Abstract
Despite the fact that the world has achieved adequate food grain production to fight the battle against caloric hunger, still, a significant fraction of population is suffering with deficiency of micronutrients like Fe and Zn. However, the dietary intake of these micronutrients could be sufficient to meet the nutritional demand if the bioavailability was not low due to the strong inhibition by phytic acid and phenolics. Another cause behind inadequate intake is the scarcity of plant-available micronutrients in soil and genetic makeup of plants impeding high accumulation. Postharvest fortification is the major strategy to enrich staple food crops with micronutrients, but biofortification of food crops using breeding and agronomic strategies is also gaining popularity. However, one important issue remained unaddressed as none of them could really increase the plant-available micronutrients like Fe or Zn which otherwise remain insoluble in soil. Microorganisms due to their enormous metabolic diversity are known to be key players in biogeochemical cycling. Their roles in improving the uptake of major nutrients by plants are well-known and understood. Enrichment of edible crops with Fe and Zn can be achieved through microorganisms by any of the three following strategies—(a) increased availability of micronutrients due to microbial activity such as production of acids, chelators, and phytohormones; (b) microbe-mediated modulation of micronutrient transporters; and (c) de-complexation of micronutrients from compounds like phytate through microbial activity during postharvest processing. Microbe-mediated biofortification can potentially complement the agronomic and genetic biofortification of staple crops.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Since the inception of green revolution, productivity of cereal crops increased more than double to sustain the growing population demand of food supply. Development of high-yielding varieties with stress-tolerant traits had been the major means to increase the crop productivity. However, most of the early works on varietal development focused solely on productivity instead of quality. Our aim to combat caloric malnutrition (acute hunger) however undermined the so-called hidden hunger caused due to deficiency of some minerals and vitamins.
Micronutrients are low in majority of the staple crops such as rice, wheat, and potato on which more than half of the global population are dependent. Bioavailability (uptake) of Fe and Zn by the plant from the soil and bioavailability (absorption) by humans from the plant food are limited. Due to their involvement in multitude of biological functions, deficiency of Fe and Zn are widely distributed especially in developing nations. Apart from inherent low bioavailability of Fe and Zn in cereals, postharvest processes, viz. polishing, milling, and pearling, also lower the amount of these micronutrients (Borg et al. 2009). Moreover, anti-nutritional factors, for example, phytates and tannins, may further lower the absorption of Fe and Zn by humans from plant foods (White and Broadley 2005; Brinch-Pedersen et al. 2007; Pfeiffer and McClafferty 2007). It is estimated that more than 2.6 billion people are iron-deficient (WHO 2019; Cacoub et al. 2020) while approximately one-third of the world human population are at risk of zinc deficiency (Lilay et al. 2020). Application of specific micronutrient-bearing chemical fertilizers had been tried but was not effective as they form complexes in soil which cannot be taken up by plants efficiently. Among other interventions to combat micronutrient deficiencies in humans are supplementation with pharmacological (high) doses for treatment of deficiencies (Fe, Zn, vit A and D), and food fortification of cereals (Fe, folic acid) or oils, and margarine (vit A, vit D) for prevention of deficiencies. These strategies are frequently successful. Dietary diversification is often recommended but has had little success because the low-income populations usually cannot afford the recommended foods (e.g., animal source foods for Fe and Zn) and lack of robust distribution systems, and crop seasonality. Biofortification has now emerged as an efficient strategy to sustainably enhance the level of micronutrients in staple food crops and is cost-effective and nutrient reaches the target people in natural form. A number of strategies like conventional and molecular breeding, genetic modification, and agronomic and/or soil management have been applied to increase the micronutrient levels in food crops. As most of the micronutrients present in soil remain in inaccessible form, breeding strategies may not always work to make them available for plants as a variety/line may not achieve its genetic potential when the nutrients are not present in bioavailable form in soil. Microorganisms which remain in close association to the plants and key to the biogeochemical cycling hold considerable promise for biofortification. Application of microorganisms as a part of soil management can make the unavailable nutrients available to plants and can also modulate the specific transporters for enhanced uptake. However, the validated and published reports in this area are very few which warrant a significant emphasis to develop microbe-based effective strategies for micronutrient biofortification. In the present review, we will discuss the status and prospects of microbe-mediated biofortification in comparison to other available strategies.
2 Iron and Zinc in Human Nutrition
Iron is an essential component of hemoglobin and myoglobin. It is also required for a number of biochemical reactions and enzyme systems including energy metabolism, cell division, production of neurotransmitters, formation of collagen, and immune system function (Edison et al. 2008). The recommended dietary allowance (RDA) of iron among non-vegetarians based on an estimated iron bioavailability of 18% for a mixed diet which includes animal products ranges from 0.27 to 10 mg/day for children, 8 to 11 mg/day for adult males, 8 to 18 mg/day for adult females, and 9 to 27 mg/day for lactating and pregnant females (Institute of Medicine 2001). On the other hand, RDA for vegetarians is almost 1.8 times that of the non-vegetarians as meat-derived heme iron is more bioavailable than non-heme iron from plant-based foods due its lower (2–10%) absorption (Trumbo et al. 2001). RDA of iron among vegetarians ranges from 11.5 to 13.7 mg/day for children, 15.1 to 27 mg/day for teens, 16.3 to 18.2 mg/day for adult males, 12.3 to 32.4 mg/day for adult females, 48.6 mg/day for pregnant women, and 16.2 mg/day for lactating women (U.S. Department of agriculture 2012). People consuming a phytate-rich diet with little animal tissue food or vitamin C from fruits and vegetables are at higher risk of iron deficiency (ID). Pregnant women and children who have higher requirements for growth are also at risk of ID. It has been reported that almost 50% of the pregnant women worldwide are anemic due to ID where in country like India, almost 88% of pregnant women are anemic (Lopez et al. 2016).
Like plants, human also require Zn for growth and development. Kumssa et al. (2015) have reported that average per capita Zn supply is ~ 16.3 mg/capita/day while more than two billion people are at risk of Zn deficiency. Children and pregnant and lactating women require higher amounts of Zn and, hence, are at higher risk of zinc deficiency (Reeves and Chaney 2008; Boonchuay et al. 2013). Along with Fe, and vitamin A and I deficiencies, Zn deficiency was incorporated as a major global risk in 2002 (WHO 2002). Zn deficiency in human causes lack of taste, decreased fertility, impaired cognitive function, decreased work capacity, and stunting of growth and increases susceptibility to infections (Prasad 2009; Barnett et al. 2010; Cakmak et al. 2010).
3 Iron and Zinc in Crop Plants
Micronutrients are required in minute quantities by plants but play a significant role in plant nutrition as they are necessary as cofactors and involved in many metabolic functions. Iron is essential for a number of cellular functions in plants, involving in photosynthesis, respiration, biosynthesis of chlorophylls, DNA, hormones etc. (Hansch and Mendel 2009; Kobayashi and Nishizawa 2012). Ferric iron (Fe3+) and ferrous iron (Fe2+) are the most common forms of iron found in the earth crust (Hori et al. 2015). Fe3+ is insoluble and its uptake is difficult; Fe2+ is soluble and readily available to plants. In general, a neutral to alkaline soil pH (7.4–8.5) causes a low solubility and slow dissolution of iron-bearing minerals. Higher bicarbonate levels, which are prevalent in calcareous soils, reduce the iron uptake by plants grown on alkaline soils. Under aerated and alkaline soils, Fe is oxidized as insoluble iron oxides, but in flooded soils where oxygen diffusion is limited, pH decreases and ferric ions are reduced to ferrous forms (Morrissey and Guerinot 2009). Besides low iron availability and uptake from soils, partitioning of iron in shoots and seeds further reduces the Fe content in seeds especially in cereals. The level of remobilization from shoot to seed varies from plant to plant; for example, in rice, only 4% of shoot Fe is mobilized to the seeds.
Majority of the cereal staple crops are low to moderate in iron content (Fig. 1a). In whole grains of wheat, Fe is present in the range of 29–73 mg/kg (Rengel et al. 1999; Cakmak et al. 2004). As only 25% of these nutrients are localized in endosperm, major fraction of the nutrients present in other parts is lost during milling (Slavin et al. 2001; Ozturk et al. 2006). Iron concentration in brown rice ranges from 6.3 to 24.4 mg/kg, and thus, the iron intake will be 3.78–14.64 mg assuming a maximum of 600-g rice consumed daily while polished rice contains only ~ 2 mg iron/kg; thus, the iron intake will be 1.2 mg/day (Lichtenstein et al. 2006). The amount needed in a country like India, with higher RDA values, would likely be even higher, making postharvest fortification of rice a more potential strategy than biofortification. Other cereal flours have a much higher Fe concentration than rice which makes biofortification with plant breeding and agronomic techniques a more plausible strategy.
Zinc is essential for a number of metabolic functions in plants such as oxidative reactions, structural and catalytic activities, membrane stability, DNA replication, translation, and energy transfer reactions (Broadley et al. 2011; Gurmani et al. 2012). A number of key enzymes, viz. hydrogenase, carbonic anhydrase, Cu/Zn super oxide dismutase (SOD), and RNA polymerase, also require Zn for their catalytic activity (McCall et al. 2000). While Zn deficiency is quite common in plants, Zn toxicity is rare. Zinc deficiency during plant growth and development results in lower Zn content in fruits and grains. To fulfill the human nutritional requirement, the optimum grain Zn concentration should be 50 μg g−1 dry weight; however, the current status is 20–30 μg g−1 dry weight (Cakmak 2008) (Fig. 1b). Therefore, biofortification strategies are required to double the zinc concentration in the grains to provide the daily amount of zinc needed. Low plant-available Zn was reported for soils of various characteristics: extreme pH, high and low organic matter, calcareous, sodic, sandy, wetland or ill-drained, limed acid soils, etc. Reducing conditions as well as low pH favors conversion of non-available (non-toxic) Fe3+ into plant-available Fe2+ ions (Genon et al. 1994) that is toxic if present at high ionic activity (Rengel 2002; Khabaz-Saberi and Rengel 2010).
4 Biofortification of Iron and Zinc Levels in Crop Plants: Strategies and Issues
Different plants show differential ability to uptake Fe and Zn, for example, rice, maize, and sorghum are known to be highly susceptible to zinc deficiency while wheat, oat etc. have been reported to be zinc-efficient. High phytic acid in food crops is reported to inhibit the absorption of Fe and Zn by human from these foods (Graham et al. 2001). Negatively charged phytic acids strongly chelate with divalent micronutrients such as Zn2+, Fe2+, Ca2+, and Mg2+ and reduce the bioavailability of these micronutrients for humans due to the absence of phytase enzyme in their digestive tract. Food processing can also decrease the level of iron and zinc in the grain when the outer layers are removed. For example, in rice, Zn and Fe are localized in aleurone layer and embryo of grains, which is removed during polishing and milling, thus reducing the amount of available Zn and Fe in the edible part of rice (endosperm) (Haas et al. 2005; Zimmermann and Hurrell 2007). However, this can also lead to better absorption of iron as phytic acid is also localized in the outer layers. The most economical approach to alleviate micronutrient deficiency in crop plants is biofortification, a process through which the content and bioavailability of essential nutrients in staple crops are increased during plant growth through agronomic approaches, conventional or molecular breeding, genetic engineering, or any other means (Petry et al. 2010; Tiwari et al. 2010; Bouis et al. 2011; El-Mekser et al. 2014). Biofortification has been mainly focused on starchy staple crops (rice, wheat, maize, sorghum, millet, sweet potato etc.) (Hussain et al. 2012; Saltzman et al. 2013). Agronomic biofortification is achieved through soil or foliar application of micronutrients in the form of chemical fertilizers while genetic biofortification involves either conventional breeding or genetic engineering to enhance micronutrient sequestration or reduction in inhibitors (Saltzman et al. 2013). Agronomic biofortification for Fe and Zn has been reviewed earlier in detail by others (Cakmak 2008; Prasad et al. 2014; de Valenca et al. 2017; Cakmak and Kutman 2018).
Crop biofortification by breeding has been a popular means to develop biofortified varieties in developing countries (Graham et al. 2007). Genotypic variation in major crops showing broad range of Fe and Zn content has been exploited through breeding programs to develop Fe- and Zn-rich crop varieties. Different genotype of various crops have been reported with varying concentration of micronutrients in grains, for example, rice genotypes vary from 6 to 24 μg/g iron in their grains (Gregorio et al. 2000; White and Broadley 2005) while in wheat, iron concentration ranges from 25 to 56 μg/g (Monasterio and Graham 2000). Table 1 presents a list of some prominent biofortified crop varieties developed and released globally. Breeding for biofortification has made a significant impact on alleviating micronutrient malnutrition in many Asian and African countries. Globally coordinated initiatives like Harvest Plus have developed and released a large number of biofortified (for Fe, Zn, and vitamin A) varieties of 13 different crops in Asia, Africa, and Latin America (HarvestPlus 2019).
In the absence of genetic variation in micronutrient content among varieties, breeding approaches are not successful. Under such conditions, genetic engineering becomes more apt for biofortification (Brinch-Pedersen et al. 2007). However, this approach requires a deep understanding of metabolic pathways, enzymes, and genes involved in micronutrient transport and sequestration. Introduction of novel genes of prokaryotic or eukaryotic origin and engineering metabolic pathways are the key approaches for genetic engineering–guided biofortification. Genetic biofortification through breeding or genetic engineering has been reviewed in details by Rawat et al. (2013), Vasconcelos et al. (2017), and Cakmak and Kutman (2018).
Plant breeding approach of biofortification is comparatively a better approach for micronutrient fortification in grains, but it still depends on soil factors such as soil pH, moisture, and soil nutrient content. Although it is a sustainable and economical approach, it is still labor- and time-intensive. Stein (2006) estimated that breeding high Zn-accumulating varieties would be three times more cost-effective than agronomic fortification but the grain accumulation of Zn depends on the amount plant-accessible Zn stores in the soil. Genetic engineering may overcome the problem of low genetic variation but has low social acceptance. Different time taking regulatory processes in different countries have also hindered the adoption of genetically engineered crops (Garg et al. 2018).
Although agronomic and genetic biofortification are still the most practiced way for micronutrient enhancement in edible crops, they cannot be universally applied for all crops and all geographical regions. For example, agronomic biofortification using Zn- and Fe-containing fertilizers is highly influenced by soil types and conditions (Cakmak 2008). Low soil moisture, high pH, high CaCO3 content, and low amount of organic matter severely decrease solubility and availability of Zn and Fe in the soil. Most of the soil-applied micronutrients are quickly fixed into plant unavailable form; as a consequence, sufficient uptake of Zn or Fe is hindered and grain mineral concentrations are significantly depressed (Cakmak 2008). Use of iron fertilizer is complicated due to low solubility of iron and low mobility through phloem. However, this can be tackled through use in large quantities or when expensively chelated to organic molecules. Many of the chelates are expensive while many are toxic and non-biodegradable. For iron, foliar applications have been reported to be the most effective. But foliar application of micronutrients is strongly influenced by factors such as wind (which may cause variability in spray deposition) and soil moisture. Furthermore, it is not always possible to target the micronutrient into fruits, seeds, or grains, and sometimes, they may accumulate in other parts, for example, leaves; therefore, this technique is only successful in certain minerals and specific plant species (Cakmak et al. 1999).
5 Microorganisms—a Promising Option for Micronutrient Biofortification
Due to their immense metabolic diversity, microorganisms are present in any habitat we can imagine. Microbial activities influence primary productivity, plant and animal diversity, and Earth’s climate (Nazaries et al. 2013). In soil, microorganisms are the key players in biogeochemical cycling of the nutrients essential for the survival of any living entity on the earth. The role of microorganisms in decomposition of organic matters, biological nitrogen fixation, denitrification, phosphate solubilization etc. is well-known. By virtue of their metabolic multiplicity, microorganisms can produce a number of metabolites such as phytohormone, antibiotics, organic acids, and siderophores, helping in plant growth directly or indirectly.
Rhizospheric soil harbors more number of microorganisms in comparison to the bulk soil due to secretion of sugars, amino acids, vitamins etc. in the root zone. The microorganisms colonizing the rhizosphere help in nutrient mobilization, root growth, protection from abiotic, and biotic stresses. Microbial production of organic acids and siderophores has been implicated as major means of nutrient solubilization in soil. Reduction of pH in microhabitats due to secretion of organic acids by microorganisms helps to solubilize nutrients such as P, K, and Zn which are complexed with other metals or ions. Siderophores produced by microorganisms can increase the solubility of Fe by chelation to form siderophore-Fe complex, and this process has been regarded as the key microbial process involved in Fe uptake by plants (Desai and Archana 2011). Production of phytohormones, for example, IAA and cytokinin, by microorganisms can significantly influence root architecture and anatomy, thereby enabling the plants to uptake nutrients efficiently. Apart from the rhizospheric microorganisms, endophytes also play similar functions which can help to enhance the uptake of nutrients in plants. Since last one decade, there are increasing numbers of reports on microbe-mediated Zn and Fe biofortification (Table 2). In comparison to other biofortification strategies, microbe-mediated biofortification is an environment-friendly, cheaper, and sustainable alternative to enrich food crops with micronutrients. Moreover, microbe-mediated fortification strategies can offer additional advantages like overall improvement in growth and protection from stresses.
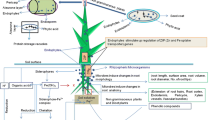
The following sections will discuss the potential of microbe-mediated biofortification with specific examples along with their possible mechanisms. Figure 2 presents different mechanisms employed by microorganisms to improve uptake of Fe and Zn in plants. The major mechanisms related to microbe-mediated Fe and Zn uptake are presented below.
5.1 Production of Siderophores and Other Chelators
Under iron-depleted conditions, microorganisms produce siderophores which are low molecular weight organic compounds with strong affinity to Fe. At first, the siderophores form complex with Fe3+, and then, this complex moves into the cell through specific receptors located in cell membrane. Siderophore-iron complex transport in the cell membranes of gram-positive bacteria is assisted by siderophore-binding proteins, permeases, and ATPases while in gram-negative bacteria, the same is mediated by an outer membrane receptor, a periplasmic binding protein, and a cytoplasmic ABC-transporter (Ahmed and Holmstrom 2014). Once this siderophore-iron reaches the cytoplasm, the ferric iron gets reduced to ferrous form and released from the siderophore. Siderophores of microbial origin have conclusively been shown to be an important factor in iron nutrition of plants (Bar-Ness et al. 1992; Desai and Archana 2011). Fe-siderophore complex can be reduced by the plants using strategy I for Fe uptake and thus the released Fe2+ becomes accessible to plant transport system. The Fe-Siderophore complex can directly be taken up by the plants and afterwards reduced by extracellular reductases (Wang et al. 1993). In some cases, the Fe-siderophore complex may be taken up directly and transported to the shoot (Manthey et al. 1996). Reports also suggested that some plants may have specific transporters for such siderophore complexes. It has also been reported that the Fe-siderophore complex may enter to the plant system through the cracks developed due to lateral root emergence. In strategy II plants where phytosiderophores are produced by plants, it has been suggested that microbial siderophores can also exchange the bound ligand with the phytosiderophores. Rasouli-Sadaghiani et al. (2014) reported that Pseudomonades could enhance the iron nutrition of wheat either by increasing the Fe supply on root surface or ligand exchange with wheat siderophores. Similarly, Scagliola et al. (2016) reported that siderophores produced by Enterobacter sp. BFD160 and Pseudomonas sp. TFD26, complex ferric ion, and via a ligand exchange with plant siderophores, make it available to the plasma membrane FCR. Sah et al. (2017) reported increased accumulation of iron in maize grains upon inoculation with siderogenic Pseudomonas aeruginosa which could enhance the amount of plant-available Fe in soil through production of siderophores. Rice endophytic Streptomyces sp. has also been reported to be involved in the iron nutrition of rice (Rungin et al. 2012). Gopalakrishnan et al. (2016) also implicated siderophore-mediated Fe solubilization in Fe fortification in chickpea and pigeon pea upon inoculation with plant growth-promoting bacteria.
Apart from siderophores, microbes are known to produce other metal chelators which can increase the availability of certain metal ions. Whiting et al. (2001) suggested that many bacteria produce Zn-chelating metallophores for increasing water-soluble Zn (bioavailable) in soil. Mastropasqua et al. (2017) reported that Pseudomonas aeruginosa produce a metallophore which is released outside the cell and mediates zinc uptake through a receptor. Lhospice et al. (2017) implicated pseupaline metallophore as primary mediator of Zn uptake in chelating environment. Chelators have also been attributed towards increased mobilization and bioavailability of Zn in rice by Azospirillum lipoferum, Pseudomonas sp., and Agrobacterium sp. (Tariq et al. 2007).
5.2 Zinc Solubilization
In soil, Zn is present in the form of sulfates, oxides, carbonates, silicates, sulfides etc. pH is an important factor which governs the proportion of plant-available zinc forms in soil. In general, Zn solubility increases with a reduction in soil pH. At alkaline pH, Zn becomes fixed in the form of insoluble carbonates, sulfides, phosphates etc. and proportion of the bioavailable zinc pool decreases. Microbe-mediated solubilization of Zn can be accomplished by a range of mechanisms such as excretion of organic acids, proton extrusion, or production of chelators (Goteti et al. 2013). Additionally, inorganic acids, viz. sulfuric acid, nitric acid, and carbonic acid, can also facilitate the solubilization process. Microorganisms can produce an array of organic acids which can reduce the soil pH in and around the rhizosphere, thereby releasing the Zn to make it bioavailable to plants (Table 3). Mostly, gluconic and ketogluconic acid has been reported to be associated with Zn solubilization (Fasim et al. 2002; Saravanan et al. 2007a; Saravanan et al. 2007b; Sunithakumari et al. 2016). Gluconic acid has been reported to be produced by bacteria across various phyla like Firmicutes, Protobacteria, and Actinobacteria (Table 3). However, production of citric acid, malic acid, oxalic acid, tartaric acid, formic acid, and acetic acid has also been reported to be associated with Zn solubilization by bacteria from different phyla (Martino et al. 2003; Li et al. 2010; Sah et al. 2017). Increased zinc uptake by plants can lead to enhancement in grain zinc accumulation. For example, organic acid production has been implicated in enhanced uptake and accumulation of zinc by Enterobacter cloaceae MDSR9 in soybean (Ramesh et al. 2014a); by Pseudomonas fluorescens strain Psd in wheat (Sirohi et al. 2015); and by Exiguobacterium aurantiacum in wheat (Shaikh and Saraf 2017).
5.3 Modulation of Plant Nutrient Uptake Systems
Microorganisms can produce an array of signals which can modulate the expression of the genes involved in nutrient uptake and transport. A number microorganisms including endophytes and mycorrhiza have been reported to possess the capability to modulate the uptake systems for a variety of plant nutrients especially N, P, and Na (Zhang et al. 2009; Saia et al. 2015; Liu et al. 2018). There are reports on microbial influence on plant transporters of micronutrients like Zn and Fe which are, however, lesser in number as compared to N and P. Zhang et al. (2009) showed that activation of iron-induced transcriptional regulator (FIT) by Bacillus subtilis GB03 in Arabidopsis resulted in upregulation of ferric chelate reductase (FRO) and iron-regulated transporter (IRT1), thereby increasing the iron absorption. Similar observations were also made by Zhou et al. (2016) in Arabidopsis plants when inoculated with Paenibacillus polymyxa BFKC01, which activated FIT leading to upregulation of FRO and IRT1. Pii et al. (2016) reported upregulation of CsFRO (ferric chelate reductase) and CsHA1 (PM H+ ATPase) in cucumber upon inoculation with Azospirrilum brasiliense which resulted in increased Fe uptake through iron reduction on root surface and rhizosphere acidification. Krithika and Balachandar (2016) reported that inoculation of Zn-solubilizing bacteria, Enterobacter cloaceae, resulted in modulation of ZIP (ZRT, IRT-like proteins) genes in rice. Likewise, endophytes have also been reported to potentially modulate the metal transporters in crop plants. For example, over expression of TaZIP (Zn-Fe transporter like protein in wheat) genes was implicated in endophytic Arthrobacter-mediated Fe and Zn uptake in wheat (Singh et al. 2017). Endophytic fungi Phomopsis liquidambari has been reported to upregulate FRO and IRT1 genes which was also associated with significant increase in Fe accumulation in the roots, stems, and leaves of groundnut (Su et al. 2019).
5.4 Microbe-Mediated Dephytinization of Food Crops
Phytates vary from ~ 0.4 to 2.0% in cereals and legumes (Reddy et al. 1982). Six phosphate groups of phytates carry twelve negative charges which can bind to cations such as Ca, Mg, Fe, Zn, Cu, and Mn to form stable complexes resulting in low bioavailability of these minerals to humans. Phytases can be used to remove the phosphate groups from phytic acid which prevents binding of Fe and Zn in the digestive tract of humans and thus making these minerals available for absorption.
Diverse microorganisms produce diverse phytases, viz. cysteine phytases (CPhy), histidine acid phosphatases (HAP), beta propeller phytases (BBP), and purple acid phosphatases (PAP). HAPs are more predominant in filamentous fungi while BBPs are most prevalent in bacteria (Jorquera et al. 2008; Singh and Satyanarayana 2015). Among bacteria, Proteobacteria have been reported to possess all different types of phytases. A number of studies have reported exogenous application of microbial phytases in feeds for enhancing mineral availability in animals, fish, and birds (Mohanna and Nys 1999; Brenes et al. 2003; Baruah et al. 2005; Nwanna et al. 2008). Similarly, it has also been used in human foods for improving mineral absorptions (Troesch et al. 2013). Hurrell et al. (2003) reported that dephytinization using Finase (a commercial phytase from Trichoderma reesei) in various cereal porridges increased iron absorption from rice, oat, maize, and wheat porridge by ~ 3-, ~ 8-, ~ 5-, and ~ 11-folds. Dephytinization of wheat- and soy-based foods also resulted in higher zinc absorption in adult human subject from Switzerland (Egli et al. 2004). However, in all of these studies, microbial phytase have been used either during food processing or as an ingredient of the food. Microorganisms such as Lactobacillus pentosus have been successfully used to remove phytates from seed coat matter (SCM) of finger millets ultimately resulting in increased bioavailability of Zn (Amritha et al. 2018). Phytate-degrading microorganisms have been reported as endophytes in different plant parts including seeds (Mehdipour-Moghaddam et al. 2010; Costa et al. 2018; Zhu et al. 2019). Especially, phytate-degrading seed endophytes can be very useful in dephytinization. Phytase activity has been reported by endophytic bacteria isolated from seeds of bean (Rhizobium endophyticum) and maize (Pantoea stewartii) (Lopez-Lopez et al. 2010; Hafsan et al. 2018). However, adequate studies have still not been carried out with specific focus on microbe (especially endophytes)-mediated dephytinization of food grains despite sufficient scientific evidences suggesting their significant potential.
5.5 Modification of Root Architecture
Root is the main nutrient-absorbing part of the plants, and mineral nutrition is a function of root growth. Higher numbers of fine roots is very important for mineral absorption. Plants with more fine roots can explore a large volume of soil to efficiently take up small amounts of immobile micronutrients. Longer and finer root systems in early growth stage have been reported as the two characters associated with Zn-efficient genotypes (Dong et al. 1995).
It is a well-known fact that microbial activity can greatly influence the root growth and development through production of phytohormones and other metabolites. Moreover, mycorrhizal infection of roots is also known to enhance the nutrient uptake capacity of the plants. Wang et al. (2014) reported modulation of root morphology as one of the mechanisms involved in endophyte-enhanced metal uptake and accumulation in rice plants which was evident from the improved architecture of rice roots in presence of endophytic bacteria. In this study, the Zn content in brown rice from inoculated plants ranged from 30.0 to 31.0 mg/kg which is ~ 25% improvement over normal Zn content (6.3–24.0 mg/kg) in brown rice. Singh et al. (2017) observed that inoculation of endophytic bacteria, viz. B. subtilis and Arthrobacter sp., resulted in significant improvement in root length, surface area, volume, and diameter which might have helped the wheat plants to accumulate more zinc in grains. They reported that the grain Zn content in inoculated plants ranged from 50.0 to 66.2 mg/kg which was well beyond the target of 50 mg Zn/kg.
Mycorrhizal association with plants and their contribution in plant nutrition are well-known. Ninety percent of plant species are known to establish a mycorrhizal association (Smith and Read 2008). The most common type of mycorrhiza is the arbuscular mycorrhiza with fungal members of Glomeromycota. The fungus forms an appressorium penetrating the root cortex where it moves through the intercellular spaces and develops a network with formation of arbuscules. Nutrients are exchanged across the periarbuscular and host cell membranes, with which arbuscules make a close contact (Rausch et al. 2001; Javot et al. 2007a; Javot et al. 2007b). It is estimated that mycorrhiza can transfer more than 90% of the P and more than 50% of the fixed N to their host in exchange of photosynthates (Smith and Smith 2011). Besides translocation of major nutrients, arbuscular mycorrhizal roots are also known to help in micronutrient transportation in plants. For instance, zinc content in tomato fruits was 50% higher in mycorrhiza-colonized plants (Cavagnaro et al. 2006). Furthermore, it has been reported that during mycorrhiza-mediated micronutrient delivery, expression of nutrient transporter genes in plant decreases. For example, cortical ZIP in Medicago truncatula gets downregulated upon mycorrhization (Burleigh et al. 2003). The contribution of the mycorrhiza in plant metal nutrition may range from 20 to 50% (Ortas 2012; Lehmann et al. 2014). In addition, AMF genome encodes several metal transporters (Tamayo et al. 2014), some of which could be involved in metal uptake. Piriformospora indica (belonging to order Sebacinales of family Serandipitaceae) which resembles AM fungi, owing to its ability to colonize and interact with a wide variety of unrelated host plants, holds a tremendous practical application as nutrient mobilizer. It offers multiple benefits to its host plants such as nutrient uptake, growth promotion, abiotic stress alleviation, growth promotion, and disease resistance (Unnikumar et al. 2013). Inoculation with P. indica under zinc supplementation has been shown to increase Zn concentration in lettuce plants (Padash et al. 2016). Improved iron uptake has been observed in a medicinal herb Chlorophytum sp. inoculated with P. indica and Pseudomonas fluorescens (Gosal et al. 2010). Although there are only two reports on biofortification using P. indica, the beneficial roles played by this endophyte such as root development and nutrient uptake indicate that P. indica can be a potential candidate to be used for biofortification.
5.6 Improving Overall Plant Growth and Nutrition
Microorganisms are well-known for their role in biogeochemical cycling of nutrients and improvement of plant growth through various direct and indirect mechanisms. Microbes are the key mediators of nutrient cycling in soil and responsible for maintaining the soil fertility. In soil, the macro- and micronutrients interact in a complex way interfering with each other’s uptake by plant. It is known that level of nitrogen in soil can significantly influence the uptake of micronutrients such as Fe and Zn. Kutman et al. (2011) reported that nitrogen nutrition was critical for uptake and allocation of Fe and Zn in wheat. Similar findings were also reported by Xue et al. (2014). Excessive application of phosphorus can reduce the micronutrient uptake and allocation (Nyoki and Ndakidemi 2014; Zhang et al. 2017). Zribi et al. (2015) showed that symbiosis of Sinorhizobium meliloti with Medicago sativa resulted in higher accumulation of zinc in roots. Studies carried out by Kumar et al. (2014) revealed that inoculation of siderogenic bacteria along with diazotrophic Arthrobacter resulted higher gain accumulation of iron in wheat as compared to inoculation only with siderogenic bacteria. Praburaman et al. (2017) showed that N2-fixing plant growth-promoting Herbaspirillum sp. GW103 can enhance Zn accumulation in maize. Rana et al. (2012) reported grain Fe content of 271.93 mg/kg in wheat upon inoculation of Providencia sp. PW5 and attributed this improvement towards better N nutrition due to inoculation. It is evident from such studies that microbial inoculation can achieve the micronutrient levels in grains well beyond the desired level.
6 Future Prospects
Deficiency of micronutrients particularly Fe and Zn is a grave concern for the entire world, especially for the Asian and African countries. Efforts are being made to enrich the foods including the staple crops with iron and zinc through agronomic or genetic biofortification. Agronomic biofortification is not any permanent solution to this problem while genetic methods including breeding strategies are time- and cost-intensive. Microorganisms due to their huge metabolic diversity, known role in biogeochemical cycling, and intricate interaction with plant and soil can be a better choice to mobilize micronutrients to the plants. From various reports of microbe-mediated biofortification around the globe, it is clear that microbial inoculation can improve grain Zn content in a range of 20–50% in rice, 30–80% in wheat, and 80–100% increase in grain Fe content in wheat depending on type of soil and plant. This level of enhancement of Fe and Zn can definitely help to combat deficiency. If we assume a conservative 30% increase in Zn content of brown rice achieved by microbial inoculation, then it can supply additional 1.05–4.35 mg Zn daily assuming consumption of 600-g rice.
Enrichment of edible crops with Fe and Zn can be achieved through microorganisms by any of the three following strategies—(a) increased availability of micronutrients due to microbial activity like production of acids, chelators, and phytohormones; (b) microbe-mediated modulation of micronutrient transporters; and (c) de-complexation of micronutrients from compounds such as phytate through microbial activity during postharvest processing. However, higher micronutrient availability alone may not be effective enough to fortify the edible parts as the activity of specific transporters should be modulated to accumulate higher amounts of micronutrients. Soil or rhizosphere inhabiting microorganisms seem to be more potent to increase the soil availability of micronutrients and their uptake by plant roots while the endophytic microorganisms may be more suitable to influence the uptake and transportation. Furthermore, endophytes can also be helpful for degradation of anti-nutritional factors like phytate in seeds/grains and thus improving the bioavailability upon consumption. Endophytes from wild plants having high Fe and Zn content need to be explored for such activities. Application of microorganisms for biofortification should be carried out after thorough examination of interaction among potential microbes, crop genotypes with varying accumulation pattern, and soils with differing micronutrient status. Breeding approaches can also be focused on selecting genotypes preferentially harboring higher population of potential endophytes or rhizobacteria with micronutrient-mobilizing capability. However, the application of microorganism cannot be the sole solution for combating the hidden hunger. Integration of genetic as well as agronomic biofortification should be explored to work out a viable, economical, and sustainable option for biofortification.
References
Ahmed E, Holmstrom SJM (2014) Siderophores in environmental research: roles and applications. Microb Biotechnol 7:196–208. https://doi.org/10.1111/1751-7915.12117
Amritha GK, Dharmaraj U, Halami PM, Venkateswaran G (2018) Dephytinization of seed coat matter of finger millet (Eleusine coracana) by Lactobacillus pentosus CFR3 to improve zinc bioavailability. Lwt 87:562–566. https://doi.org/10.1016/j.lwt.2017.09.024
Baloch QB, Mujahid MY, Noreen S, Makhdum MI (2018) Biofortified high zinc wheat: the traditional staple dietary food to address malnutrition in Pakistan. Int J Endorsing Health Sci Res 6:31–38. https://doi.org/10.29052/IJEHSR.v6.i1.2018.31-38
BARI (2017) Proforma for obtaining approval of the National Seed Board of Bangladesh (NSB) for a new crop variety/cultivar. Bangladesh Agricultural Research Institute (BARI), and National Seed Board of Bangladesh (NSB), Dhaka
Bar-Ness E, Hadar Y, Chen Y, Shanzer A, Libman J (1992) Iron uptake by plants from microbial siderophores: a study with 7-nitrobenz-2-oxa-1, 3-diazole-desferrioxamine as fluorescent ferrioxamine B analog. Plant Physiol 99:1329–1335. https://doi.org/10.1104/pp.99.4.1329
Barnett JB, Hamer DH, Meydani SN (2010) Low zinc status: a new risk factor for pneumonia in the elderly? Nutr Rev 68:30–37. https://doi.org/10.1111/j.1753-4887.2009.00253.x
Baruah K, Pal AK, Sahu NP, Jain KK, Mukherjee SC, Debnath D (2005) Dietary protein level, microbial phytase, citric acid and their interactions on bone mineralization of Labeo rohita (Hamilton) juveniles. Aquac Res 36:803–812. https://doi.org/10.1111/j.1365-2109.2005.01290.x
Bashar KM (2018) Moving from agriculture to food through biofortification. HarvestPlus – Bangladesh, In SAFANSI Round Table, Colombo
Boonchuay P, Cakmak I, Rerkasem B, Prom-U-Thai C (2013) Effect of different foliar zinc application at different growth stages on seed zinc concentration and its impact on seedling vigor in rice. Soil Sci Plant Nutr 59:180–188. https://doi.org/10.1080/00380768.2013.763382
Borg S, Brinch-Pedersen H, Tauris B, Holm PB (2009) Iron transport, deposition and bioavailability in the wheat and barley grain. Plant Soil 325:15–24. https://doi.org/10.1007/s11104-009-0046-6
Bouis HE, Hotz C, McClafferty B, Meenakshi JV, Pfeiffer WH (2011) Biofortification: a new tool to reduce micronutrient malnutrition. Food Nutr Bull 32:S31–S40. https://doi.org/10.1177/15648265110321S105
Brenes A, Viveros A, Arija I, Centeno C, Pizarro M, Bravo C (2003) The effect of citric acid and microbial phytase on mineral utilization in broiler chicks. Anim Feed Sci Technol 110:201–219. https://doi.org/10.1016/S0377-8401(03)00207-4
Brinch-Pedersen H, Borg S, Tauris B, Holm PB (2007) Molecular genetic approaches to increasing mineral availability and vitamin content of cereals. J Cereal Sci 46:308–326. https://doi.org/10.1016/j.jcs.2007.02.004
Broadley M, Brown P, Cakmak I, Rengel Z, Zhao F (2011) Function of nutrients: micronutrients. In: Marschner’s mineral nutrition of higher plants, 3rd edn. Academic Press, pp 191–248. https://doi.org/10.1016/B978-0-12-384905-2.00007-8
Burleigh SH, Kristensen BK, Bechmann IE (2003) A plasma membrane zinc transporter from Medicago truncatula is up-regulated in roots by Zn fertilization, yet down-regulated by arbuscular mycorrhizal colonization. Plant Mol Biol 52:1077–1088. https://doi.org/10.1023/A:1025479701246
Cacoub P, Nicolas G, Peoch K (2020) Iron deficiency markers in patients undergoing iron replacement therapy: a 9-year retrospective real-world evidence study using healthcare databases. Sci Rep 10:14983. https://doi.org/10.1038/s41598-020-72057-9
Cakmak I (2008) Enrichment of cereal grains with zinc: agronomic or genetic biofortification? Plant Soil 302:1–17. https://doi.org/10.1007/s11104-007-9466-3
Cakmak I, Kutman UB (2018) Agronomic biofortification of cereals with zinc: a review. Eur J Soil Sci 69:172–180. https://doi.org/10.1111/ejss.12437
Cakmak I, Kalaycı M, Ekiz H, Braun HJ, Kılınc Y, Yılmaz A (1999) Zinc deficiency as a practical problem in plant and human nutrition in Turkey: a NATO-science for stability project. Field Crops Res 60:175–188. https://doi.org/10.1016/S0378-4290(98)00139-7
Cakmak I, Torun A, Ozkan H, Millet E, Feldman M, Fahima T, Korol A, Nevo E, Braun HJ (2004) Triticum dicoccoides: an important genetic resource for increasing zinc and iron concentration in modern cultivated wheat. Soil Sci Plant Nutr 50:1047–1054. https://doi.org/10.1080/00380768.2004.10408573
Cakmak I, Pfeiffer WH, McClafferty B (2010) Biofortification of durum wheat with zinc and iron. Cereal Chem 87:10–20. https://doi.org/10.1094/CCHEM-87-1-0010
Cavagnaro TR, Jackson LE, Six J, Ferris H, Goyal S, Asami D, Scow KM (2006) Arbuscular mycorrhizas, microbial communities, nutrient availability, and soil aggregates in organic tomato production. Plant Soil 282:209–225. https://doi.org/10.1007/s11104-005-5847-7
Costa LE d O, Correa TLR, Teixeira JA, Araujo EF d, Queiroz MV d (2018) Endophytic bacteria isolated from Phaseolus vulgaris produce phytases with potential for biotechnology application. Braz J Biol Sci 5:657–671. https://doi.org/10.21472/bjbs.051105
Costerousse B, Schonholzer-Mauclaire L, Frossard E, Thonar C (2018) Identification of heterotrophic zinc mobilization processes among bacterial strains isolated from wheat rhizosphere (Triticum aestivum L.). Appl Environ Microbiol 84:01715–01717. https://doi.org/10.1128/AEM.01715-17
de Valenca AW, Bake A, Brouwer ID, Giller KE (2017) Agronomic biofortification of crops to fight hidden hunger in sub-Saharan Africa. Global Food Secur 12:8–14. https://doi.org/10.1016/j.gfs.2016.12.001
Desai A, Archana G (2011) Role of siderophores in crop improvement. (2011). In: Maheshwari DK (ed) Bacteria in agrobiology: plant nutrient management, 3rd edn. Springer, Heidelberg, New York, pp 109–139. https://doi.org/10.1007/978-3-642-21061-7_6
Di Simine CD, Sayer JA, Gadd GM (1998) Solubilization of zinc phosphate by a strain of Pseudomonas fluorescens isolated from a forest soil. Biol Fertil Soils 28:87–94. https://doi.org/10.1007/s003740050467
Dinesh R, Srinivasana V, Hamza S, Sarathambal C, Ankegowda SJ, Ganeshamurthy AN, Gupta SB, Nair VA, Subila KP, Lijina A (2018) Isolation and characterization of potential Zn solubilizing bacteria from soil and its effects on soil Zn release rates, soil available Zn and plant Zn content. Geoderma 321:173–186. https://doi.org/10.1016/j.geoderma.2018.02.013
Dong B, Rengel Z, Graham RD (1995) Root morphology of wheat genotypes differing in zinc efficiency. J Plant Nutr 18:2761–2773. https://doi.org/10.1080/01904169509365098
Edison P, Rowe CC, Rinne JO, Ng S, Ahmed I, Kemppainen N, Villemagne VL, O’Keefe G, Nagren K, Chaudhuri R (2008) Amyloid load in Parkinson’s disease dementia and Lewy body dementia measured with [11C] PIB-PET. J Neurol Neurosur Ps 79:1331–1338. https://doi.org/10.1136/jnnp.2007.127878
Egli I, Davidsson L, Zeder C, Walczyk T, Hurrell R (2004) Dephytinization of a complementary food based on wheat and soy increases zinc, but not copper, apparent absorption in adults. J Nutr 134:1077–1080. https://doi.org/10.1093/jn/134.5.1077
El-Mekser HKA, Mohamed ZEOM, Ali MAM (2014) Influence of humic acid and some micronutrients on yellow corn yield and quality. World Appl Sci J 32:1–11. https://doi.org/10.5829/idosi.wasj.2014.32.01.14504
Fasim F, Ahmed N, Parsons R, Gadd GM (2002) Solubilization of zinc salts by a bacterium isolated from the air environment of a tannery. FEMS Microbiol Lett 213:1–6. https://doi.org/10.1111/j.1574-6968.2002.tb11277.x
Garg M, Sharma N, Sharma S, Kapoor P, Kumar A, Chunduri V, Arora P (2018) Biofortified crops generated by breeding, agronomy, and transgenic approaches are improving lives of millions of people around the world. Front Nutr 5:12. https://doi.org/10.3389/fnut.2018.00012
Genon JG, de Hepcee N, Delvaux B, Dufey JE, Hennebert PA (1994) Redox conditions and iron chemistry in highland swamps of Burundi. Plant Soil 166:165–171. https://doi.org/10.1007/BF00008329
Gopalakrishnan S, Vadlamudi S, Samineni S, Sameer Kumar CV (2016) Plant growth-promotion and biofortification of chickpea and pigeonpea through inoculation of biocontrol potential bacteria, isolated from organic soils. SpringerPlus 5:1882. https://doi.org/10.1186/s40064-016-3590-6
Gosal SK, Karlupia A, Gosal SS, Chhibba IM, Varma A (2010) Biotization with Piriformospora indica and Pseudomonas fluorescens improves survival rate, nutrient acquisition, field performance and saponin content of micropropagated Chlorophytum sp. Indian J Biotechnol 9:289–297. https://doi.org/10.1111/j.1600-0625.2006.00439.x
Goteti PK, Emmanuel LDA, Desai S, Shaik MHA (2013) Prospective zinc solubilizing bacteria for enhanced nutrient uptake and growth promotion in maize (Zea mays L.). Int J Microbiol 2013:869697. https://doi.org/10.1155/2013/869697
Govindaraj M, Rai KN (2016) Breeding biofortified pearl millet cultivars with high iron density. Indian Farm 65:53–55
Graham RD, Welch RM, Bouis HE (2001) Addressing micronutrient malnutrition through enhancing the nutritional quality of staple foods: principles, perspectives and knowledge gaps. Adv Agron 70:77–142. https://doi.org/10.1016/S0065-2113(01)70004-1
Graham RD, Welch RM, Saunders DA, Ortiz-Monasterio I, Bouis HE, Bonierbale M, de Haan S, Burgos G, Thiele G, Liria R, Meisner CA, Beebe SE, Potts MJ, Kadian M, Hobbs PR, Gupta RK, Twomlow S (2007) Nutritious subsistence food systems. Adv Agron 92:1–74. https://doi.org/10.1016/S0065-2113(04)92001-9
Gregorio GB, Senadhira D, Htut H, Graham RD (2000) Breeding for trace mineral density in rice. Food Nutr Bull 21:382–386. https://doi.org/10.1177/156482650002100407
Gurmani AR, Jalal-Ud-Din Khan SU, Andaleep R, Waseem K, Khan A, Hadyatullah (2012) Soil application of zinc improves growth and yield of tomato. Int J Agri Biol 14:91–96
Haas JD, Beard JL, Murray-Kolb LE, del Mundo AM, Felix A, Gregorio GB (2005) Iron-biofortified rice improves the iron stores of nonanemic Filipino women. J Nutr 135:2823–2830. https://doi.org/10.1093/jn/135.12.2823
Hafsan H, Masri M, Ahmad A, Agustina L, Natsir N (2018) Phytase activity of four endophytes bacteria from Zea Mays L. 11th international conference on chemical, agricultural, biological and environmental sciences (CABES-2018), Kyoto (Japan). https://doi.org/10.17758/IICBE1.C0418153
Hansch R, Mendel RR (2009) Physiological functions of mineral micronutrients (cu, Zn, Mn, Fe, Ni, Mo, B, cl). Curr Opin Plant Biol 12:259–266. https://doi.org/10.1016/j.pbi.2009.05.006
HarvestPlus (2019) Getting biofortified food on everyone’s plate with 2019 annual report. Published online at https://www.harvestplus.org/knowledge-market/publications. HarvestPlus, Washington, DC, 2019
Hori T, Aoyagi T, Itoh H, Narihiro T, Oikawa A, Suzuki K, Ogata A, Friedrich MW, Conrad R, Kamagata Y (2015) Isolation of microorganisms involved in reduction of crystalline iron(III) oxides in natural environments. Front Microbiol 6:386. https://doi.org/10.3389/fmicb.2015.00386
Hurrell RF, Reddy MB, Juillerat MA, Cook JD (2003) Degradation of phytic acid in cereal porridges improves iron absorption by human subjects. Am J Clin Nutr 77:1213–1219. https://doi.org/10.1093/ajcn/77.5.1213
Hussain S, Maqsood MA, Rengel Z, Aziz T (2012) Biofortification and estimated human bioavailability of zinc in wheat grains as influenced by methods of zinc application. Plant Soil 361:279–290. https://doi.org/10.1007/s11104-012-1217-4
Hussain A, Arshad M, Zahir ZA, Asghar M (2015) Prospects of zinc solubilizing bacteria for enhancing growth of maize. Pakistan J Agric Sci 52:915–922
Institute of Medicine (2001) Food and Nutrition Board. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc: a report of the panel on micronutrients. National Academy Press, Washington DC
Intorne AC, de Oliveira MVV, Lima ML, da Silva JF, Olivares FL, de Souza Filho GA (2009) Identification and characterization of Gluconacetobacter diazotrophicus mutants defective in the solubilization of phosphorus and zinc. Arch Microbiol 191:477–483. https://doi.org/10.1007/s00203-009-0472-0
Islam MS, Rahman MJ, Karim MR, Kabir MA, Qurashi TA (2016) Agronomic performance and farmers perception on zinc enriched rice BRRI Dhan62. Int J Agron Agric Res 9:198–204
Jaivel N, Sivakumar U, Marimuthu P (2017) Characterization of zinc solubilization and organic acid detection in Pseudomonas sp. RZ1 from rice phyllosphere. Int J Chem Stud 5:272–277
Javot H, Penmetsa RV, Terzaghi N, Cook DR, Harrison MJ (2007a) A Medicago truncatula phosphate transporter indispensable for the arbuscular mycorrhizal symbiosis. Proc Natl Acad Sci 104:1720–1725. https://doi.org/10.1073/pnas.0608136104
Javot H, Pumplin N, Harrison MJ (2007b) Phosphate in the arbuscular mycorrhizal symbiosis: transport properties and regulatory roles. Plant Cell Environ 30:310–322. https://doi.org/10.1111/j.1365-3040.2006.01617.x
Jorquera MA, Hernandez MT, Rengel Z, Marschner P, de la Luz MM (2008) Isolation of culturable phosphobacteria with both phytate-mineralization and phosphate-solubilization activity from the rhizosphere of plants grown in a volcanic soil. Biol Fertil Soils 44:1025–1034. https://doi.org/10.1007/s00374-008-0288-0
Joshi D, Negi G, Vaid S, Sharma A (2013) Enhancement of wheat growth and Zn content in grains by zinc solubilizing bacteria. Int J Agric Environ Biotechnol 6:363–370. https://doi.org/10.5958/j.2230-732X.6.3.004
Kamran S, Shahid I, Baig DN, Rizwan M, Malik KA, Mehnaz S (2017) Contribution of zinc solubilizing bacteria in growth promotion and zinc content of wheat. Front Microbiol 8:2593. https://doi.org/10.3389/fmicb.2017.02593
Khabaz-Saberi H, Rengel Z (2010) Aluminum, manganese, and iron tolerance improves performance of wheat genotypes in waterlogged acidic soils. J Plant Nutr Soil Sci 173:461–468. https://doi.org/10.1002/jpln.200900316
Kobayashi T, Nishizawa NK (2012) Iron uptake, translocation, and regulation in higher plants. Annu Rev Plant Biol 63:131–152. https://doi.org/10.1146/annurev-arplant-042811-105522
Krithika S, Balachandar D (2016) Expression of zinc transporter genes in rice as influenced by zinc-solubilizing Enterobacter cloacae strain ZSB14. Front Plant Sci 7:446. https://doi.org/10.3389/fpls.2016.00446
Kumar A, Maurya BR, Raghuwanshi R (2014) Isolation and characterization of PGPR and their effect on growth, yield and nutrient content in wheat (Triticum aestivum L.). Biocatalysis Agric Biotechnol 3:121–128. https://doi.org/10.1016/j.bcab.2014.08.003
Kumssa DB, Joy EJM, Ander EL, Watts MJ, Young SD, Walker S, Broadley MR (2015) Dietary calcium and zinc deficiency risks are decreasing but remain prevalent. Sci Rep 5:10974. https://doi.org/10.1038/srep10974
Kutman UB, Yildiz B, Cakmak I (2011) Effect of nitrogen on uptake, remobilization and partitioning of zinc and iron throughout the development of durum wheat. Plant Soil 342:149–164. https://doi.org/10.1007/s11104-010-0679-5
Lehmann A, Veresoglou SD, Leifheit EF, Rillig MC (2014) Arbuscular mycorrhizal influence on zinc nutrition in crop plants–a meta-analysis. Soil Biol Biochem 69:123–131. https://doi.org/10.1016/j.soilbio.2013.11.001
Lhospice S, Gomez NO, Ouerdane L, Brutesco C, Ghssein G, Hajjar C, Liratni A, Wang S, Richaud P, Bleves S (2017) Pseudomonas aeruginosa zinc uptake in chelating environment is primarily mediated by the metallophore pseudopaline. Sci Rep 7:17132. https://doi.org/10.1038/s41598-017-16765-9
Li WC, Ye ZH, Wong MH (2010) Metal mobilization and production of short-chain organic acids by rhizosphere bacteria associated with a Cd/Zn hyperaccumulating plant, Sedum alfredii. Plant Soil 326:453–467. https://doi.org/10.1007/s11104-009-0025-y
Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, Howard B, Karanja N, Lefevre M, Rudel L, Sacks F, Van Horn L, Winston M, Wylie-Rosett J (2006) Diet and lifestyle recommendations revision 2006: a scientific statement from the American heart association nutrition committee. Circulation 114:82–96. https://doi.org/10.1161/CIRCULATIONAHA.106.176158
Lilay GH, Castro PH, Guedes JG, Almeida DM, Campilho A, Azevedo H, Aarts MG, Saibo NJ, Assunção AG (2020) Rice F-bZIP transcription factors regulate the zinc deficiency response. J Exp Bot 71:3664–3677. https://doi.org/10.1093/jxb/eraa115
Liu C, Ravnskov S, Liu F, Rubaek GH, Andersen MN (2018) Arbuscular mycorrhizal fungi alleviate abiotic stresses in potato plants caused by low phosphorus and deficit irrigation/partial root-zone drying. J Agric Sci 156:46–58. https://doi.org/10.1017/S0021859618000023
Lopez A, Cacoub P, Macdougall IC, Peyrin-Biroulet L (2016) Iron deficiency anemia. Lancet 387:907–916. https://doi.org/10.1016/S0140-6736(15)60865-0
Lopez-Lopez A, Rogel MA, Ormeno-Orrillo E, Martinez-Romero J, Martinez-Romero E (2010) Phaseolus vulgaris seed-borne endophytic community with novel bacterial species such as Rhizobium endophyticum sp. Nov. Syst Appl Microbiol 33:322–327. https://doi.org/10.1016/j.syapm.2010.07.005
Manthey JA, Tisserat B, Crowley DE (1996) Root responses of sterile-grown onion plants to iron deficiency. J Plant Nutr 19:145–161. https://doi.org/10.1080/01904169609365113
Martino E, Perotto S, Parsons R, Gadd GM (2003) Solubilization of insoluble inorganic zinc compounds by ericoid mycorrhizal fungi derived from heavy metal polluted sites. Soil Biol Biochem 35:133–141. https://doi.org/10.1016/S0038-0717(02)00247-X
Mastropasqua MC, D’orazio M, Cerasi M, Pacello F, Gismondi A, Canini A, Canuti L, Consalvo A, Ciavardelli D, Chirullo B (2017) Growth of Pseudomonas aeruginosa in zinc poor environments is promoted by a nicotianamine-related metallophore. Mol Microbiol 106:543–561. https://doi.org/10.1111/mmi.13834
McCall KA, Huang C, Fierke CA (2000) Function and mechanism of zinc metalloenzymes. J Nutr 130:1437S–1446S. https://doi.org/10.1093/jn/130.5.1437S
Mehdipour-Moghaddam MJ, Emtiazi G, Bouzari M, Mostajeran A, Salehi Z (2010) Novel phytase and cellulase activities in endophytic Azospirilla. W Appl Sci J 10:1129–1135
Mohanna C, Nys Y (1999) Changes in zinc and manganese availability in broiler chicks induced by vegetal and microbial phytases. Anim Feed Sci Technol 77:241–253. https://doi.org/10.1016/S0377-8401(98)00254-5
Monasterio I, Graham RD (2000) Breeding for trace minerals in wheat. Food Nutr Bull 21:392–396. https://doi.org/10.1177/156482650002100409
Morrissey J, Guerinot ML (2009) Iron uptake and transport in plants: the good, the bad, and the ionome. Chem Rev 109:4553–4567. https://doi.org/10.1021/cr900112r
Mottaleb KA, Govindan V, Singh PK, Sonder K, He X, Singh RP, Joshi AK, Barma NC, Kruseman G, Erenstein O (2019) Economic benefits of blast-resistant biofortified wheat in Bangladesh: the case of BARI Gom 33. Crop Prot 123:45–58. https://doi.org/10.1016/j.cropro.2019.05.013
Mulambu J, Andersson M, Palenberg M, Pfeiffer W, Saltzman A, Birol E, Oparinde A, Boy E, Asare-Marfo D, Lubobo A, Mukankusi C (2017) Iron beans in Rwanda: crop development and delivery experience. Afr J Food Agric Nutr Dev 17:12026–12050. https://doi.org/10.18697/ajfand.78.HarvestPlus010
Mumtaz MZ, Ahmad M, Jamil M, Hussain T (2017) Zinc solubilizing Bacillus spp. potential candidates for biofortification in maize. Microbiol Res 202:51–60. https://doi.org/10.1016/j.micres.2017.06.001
Nazaries L, Pan Y, Bodrossy L, Baggs EM, Millard P, Murrell JC, Singh BK (2013) Evidence of microbial regulation of biogeochemical cycles from a study on methane flux and land use change. Appl Env Microb 79:4031–4040. https://doi.org/10.1128/AEM.00095-13
Nwanna LC, Kolahsa M, Eisenreich R, Schwarz FJ (2008) Pre-treatment of dietary plant feedstuffs with phytase and its effect on growth and mineral concentration in common carp (Cyprinus carpio L.). J Anim Physiol Anim Nutr (Berl) 92:677–682. https://doi.org/10.1111/j.1439-0396.2007.00764.x
Nyoki D, Ndakidemi PA (2014) Influence of Bradyrhizobium japonicum and phosphorus on micronutrient uptake in cowpea. A case study of zinc (Zn), iron (Fe), copper (Cu) and manganese (Mn). Am J Plant Sci 5:427. https://doi.org/10.4236/ajps.2014.54056, 435
Ortas I (2012) Mycorrhiza in citrus: growth and nutrition. In: Srivastava A (ed) Advances in citrus nutrition. Springer, Dordrecht, pp 333–351. https://doi.org/10.1007/978-94-007-4171-3_23
Ozturk L, Yazici MA, Yucel C, Torun A, Cekic C, Bagci A, Ozkan H, Braun HJ, Sayers Z, Cakmak I (2006) Concentration and localization of zinc during seed development and germination in wheat. Physiol Plantarum 128:144–152. https://doi.org/10.1111/j.1399-3054.2006.00737.x
Padash A, Shahabivand S, Behtash F, Aghaee A (2016) A practicable method for zinc enrichment in lettuce leaves by the endophyte fungus Piriformospora indica under increasing zinc supply. Sci Hortic (Amsterdam) 213:367–372. https://doi.org/10.1016/j.scienta.2016.10.040
Pellegrino E, Bedini S (2014) Enhancing ecosystem services in sustainable agriculture: biofertilization and biofortification of chickpea (Cicer arietinum L.) by arbuscular mycorrhizal fungi. Soil Biol Biochem 68:429–439. https://doi.org/10.1016/j.soilbio.2013.09.030
Petry N, Egli I, Zeder C, Walczyk T, Hurrell R (2010) Polyphenols and phytic acid contribute to the low iron bioavailability from common beans in young women. J Nutr 140:1977–1982. https://doi.org/10.3945/jn.110.125369
Pfeiffer WH, McClafferty B (2007) HarvestPlus: breeding crops for better nutrition. Crop Sci 47:S-88. https://doi.org/10.2135/cropsci2007.09.0020IPBS
Pii Y, Marastoni L, Springeth C, Fontanella MC, Beone GM, Cesco S, Mimmo T (2016) Modulation of Fe acquisition process by Azospirillum brasilense in cucumber plants. Environ Exp Bot 130:216–225. https://doi.org/10.1016/j.envexpbot.2016.06.011
Praburaman L, Park SH, Cho M, Lee KJ, Ko JA, Han SS, Lee SH, Kamala-Kannan S, Oh BT (2017) Significance of diazotrophic plant growth-promoting Herbaspirillum sp. GW103 on phytoextraction of Pb and Zn by Zea mays L. Env Sci Pollut Res 24:3172–3180. https://doi.org/10.1007/s11356-016-8066-2
Prasad AS (2009) Impact of the discovery of human zinc deficiency. Am Coll Nutr 28:257–265. https://doi.org/10.1080/07315724.2009.10719780
Prasad R, Shivay YS, Kumar D (2014) Agronomic biofortification of cereal grains with iron and zinc. Adv Agron 125:55–91. https://doi.org/10.1016/B978-0-12-800137-0.00002-9
Rai KN, Patil HT, Yadav OP, Govindaraj M, Khairwal IS, Cherian B, Rajpurohit BS, Rao AS, Shivade H, Kulkarni MP (2014) Dhanashakti: a high-iron pearl millet variety. Indian Farm 64:32–34
Ramesh A, Sharma SK, Sharma MP, Yadav N, Joshi OP (2014a) Plant growth-promoting traits in Enterobacter cloacae subsp. dissolvens MDSR9 isolated from soybean rhizosphere and its impact on growth and nutrition of soybean and wheat upon inoculation. Agric Res 3:53–66. https://doi.org/10.1007/s40003-014-0100-3
Ramesh A, Sharma SK, Sharma MP, Yadav N, Joshi OP (2014b) Inoculation of zinc solubilizing Bacillus aryabhattai strains for improved growth, mobilization and biofortification of zinc in soybean and wheat cultivated in Vertisols of central India. Appl Soil Ecol 73:87–96. https://doi.org/10.1016/j.apsoil.2013.08.009
Rana A, Joshi M, Prasanna R, Shivay YS, Nain L (2012) Biofortification of wheat through inoculation of plant growth promoting rhizobacteria and cyanobacteria. Eur J Soil Biol 50:118–126. https://doi.org/10.1016/j.ejsobi.2012.01.005
Rasouli-Sadaghiani M, Malakouti MJ, Khavazi K, Miransari M (2014) Siderophore efficacy of fluorescent Pseudomonades affecting labeled iron (59Fe) uptake by wheat (Triticum aestivum L.) genotypes differing in Fe efficiency. In: Use of microbes for the alleviation of soil stresses, vol 2. Springer, New York, pp 121–132. https://doi.org/10.1007/978-1-4939-0721-2_7
Rausch C, Daram P, Brunner S, Jansa J, Laloi M, Leggewie G, Amrhein N, Bucher M (2001) A phosphate transporter expressed in arbuscule-containing cells in potato. Nat 414:462–465. https://doi.org/10.1038/35106601
Rawat N, Neelam K, Tiwari VK, Dhaliwal HS (2013) Biofortification of cereals to overcome hidden hunger. Plant Breed 132:437–445. https://doi.org/10.1111/pbr.12040
Reddy NR, Sathe SK, Salunkhe DK (1982) Phytates in legumes and cereals. Adv F Res 28:1–92. https://doi.org/10.1016/s0065-2628(08)60110-x
Reeves PG, Chaney RL (2008) Bioavailability as an issue in risk assessment and management of food cadmium: a review. Sci Total Env 398:13–19. https://doi.org/10.1016/j.scitotenv.2008.03.009
Rehman A, Farooq M, Naveed M, Nawaz A, Shahzad B (2018) Seed priming of Zn with endophytic bacteria improves the productivity and grain biofortification of bread wheat. Eur J Agron 94:98–107. https://doi.org/10.1016/j.eja.2018.01.017
Rengel Z (2002) Breeding for better symbiosis. Plant Soil 245:147–162. https://doi.org/10.1023/A:1020692715291
Rengel Z, Batten GD, Crowley DE (1999) Agronomic approaches for improving the micronutrient density in edible portions of field crops. F Crop Res 60:27–40. https://doi.org/10.1016/S0378-4290(98)00131-2
Rungin S, Indananda C, Suttiviriya P, Kruasuwan W, Jaemsaeng R, Thamchaipenet A (2012) Plant growth enhancing effects by a siderophore-producing endophytic Streptomycete isolated from a Thai jasmine rice plant (Oryza sativa L. cv. KDML105). Antonie Van Leeuwenhoek 102:463–472. https://doi.org/10.1007/s10482-012-9778-z
Sah S, Singh N, Singh R (2017) Iron acquisition in maize (Zea mays L.) using Pseudomonas siderophore. 3 Biotech 7:121. https://doi.org/10.1007/s13205-017-0772-z
Saia S, Rappa V, Ruisi P, Abenavoli MR, Sunseri F, Giambalvo D, Frenda AS, Martinelli F (2015) Soil inoculation with symbiotic microorganisms promotes plant growth and nutrient transporter genes expression in durum wheat. Front Plant Sci 6:815. https://doi.org/10.3389/fpls.2015.00815
Saltzman A, Birol E, Bouis HE, Boy E, De Moura FF, Islam Y, Pfeiffer WH (2013) Biofortification: Progress toward a more nourishing future. Global Food Secur 2:9–17. https://doi.org/10.1016/j.gfs.2012.12.003
Saravanan VS, Kalaiarasan P, Madhaiyan M, Thangaraju M (2007a) Solubilization of insoluble zinc compounds by Gluconacetobacter diazotrophicus and the detrimental action of zinc ion (Zn2+) and zinc chelates on root knot nematode Meloidogyne incognita. Lett Appl Microbiol 44:235–241. https://doi.org/10.1111/j.1472-765X.2006.02079.x
Saravanan VS, Madhaiyan M, Thangaraju M (2007b) Solubilization of zinc compounds by the diazotrophic, plant growth promoting bacterium Gluconacetobacter diazotrophicus. Chemosphere 66:1794–1798. https://doi.org/10.1016/j.chemosphere.2006.07.067
Scagliola M, Pii Y, Mimmo T, Cesco S, Ricciuti P, Crecchio C (2016) Characterization of plant growth promoting traits of bacterial isolates from the rhizosphere of barley (Hordeum vulgare L.) and tomato (Solanum lycopersicon L.) grown under Fe sufficiency and deficiency. Plant Physiol Biochem 107:187–196. https://doi.org/10.1016/j.plaphy.2016.06.002
Shaikh S, Saraf M (2017) Biofortification of Triticum aestivum through the inoculation of zinc solubilizing plant growth promoting rhizobacteria in field experiment. Biocatal Agricl Biotechnol 9:120–126. https://doi.org/10.1016/j.bcab.2016.12.008
Shakeel M, Rais A, Hassan MN, Hafeez FY (2015) Root associated Bacillus sp. improves growth, yield and zinc translocation for basmati rice (Oryza sativa) varieties. Front Microbiol 6:1286. https://doi.org/10.3389/fmicb.2015.01286
Singh BB (2014) Iron cowpea. G.B. Pant University of Agriculture and Technology. Crop Development, Harvest plus, Biofortification Progress Brief 11, pp. 21–22
Singh B, Satyanarayana T (2015) Fungal phytases: characteristics and amelioration of nutritional quality and growth of non-ruminants. J Anim Physiol Anim Nutr (Berl) 99:646–660. https://doi.org/10.1111/jpn.12236
Singh D, Rajawat MVS, Kaushik R, Prasanna R, Saxena AK (2017) Beneficial role of endophytes in biofortification of Zn in wheat genotypes varying in nutrient use efficiency grown in soils sufficient and deficient in Zn. Plant Soil 416:107–116. https://doi.org/10.1007/s11104-017-3189-x
Sirohi G, Upadhyay A, Srivastava PS, Srivastava S (2015) PGPR mediated zinc biofertilization of soil and its impact on growth and productivity of wheat. J Soil Sci Plant Nutr 15:202–216. https://doi.org/10.4067/S0718-95162015005000017
Slavin JL, Jacobs D, Marquart L (2001) Grain processing and nutrition. Crit Rev Biotechnol 40:309–326. https://doi.org/10.1080/20013891081683
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic Press, London, pp 1–787
Smith FA, Smith SE (2011) What is the significance of the arbuscular mycorrhizal colonization of many economically important crop plants? Plant Soil 348:63–79. https://doi.org/10.1007/s11104-011-0865-0
Stein AJ (2006) Micronutrient malnutrition and the impact of modern plant breeding on public health in India: how cost-effective is biofortification? Cuvillier Verlag, Germany, Göttingen, pp 1–162
Su CL, Zhang FM, Sun K, Zhang W, Dai CC (2019) Fungal endophyte Phomopsis liquidambari improves iron and molybdenum nutrition uptake of peanut in consecutive monoculture soil. J Soil Sci Plant Nutr 19:71–80. https://doi.org/10.1007/s42729-019-0011-2
Subramanian KS, Balakrishnan N, Senthil N (2013) Mycorrhizal symbiosis to increase the grain micronutrient content in maize. Aust J Crop Sci 7:900–910
Sunithakumari K, Padma Devi SN, Vasandha S (2016) Zinc solubilizing bacterial isolates from the agricultural fields of Coimbatore, Tamil Nadu, India. Curr Sci 110:196–205. https://doi.org/10.18520/cs/v110/i2/196-205
Tamayo E, Gomez-Gallego T, Azcon-Aguilar C, Ferrol N (2014) Genome-wide analysis of copper, iron and zinc transporters in the arbuscular mycorrhizal fungus Rhizophagus irregularis. Front Plant Sci 5:547. https://doi.org/10.3389/fpls.2014.00547
Tariq M, Hameed S, Malik KA, Hafeez FY (2007) Plant root associated bacteria for zinc mobilization in rice. Pakistan J Bot 39:245–253
Tiwari VK, Rawat N, Neelam K, Kumar S, Randhawa GS, Dhaliwal HS (2010) Substitutions of 2S and 7U chromosomes of Aegilops kotschyi in wheat enhance grain iron and zinc concentration. Theor Appl Genet 121:259–269. https://doi.org/10.1007/s00122-010-1307-8
Troesch B, Jing H, Laillou A, Fowler A (2013) Absorption studies show that phytase from Aspergillus niger significantly increases iron and zinc bioavailability from phytate-rich foods. Food Nutr Bull 34(2_suppl1):S90–S101. https://doi.org/10.1177/15648265130342S111
Trumbo P, Yates AA, Schlicker S, Poos M (2001) Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Acad Nutr Diet 101:294–301
U.S. Department of Agriculture (2012) Agricultural Research Service. What we eat in America, 2009–2010
Unnikumar KR, Sree KS, Varma A (2013) Piriformospora indica: a versatile root endophytic symbiont. Symbiosis 60:107–113. https://doi.org/10.1007/s13199-013-0246-y
Vaid SK, Kumar B, Sharma A, Shukla AK, Srivastava PC (2014) Effect of Zn solubilizing bacteria on growth promotion and Zn nutrition of rice. J Soil Sci Plant Nutr 14:889–910. https://doi.org/10.4067/S0718-95162014005000071
Vasconcelos MW, Gruissem W, Bhullar NK (2017) Iron biofortification in the 21st century: setting realistic targets, overcoming obstacles, and new strategies for healthy nutrition. Curr Opin Biotechnol 44:8–15. https://doi.org/10.1016/j.copbio.2016.10.001
Wang Y, Brown HN, Crowley DE, Szaniszlo PJ (1993) Evidence for direct utilization of a siderophore, ferrioxamine B, in axenically grown cucumber. Plant Cell Env 16:579–585. https://doi.org/10.1111/j.1365-3040.1993.tb00906.x
Wang Y, Yang X, Zhang X, Dong L, Zhang J, Wei Y, Feng Y, Lu L (2014) Improved plant growth and Zn accumulation in grains of rice (Oryza sativa L.) by inoculation of endophytic microbes isolated from a Zn hyperaccumulator, Sedum alfredii H. J Agric Food Chem 62:1783–1791. https://doi.org/10.1021/jf404152u
White PJ, Broadley MR (2005) Biofortifying crops with essential mineral elements. Trends Plant Sci 10:586–593. https://doi.org/10.1016/j.tplants.2005.10.001
Whiting SN, De Souza MP, Terry N (2001) Rhizosphere bacteria mobilize Zn for hyperaccumulation by Thlaspi caerulescens. Env Sci Technol 35:3144–3150. https://doi.org/10.1021/es001938v
WHO (2002) The world health report 2002: reducing risks, promoting healthy life. World Health Organization
WHO (2019) Nutrition: micronutrient deficiencies. Iron deficiency anaemia, World Health Organisation https://www.who.int/nutrition/topics/ida/fr/
Xue Y, Yue S, Zhang W, Liu D, Cui Z, Chen X, Ye Y, Zou C (2014) Zinc, iron, manganese and copper uptake requirement in response to nitrogen supply and the increased grain yield of summer maize. PLoS One 9:e93895. https://doi.org/10.1371/journal.pone.0093895
Yadava DK, Choudhury PR, Hossain F, Kumar D (2017) Biofortified varieties: sustainable way to alleviate malnutrition. Indian Council of Agricultural Research, New Delhi, 18p
Yadava DK, Hossain F, Mohapatra T (2018) Nutritional security through crop biofortification in India: status & future prospects. Indian J Med Res 148:621–631. https://doi.org/10.4103/ijmr.IJMR_1893_18
Yadava DK, Choudhury PR, Hossain F, Kumar D Mohapatra T (2019) Biofortified varieties: sustainable way to alleviate malnutrition (2nd Edn). Indian Council of Agricultural Research, New Delhi. 44p. 3
Yadava DK, Choudhury PR, Hossain F, Kumar D, Mohapatra T (2020) Biofortified varieties: sustainable way to alleviate malnutrition, 3rd edn. Indian Council of Agricultural Research, New Delhi, 86p
Zhang H, Sun Y, Xie X, Kim M, Dowd SE, Pare PW (2009) A soil bacterium regulates plant acquisition of iron via deficiency-inducible mechanisms. Plant J 58:568–577. https://doi.org/10.1111/j.1365-313X.2009.03803.x
Zhang W, Liu DY, Li C, Chen XP, Zou CQ (2017) Accumulation, partitioning, and bioavailability of micronutrients in summer maize as affected by phosphorus supply. Eur J Agron 86:48–59. https://doi.org/10.1016/j.eja.2017.03.005
Zhou C, Guo J, Zhu L, Xiao X, Xie Y, Zhu J, Ma Z, Wang J (2016) Paenibacillus polymyxa BFKC01 enhances plant iron absorption via improved root systems and activated iron acquisition mechanisms. Plant Physiol Biochem 105:162–173. https://doi.org/10.1016/j.plaphy.2016.04.025
Zhu A, Tan H, Cao L (2019) Isolation of phytase-producing yeasts from rice seedlings for prospective probiotic applications. 3 Biotech 9:216. https://doi.org/10.1007/s13205-019-1746-0
Zimmermann MB, Hurrell RF (2007) Nutritional iron deficiency. Lancet 370:511–520. https://doi.org/10.1016/S0140-6736(07)61235-5
Zribi K, Nouairi I, Slama I, Talbi-Zribi O, Mhadhbi H (2015) Medicago sativa-Sinorhizobium meliloti symbiosis promotes the bioaccumulation of zinc in nodulated roots. Int J Phytorem 17:49–55. https://doi.org/10.1080/15226514.2013.828017
Acknowledgements
Infrastructural facilities provided by ICAR-National Bureau of Agriculturally Important Microorganisms (NBAIM) are duly acknowledged.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Funding
Authors are thankful to the National Agricultural Science Fund (NASF) for financial assistance provided to carry out the project entitled “Biofortification of Wheat and Maize with Zinc and Iron using endophytic microorganisms” (NASF/Mn-5019/2016-17).
Author information
Authors and Affiliations
Contributions
HC contributed in conceptualization of the work. SV, HC, and KM have collected the literature and contributed in writing-reviewing the original draft. AV and AKS have edited the final draft and given final approval of this version to be published. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Verma, S., Chakdar, H., Kumar, M. et al. Microorganisms as a Sustainable Alternative to Traditional Biofortification of Iron and Zinc: Status and Prospect to Combat Hidden Hunger. J Soil Sci Plant Nutr 21, 1700–1717 (2021). https://doi.org/10.1007/s42729-021-00473-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-021-00473-5