Abstract
Nowadays, nanoparticles (NPs), especially silver nanoparticles (AgNPs), are introduced in a growing number of commercial products and their production is released into the environment and may adversely influence on organisms. Up to now, limited studies are available about toxicity effects of NPs on higher plants. In this work, the effects of AgNPs in comparison with silver nitrate (AgNO3) on some physiological parameters of the wheat (Triticum aestivum) were investigated. Silver nanoparticles and AgNO3 at 10 and 100 mg−1 L concentrations significantly decreased the fresh and dry weight of roots and shoots. The results showed that AgNPs and AgNO3 decreased plant tissue chlorophyll “a” and “b,” carotenoid and total protein contents of the leaves significantly. Both AgNO3 and AgNPs treatments also increased the amount of proline, lipid peroxidation and catalase activity of wheat seedling tissues. Results of this work revealed that exposure to silver nanoparticles and silver ions might cause negative aspects and toxicity problems in plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Heavy metal toxicity has become a universal threat to all life forms including plants, animals and eventually humans. The unwanted growth of toxic heavy metals, mainly due to different anthropogenic activities, leads to heavy metal pollution that can have disastrous and unexpected effects on ecosystems (Das et al. 1997; Duruibe et al. 2007; Foy et al. 1978; Jarup 2003; Prasad 2004; Roesijadi 1992). Today design, production, optimization, and application of nanoparticles are interesting areas of research. The reason for this interest is the different physical and chemical properties of particles in the nanoscale (Stone et al. 2010) that are increasingly used in diverse fields such as biomedical sciences, medicine, drug delivery, gene therapy, cell targeting, magnetic, optic, mechanic, catalysis and electric devices (Bao et al. 2013; Daniel and Astruc 2004; Luo et al. 2006; Ramalingam et al. 2012; Sharma et al. 2012; Yoo et al. 2011). As for improvement of any kind of new technology, there are concerns about the potentially adverse effects of nanoparticles on human, animal, plant and the general environment. Existing investigations suggest that a complete understanding of the potential for health or environmental risks of nanoparticles does not exist (Morgan 2005). Increasing applications of nanoparticles highlights require elucidating biological effects and nanotoxicity of nanoparticles in organisms (Fabrega et al. 2011; Shaw and Handy 2011; Stampoulis et al. 2009). Indeed, in spite of the considerable number of studies on the toxicity of nanoparticles in animal and bacteria, limited studies are available in higher plants (Ma et al. 2010; Monica and Cremonini 2009). Silver ions are one of the most toxic heavy metals and silver nanoparticles (AgNPs) are one of the most common nanomaterials that have a tendency to be released into the environment. Both silver ions and silver nanoparticles are toxic, but there is some evidence to show that AgNPs toxicity depends on the release of silver ions (Beer et al. 2012; Kittler et al. 2010). Nanomaterials risk assessment of the environment must be estimated based on investigations to clarify all related aspects of the concern, but it is difficult for nanomaterials as few studies have been done. Many effects of nanomaterials on ecosystems remain unknown (Dupuy and Mills 2004; Holden et al. 2012). Negative effects of AgNPs the same as Ag ions in plant are significant, such as degradation of the plasma membrane and changes in membrane permeability; failure of the proton motive force and inhibition of the ATP synthesis; inhibition of enzyme activity by binding to –SH groups of amino acids; denature ribosome and inhibiting protein synthesis; creation of reactive oxygen species (ROS) and damaging vital macromolecules (Kaegi et al. 2011; Kumari et al. 2009a; Monica and Cremonini 2009; Nair et al. 2010). Toxicity of silver nanoparticles on plants depends on particle’s properties such as size, shape, aggregation state, surface coatings, concentration, exposure time and the types of compounds. In addition, type, age and development stage of plants have significant impacts on plant resistance against toxicity of nanoparticles. To date, there have been only a few reported studies of the impact of AgNPs on plants such as Phaseolus vulgaris (Najafi et al. 2014), Sorghum bicolor (Lee et al. 2012), Lemna gibba (Oukarroum et al. 2013), Lolium multiflorum (Yin et al. 2011), Arabidopsis thaliana (Geisler-Lee et al. 2012; Kaveh et al. 2013), Allium cepa (Kumari et al. 2009b), Eruca sativa (Vannini et al. 2013) and Oryza sativa (Mazumdar and Ahmed 2011). It has been shown that AgNPs can have positive and negative effects on plant growth. Recently, more and more innovative applications for nanomaterials have been proposed and evaluated. In recent years, silver nanoparticles have become more widely used in various technologies and incorporated into a wide range of consumer products that take advantage of their attractive optical, conductive and antibacterial properties. In the present study, we experienced some physiological parameters of young wheat plants affected by two concentrations of AgNPs and AgNO3.
2 Materials and Methods
2.1 Plant Materials and Growth Conditions
Seeds of wheat (Triticum aestivum L. var. Chamran) were obtained from Zarghan Agricultural Research Center, Iran. These seeds were kept in a cool and dark place in the laboratory. Seeds were surface-sterilized by soaking in 5% (w/v) sodium hypochlorite (NaOCl) for 10 min. They were washed three times with distilled water and air-dried on filter papers. Seeds were allowed to germinate in the dark at 25 °C on wet papers in the plate. Five-day-old seedlings (20) were transferred into small plastic containers filled with perlite and Hoagland nutrient solution (pH 6.2). Wheat seedlings were grown in the growth chamber set at 16 h/8 h light–dark periods. Three replicates were used for each treatment.
2.2 Silver Nitrate and Silver Nanoparticle Treatments
Silver nanoparticles with average sizes of 20 nm and 99.99% purity were purchased from US Research Nanomaterials, Inc. [USA] (Fig. 1). Using Hoagland nutrient solution as a solvent, two concentrations (10 and 100 mg−1 L) of AgNPs and AgNO3 were prepared. The dissolved particles were dispersed by a high-power probe-type Sonicator (Misonix, QSonica LLC, Newton, USA) for 30 min. Hoagland nutrient solution was used as a control. Wheat seedlings (21-day old) were collected one week after the beginning of treatments, washed with double-distilled water and used for analyses. Parameters analyzed were roots and shoots, fresh and dry weight, chlorophyll and carotenoid pigments, catalase activity, lipid peroxidation, proline and leaves total protein contents.
2.3 Seedlings Fresh and Dry Weight
After washing with distilled water, wheat seedlings were blotted dry on tissue papers, and after taking their fresh weights, they were dried at 70 °C for 48 h for dry weight analysis.
2.4 Photosynthetic Pigment Measurement
The contents of photosynthetic pigments were determined according to Wellburn and Lichtenthaler (1984). Fresh leaf tissue (200 mg) was weighted and powdered using liquid nitrogen. After adding 80% acetone, the volume was brought to 25 mL. The resulting solution was centrifuged at 4800 rpm for 20 min. The supernatant was used for measuring the chlorophyll a, b and carotenoid. The absorbance of the clear supernatant was read at 645 nm (chlorophyll b), 663 nm (chlorophyll a), and 470 nm (carotenoid).
2.5 Protein Determination
Soluble protein was quantified according to Bradford (1976). Samples were homogenized in 0.1 M Na-phosphate buffer (pH 7; 1:5 w/v). After adding the reagent, absorbance was recorded at 595 nm and the concentration was calculated using a calibration curve made with bovine serum albumin. Protein concentrations were determined after realizing a standard curve.
2.6 Proline Determination
Free proline contents were measured by the method of Bates et al. (1973). Fresh leaf tissue (100 mg) was homogenized in 3% (w/v) sulphosalicylic acid, and proline was estimated by ninhydrin reagent (0.125 g of ninhydrin in 2 mL orthophosphoric acid 6 M, and 3 mL of acetic acid). The earned chromophore was extracted from liquid phase with toluene, and remarking organic layer was read at 520 nm. Proline concentrations were determined after realizing a standard curve.
2.7 Catalase Determination
Catalase (CAT) activity was determined by decomposition of H2O2 and was measured by assessing the decrease in absorbance at 240 nm (Aebi 1984). The reaction mixture contained 200 mM KPO4 buffer (pH 7.0), 30 mM H2O2 and enzyme extract. Catalase activity was calculated through Aebi formula, and H2O2 decomposed g−1 FW min−1 was defined as a unit of CAT.
2.8 Lipid Peroxidation
The lipid peroxidation was measured in the leaf tissues by estimating the malondialdehyde (MDA) as an indicator of lipid peroxidation. Malondialdehyde was assayed by thiobarbituric acid reactive substances contents (Heath and Packer 1968).
2.9 Statistical Analysis
The experimental designs were randomized complete block, and each value reported is the average of three repeats. The raw data were imported into Microsoft Excel 2007 program for calculations and graphic representation. SPSS (version 16.0) software was used for analysis of variance. Quantitative changes in parameters were analyzed through analysis of variance (one-way ANOVA), with Duncan’s multiple range tests at p ≤ 0.05 to find out significant differences among treatments. All results are presented as the mean ± standard deviation (SD).
3 Results and Discussion
3.1 Effects of AgNPs and AgNO3 on Plant Growth
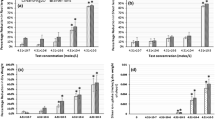
After 1 week of silver ions and silver nanoparticles exposure, the fresh and dry weights of root and shoot of T. aestivum L. were measured (Tables 1, 2 or Figs. 2, 3). Data represent the mean ± standard deviation (SD). Data with different letters are significantly different.
A clear and significant growth inhibition was observed in wheat plants exposed to AgNO3 and AgNPs treatments. Results showed that shoot fresh weight, root fresh weight and shoot + root fresh weight in all treatments led to a significant decrease according to the increase in the concentration of AgNO3 and AgNPs compared with the control, with the exception of treated plants at 10 mg−1 L AgNO3 concentration. Shoot + root fresh weight of AgNO3- and AgNPs-treated plants decreased about 52 and 51%, at 10 mg−1 L concentration and 68 and 66% at 100 mg−1 L concentration compared with the control, respectively. The maximum reduction in the root fresh weight was observed at 100 mg−1 L AgNO3. Shoot + root dry weight of AgNO3- and AgNPs-treated plants was decreased about 57 and 46%, at 10 mg−1 L concentration and 69 and 58% at 100 mg−1 L concentration compared to the control, respectively. Maximum reduction in root dry weight was observed at 100 mg−1 L concentration of both AgNO3 and AgNPs, and its value was not significantly different.
As the results showed, AgNPs and AgNO3 reduced the fresh and dry weights (biomass) of wheat approximately in all concentrations (Tables 1, 2 or Figs. 3, 4). Our results supported the outcomes found in the study of the effects of heavy metals and nanoparticles on other plant (Fritioff et al. 2005; Glick 2003; Jiang et al. 2012; Oukarroum et al. 2012). However, for fresh and dry weight, the values of AgNO3 were lower than AgNPs; therefore, growth inhibition of AgNO3 was significantly stronger than AgNPs. AgNO3 and AgNPs possibly by the decrease in leaf chlorophyll and photosynthesis led to a significant decrease in dry and fresh weights of wheat root and shoot.
3.2 Effects of AgNPs and AgNO3 on Contents of Photosynthetic Pigments
Contents of photosynthetic pigments (chlorophyll a, chlorophyll b and carotenoid) in wheat are presented in Table 3 and Figs. 5 and 6. A significant decrease in chlorophyll a, b and carotenoid contents in plants (1-week exposed to silver ions and silver nanoparticles treatments) compared with the control was observed. Wheat leaves showed signs of chlorosis after exposure to treatments according to the increase in silver ions and silver nanoparticles concentration, while the control plant appeared to be healthy and green (Fig. 2). The total chlorophyll contents of AgNO3- and AgNPs-treated plants decreased around 48 and 31%, at 10 mg−1 concentration and 77 and 64% at 100 mg−1 concentration compared with the control, respectively. The same result was observed in the contents of carotenoids. The carotenoid contents of AgNO3- and AgNPs-treated plants decreased approximately 67 and 50%, at 10 mg−1 concentration and 79 and 68% at 100 mg−1 concentration compared with the control, respectively. The decrease in the chlorophyll and carotenoid in AgNO3 treatments was significantly more than that of the AgNPs at the same concentration.
As shown in Table 3 and Figs. 5 and 6, photosynthetic pigments (chlorophyll a, chlorophyll b and carotenoid) decrease significantly in wheat on exposure to AgNO3 and AgNPs. In addition, declines in leaf total chlorophyll content lead to chlorosis. Chlorosis is the most common sign of toxicity of heavy metals (Pandey and Sharma 2002). Also, the declines in total chlorophyll and carotenoid contents can be regarded as general responses related to metal toxicity (Chandra et al. 2009; MacFarlane and Burchett 2001; Radic et al. 2010; Ralph and Burchett 1998). It seems that the decrease in chlorophyll contents in tissues of metal treated plants is dependent on several factors such as disturbance in the synthesis of pigments (Shweta and Agrawal 2006), pigments degradation (Prasad et al. 2001; Somashekaraiah et al. 1992), direct inhibition of enzymatic steps coupled with chlorophyll biosynthesis, protein composition of photosynthetic membranes (Mysliwa-Kurdziel et al. 2004; Prasad and Strzałka 1999) and avoiding the arrangement of photoactive protochlorophyll reductase enzyme complex and aminolevulinic acid (ALA) synthesis (Oncel et al. 2000; Stobart et al. 1985). These results supported the findings of other researchers (Lagriffoul et al. 1998; Oukarroum et al. 2012; Ralph and Burchett 1998; Saison et al. 2010; Wei et al. 2010).
3.3 Effects of AgNPs and AgNO3 on Contents of Proline
Proline contents of treated and untreated wheat are shown in Table 4 and Fig. 7. According to the increase in AgNO3 and AgNPs concentration, the proline contents of leaf increased significantly compared to the control. The maximum increase in proline contents was observed at 100 mg−1 L in both AgNO3 and AgNPs. Proline contents of AgNO3- and AgNPs-treated plants increased approximately 25 and 8%, at 10 mg−1 L concentration and 39 and 32% at 100 mg−1 L concentration compared with the control, respectively.
Results indicated that the accumulation of proline in leaves of wheat increases significantly with rising AgNO3 and AgNPs concentration. Proline as an amino acid is an important osmolyte and accumulates in a broad range of organisms ranging from bacteria to higher plants on exposure to abiotic stress throughout the adaptation to a diversity of types of environmental stress, such as drought, cold, salinity, high temperature, nutrient lack and exposure to heavy metals (Ashraf and Foolad 2007b). Proline in various ways, including acting as a metal chelator (Sharma and Dubey 2005), detoxification of reactive oxygen species (ROS) such as hydroxyl radical and singlet oxygen (Szabados and Savouré 2010) and osmoprotectant (Ashraf and Foolad 2007a; Tamayo and Bonjoch 2001), protection for the enzymes against denaturation and stabilization of protein synthesis (Sanchez-Partida et al. 1992; Shah and Dubey 1997) alleviates heavy metal toxicity.
In addition, proline supports mitochondrial oxidative phosphorylation for protecting natural generation of ATP (Ashraf and Foolad 2007b; Siripornadulsil et al. 2002) and acts as an inhibitor of lipid peroxidation (Hara et al. 2003; Mehta and Gaur 1999). Our results were also similar to results obtained by other investigators (Jiang et al. 2012; John et al. 2009; Kastori et al. 1992; Mehta and Gaur 1999).
3.4 Effects of AgNPs and AgNO3 on Lipid Peroxidation
The effect of special treatment of silver ions and silver nanoparticles with respect to the amount of lipid peroxidation was significant (Table 4; Fig. 8). The level of MDA formation indicates the level of free radical production and lipid peroxidation (Dexter et al. 1989; Mak and Weglicki 1988). The smallest amount of lipid peroxidation was realized on control, most of which was at concentration 100 mg−1 L of AgNO3. The increase in the lipid peroxidation in AgNO3 treatments was significantly more than that of the AgNPs of the same concentration. The difference between lipid peroxidation of concentration 10 and 100 mg−1 L of silver ions and 100 mg−1 L of silver nanoparticles was significant compared with the control. Results showed no significant effect on lipid peroxidation of low concentration (10 mg−1 L) of AgNPs. The amount of lipid peroxidation of AgNO3 and AgNPs treated plants increased approximately 36 and 12%, at 10 mg−1 L concentration and 87 and 39% at 100 mg−1 L concentration compared with the control, respectively.
Malondialdehyde (MDA) contents, a product of lipid peroxidation, are considered as an indicator of oxidative damage and peroxidation of membrane lipids in plants (Nacif de Abreu and Mazzafera 2005; Xu et al. 2006). The cell membrane is usually the main site of the attack by any heavy metal in a plant cell. In our experiments, significant increases in MDA concentration with increasing the AgNO3 and AgNPs concentration were observed that indicate a negative effect of heavy metals on membrane integrity and permeability. Produced free radicals as a result of AgNO3 and AgNPs can attack the unsaturated fatty acid side chains of membrane lipids and cause the formation of lipid hydroperoxides (Halliwell and Chirico 1993). This result was similar to the consequences obtained by other investigators (Gallego et al. 1996; Ghosh et al. 2010; Panda et al. 2003; Sayes et al. 2005; Zhang et al. 2007).
3.5 Effects of AgNPs and AgNO3 on Catalase Activity
A significant increase in catalase activity was observed in response to the increase of AgNO3 and AgNPs concentrations (Table 4; Fig. 9). The highest value of catalase activity was recorded at 100 mg−1 L AgNO3. Catalase activity contents of AgNO3- and AgNPs-treated plants increased approximately 60 and 30%, at 10 mg−1 L concentration and 240 and 80% at 100 mg−1 L concentration compared with the control, respectively. The increase in the amount of catalase activity in treatments was significantly more than (twofold at low concentration and threefold at high concentration) that of the AgNPs at the same concentration.
The result showed that the catalase activity of leaves significantly increased in response to the increase of AgNO3 and AgNPs concentrations. Both AgNPs and AgNO3 induce antioxidant enzyme, but this induction in AgNO3 treatments was significantly more than the AgNPs at the same concentration. The activities of antioxidant enzyme have generally increased during abiotic stress, such as chilling, drought, high temperature, salt and heavy metal stress (Baker and Orlandi 1995; Mittler 2002), and correlated with enhanced cellular protection of reactive oxygen species. Catalase is an important antioxidant which protects plants by suppressing oxidative injury and assists as a reactive species scavenger. These consequences were similar to the results obtained by other researchers (Du et al. 2011; Gallego et al. 1996; Krishnaraj et al. 2012; Zhang et al. 2007).
3.6 Effects of AgNPs and AgNO3 on Contents of Total Protein
According to increasing concentrations of AgNO3 and AgNPs, total protein content was decreased. Protein contents of AgNO3- and AgNPs-treated plants decreased by 28 and 23%, at 10 mg−1 L concentration and by 49 and 31% at 100 mg−1 L concentration compared with the control, respectively. Amount of total protein in response to the AgNPs at 10 mg−1 L concentration showed no significant change as compared to control. The decrease in the total protein by AgNO3 was more than by AgNPs at the same concentration (Table 4; Fig. 10).
Under heavy metals and nanoparticles, oxidative stresses lead to generation of reactive oxygen species and degeneration of protein (Choi and Hu 2008; Rana 2008; Wan et al. 2012; Xia et al. 2008). The results showed that in most cases, AgNO3 had more negative effects than AgNPs. Although both dissolved silver and AgNPs can provoke the production of reactive oxygen species, AgNPs may have direct toxic effects (Yin et al. 2011). While toxicity of AgNPs to plants is obvious, their negative effects and mechanisms on higher plants have not been completely characterized (Jiang et al. 2012).
4 Conclusion
Our study focused on the potential effect of AgNO3 and silver nanoparticles on wheat. Overall, findings of this work revealed that exposure to silver nanoparticles and silver ions might cause negative aspects and toxicity problems in plants. Although silver ions and nanoparticles have many positive aspects in life, overuse and lack of knowledge about the environmental impacts can cause damage in the environment, especially to human, animals and plant health. Therefore, to better understand the toxicity effects of Ag ions and AgNPs further experiments should be performed and, at the same time, essential precautions whether in the production or consumption of these materials must be taken.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Ashraf M, Foolad M (2007a) Improving plant abiotic-stress resistance by exogenous application of osmoprotectants glycine betaine and proline. Environ Exp Bot 59:206–216
Ashraf M, Foolad M (2007b) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Bao G et al (2013) Multifunctional nanoparticles for drug delivery and molecular imaging. Annu Rev Biomed Eng 15:253–282
Baker CJ, Orlandi EW (1995) Active oxygen in plant pathogenesis. Annu Rev Phytopathology 33(1):299–321
Bates L et al (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Beer C et al (2012) Toxicity of silver nanoparticles—nanoparticle or silver ion? Toxicol Lett 208:286–292
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chandra R et al (2009) Accumulation and distribution of toxic metals in wheat (Triticum aestivum L.) and Indian mustard (Brassica campestris L.) irrigated with distillery and tannery effluents. J Hazard Mater 162:1514–1521
Choi O, Hu Z (2008) Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ Sci Technol 42:4583–4588
Daniel M-C, Astruc D (2004) Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 104:293–346
Das P et al (1997) Studies on cadmium toxicity in plants: a review. Environ Pollut 98:29–36
Dexter D et al (1989) Basal lipid peroxidation in substantia nigra is increased in Parkinson’s disease. J Neurochem 52:381–389
Du W et al (2011) TiO2 and ZnO nanoparticles negatively affect wheat growth and soil enzyme activities in agricultural soil. J Environ Monit 13:822–828
Dupuy JP, Mills K (2004) Benefits, risks, ethical, legal and social aspects of nanotechnology
Duruibe J et al (2007) Heavy metal pollution and human biotoxic effects. Int J Phys Sci 2:112–118
Fabrega J et al (2011) Silver nanoparticles: behaviour and effects in the aquatic environment. Environ Int 37:517–531
Foy C et al (1978) The physiology of metal toxicity in plants. Ann Rev Plant Physiol 29:511–566
Fritioff A et al (2005) Influence of temperature and salinity on heavy metal uptake by submersed plants. Environ Pollut 133:265–274
Gallego SM et al (1996) Effect of heavy metal ion excess on sunflower leaves: evidence for involvement of oxidative stress. Plant Sci 121:151–159
Geisler-Lee J et al (2012) Phytotoxicity, accumulation and transport of silver nanoparticles by Arabidopsis thaliana. Nanotoxicology 7:323–337
Ghosh M et al (2010) Genotoxicity of titanium dioxide (TiO2) nanoparticles at two trophic levels: plant and human lymphocytes. Chemosphere 81:1253–1262
Glick BR (2003) Phytoremediation: synergistic use of plants and bacteria to clean up the environment. Biotechnol Adv 21:383–393
Halliwell B, Chirico S (1993) Lipid peroxidation: its mechanism, measurement, and significance. Am J Clin Nutr 57:715S–724S
Hara M et al (2003) Enhancement of cold tolerance and inhibition of lipid peroxidation by citrus dehydrin in transgenic tobacco. Planta 217:290–298
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Holden PA et al (2012) Ecological nanotoxicology: integrating nanomaterial hazard considerations across the subcellular, population, community, and ecosystems levels. Acc Chem Res 46:813–822
Jarup L (2003) Hazards of heavy metal contamination. Br Med Bull 68:167–182
Jiang HS et al (2012) Physiological analysis of silver nanoparticles and AgNO3 toxicity to Spirodela polyrhiza. Environ Toxicol Chem 31:1880–1886
John R et al (2009) Heavy metal toxicity: effect on plant growth, biochemical parameters and metal accumulation by Brassica juncea L. Int J Plant Prod 3:65–75
Kaegi R et al (2011) Behavior of metallic silver nanoparticles in a pilot wastewater treatment plant. Environ Sci Technol 45:3902–3908
Kastori R et al (1992) Effect of excess lead, cadmium, copper, and zinc on water relations in sunflower. J Plant Nutr 15:2427–2439
Kaveh R et al (2013) Changes in Arabidopsis thaliana gene expression in response to silver nanoparticles and silver ions. Environ Sci Technol 47:10637–10644
Kittler S et al (2010) Toxicity of silver nanoparticles increases during storage because of slow dissolution under release of silver ions. Chem Mater 22:4548–4554
Krishnaraj C et al (2012) Effect of biologically synthesized silver nanoparticles on Bacopa monnieri (Linn.) Wettst. plant growth metabolism. Process Biochem 47:651–658
Kumari M et al (2009a) Genotoxicity of silver nanoparticles in Allium cepa. Sci Total Environ 407:5243–5246
Kumari M et al (2009b) Genotoxicity of silver nanoparticles in Allium cepa. Sci Total Environ 407:5243–5246
Lagriffoul A et al (1998) Cadmium toxicity effects on growth, mineral and chlorophyll contents, and activities of stress related enzymes in young maize plants (Zea mays L.). Plant Soil 200:241–250
Lee W-M et al (2012) Effect of silver nanoparticles in crop plants Phaseolus radiatus and Sorghum bicolor: media effect on phytotoxicity. Chemosphere 86:491–499
Luo X et al (2006) Application of nanoparticles in electrochemical sensors and biosensors. Electroanalysis 18:319–326
Ma Y et al (2010) Effects of rare earth oxide nanoparticles on root elongation of plants. Chemosphere 78:273–279
MacFarlane G, Burchett M (2001) Photosynthetic pigments and peroxidase activity as indicators of heavy metal stress in the grey mangrove, Avicennia marina (Forsk.) Vierh. Mar Pollut Bull 42:233–240
Mak IT, Weglicki WB (1988) Protection by beta-blocking agents against free radical-mediated sarcolemmal lipid peroxidation. Circ Res 63:262–266
Mazumdar H, Ahmed G (2011) Phytotoxicity effect of silver nanoparticles on Oryza sativa. Int J ChemTech Res 3:1494–1500
Mehta S, Gaur J (1999) Heavy-metal-induced proline accumulation and its role in ameliorating metal toxicity in Chlorella vulgaris. New Phytol 143:253–259
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7(9):405–410
Monica RC, Cremonini R (2009) Nanoparticles and higher plants. Caryologia 62:161–165
Morgan K (2005) Development of a preliminary framework for informing the risk analysis and risk management of nanoparticles. Risk Anal 25:1621–1635
Myśliwa-Kurdziel et al (2004) Photosynthesis in heavy metal stressed plants. In: Heavy metal stress in plants. Springer, Berlin, Heidelberg, pp 146–181
Nacif de Abreu I, Mazzafera P (2005) Effect of water and temperature stress on the content of active constituents of Hypericum brasiliense Choisy. Plant Physiol Biochem 43:241–248
Nair R et al (2010) Nanoparticulate material delivery to plants. Plant Sci 179:154–163
Najafi S et al (2014) Photosynthetic characteristics, membrane lipid levels and protein content in the Phaseolus vulgaris L. (cv. Sadri) exposed to magnetic field and silver nanoparticles. Bull Environ Pharmacol Life Sci 3:72–76
Oncel I et al (2000) Interactive effects of temperature and heavy metal stress on the growth and some biochemical compounds in wheat seedlings. Environ Pollut 107:315–320
Oukarroum A et al (2012) Inhibitory effects of silver nanoparticles in two green algae Chlorella vulgaris and Dunaliella tertiolecta. Ecotoxicol Environ Saf 78:80–85
Oukarroum A et al (2013) Silver nanoparticle toxicity effect on growth and cellular viability of the aquatic plant Lemna gibba. Environ Toxicol Chem 32:902–907
Panda S et al (2003) Heavy metals induce lipid peroxidation and affect antioxidants in wheat leaves. Biol Plant 46:289–294
Pandey N, Sharma CP (2002) Effect of heavy metals Co2+, Ni2+ and Cd2+ on growth and metabolism of cabbage. Plant Sci 163:753–758
Prasad MNV (2004) Heavy metal stress in plants: from biomolecules to ecosystems. Springer, Berlin
Prasad M, Strzałka K (1999) Impact of heavy metals on photosynthesis. In: Heavy metal stress in plants. Springer, Berlin, Heidelberg, pp 117–138
Prasad M et al (2001) Physiological responses of Lemna trisulca L. (duckweed) to cadmium and copper bioaccumulation. Plant Sci 161:881–889
Radic S et al (2010) Ecotoxicological effects of aluminum and zinc on growth and antioxidants in Lemna minor L. Ecotoxicol Environ Saf 73:336–342
Ralph P, Burchett M (1998) Photosynthetic response of Halophila ovalis to heavy metal stress. Environ Pollut 103:91–101
Ramalingam M et al (2012) Integrated biomaterials for biomedical technology. Wiley, Berlin
Rana SVS (2008) Metals and apoptosis: recent developments. J Trace Elem Med Biol 22:262–284
Roesijadi G (1992) Metallothioneins in metal regulation and toxicity in aquatic animals. Aquat Toxicol 22:81–113
Saison C et al (2010) Effect of core–shell copper oxide nanoparticles on cell culture morphology and photosynthesis (photosystem II energy distribution) in the green alga Chlamydomonas reinhardtii. Aquat Toxicol 96:109–114
Sanchez-Partida L et al (1992) Proline and glycine betaine in cryoprotective diluents for ram spermatozoa. Reprod Fertil Dev 4:113–118
Sayes CM et al (2005) Nano-C 60 cytotoxicity is due to lipid peroxidation. Biomaterials 26:7587–7595
Shah K, Dubey R (1997) Effect of cadmium on proline accumulation and ribonuclease activity in rice seedlings: role of proline as a possible enzyme protectant. Biol Plant 40:121–130
Sharma P, Dubey RS (2005) Lead toxicity in plants. Braz J Plant Physiol 17:35–52
Sharma A et al (2012) Toxicological considerations when creating nanoparticle-based drugs and drug delivery systems. Expert Opin Drug Metab Toxicol 8:47–69
Shaw BJ, Handy RD (2011) Physiological effects of nanoparticles on fish: a comparison of nanometals versus metal ions. Environ Int 37:1083–1097
Shweta M, Agrawal S (2006) Interactive effects between supplemental ultraviolet-B radiation and heavy metals on the growth and biochemical characteristics of Spinacia oleracea L. Braz J Plant Physiol 18:307–314
Siripornadulsil S et al (2002) Molecular mechanisms of proline-mediated tolerance to toxic heavy metals in transgenic microalgae. Plant Cell Online 14:2837–2847
Somashekaraiah B et al (1992) Phytotoxicity of cadmium ions on germinating seedlings of mung bean (Phaseolus vulgaris): involvement of lipid peroxides in chlorphyll degradation. Physiol Plant 85:85–89
Stampoulis D et al (2009) Assay-dependent phytotoxicity of nanoparticles to plants. Environ Sci Technol 43:9473–9479
Stobart AK et al (1985) The effect of Cd2+ on the biosynthesis of chlorophyll in leaves of barley. Physiol Plant 63:293–298
Stone V et al (2010) Nanomaterials for environmental studies: classification, reference material issues, and strategies for physico-chemical characterisation. Sci Total Environ 408:1745–1754
Szabados L, Savouré A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97
Tamayo PR, Bonjoch NP (2001) Free proline quantification. In: Handbook of plant ecophysiology techniques. Springer, Netherlands, pp 365–382
Vannini C et al (2013) Morphological and proteomic responses of Eruca sativa exposed to silver nanoparticles or silver nitrate. PLoS ONE 8:e68752
Wan R et al (2012) DNA damage caused by metal nanoparticles: involvement of oxidative stress and activation of ATM. Chem Res Toxicol 25:1402–1411
Wei C et al (2010) Effects of silica nanoparticles on growth and photosynthetic pigment contents of Scenedesmus obliquus. J Environ Sci 22:155–160
Wellburn AR, Lichtenthaler H (1984) Formulae and program to determine total carotenoids and chlorophylls a and b of leaf extracts in different solvents. In: Advances in photosynthesis research. Springer, Netherlands, pp 9–12
Xia T et al (2008) Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano 2:2121–2134
Xu S et al (2006) Effects of heat acclimation pretreatment on changes of membrane lipid peroxidation, antioxidant metabolites, and ultrastructure of chloroplasts in two cool-season turfgrass species under heat stress. Environ Exp Bot 56:274–285
Yin L et al (2011) More than the ions: the effects of silver nanoparticles on Lolium multiflorum. Environ Sci Technol 45:2360–2367
Yoo D et al (2011) Theranostic magnetic nanoparticles. Acc Chem Res 44:863–874
Zhang F-Q et al (2007) Effect of heavy metal stress on antioxidative enzymes and lipid peroxidation in leaves and roots of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza). Chemosphere 67:44–50
Acknowledgements
The authors thank the Biology Department of Shiraz University for support of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Karimi, J., Mohsenzadeh, S. Physiological Effects of Silver Nanoparticles and Silver Nitrate Toxicity in Triticum aestivum . Iran J Sci Technol Trans Sci 41, 111–120 (2017). https://doi.org/10.1007/s40995-017-0200-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40995-017-0200-6