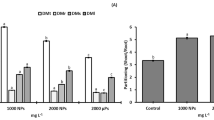
TiO2 NPs were applied to the leaves at different concentration. After 7 days of exposure, Ti concentration was increased in leaves and stem in the 160 mg L−1 treatment (Fig. 2). Significantly, higher Ti concentration was observed in leaves compared to stem and roots. With respect to the control, significant differences in Ti concentration (p < 0.05) for leaves and stem were observed at 160 mg L−1 dose. The Ti accumulation was not observed in roots regardless of the dose applied (Fig. 2).
Titanium concentration in leaves (L), stem (S), and roots (R) of S. lycopersicum plants 7 days after TiO2 NPs foliar application at various doses. Different uppercase letters indicate a significant difference (p ≤ 0.05) among treatments for the same organ by Tukey test (p ≤ 0.05). Different lowercase letters indicate a significant difference among organs for the same treatment by Tukey test (p ≤ 0.05). Means ± SE, n = 3
3.3 Biomass Production
Dry matter was measured after 7 days of the treatment (Fig. 3). Compared to the control, in the root dry matter (DMr), a significant decline occurred in the treatments with 5 and especially 10 mg L−1. Stem dry matter (DMs) was higher at 10 mg L−1 compared to the control, and was about 40% lower at 20 mg L−1 compared to the 10 mg L−1 treatment. Leaf dry matter (DMl) and total dry matter (DMt) showed a decreasing trend with the doses from 10 to 160 mg L−1. The high values for DMl and DMt were observed in the 10 mg L−1 treatment, being 33% and 40% higher (respectively) than the low values measured at 20 mg L−1.
3.4 Lipid Peroxidation and Antioxidant System
Lipid peroxidation was measured in leaves after 7 days of exposure (Fig. 4A). There was an increasing trend from control to higher doses. However, significantly higher lipid peroxidation was recorded only at 20 mg L−1 (Fig. 4A).
Antioxidant system in leaves of S. lycopersicum 7 days after TiO2 foliar application at various doses. (A) lipid peroxidation, (B) superoxide dismutase activity, (C) antioxidant activity, and (D) total reducing capacity. Different lowercase letters indicate a significant difference (p < 0.05) among treatments by Tukey test (p ≤ 0.05). Means ± SE, n = 3
Regarding the enzymatic antioxidant system, the SOD activity showed an increase in the treatment with 20 mg L−1 (about 2-fold compared with the control) and a significant decrease between 20 and 160 mg L−1 (Fig. 4B). Similar trends were observed for antioxidant activity and total reducing capacity across the treatments (Fig. 4C and D). Compared with the control, the dose of 40 mg L−1 produced significantly higher antioxidant activity (1.5-fold) and total reducing capacity (1.7-fold). The dose of 80 mg L−1 showed a slight increase compared to the control.
4 Discussion
TiO2 NPs used in this work had a rutile crystalline structure based on the two main peaks in the Raman spectrum near 446 and 610 (cm−1), characteristic for rutile TiO2 NPs (Choi et al. 2005; Moreno et al. 2019). These NPs had a moderate-to-high tendency to agglomerate, duplicating its initial size in suspension. These NPs were also characterized by García-Rodríguez et al. (2018), who observed similar properties using TEM, with rod sizes ranging from 70 to 40 nm, similar to our values 77.4 nm (length) and 52 nm (width) in suspension. The TiO2 NPs agglomeration is an intrinsic property in aqueous media, where the hydrodynamic diameter of particles can be increased 50-fold (Jiang et al. 2009). This natural trend for NPs to agglomerate varies with the NPs type, its crystalline form, and the suspension medium (Reches et al. 2018).
In our work, the hydrodynamic size was a relevant parameter because the NPs TiO2 were used in an aqueous suspension. The aggregation would influence absorption and Ti translocation inside the plant. This could be a reason why Ti was found in leaves at higher concentration than in stem and roots, especially at the high doses. Our data showed Ti concentration in S. lycopersicum exposed to 160 mg L−1 being around 3.7-fold higher in leaves and 2-fold higher in stem but only 1.08-fold higher in roots compared with the control. The presence of Ti in roots might have been an effect of Ti present in the substrate and not due to the foliar application of TiO2 because root Ti concentration did not differ significantly among the treatments, except being slightly lower in the 40 mg L−1 treatment. In the other study, the tissue distribution of Ti applied at 1000 mg kg−1 as foliar spray to S. lycopersicum resulted in around 33-fold higher concentration in leaves, around 8-fold in roots, and around 5-fold higher in stem compared with the control (Raliya et al. 2015). Thus, we observed lower translocation in our work compared to that reported by Raliya et al. (2015), which can be explained by the lower maximum dose (160 mg L−1) we used. In contrast, the distribution of Ti in O. basilicum plants was around 5-fold greater in roots and 1.1-fold greater in the shoot with respect to the control, using the dose of 750 mg kg−1 applied to soil (Tan et al. 2017). Likewise, H. vulgare seedlings showed Ti concentration in roots fluctuating from 35- to 412-fold with respect to the control and from 7.8- to 26-fold in the shoot at 1000 and 2000 mg L−1 of TiO2 applied to seeds (Mattiello et al. 2015). These variations in Ti transport and concentration in plant tissues are related mainly to the dose and the mode of application, physicochemical properties of NPs, and the plant physiology (Tan et al. 2018; Verano-Braga et al. 2014). Moreover, the species-specific relationships between plant and NPs have been reported Gruyer et al. (2014).
The transport and the availability of NPs within organisms are associated with biotransformation processes (Montes et al. 2012). In S. lycopersicum in the present study, although we did not evaluate biotransformation, this phenomenon could explain in part the TiO2 immobilization in leaves and its poor Ti transport toward stem and roots. The only work that evaluated TiO2 biotransformation in crop plants did not found any evidence for it in Cucumis sativus L., with TiO2 staying in that form during its transport from roots to trichomes (Servin et al. 2012).
In our study, the high Ti content in the substrate (37.4 mg kg−1) may have resulted in plant absorption of Ti, which would explain the small difference in Ti concentration in the specific organ among the treatments, and among plant organs for the same treatment, with such differences being accentuated only at the high foliar doses (e.g., between 80 and 160 mg L−1). It is important to bear in mind that Ti content in the substrate we used, mainly in peat (mosses of the genus Sphagnum), probably was due to the natural absorption, given that Ti is the ninth most abundant element in the earth’s crust, and the second most abundant transition metal (after Fe) (Buettner and Valentine 2012).
In our work, the exposure to TiO2 NPs for 7 days was sufficient to generate a significant decreasing trend in biomass of leaves and the whole plant, concomitant with an increase in the doses applied, mainly from 10 to 160 mg L−1. This decrease was negatively correlated (Pearson’s correlation) with Ti concentration in leaf biomass (r = −0.71; p = 0.045) and total biomass (r = −0.62; p = 0.048). Similar studies by Raliya et al. (2015) in the same species over the whole phenological cycle found the opposite results, with a biomass increment in the treatment with 250 mg kg−1 TiO2 NPs. This opposite plant response may be associated with TiO2 application to the root medium and/or the higher dose (56% greater than the highest dose used in our work) used by Raliya et al. (2015).
Activation of SOD was recorded at low dose (20 mg L−1) (Fig. 4B), and high total reducing capacity (non-enzymatic mechanism) was noted at high doses (40 and 80 mg L−1) (Fig. 4D). Only SOD was negatively correlated (Pearson’s correlation) with leaf Ti concentration (r = −0.70; p = 0.01). An increase in antioxidant parameters is a common response in plants treated with TiO2 NPs. For example, in S. lycopersicum, an increase in SOD activity was observed in plants treated with 5000 mg kg−1 TiO2 NPs (Song et al. 2013). Also, in Vicia narbonensis, TiO2 NPs gradually and differentially activated non-enzymatic and enzymatic antioxidant responses (Castiglione et al. 2014), which is in accordance with our observations. Nevertheless, despite a rising trend of oxidative stress in leaves (Fig. 4A) with an increase in the TiO2 NPs dose in our study, no significant correlation was observed. Little information exists in the literature regarding oxidative stress induced by TiO2 NPs; however, some reports with application of bulk TiO2 showed an increase in oxidative stress in Vicia faba L. (Castiglione et al. 2016). However, several reports in animal models have noted oxidative stress caused by TiO2 NPs in specific tissues of rats (Liang et al. 2009; Chen et al. 2020), fish (Federici et al. 2007), mussels (Huang et al. 2018), etc.
In the present study, the integrated metabolic response of S. lycopersicum to the TiO2 NPs treatments apparently were triggered at the exposure doses from 10 to 80 mg L−1, with a rise at 20 mg L−1 and then a decrease at the highest dose. This response was potentially due to an unavoidable trend of NPs agglomeration, which was greater at higher concentrations. However, it should be borne in mind that Degabriel et al. (2018) pointed out that the rod-shaped TiO2 NPs (similar to those used in the present work) had a lower critical coagulation (aggregation) concentration than other crystalline shapes (such as spheroids).
5 Conclusions
The titanium dioxide nanoparticles used in this work were rod-shaped; applied foliarly, they generated an increase in leaf concentration of Ti, but also translocation from leaves to stem. After 7 days of plant exposure to TiO2, there was a decrease in biomass production at intermediate and high doses; however, the activation of the antioxidant metabolism was present only at intermediate doses (20 mg L−1 regarding superoxide dismutase activity and at 40 mg L−1 for antioxidant activity) suggesting an increase of toxicity in this range. The moderate trend of agglomeration of these nanoparticles could explain these observations partly. However, further studies are necessary to verify titanium dioxide translocation (by transmission electron microscope analysis) and its particle sizes inside plant tissue, as well as to evaluate the effects of long-term titanium dioxide exposure at higher doses.
References
Bakshi M, Liné C, Bedolla DE, Stein RJ, Kaegi R, Sarret G, Pradas del Real AE, Castillo-Michel H, Larue C (2019) Assessing the impacts of sewage sludge amendment containing nano-TiO2 on tomato plants: a life cycle study. J Hazard Mater 369:191–198. https://doi.org/10.1016/j.jhazmat.2019.02.036
Balestri M, Bottega S, Spanò C (2014) Response of Pteris vittata to different cadmium treatments. Acta Physiol Plant 36:767–775. https://doi.org/10.1007/s11738-013-1454-z
Bradford MM (1976) A rapid and sensitive method for the quantitation microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Buettner KM, Valentine AM (2012) Bioinorganic chemistry of titanium. Chem Rev 112:1863–1881. https://doi.org/10.1021/cr1002886
Castiglione MR, Giorgetti L, Cremonini R, Bottega S, Spanò C (2014) Impact of TiO2 nanoparticles on Vicia narbonensis L.: potential toxicity effects. Protoplasma 251:1471–1479. https://doi.org/10.1007/s00709-014-0649-5
Castiglione MR, Giorgetti L, Bellani L, Muccifora S, Bottega S, Spanò C (2016) Root responses to different types of TiO2 nanoparticles and bulk counterpart in plant model system Vicia faba L. Environ Exp Bot 130:11–21. https://doi.org/10.1016/j.envexpbot.2016.05.002
Chen Z, Zheng P, Han S, Zhang J, Li Z, Zhou S, Jia G (2020) Tissue-specific oxidative stress and element distribution after oral exposure to titanium dioxide nanoparticles in rats. Nanoscale 12:20033–20046. https://doi.org/10.1039/D0NR05591C
Chinnici F, Bendini A, Gaiani A, Riponi C (2004) Radical scavenging activities of peels and pulps from cv. Golden delicious apples as related to their phenolic composition. J Agr Food Chem 52:4684–4689. https://doi.org/10.1021/jf049770a
Choi HC, Jung YM, Kim S (2005) Size effects in the Raman spectra of TiO2 nanoparticles. Vib Spectros 37:33e38–33e38. https://doi.org/10.1016/j.vibspec.2004.05.006
Conway JR, Beaulieu AL, Beaulieu NL, Mazer SJ, Keller AA (2015) Environmental stresses increase photosynthetic disruption by metal oxide nanomaterials in a soil-grown plant. ACS Nano 9:11737–11749. https://doi.org/10.1021/acsnano.5b03091
Cox A, Venkatachalam P, Sahi S, Sharma N (2017) Reprint of: silver and titanium dioxide nanoparticle toxicity in plants: a review of current research. Plant Physiol Bioch 110:33–49. https://doi.org/10.1016/j.plaphy.2016.08.007
Da Costa MVJ, Sharma PK (2016) Effect of copper oxide nanoparticles on growth, morphology, photosynthesis, and antioxidant response in Oryza sativa. Photosynthetica 54:110–119. https://doi.org/10.1007/s11099-015-0167-5
Degabriel T, Colaço E, Domingos RF, El Kirat K, Brouri D, Casale S, Landoulsi L, Spadavecchia J (2018) Factors impacting the aggregation/agglomeration and photocatalytic activity of highly crystalline spheroid-and rod-shaped TiO2 nanoparticles in aqueous solutions. Phys Chem Chem Phys 20:12898–12907. https://doi.org/10.1039/C7CP08054A
Federici G, Shaw BJ, Handy RD (2007) Toxicity of titanium dioxide nanoparticles to rainbow trout (Oncorhynchus mykiss): gill injury, oxidative stress, and other physiological effects. Aquat Toxicol 84:415–430. https://doi.org/10.1016/j.aquatox.2007.07.009
Gao J, Xu G, Qian H, Liu P, Zhao P, Hu Y (2013) Effects of nano-TiO2 on photosynthetic characteristics of Ulmus elongata seedlings. Environ Pollut 176:63–70. https://doi.org/10.1016/j.envpol.2013.01.027
García-Rodríguez A, Vila L, Cortés C, Hernández A, Marcos R (2018) Effects of differently shaped TiO2 NPs (nanospheres, nanorods and nanowires) on the in vitro model (Caco-2/HT29) of the intestinal barrier. Part Fibre Toxicol 15:33. https://doi.org/10.1186/s12989-018-0269-x
Ghosh SK, Pal T (2007) Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: from theory to applications. Chem Rev 107:4797–4862. https://doi.org/10.1021/cr0680282
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I. occurrence in higher plants. Plant Physiol 59:309–314. https://doi.org/10.1104/pp.59.2.309
Gogos A, Knauer K, Bucheli TD (2012) Nanomaterials in plant protection and fertilization: current state, foreseen applications, and research priorities. J Agr Food Chem 60:9781–9792. https://doi.org/10.1021/jf302154y
Gruyer N, Dorais M, Bastien C, Dassylva N (2014) Interaction between silver nano-particles and plant growth. Acta Hortic 1037:795e800
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198. https://doi.org/10.1016/0003-9861(68)90654-1
Hong F, Zhou J, Liu C, Yang F, Wu C, Zheng L, Yang P (2005) Effect of nano-TiO2 on photochemical reaction of chloroplasts of spinach. Biol Trace Elem Res 105:269–279. https://doi.org/10.1385/BTER:105:1-3:269
Huang X, Liu Z, Xie Z, Dupont S, Huang W, Wu F, Kong H, Liu L, Sui Y, Lin D, Lu W, Hu M, Wang Y (2018) Oxidative stress induced by titanium dioxide nanoparticles increases under seawater acidification in the thick shell mussel Mytilus coruscus. Mar Environ Res 137:49–59. https://doi.org/10.1016/j.marenvres.2018.02.029
Jacob DL, Borchardt JD, Navaratnam L, Otte ML, Bezbaruah AN (2013) Uptake and translocation of Ti from nanoparticles in crops and wetland plants. Int J Phytoremediat 15:142–153. https://doi.org/10.1080/15226514.2012.683209
Jiang J, Oberdörster G, Biswas P (2009) Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J Nanopart Res 11:77–89. https://doi.org/10.1007/s11051-008-9446-4
Jiang F, Shen Y, Ma C, Zhang X, Cao W, Rui Y (2017) Effects of TiO2 nanoparticles on wheat (Triticum aestivum L.) seedlings cultivated under super-elevated and normal CO2 conditions. PloS One 12:e0178088. https://doi.org/10.1371/journal.pone.0178088
Karimi J, Mohsenzadeh S (2017) Physiological effects of silver nanoparticles and silver nitrate toxicity in Triticum aestivum. Iran J of Sci and Technol A 41:111–120. https://doi.org/10.1007/s40995-017-0200-6
Khan MR, Adam V, Rizvi TF, Zhang B, Ahamad F, Jośko I, Yang M, Mao (2019) Nanoparticle–plant interactions: two-way traffic. Small 15:1901794. https://doi.org/10.1002/smll.201901794
Kőrösi L, Bouderias S, Csepregi K, Bognár B, Teszlák P, Scarpellini A, Castelli A, Eideg E, Jakab G (2019) Nanostructured TiO2-induced photocatalytic stress enhances the antioxidant capacity and phenolic content in the leaves of Vitis vinifera on a genotype-dependent manner. J Photoch Photobio B 190:137–145. https://doi.org/10.1016/j.jphotobiol.2018.11.010
Larue C, Veronesi G, Flank AM, Surble S, Herlin-Boime N, Carrière M (2012) Comparative uptake and impact of TiO2 nanoparticles in wheat and rapeseed. J Toxicol Environ Health A 75:722–734. https://doi.org/10.1080/15287394.2012.689800
Li J, Naeem MS, Wang X, Liu L, Chen C, Ma N, Zhang C (2015) Nano-TiO2 is not phytotoxic as revealed by the Oilseed rape growth and photosynthetic apparatus ultra-structural response. PLoS One 10:e0143885. https://doi.org/10.1371/journal.pone.0143885
Liang G, Pu Y, Yin L, Liu R, Ye B, Su Y, Li Y (2009) Influence of different sizes of titanium dioxide nanoparticles on hepatic and renal functions in rats with correlation to oxidative stress. J Toxicol Environ Health Part A 72(11–12):740–745. https://doi.org/10.1080/15287390902841516
Manesh RR, Grassi G, Bergami E, Marques-Santos LF, Faleri C, Liberatori G, Corsi I (2018) Co-exposure to titanium dioxide nanoparticles does not affect cadmium toxicity in radish seeds (Raphanus sativus). Ecotox Environ Safety 148:359–366. https://doi.org/10.1016/j.ecoenv.2017.10.051
Marchiol L, Mattiello A, Pošćić F, Fellet G, Zavalloni C, Carlino E, Musetti R (2016) Changes in physiological and agronomical parameters of barley (Hordeum vulgare) exposed to cerium and titanium dioxide nanoparticles. Int J Environ Res Public Health 13:332. https://doi.org/10.3390/ijerph13030332
Mattiello A, Filippi A, Pošćić F, Musetti R, Salvatici MC, Giordano C, Vischi M, Bertolni A, Marchiol L (2015) Evidence of phytotoxicity and genotoxicity in Hordeum vulgare L. exposed to CeO2 and TiO2 nanoparticles. Front Plant Sci 6:1043. https://doi.org/10.3389/fpls.2015.01043
Moaveni P, Talebi AH, Farahani A, Maroufi K (2011) Study of nano particles TiO2 spraying on some yield components in barley (Hordem vulgare L.). In: International Conference on Environmental and Agriculture Engineering, pp 115–119
Montes MO, Hanna SK, Lenihan HS, Keller AA (2012) Uptake, accumulation, and biotransformation of metal oxide nanoparticles by a marine suspension-feeder. J Hazard Mater 225:139–145. https://doi.org/10.1016/j.jhazmat.2012.05.009
Mora ML, Rosas A, Ribera A, Rengel Z (2009) Differential tolerance to Mn toxicity in perennial ryegrass genotypes: involvement of antioxidative enzymes and root exudation of carboxylates. Plant Soil 320:79–89. https://doi.org/10.1007/s11104-008-9872-1
Moreno V, Zougagh M, Ríos Á (2019) Analytical nanometrological approach for screening and confirmation of titanium dioxide nano/micro-particles in sugary samples based on Raman spectroscopy–capillary electrophoresis. Anal Chim Acta 1050:169–175. https://doi.org/10.1016/j.aca.2018.10.067
Muscat J, Swamy V, Harrison NM (2002) First-principles calculations of the phase stability of TiO2. Phys Rev B 65:224112. https://doi.org/10.1103/PhysRevB.65.224112
Nanogenotox (2011) http://www.nanogenotox.eu/files/PDF/Deliverables/nanogenotox%20%20%20deliverable%25%20203_wp4%20dispersion%20protocol.pdf
Organization for Economic Cooperation and Development (OECD) (2003) OECD Guide-lines for the testing of chemicals: proposals for updating guideline 208—terrestrial plant test: seedling emergence and seedling growth test. Available from: http:// www.oecd.org/dataoecd/11/31/33653757.pdf
Pacheco D, Rico J, Díaz JH, Espitia MJ (2014) Estudio DFT de propiedades estructurales y electrónicas del óxido de titanio en sus fases: rutilo, anatasa y brookita. Revista Colombiana de Materiales 5:179–185
Qi M, Liu Y, Li T (2013) Nano-TiO2 improve the photosynthesis of tomato leaves under mild heat stress. Biol Trace Elem Res 156:323–328. https://doi.org/10.1007/s12011-013-9833-2
Raliya R, Nair R, Chavalmane S, Wang WN, Biswas P (2015) Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant. Metallomics 7:1584–1594. https://doi.org/10.1039/c5mt00168d
Reches Y, Thomson K, Helbing M, Kosson DS, Sanchez F (2018) Agglomeration and reactivity of nanoparticles of SiO2, TiO2, Al2O3, Fe2O3, and clays in cement pastes and effects on compressive strength at ambient and elevated temperatures. Constr Buil Mater 167:860–873. https://doi.org/10.1016/j.conbuildmat.2018.02.032
Rodríguez-González V, Terashima C, Fujishima A (2019) Applications of photocatalytic titanium dioxide-based nanomaterials in sustainable agriculture. J Photochem Photobiol C 40:49–67. https://doi.org/10.1016/j.jphotochemrev.2019.06.001
Sarmast MK, Salehi H (2016) Silver nanoparticles: an influential element in plant nanobiotechnology. Mol Biotechnol 58:441–449. https://doi.org/10.1007/s12033-016-9943-0
Schmid G (1994) Clusters and colloids: from theory to applications. Weinheim, Germany, VCH, 558 p
Servin AD, Castillo-Michel H, Hernandez-Viezcas JA, Diaz BC, Peralta-Videa JR, Gardea-Torresdey JL (2012) Synchrotron micro-XRF and micro-XANES confirmation of the uptake and translocation of TiO2 nanoparticles in cucumber (Cucumis sativus) plants. Environ Sci Technol 46:7637–7643. https://doi.org/10.1021/es300955b
Silva S, Craveiro SC, Oliveira H, Calado AJ, Pinto RJ, Silva AM, Santos C (2017) Wheat chronic exposure to TiO2-nanoparticles: cyto-and genotoxic approach. Plant Physiol Biochem 121:89–98. https://doi.org/10.1016/j.plaphy.2017.10.013
Silva S, de Oliveira JMPF, Dias MC, Silva AM, Santos C (2019) Antioxidant mechanisms to counteract TiO2-nanoparticles toxicity in wheat leaves and roots are organ dependent. J Hazard Mater 380:120889. https://doi.org/10.1016/j.jhazmat.2019.120889
Singh P, Singh R, Borthakur A, Srivastava P, Srivastava N, Tiwary D, Mishra PK (2016) Effect of nanoscale TiO2-activated carbon composite on Solanum lycopersicum (L.) and Vigna radiata (L.) seeds germination. Energ Ecol Environ 1:131–140. https://doi.org/10.1007/s40974-016-0009-8
Slinkard K, Singleton VL (1977) Total phenol analysis: automation and comparison with manual methods. Am J Enol Viticult 28:49–55
Song U, Jun H, Waldman B, Roh J, Kim Y, Yi J, Lee EJ (2013) Functional analyses of nanoparticle toxicity: a comparative study of the effects of TiO2 and Ag on tomatoes (Lycopersicon esculentum). Ecotox Environ Safe 93:60–67. https://doi.org/10.1016/j.ecoenv.2013.03.033
Tan W, Du W, Darrouzet-Nardi AJ, Hernandez-Viezcas JA, Ye Y, Peralta-Videa JR, Gardea-Torresdey JL (2017) Effects of the exposure of TiO2 nanoparticles on basil (Ocimum basilicum) for two generations. Sci Total Environ 636:240–248. https://doi.org/10.1016/j.scitotenv.2018.04.263
Tan W, Peralta-Videa JR, Gardea-Torresdey JL (2018) Interaction of titanium dioxide nanoparticles with soil components and plants: current knowledge and future research needs–a critical review. Environ Sci Nano 5:257–278. https://doi.org/10.1039/C7EN00985B
Tighe-Neira R, Carmora E, Recio G, Nunes-Nesi A, Reyes-Diaz M, Alberdi M, Rengel Z, Inostroza-Blancheteau C (2018) Metallic nanoparticles influence the structure and function of the photosynthetic apparatus in plants. Plant Physiol Biochem 130:408–417. https://doi.org/10.1016/j.plaphy.2018.07.024
Tiwari M, Sharma NC, Fleischmann P, Burbage J, Venkatachalam P, Sahi SV (2017) Nanotitania exposure causes alterations in physiological, nutritional and stress responses in tomato (Solanum lycopersicum). Front Plant Sci 8:633. https://doi.org/10.3389/fpls.2017.00633
Tripathi DK, Singh S, Singh S, Pandey R, Singh VP, Sharma NC, Prasad SM, Dubey NK, Chauhan DK (2017) An overview on manufactured nanoparticles in plants: uptake, translocation, accumulation and phytotoxicity. Plant Physiol Biochem 110:2–12. https://doi.org/10.1016/j.plaphy.2016.07.030
Verano-Braga T, Miethling-Graff R, Wojdyla K, Rogowska-Wrzesinska A, Brewer JR, Erdmann H, Kjeldsen F (2014) Insights into the cellular response triggered by silver nanoparticles using quantitative proteomics. ACS Nano 8:2161–2175. https://doi.org/10.1021/nn4050744
Wu B, Zhu L, Le C (2017) Metabolomics analysis of TiO2 nanoparticles induced toxicological effects on rice (Oryza sativa L.). Environ Pollut 23:302–310. https://doi.org/10.1016/j.envpol.2017.06.062
Zahra Z, Waseem N, Zahra R, Lee H, Badshah MA, Mehmood A, Arshad M (2017) Growth and metabolic responses of rice (Oryza sativa L.) cultivated in phosphorus-deficient soil amended with TiO2 nanoparticles. J Agric Food Chem 65:5598–5606. https://doi.org/10.1021/acs.jafc.7b01843
Acknowledgements
We appreciate the technical assistance in nanoparticle characterization by Loreto Troncoso (Universidad Austral de Chile) and Alba García (Universitat Autonòma de Barcelona).
Funding
We acknowledge funding from the project FONDEQUIP EQM160050, and the CONICYT-PCHA/Doctorado Nacional/2016-21160984 scholarships.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tighe-Neira, R., Reyes-Díaz, M., Nunes-Nesi, A. et al. Titanium Dioxide Nanoparticles Increase Tissue Ti Concentration and Activate Antioxidants in Solanum lycopersicum L.. J Soil Sci Plant Nutr 21, 1881–1889 (2021). https://doi.org/10.1007/s42729-021-00487-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-021-00487-z