Abstract
The objectives of this study were to characterize the genetic diversity and evaluate the ability to tolerate stress as well as to assess the symbiotic efficiency of bacteria from cowpea nodules in agricultural soils with different uses in the semiarid region of Bahia state (Brazil). Soil samples were collected from six crop lands and one from the pristine Caatinga biome. After a trap-host experiment, the bacteria were isolated and culturally characterized. Isolates with typical characteristics of Bradyrhizobium were subjected to the nodC symbiotic gene amplification and those positive were evaluated by 16S-23S IGS-RFLP. Twenty-seven isolates belonging to different genetic clusters were selected for 16S-23S IGS sequencing. In additions, the selected bacteria were characterized biochemically and symbiotically. Among 420 characterized isolates, approximately 60% (251 isolates) displayed typical Bradyrhizobium cultural features. A total of 161, out of 251 isolates, showed positive amplification of the nodC gene fragment. The IGS-RFLP profiles analysis generated 33 groups and 27 were selected for further analysis. The fertility of the soils influenced the distribution of the isolates in the IGS-RFLP clusters. The bacteria were assigned to two genera, Bradyrhizobium and Microvirga, with 26 and 1 representative bacteria, respectively. Some isolates were able to tolerate NaCl as well as acidic and alkaline pH. In addition, isolates showed the abilities to produce biofilm under stress and to produce indole compounds, as well as efficient nodulation and nitrogen fixation. The isolates displayed great genetic, biochemical, and symbiotic variability with promising biotechnological potential.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The lower half São Francisco River Valley is an important center of irrigated agriculture in the semiarid region of Brazil. The high temperatures and solar radiation incidence along with the use of irrigation allow for the cultivation of a wide range of plant species of economic interest. Approximately 120,000 ha are currently irrigated, and the region has the potential to reach about 360,000 ha (Leão and Moutinho 2014). The intensification and expansion of crop areas directly affect soil characteristics and impact the diversity of the microbial populations (Bissett et al. 2013; Lacerda-Júnior et al. 2019). Combined with the modification of natural vegetation, the types of agricultural use can strongly influence native microorganisms with great biotechnological potential, such as the legume-associated nitrogen-fixing bacteria, collectively known as rhizobia.
In legumes roots and/or stems, these bacteria induce the formation of anatomical changes and the establishment of nodules as a high specialized structure where the N2-fixation occurs. Into the nodules, the atmospheric N2 is fixed and the releasing ammonium, a readily available N source to plants, occurs and helps the plant N nutrition. Given the contribution of these bacteria to legume development and production, the biological fixation of atmospheric nitrogen is considered one of the most important processes mediated by soil microorganisms (Boyd and Peters 2013). In agriculture this process is exploited by means of inoculating the seeds with selected bacteria by means of rhizobial inoculant application.
Cowpea [Vigna unguiculata (L.) Walp] is a prominent legume for drylands because it tolerates high temperatures and low water availability conditions (Dutra et al. 2017). Cowpea ability to associate with a wide range of nitrogen-fixing bacteria is another feature of great importance for this crop, since the majority of smallholders, the main part of cowpea growers in Brazil, do not use rhizobial inoculants or N fertilization. So, this characteristic allows cowpea to fix high amounts of N (up to 45 kg N ha−1) under rainfed conditions in Brazilian drylands (Freitas et al. 2012). The adaptation of cowpea to harsh climatic conditions and the low specificity of the symbiotic association with rhizobia evidence the technological potential of this crop for drylands. In addition, identifying bacteria that associate with the cowpea is a valuable strategy for semiarid regions because it is the first step in developing innovations such as efficient and well-adapted commercial inoculants (Martins et al. 2003; Marinho et al. 2017).
Many studies have sought to determine the diversity of native nitrogen-fixing bacteria in Brazilian dry regions (Martins et al. 2003; Leite et al. 2009; Marinho et al. 2017; Santos et al. 2017; Rodrigues et al. 2018), such as in the Lower Half São Francisco River Valley (Leite et al. 2009; Nunes et al. 2018). However, these studies did not consider the heterogeneity of the agricultural systems in the region. Probably, due to this reason, these studies reported the predominance of bacteria with phenotypic characteristics associated to low symbiotic efficiency and the inability to renodulate the original host. The influence of different land uses on rhizobia diversity has been highlighted in several studies investigating the impact of agriculture on these important microorganisms in natural and/or agricultural systems (Leite et al. 2009; Yan et al. 2014; Vuong et al. 2017). Even though, these evaluations were not conducted to Brazilian semiarid region.
Thus, considering the heterogeneity of the agricultural land uses in Brazilian semiarid region, we showed different agricultural systems in the municipality of Juazeiro, Bahia State, Brazil, to collect the soil samples to conduce the present study. Thus, we hypothesized that the different agricultural use systems influence in the diversity and efficiency of cowpea rhizobia in Juazeiro, Bahia State. The aim of this study was to isolate and characterize molecularly, biochemically, and symbiotically the cowpea rhizobia inhabiting soils submitted to different agricultural practices in Brazilian drylands.
2 Materials and Methods
2.1 Soil Sampling for Trap-Host Experiment and Bacterial Isolation
The soil sampling was conducted in seven areas. Six out of the seven sampling sites were agricultural areas and one was an undisturbed native Caatinga, the Stepic-Savanna native to the region. All sites were located in Juazeiro municipality (Bahia State) under a semiarid climate, BSwh according to Köeppen. The characteristics of each sampling site, as well as the soil fertility parameters are shown in Table 1.
For the trap-host experiment, all samples were manually homogenized, sieved (5 mm sieve), and distributed into 3 L pots, with approximately 3.5 kg of soil sample per pot. Two genotypes of cowpea were used in this study. BRS Pujante was bred for grown in the semiarid belt (Santos 2011) and of the local landrace Canapu Ligeiro widely, used by the growers in the region. The seeds were surface disinfected (Vincent 1970) and sown in the pots, with three replicates for each genotype (2) and soil (7) combination, totaling 42 pots. Ten days after the emergence (DAE), the spare seedlings were cut at the level of the substrate and one plant was left per pot. The experiment was conducted in a greenhouse and the plants were irrigated daily with 150 mL of distilled autoclaved water (DAW). The experiment was harvested at 35 DAE, and 10 nodules from each pot were randomly selected for isolation. Soon after the harvest, the nodules were surface disinfected [30 s in 70% ethanol, 5 min in sodium hypochlorite (4–6% v/v)] and washed 10 times in DAW, crushed and inoculated in yeast mannitol agar YMA applied with Congo red (Vincent 1970), and incubated in a growth chamber at 28 °C for 10 days.
The typical of Bradyrhizobium colonies were streaked in Petri dishes with YMA medium with bromothymol blue and incubated as described above. The typical bradyrhizobial colonies were purified in the same medium. The isolates were preserved in centrifuge microtubes containing 1 mL YM culture medium supplemented with 25% (v/v) glycerol at − 80 °C and in the Culture Collection of Microorganisms of Agricultural Interest of Embrapa Semiárido (CMISA).
2.2 Molecular Analysis
The bacterial isolates were grown in YM liquid medium. The DNA was extracted using the Wizard® genomic DNA purification kit (Promega, USA) following the manufacturer’s instructions. First of all, we amplified the nodC gene as strategy to preliminary selection of putative rhizobia (Mothapo et al. 2013; Silva et al. 2019). The 16S-23S rRNA intergenic spacer (IGS) region was amplified and subjected to endonuclease digestion for the restriction length fragment polymorphism (RFLP) technique on the IGS fragment (IGS-RFLP) using HaeIII, AluI, and HindIII (Thermo Scientific, USA) restriction enzymes. The primers used and the PCR conditions are described in Table S1.
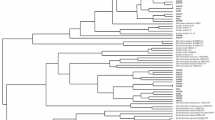
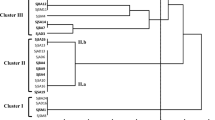
The amplification products were stained with GelRed® (Biotium, Hayward, CA, USA) and submitted horizontal electrophoresis in 1% (w/v) agarose gel in the presence of 0.5× TBE for electrophoresis with a voltage of 150 V for 1 h. The endonuclease digestion products were stained and separated by electrophoresis as abovementioned with 80 V for 3 h. The IGS-RFLP profiles were imported into BioNumerics v. 7.6 (Applied Maths, Kortrijk, Belgium), where they were standardized and used to construct similarity dendrograms by the unweighted pair-group and arithmetic average (UPGMA) methods using the Dice coefficient. Based on this clustering, 27 isolates were selected for further analysis.
The 16S-23S rRNA IGS was amplified as described above and the PCR products were purified with the QIAquick PCR purification kit (Qiagen, Hilden, Germany) and sequenced at Macrogen (Seoul, South Korea) in a 3037xl genetic analyzer (Thermo Fisher, Waltham, MA, USA). The quality of the sequences was evaluated in the SeqScanner v 2.0 software (Thermo Fisher, Waltham, MA, USA). Through this program, high-quality (QV > 20) contiguous sequences were extracted and used for comparison in the database. The similarity of the sequences obtained was compared with those deposited in the GenBank database of the National Center for Biotechnology Information (NCBI) using Basic Local Alignment Search Tool (BLASTn) (Altschul et al. 1990). The IGS sequences were deposited in GenBank database under the accession numbers MN160571 to MN160597.
2.3 Production of Indole Compounds, Tolerance to Different NaCl Concentrations and pH Levels, and Biofilm Formation
To evaluate the production of indolic compounds, the colorimetric assay described by Sarwar and Kremer (1995) was adapted as briefly described below. All selected bacteria (27 cowpea isolates and 1 reference strains) grew on YMA dishes to assure their purity. Each strain was inoculated in glass tubes containing 5 mL of Dyg’s liquid medium (Rodrigues Neto et al. 1986), supplied with 5 g L−1 of mannitol, and incubated with agitation for 48 h. A 1-mL aliquot of each pre-inoculum was transferred in triplicate to glass tubes with Dyg’s medium with mannitol supplemented with L-tryptophan (L-trp) and incubated under constant mixing (100 rpm) for 6 days, at room temperature, mean 28 °C. Afterward, the optical density (OD) of the cultures was measured at 540 nm and adjusted to 0.5.
Aliquots (1 mL) of the OD540 adjusted broth were centrifuged for 3 min at 1600×g. One hundred microliters of the Salkowski reagent (2% of 0.5 M FeCl3 in 35% v/v perchloric acid) was added to 150 μL of the supernatant, and the mixture was incubated in the dark for 30 min, followed by reading on spectrophotometer Multiskan GO (Thermo Fisher, Waltham, MA, USA) at 530 nm. Production of indolic compounds was quantified comparing the absorbance data to a standard curve made using different concentrations of indole-3-acetic acid.
To evaluate their tolerance to different NaCl concentrations and pH levels, as well as the biofilm formation capabilities, bacteria were grown in glass tubes containing 3 mL of YM medium for 5 days under constant mixing, followed by adjustment of the OD540 to 0.1. Tolerance to different NaCl concentrations and pH values was tested separately by growing isolates in a modified YM medium under acidic or alkaline conditions (pHs 4.0 or 10.0, respectively) and with NaCl 3% (w/v) and the treatment in standard YM medium (pH = 7.0 and without NaCl supply) was used as control for both evaluations. The assay was conducted in 96 wells of an ELISA microplate. In each well, we inoculated 10 μL of OD540 adjusted broth to 150 μL of liquid YM culture medium. The microplates were incubated at 28 °C in a growth chamber stationary. To assure the absence of contamination, all microplates evaluated had a negative control, with the standard culture medium without inoculation.
To evaluate the bacterial growth in the NaCl or pH altered media, the readings were conducted in spectrophotometer as 540 nm after 5 days of incubation period. For the negative control, just before reading, 10 μL of an autoclaved broth (OD540 = 0.1) was added.
Biofilm production was evaluated following the method of Nostro et al. (2007), with modifications, as briefly described below. After reading the plates in the NaCl and pH experiments, as described above, the culture broths were discarded, and each microplate well was washed three times with 200 μL of DAW. Afterward, the plates were dried at room temperature and 100 μL of 0.25% (w/v) gentian violet was added. After 5-min incubation at room temperature, the plates were washed again with DAW, and an ethanol/acetone solution (80:20 v/v) was added. The biofilm formation was quantified by measuring the intensity of the purple-blue color in a spectrophotometer at 620 nm. A standard curve was constructed using different concentrations of gentian/crystal violet (ranging from 0 to 0.6 mmol L−1) and an ethanol/acetone (80:20) solution.
2.4 Symbiotic Efficiency of Bacterial Isolates
The symbiotic efficiency of the 27 isolates selected in the IGS-RFLP clustering was determined under greenhouse conditions in sterilized substrate. In addition to these 27 isolates, we analyzed two reference strains, BR 3267 (Martins et al. 2003) and ESA 17 (Marinho et al. 2017), an absolute control treatment, without inoculation or N application, and a nitrogen supplied treatment, with application of 80 mg of N week−1, using NH4NO3, the equivalent of 80 kg ha−1.
Properly disinfected 500-mL polystyrene pots were filled with approximately 700 g of sterilized sand per pot. Previously the sand was autoclaved twice at 120 °C, 1.5 atm for 1 h, with 72 h between the sterilizations. Three cowpea seeds (BRS Pujante) were sown in each pot; 1 mL bacterial broth (incubated in YM medium for 5 days) was applied on each seed. To avoid contaminations, the inoculated seeds were covered with sterile sand. At the 5th DAE, the spare plants were cut at the cotyledon level and one plant per pot was left. A sterile, nitrogen-free nutrient solution (Norris and Mannetje 1964) was applied once a week starting after the cotyledons dropped until the last week of the experiment. Irrigation with DAW was performed when necessary.
The experiment was harvested at 45 DAE. The shoots were separated from the roots, which were washed with tap water, and the nodules were detached and counted. Roots, shoots, and nodules were packed in paper bags and dried in a forced air oven at 65 °C until constant weight, after which they were weighed. Then, the shoots were ground in a mill to determine their N content by the dry combustion method in a Vario EL TruSpec Elemental Analyzer (LECO, St. Joseph, MI, USA).
For this assay, total dry mass (TDM), root dry mass (RDM), shoot dry mass (SDM), number of nodules (NN), nodule dry mass (NDM), shoot nitrogen content (SNC), and shoot nitrogen accumulation (SNA) were evaluated. The relationship between SNA and NDM was used to estimate the nodulation efficiency (NE).
2.5 Statistical Analysis
For evaluation of NaCl and pH tolerance, as well as biofilm formation, the experimental designs were completely randomized, with three replicates for each treatment. The greenhouse inoculation experiment was conducted in completely randomized blocks design with four replications.
The data were submitted to an analysis of variance, and the means were compared by the Scott-Knott average range test at the 5% significance level. All data of biochemical and greenhouse experiments (except RDM and TDM) were transformed by (Y + 0.5)0.5, prior to ANOVA. The analyses were conducted using the SISVAR 5.0 statistical package (Ferreira 2011).
The Pearson’s correlation between the soil chemical dataset and the genetic clustering dataset was made using PaSt 4.0 statistical package (Hammer et al. 2001).
3 Results
3.1 Bacterial Isolation, IGS-RFLP Genetic Diversity and Identification by Analysis of IGS Sequences
A total of 251 isolates with typical bradyrhizobial cultural characteristics were retrieved. Out of 251, 161 (64%) were positive for the amplification of nodC gene and were used in the other evaluations. Digestion with the restriction enzymes HaeIII, AluI, and HindIII showed specific cut patterns, and after clustering using the Dice coefficient, the restriction digestion of the IGS fragment generated 99 profiles, grouped into 33 clusters at 85% similarity (Table 2). The clusters observed in this study ranged from including 1 isolate (rare) to an abundant group with 48 isolates. A total of 27 isolates were selected from non-rare and rare clusters according to the IGS-RFLP clustering. This isolates were selected covering all origins both to the land use systems and the host genotype.
The IGS sequencing of the selected isolates was performed from amplicons ranging in size from 850 to 1075 bp, and all sequences obtained were aligned with sequences type and non-type strains in the GenBank database. The comparison classified 26 bacteria within as Bradyrhizobium genus and one bacterium within Microvirga genus (Table 3). Among the 26 isolates identified within the genus Bradyrhizobium by evaluation of the sequences of the IGS region, 21 were classified as belonging to B. japonicum superclade and only 5 were classified within B. elkanii superclade.
The bacteria include ESA 382, ESA 365, ESA 369, ESA 366, ESA 380, ESA 385, ESA 388, ESA 386, ESA 376, ESA 381, ESA 384, ESA 368, ESA 379, ESA 372, ESA 371, ESA 387, ESA 389, ESA 390, ESA 383, ESA 375, and ESA 373. Isolates ESA 382, ESA 366, ESA 369, ESA 385, ESA 386, ESA 381, ESA 376, ESA 380, and ESA 371 were found to be close to the B. yuanmingense strain CCBAU 10071T, with similarities ranging from 98 to 99%, been classified within the B. japonicum superclade included. The isolates with high similarity to B. yuanmingense BR 3267 showed similarities ranging from 99 to 100%; however, none of the isolates presented sequence coverage greater than 89%, with the possibility that the isolates were variations of BR 3267, because this strain was isolated from the same region of origin as the studied isolates. Also, in the B. japonicum superclade, the isolate ESA 373 had the best correspondence to the B. vignae 7-2T strain, with 91% similarity. Thus far, strains similar to B. vignae have not been isolated in Brazil.
Considering the cluster distribution of the IGS-RFLP dendrogram (summarized in Table 2) and the soil chemical characteristics of the origin of each isolate, we observed significative Pearson’s correlations between the genetic cluster distribution and soil chemical characteristics (Fig. 1). The remarkable correlations were observed in the cluster XIX (6 isolates), positively correlated to the soil characteristics pH, P, K, Na, Ca, Mg, SB, and CEC. Also, in the cluster XVIII (48 isolates), the bacterial occurrence was positively correlated to pH, K, and Mg. Other positive correlations were observed in genetic clusters with less bacterial isolates.
Pearson’s correlation between the soil chemical characteristics dataset and IGS-RFLP cluster distribution dataset. Boxed circles are significative correlations (p < 0.05). In the vertical axis, IGS-RFLP genetic clusters; in the horizontal axis, soil chemical characteristics. EC, electrical conductivity; SB, sum of bases; CEC, cation exchange capacity; SatB, base saturation
Only five isolates evaluated in this study were classified within the B. elkanii superclade. The isolates ESA 377, ESA 378, ESA 364, ESA 374, and ESA 370 were associated within this clade and belong to three different IGS-RFLP groups, namely, groups 11, 12, and 1, with only ESA 370 belonging to group 1. The isolate ESA 377 showed 99% similarity with the reference strain B. elkanii CI-19F. Furthermore, ESA 378, ESA 364, ESA 374, and ESA 370 isolates showed high similarity with the type strain B. ferriligni CCBAU 51502T, the reference strain B. elkanii CI-1A, and the type strain B. elkanii USDA 76T.
As for the other genus identified in this study, the low similarity of the ESA 367 isolate with the Russian type strain of Microvirga ossetica (87%) and the low sequence coverage of other Microvirga spp. and other bacteria were due to the limited sequences of the 16-23S intergenic region in the database, such that the sequence of the ESA 367 isolate aligned with the intergenic region of M. ossetica V5/3MT, which was deposited from the complete genome in GenBank. The low coverage (approximately 300 bp) is due to the alignment of the incomplete 16S rRNA gene in the 5′ region of the aligned sequence. However, the possibility of identifying an isolate belonging to a new species of Microvirga in our collection should not be discarded; further investigation is needed to fully understand its taxonomy.
3.2 Tolerance to NaCl and pH, Formation of Biofilm, and Production of Indole Compounds by Bacterial Isolates of Cowpea Nodules
At 3% NaCl, the isolates ESA 368, BR 3267, and ESA 379 showed the best results and were considered highly salt tolerant. Inoculation of the isolates into YM medium at pH 4 resulted in lower growth when compared with pH 10. The treatments ESA 379, ESA 375, and BR 3267 presented higher values of OD540. To be classified in the highest cluster according to the Scott-Knott mean range test, these bacteria were assigned as acid tolerant. The pH = 10 did not affect the growth of the majority of the isolates and significant difference was observed among the bacteria. Eleven bacteria (ESA 376, ESA 379, ESA 385, ESA 390, ESA 378, ESA 389, ESA 369, ESA 383, ESA 375, ESA 387, and ESA 377) out of the 27 tested bacteria showed higher values of OD540 than the other bacteria and were assigned as alkali-tolerant isolates (Table 4).
All isolates formed biofilm in YM medium at pH 7.0 and without NaCl supplement. The treatments ESA 371, ESA 369, ESA 380, and ESA 377 produced more biofilm and were higher than the other treatments. ESA 372 was statistically identical to BR 3267, and the other treatments presented lower biofilm production (Table S2). Biofilm formation under abiotic stress conditions (NaCl and pH) was tested for all isolates. Under an acidic pH (4.0), there was no significant difference among the treatments. When the pH was increased to 10, the treatments showed differences, especially isolates ESA 377 and ESA 369. These isolates were the best biofilm producers, followed by the isolates ESA 367, ESA 368, ESA 380, ESA 379, and BR 3267.
At 3% (w/v) of NaCl concentrations, biofilm formation was statistically identical in all treatments.
There was no significant difference among the treatments at the 3% NaCl concentration; however, the majority of the isolates under this concentration had lower mean biofilm production values than those observed at the control treatment, in spite of the positive growth observed .
The tested isolates and the reference strain synthesized indole compounds in the presence of L-Trp. The treatments ESA 281, ESA 384, ESA 365, ESA 369, ESA 383, ESA 378, ESA 389, ESA 366, ESA 375, ESA 390, ESA 380, ESA 385, and ESA 364 showed increased production of indole compounds, as well as the reference strain BR 3267, compared to the other treatments, with values equivalents to the varying from 69.53 to 614.42 mg of AIA L−1 (Fig. 2).
Production of indolic compounds (indole acetic acid standard curve) in the presence of tryptophan by Bradyrhizobium and Microvirga isolates from cowpea nodules. Columns with the same letter do not differ by the Scott-Knott test at 5% probability (p < 0.05). Bars represent the standard error of the mean
3.3 Symbiotic Efficiency of Bacterial Isolates
In the symbiotic efficiency assay, all variables analyzed were significant (p < 0.05) by the Scott-Knott mean range test, except for the number of nodules. The 27 inoculated treatments were able to nodulate without apparent limitation. None of the non-inoculated control treatments showed nodulation, confirming the aseptic conditions of the experiment. Only 7 out of 27 isolates presented lower TDM than the reference strain B. yuanmingense BR 3267 (Table 5). Among them, only ESA 377 was equal to the absolute control. In general, the new native bacteria had a TDM equal to that of the strain officially recommended by the Brazilian Ministry of Agriculture as a cowpea inoculant.
The treatments with increased SDM did not show significant differences when compared to the treatments with the reference strains BR 3267, ESA 17, and the treatment supplemented with nitrogen. Only eight of the isolates tested showed lower SDM than the nitrogen supplementation control. Nineteen out of 27 bacteria tested did not differ from the control treatments cited above. The SDM ranged from 0.79 to 1.62 g plant−1, with the lowest mean corresponding to the absolute control treatment and the highest to the treatment inoculated with the ESA 369 isolate (from RAIN-N area).
Regarding the NDM, there were significant differences between the inoculated treatments. The treatments ESA 377, ESA 364, ESA 378, ESA 386 (C-oni), ESA 381, ESA 384, ESA 379 (UNEB), ESA 383, ESA 390, ESA 375, ESA 367 (AGROE), ESA 366 (RAIN) ESA 370, ESA 374 (RAIN-N), and BR 3267 presented increased values compared to the other treatments, including the reference strain ESA 17.
Regarding shoot nitrogen accumulation and content (SNA and SNC), both the treatments inoculated with the BR 3267 and ESA 17 reference strains and with N supplementation did not differ significantly from 23 out of the 27 new bacteria assessed. The highest mean SNC and SNA were observed for the ESA 378 isolate, which was approximately 14% higher than the treatment supplemented with NH4NO3.
The treatments ESA 378, ESA 376 (C-oni), ESA 368, ESA 372 (UNEB), ESA 369, ESA 382 (RAIN-N), ESA 371, ESA 387 (AGROE), ESA 380 (RAIN), ESA 388 (CAAT), and ESA 17 presented significantly increased nodulation efficiency. The isolates ESA 377, ESA 374, and ESA 370 presented lower nodulation efficiency. Intermediate nodulation efficiency was observed in most of the treatments tested, as well as in the reference strain BR 3267, according to the mean range test.
4 Discussion
The preliminary amplification of nodC fragment is a promising strategy to increase the probability to select rhizobia rather than non-rhizobial isolates. This strategy as already applied to rhizobial culture collections with good results (Mothapo et al. 2013; Hollowell et al. 2016; Silva et al. 2019) and along with the nifH primer was recently proposed as criterion for separation of rhizobia to non-rhizobia in culture collections (Silva et al. 2019).
The non-nodC harboring slow growing bacteria indicated should be bradyrhizobial isolates that lack the presence of nod genes but are effective plant nodulating bacteria (Giraud et al. 2007; Okazaki et al. 2016) or they are non-symbiotic bradyrhizobia (Hollowell et al. 2016). The use of this strategy fits to the selection of bacterial isolates for biotechnological applications as inoculants, due to the increasing of the selection of putative rhizobial over the non-rhizobial strains. In our collection, we obtained a total of 64% of all slow growing isolates as positive for nodC amplification, with 161 positive isolates, in agreement to the isolation of non-rhizobial isolate from nodules in the Brazilian semiarid region (Nunes et al. 2018) and in African continent (Mohammed et al. 2018).
To assess the genetic diversity of rhizobial collections, several molecular tools have been applied such as the use of the intergenic region (IGS). Analysis of the intergenic region has been the subject of several rhizobial studies (Riah et al. 2014; Jaiswal et al. 2017; Tampakaki et al. 2017; Menezes Júnior et al. 2019), due to the ability to differentiate microorganisms with a very high degree of similarity. This level of heterogeneity may indicate a high diversity among the isolates, which was also reported by Jaiswal et al. (2017) who evaluated the genetic variability of peanut rhizobia in South Africa.
Based on the dendrogram generated from the analysis of the IGS-RFLP profiles, the host genotype influenced the clustering. However, for some groups, such as group XVIII, the sampling site had higher influence in the clustering than the genotype, as well as observed in the Pearson’s correlation analysis. Areas with irrigation had grater abundance of isolates on the largest groups. The influence of soil characteristics on the rhizobial diversity was already observed for cowpea rhizobial (Oliveira et al. 2020) and non-rhizobial endophytes in Brazilian semiarid region (Leite et al. 2017). In addition to cowpea, Mimosa spp. rhizobia are also influenced by soil conditions in Brazil (Pires et al. 2018; de Oliveira et al. 2019).
In the present study, some groups were positively related to soil fertility characteristics, such as the highest concentrations of P, Mg2+, and K+, showing that, in addition to the management, the distribution of genetics groups is well correlated with the soil fertility characteristics. Oliveira et al. (2020) studied the genetic diversity of cowpea bradyrhizobia from Brazilian drylands in two contrasting soils amended or not with biochar. In their study, the authors observed the influence of the soil where the bacteria were obtained rather than the biochar management, showing the importance of the soil chemical characteristics in the diversity of cowpea rhizobia. Although, the soil properties are influenced by the agricultural land use, and rhizobial diversity has been highlighted in studies investigating the impact of agriculture on the population of this group of microorganisms, which are important for natural and/or agricultural systems (Leite et al. 2009; Yan et al. 2014; Vuong et al. 2017).
In this study, bacteria of the genus Bradyrhizobium of the clades B. japonicum and B. elkanii were found, in agreement to previous studies (Menna et al. 2009; Sarr et al. 2011; Marinho et al. 2017). At least nine bacteria were shown to be close to the type strain B. yuanmingense CCBAU 10071T. This strain was isolated from the drought-tolerant legume Lespedeza sp. and described as able to nodulate cowpeas (Boakye et al. 2016). Several strains of this species are able to nodulate cowpeas, including the strain BR 3267 (Martins et al. 2003), which is used as a commercial inoculant for cowpeas in Brazil since 2006. Isolates with high similarity to Bradyrhizobium yuanmingense BR 3267 showed similarities ranging from 99 to 100%. It was possible that the isolates were variations of BR 3267 because they were isolated from the same region of origin as the studied isolates.
Within the B. japonicum clade, the isolate ESA 373 had the highest similarity to the B. vignae 7-2T strain, with 91% similarity. This strain was isolated from cowpeas in Namibian soil (Grönemeyer et al. 2016). According to this study, the agricultural areas of this region have low fertility, and the intercropping of grain legumes with cereals is practiced to maximize production. The ESA 373 strain was isolated from the Canapu ligeiro genotype, a cowpea landrace popular among the growers of Juazeiro (Bahia State) and the surrounding towns. This genotype is intercropped with mango, an agroecosystem that where not evaluated regarding the rhizobial diversity in soils from Brazilian region. To date, strains similar to B. vignae have not been isolated in Brazil. The geographic isolation between these regions, which exhibit relatively similar bacteria (91% similarity), indicates that the ESA 373 isolate may belong to a new Brazilian Bradyrhizobium species.
In general, the prevalence of B. japonicum superclade among the evaluated isolates is clear, as reported by other authors who studied the diversity of rhizobia associated with cowpeas (Sarr et al. 2011; Chidebe et al. 2018; Jaiswal and Dakora 2019), and has been observed in other Bradyrhizobium diversity studies in the Brazilian semiarid region (Santos et al. 2017) in agricultural managed soils.
In the rainfed area without mineral fertilization, we identified isolates similar to B. yuanmingense CCBAU 10071T and to the reference strain B. yuanmingense BR 3267. In the agroecological cultivation area with Crotalaria, there were two genera, Bradyrhizobium and Microvirga; however, most of the isolates were identified as Bradyrhizobium. The Microvirga genus has legume-nodulating nitrogen-fixing species, including those that establish symbiosis with trees (Ardley et al. 2012) and grain legumes such as M. vignae that have been isolated from cowpea nodules in soils in Canindé São Francisco municipality in Sergipe State (Northeast region of Brazil) (Radl et al. 2014). Although this species was isolated from soil samples from the region, ESA 367, which was isolated from an agroecological management area, was 87% similar to the Russian type strain M. osseptica V5/3MT (Safronova et al. 2017). As well as observed to the B. vignae related bacteria, the affiliation of ESA 367 indicates that this strain can be member of a new species within Microvirga genus. In addition to M. vignae, other members of Microvirga genus had been recently isolated from soils of the São Francisco River Valley, region where the municipality of Juazeiro is located (Nunes et al. 2018; Oliveira et al. 2020), showing that members of nodulating clade of Microvirga genus are widespread in these soils.
In the native vegetation (Caatinga) area, the two representative isolates (ESA 388 and ESA 385) were related to Bradyrhizobium canariense CCBAU 51257T and Bradyrhizobium yuanmingense CCBAU 10071T. The relatedness of the newly isolated bacteria with several species of Bradyrhizobium and a species of Microvirga indicates the large genetic diversity of the rhizobia identified in the different land use systems in Brazilian semiarid region.
The success of a microorganism is linked to its ability to survive under the conditions imposed by its environment. Raza and colleagues (Raza et al. 2001) reported a decrease in the growth of Bradyrhizobium isolates with increasing NaCl concentrations, the same behavior observed by fast growing rhizobia from pigeon pea (Cajanus cajan) by Fernandes Junior et al. (2012). This behavior was observed in some isolates of the present study, although several bacteria grew in the medium with 3% of NaCl indicating that those bacteria can tolerate higher salt concentration in the medium and only 3% was not enough to evaluate the tolerance of these isolates to salt stress.
The ability to tolerate salt stress conditions is often correlated with the origin of the isolates (Xavier et al. 2007; Cardoso et al. 2015). However, ESA 379, ESA 368, ESA 381, ESA 384, and ESA 372, which were isolated from the same land use area (UNEB), showed different behavior under NaCl stress conditions. Within this area, the best performances were found for isolates ESA 379 and ESA 368, indicating that despite originating from the same agricultural system, they exhibited different behavior under stress, corroborant to the hypothesis that the salt stress tolerance is a result of the specific characteristics of each isolate (Dong et al. 2017).
The results of this study do not indicate that there is a direct link between the use of the soil from which the bacteria were isolated and the tolerance of these isolates to salt stress isolates ESA 388, ESA 379, ESA 365, and BR 3267 showed lower growth reduction under salt stress, demonstrating increased adaptability to salt stress. Rhizobia with higher tolerance to salt stress may exhibit greater symbiotic efficiency under stress conditions (Dong et al. 2017). But negative correlations between growth promotion and salt stress tolerance for plant growth-promoting bacteria of non-legumes (de Lima et al. 2018). Thus, the evaluation of tolerance to abiotic stresses should be carefully related to symbiotic efficiency.
Biofilm production by microorganisms may be a strategy used to protect the cells against stress conditions (Bomfeti et al. 2011; Nocelli et al. 2016), in addition to being essential for triggering several ecological processes in plant-microbe interactions, such as nodule formation (Wang et al. 2008; Bomfeti et al. 2011). The change in growth rate, observed from a reduction in the optical densities of the bacteria in standard YM medium, should be result of the increasing of the production of exopolysaccharides in the media with altered pHs and NaCl concentrations. This phenomenon is a survival strategy triggered under environmental stressful conditions (Bogino et al. 2013) such as salinity or pH (acid or alkaline media). This phenomenon was evidenced in the biofilm formation tests at different pHs and with NaCl.
The production of growth-promoting compounds is also a feature of rhizobia. The production of indole compounds in culture medium by rhizobia may be related to the growth promotion ability in inoculation experiments (Brígido et al. 2017). Thus, the high production of indole compounds by the 13 isolates obtained in this study indicates their potential for cowpea growth promotion. Costa et al. (2013) reported that bacteria isolated from cowpeas are able to synthesize indole compounds in the presence of L-tryptophan and that synthesis does not depend only on the isolates but also on the medium used to grow them. They observed a reduction in the synthesis of indole compounds when the test was performed in DYGS medium. Thus, it is possible that the results found in this study may not have revealed the potential of the isolates for this growth promotion mechanism.
The capacity to tolerate the salt and pH stressful conditions was not related to the ability to produce auxins in this culture collection. For example, the B. yuanmingense-like isolate ESA 379 was considered tolerant to salt and pH stresses, but this bacterium failed to produce high amounts of auxins. On the other hand, the B. yuanmingense-like ESA 366 and the B. elkanii-like ESA 366 (among other isolates) were considered low tolerant both to salt and pH stresses but were remarkable to the auxin biosynthesis. This behavior is commonly observed in rhizobia and other plant growth-promoting bacteria (Brigido et al. 2017; de Lima et al. 2018) reinforcing the necessity for continuous surveys to obtain both stress-tolerant and plant growth-promoting rhizobia.
All isolates tested in this study showed high symbiotic efficiency with cowpeas in the greenhouse conditions experiments. Thus, as reported by Rufini et al. (2014), the native isolates evaluated showed great symbiotic potential when compared to the recommended inoculant and exhibited higher nitrogen accumulation than the control, which contained chemical nitrogen. Isolates with nodulation efficiency identical to the treatment with the officially recommended strain BR 3267 can be considered to have high N-fixation potential because they presented similar results to a recommended strain for the crop. Low nodulation efficiency may indicate low specificity between the host plant and inoculant or compromised biological nitrogen fixation under the tested conditions.
All isolates evaluated in the symbiotic efficiency experiment led to increases in nitrogen accumulation compared to the absolute control. The inoculation of 24 out of 27 isolates induced the same nitrogen accumulation in the shoots than observed by the BR 3267 and ESA 17 reference strains. The bacterial isolates ESA 377 (B. elkanii-like), ESA 365 (B. canariensis-like), and ESA 370 (B. ferriglini-like) were the isolates that showed induced to lower NAS, but the averages were higher than the observed in the absolute control treatment. The treatment inoculated with the B. elkanii related ESA 378 strain increased total nitrogen in the shoots by more than 650% when compared to the absolute control treatment and by more than 14% when compared to the nitrogen supplementation treatment. No correlations were observed regarding the stress tolerance and/or auxin production and plant growth promotion and N fixation in vivo. Considering the isolates pointed in this paragraph, the low symbiotic efficient bacteria ESA 377 and the high symbiotic efficient isolate ESA 38 produced the same amounts of auxins and showed the same profile of in vitro stress tolerance (alkali tolerant, salt and acid susceptible). Although, despite to the absence of this correlation, the studies about the rhizobial physiological variability and tolerance to stressful conditions are important to get a better understand of the microbial physiology and potential behavior in different field conditions.
Considering the nodule efficiency parameter, the B. yuanmingense related ESA 17 along with other 10 new bacteria induced the formation of nodules with outstanding efficiency to N accumulation in the cowpea shoots. For the new isolates, 7 out of 10 were related to B. yuanmingense corroborant to the high efficiency of cowpea B. yuanmingense-like isolates in Brazil (Rufini et al. 2014), especially in Brazilian drylands (Martins et al. 2003; Marinho et al. 2017). The seven efficient B. yuanmingense-like bacteria were distributed to 6 out of 7 different lands use systems evaluated, confirming the high versatility of the bacteria closest relates do this species and their establishment in different land use systems (Mohammed et al. 2018; Ndungu et al. 2018; Jaiswal and Dakora 2019) and also their high symbiotic efficiency with cowpea.
5 Conclusions
The fertility characteristics of the soils with different agricultural uses in Brazilian semiarid region influences in the cowpea rhizobia genetic diversity. Bradyrhizobium and Microvirga with high symbiotic efficiency and metabolic variability were isolated indicating that these soils are a repository of symbiotically efficient bacteria with unique biochemical characteristics.
References
Altschul SF, Gish W, Miller W et al (1990) Basic local alignment search tool. J Mol Biol 215:403–410. https://doi.org/10.1016/S0022-2836(05)80360-2
Ardley JK, Parker MA, De Meyer SE et al (2012) Microvirga lupini sp. nov., Microvirga lotononidis sp. nov. and Microvirga zambiensis sp. nov. are alphaproteobacterial root-nodule bacteria that specifically nodulate and fix nitrogen with geographically and taxonomically separate le. Int J Syst Evol Microbiol 62:2579–2588. https://doi.org/10.1099/ijs.0.035097-0
Bissett A, Brown MV, Siciliano SD, Thrall PH (2013) Microbial community responses to anthropogenically induced environmental change: towards a systems approach. Ecol Lett 16:128–139. https://doi.org/10.1111/ele.12109
Boakye EY, Lawson IYD, Danso SKA, Offei SK (2016) Characterization and diversity of rhizobia nodulating selected tree legumes in Ghana. Symbiosis 69:89–99. https://doi.org/10.1007/s13199-016-0383-1
Bogino PC, Oliva MM, Sorroche FG, Giordano W (2013) The role of bacterial biofilms and surface components in plant-bacterial associations. Int J Mol Sci 14:15838–15859. https://doi.org/10.3390/ijms140815838
Bomfeti CA, Florentino LA, Guimarães AP et al (2011) Exopolysaccharides produced by the symbiotic nitrogen-fixing bacteria of leguminosae. Rev Bras Ciência do Solo 35:657–671. https://doi.org/10.1590/s0100-06832011000300001
Boyd ES, Peters JW (2013) New insights into the evolutionary history of biological nitrogen fixation. Front Microbiol 4:201. https://doi.org/10.3389/fmicb.2013.00201
Brígido C, Glick BR, Oliveira S (2017) Survey of plant growth-promoting mechanisms in native Portuguese chickpea Mesorhizobium isolates. Microb Ecol 73:900–915. https://doi.org/10.1007/s00248-016-0891-9
Cardoso P, Freitas R, Figueira E (2015) Salt tolerance of rhizobial populations from contrasting environmental conditions: understanding the implications of climate change. Ecotoxicology 24:143–152. https://doi.org/10.1007/s10646-014-1366-8
Chidebe IN, Jaiswal SK, Dakora FD (2018) Distribution and phylogeny of microsymbionts associated with cowpea (Vigna unguiculata) nodulation in three agroecological regions of Mozambique. Appl Environ Microbiol 84:1–25. https://doi.org/10.1128/AEM.01712-17
Costa EM, Nóbrega RSA, Carvalho F et al (2013) Plant growth promotion and genetic diversity of bacteria isolated from cowpea nodules. Pesqui Agropecu Bras 48:1275–1284. https://doi.org/10.1590/S0100-204X2013000900012
Silva VB, Silva AF, Silva TR et al (2019) Fast and efficient symbiotic gene-based duplex PCR approach for the preliminary selection of legume root nodule bacteria. Rhizosphere 10:100144. https://doi.org/10.1016/j.rhisph.2019.100144
Freitas ADS, Silva AF, Sampaio EVSB (2012) Yield and biological nitrogen fixation of cowpea varieties in the semi-arid region of Brazil. Biomass Bioenergy 45: 109-114. https://doi.org/10.1016/j.biombioe.2012.05.017
de Lima DRM, dos Santos IB, Oliveira JTC et al (2018) Tolerance of potentially diazotrophic bacteria to adverse environmental conditions and plant growth-promotion in sugarcane. Arch Agron Soil Sci 64:1534–1548. https://doi.org/10.1080/03650340.2018.1443212
de Oliveira ISR, Jesus EC, Ribeiro TG (2019) et al Mimosa caesalpiniifolia Benth. adapts to rhizobia populations with differential taxonomy and symbiotic effectiveness outside of its location of origin. FEMS Microbiol Ecol 95:fiz109. https://doi.org/10.1093/femsec/fiz109
Dong R, Zhang J, Huan H et al (2017) High salt tolerance of a Bradyrhizobium strain and its promotion of the growth of Stylosanthes guianensis. Int J Mol Sci 18:1625. https://doi.org/10.3390/ijms18081625
Dutra WF, de Melo AS, Suassuna JF et al (2017) Antioxidative responses of cowpea cultivars to water deficit and salicylic acid treatment. Agron J 109:895–905. https://doi.org/10.2134/agronj2015.0519
Fernandes Júnior PI, de Lima AA, Passos SR et al (2012) Phenotypic diversity and amylolytic activity of fast growing rhizobia from pigeonpea [Cajanus cajan (L.) Millsp.]. Braz J Microbiol 43:1604–1612. https://doi.org/10.1590/S1517-83822012000400045
Ferreira DF (2011) Sisvar: a computer statistical analysis system. Cienc Agrotecnol 35:1039–1042
Giraud E, Moulin L, Vallenet D et al (2007) Legumes symbioses: absence of nod genes in photosynthetic Bradyrhizobia. Science 316:1307–1312. https://doi.org/10.1126/science.1139548
Grönemeyer JL, Hurek T, Bünger W, Reinhold-Hurek B (2016) Bradyrhizobium vignae sp. nov., a nitrogen-fixing symbiont isolated from effective nodules of Vigna and Arachis. Int J Syst Evol Microbiol 66:62–69. https://doi.org/10.1099/ijsem.0.000674
Hammer Ø, Harper DAT, Ryan PD, Ryan DD, Ryan PD (2001) PaSt: paleontological statistics software package for education and data analysis. Palaentol Electron 4:5–7
Hollowell AC, Regus JU, Gano KA et al (2016) Epidemic spread of symbiotic and non-symbiotic Bradyrhizobium genotypes across California. Microb Ecol 71:700–710. https://doi.org/10.1007/s00248-015-0685-5
Jaiswal SK, Dakora FD (2019) Widespread distribution of highly adapted Bradyrhizobium species nodulating diverse legumes in Africa. Front Microbiol 10:310. https://doi.org/10.3389/fmicb.2019.00310
Jaiswal SK, Msimbira LA, Dakora FD (2017) Phylogenetically diverse group of native bacterial symbionts isolated from root nodules of groundnut (Arachis hypogaea L.) in South Africa. Syst Appl Microbiol. https://doi.org/10.1016/j.syapm.2017.02.002
Lacerda-Júnior GV, Noronha MF, Cabral L et al (2019) Land use and seasonal effects on the soil microbiome of a Brazilian dry forest. Front Microbiol 10:1–14. https://doi.org/10.3389/fmicb.2019.00648
Leão ÉLS, Moutinho LMG (2014) O arranjo produtivo local de fruticultura irrigada do Vale do Submédio do São Francisco como objeto de política. RACE - Rev Adm Contab Econ 13:829–858
Leite J, Seido SL, Passos SR et al (2009) Biodiversity of rhizobia associated with cowpea cultivars in soils of the lower half of the São Francisco River Valley. Rev Bras Cienc Solo 33:1215–1226. https://doi.org/10.1590/S0100-06832009000500015
Leite J, Fischer D, Rouws LFM et al (2017) Cowpea nodules harbor non-rhizobial bacterial communities that are shaped by soil type rather than plant genotype. Front Plant Sci 7:1–11. https://doi.org/10.3389/fpls.2016.02064
Marinho RCN, Ferreira LVM, Silva AF et al (2017) Symbiotic and agronomic efficiency of new cowpea rhizobia from Brazilian semi-arid. Bragantia 71:273–281. https://doi.org/10.1590/1678-4499.003
Martins LMV, Xavier GR, Rangel FW et al (2003) Contribution of biological nitrogen fixation to cowpea: a strategy for improving grain yield in the semi-arid region of Brazil. Biol Fertil Soils 38:333–339. https://doi.org/10.1007/s00374-003-0668-4
Menezes Júnior IA, de Matos GF, de Freitas KM et al (2019) Occurrence of diverse Bradyrhizobium spp. in roots and rhizospheres of two commercial Brazilian sugarcane cultivars. Brazilian J Microbiol. https://doi.org/10.1007/s42770-019-00090-6
Menna P, Barcellos FG, Hungria M (2009) Phylogeny and taxonomy of a diverse collection of Bradyrhizobium strains based on multilocus sequence analysis of the 16S rRNA gene, ITS region and glnII, recA, atpD and dnaK genes. Int J Syst Evol Microbiol 59:2934–2950. https://doi.org/10.1099/ijs.0.009779-0
Mohammed M, Jaiswal S, Dakora F (2018) Distribution and correlation between phylogeny and functional traits of cowpea (Vigna unguiculata L. Walp.)-nodulating microsymbionts from Ghana and South Africa. Sci Rep 12:1–19. https://doi.org/10.1038/s41598-018-36324-0
Mothapo NV, Grossman JM, Maul JE et al (2013) Genetic diversity of resident soil rhizobia isolated from nodules of distinct hairy vetch (Vicia villosa Roth) genotypes. Appl Soil Ecol. https://doi.org/10.1016/j.apsoil.2012.12.010
Ndungu SM, Messmer MM, Ziegler D et al (2018) Cowpea (Vigna unguiculata L. Walp) hosts several widespread bradyrhizobial root nodule symbionts across contrasting agro-ecological production areas in Kenya. Agric Ecosyst Environ 261:161–171. https://doi.org/10.1016/j.agee.2017.12.014
Nocelli N, Bogino PC, Banchio E, Giordano W (2016) Roles of extracellular polysaccharides and biofilm formation in heavy metal resistance of rhizobia. Materials (Basel). https://doi.org/10.3390/ma9060418
Norris DO, Mannetje L (1964) The symbiotic specialization of African Trifolium spp. in relation to their taxonomy and their agronomic use. East African Agric For J 29:214–235. https://doi.org/10.1080/00128325.1964.11661928
Nostro A, Procopio F, Pizzimenti FC et al (2007) Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. J Med Microbiol 56:519–523. https://doi.org/10.1099/jmm.0.46804-0
Nunes GFO, Menezes KAS, Sampaio AA et al (2018) Polyphasic characterization of forage legumes root nodule bacteria isolated from semiarid region in Brazil. Rev Ciênc Agrár 41:612–624. https://doi.org/10.19084/RCA17339
Okazaki S, Tittabutr P, Teulet A et al (2016) Rhizobium-legume symbiosis in the absence of nod factors: two possible scenarios with or without the T3SS. ISME J. https://doi.org/10.1038/ismej.2015.103
Oliveira GS, Sena PTS, Nascimento TR et al (2020) Are cowpea-nodulating bradyrhizobial communities influenced by biochar amendments in soils? Genetic diversity and symbiotic effectiveness assessment of two agricultural soils of Brazilian drylands. J Soil Sci Pl Nut. https://doi.org/10.1007/s42729-019-00128-6
Pires RC, Reis Júnior FB, Zilli JE et al (2018) Soil characteristics determine the rhizobia in association with different species of Mimosa in central Brazil. Plant Soil 423:411–428. https://doi.org/10.1007/s11104-017-3521-5
Radl V, Simões-Araújo JL, Leite J et al (2014) Microvirga vignae sp. nov., a root nodule symbiotic bacterium isolated from cowpea grown in semi-arid Brazil. Int J Syst Evol Microbiol 64:725–730. https://doi.org/10.1099/ijs.0.053082-0
Raza S, Jørnsgård B, Abou-Taleb H, Christiansen JL (2001) Tolerance of Bradyrhizobium sp. (Lupini) strains to salinity, pH, CaCO3 and antibiotics. Lett Appl Microbiol 32:379–383. https://doi.org/10.1046/j.1472-765X.2001.00925.x
Riah N, Béna G, Djekoun A et al (2014) Genotypic and symbiotic diversity of rhizobium populations associated with cultivated lentil and pea in sub-humid and semi-arid regions of eastern Algeria. Syst Appl Microbiol 37:368–375. https://doi.org/10.1016/j.syapm.2013.12.008
Rodrigues Neto J, Malavolta Jr VA, Victor O (1986) Meio simples para o isolamento e cultivo de Xanthomonas campestris pv. citri tipo B. Summa Phytopathol 12:32
Rodrigues DR, da Silva AF, Cavalcanti MIP et al (2018) Phenotypic, genetic and symbiotic characterization of Erythrina velutina rhizobia from Caatinga dry forest. Brazilian J Microbiol:1–10. https://doi.org/10.1016/j.bjm.2017.09.007
Rufini M, Pereira da Silva MA, Avelar Ferreira PA et al (2014) Symbiotic efficiency and identification of rhizobia that nodulate cowpea in a Rhodic Eutrudox. Biol Fertil Soils 50:115–122. https://doi.org/10.1007/s00374-013-0832-4
Safronova VI, Kuznetsova IG, Sazanova AL et al (2017) Microvirga ossetica sp. nov., a species of rhizobia isolated from root nodules of the legume species Vicia alpestris Steven. Int J Syst Evol Microbiol 67:94–100. https://doi.org/10.1099/ijsem.0.001577
Santos CAF (2011) Melhoramento do feijão-caupi para temperaturas moderadas e elevadas no Vale do São Francisco. Rev Bras Geogr Física 6:1151–1162
Santos JW, Silva JFD, Ferreira TDS et al (2017) Molecular and symbiotic characterization of peanut bradyrhizobia from the semi-arid region of Brazil. Appl Soil Ecol. https://doi.org/10.1016/j.apsoil.2017.09.033
Sarr PS, Yamakawa T, Saeki Y, Guisse A (2011) Phylogenetic diversity of indigenous cowpea bradyrhizobia from soils in Japan based on sequence analysis of the 16S-23S rRNA internal transcribed spacer (ITS) region. Syst Appl Microbiol 34:285–292. https://doi.org/10.1016/j.syapm.2010.11.021
Sarwar M, Kremer RJ (1995) Determination of bacterially derived auxins using a microplate method. Lett Appl Microbiol 20:282–285. https://doi.org/10.1111/j.1472-765X.1995.tb00446.x
Tampakaki AP, Fotiadis CT, Ntatsi G, Savvas D (2017) Phylogenetic multilocus sequence analysis of indigenous slow-growing rhizobia nodulating cowpea (Vigna unguiculata L.) in Greece. Syst Appl Microbiol 40:179–189. https://doi.org/10.1016/j.syapm.2017.01.001
Vincent JM (1970) A manual for the practical study of root-nodule bacteria. [published for the] International Biological Programme [by] Blackwell Scientific
Vuong HB, Thrall PH, Barrett LG (2017) Host species and environmental variation can influence rhizobial community composition. J Ecol 105:540–548. https://doi.org/10.1111/1365-2745.12687
Wang P, Zhong Z, Zhou J et al (2008) Exopolysaccharide biosynthesis is important for Mesorhizobium tianshanense: plant host interaction. Arch Microbiol 189:525–530. https://doi.org/10.1007/s00203-007-0345-3
Xavier GR, Martins LMV, Rumjanek NG, Neves MCP (2007) Tolerância de rizóbio de feijão-caupi à salinidade e à temperatura em condição in vitro. Caatinga 20:1–9
Yan J, Han XZ, Ji ZJ et al (2014) Abundance and diversity of soybean-nodulating rhizobia in black soil are impacted by land use and crop management. Appl Environ Microbiol 80:5394–5402. https://doi.org/10.1128/aem.01135-14
Funding
The authors are grateful to the Brazilian Agricultural Research Corporation (Embrapa 23.16.05.016.00.00) and to the Brazilian Council for Scientific and Technological Development (CNPq 406327/2013-8), and to INCT—Plant Growth Promoting Microorganisms for Agricultural Sustainability and Environmental Responsibility (CNPq/Fundação Araucária INCT-MPCPAgro 465133/2014-4) for the financial support. Acknowledgments are also given to Coordination of Improvement of Higher Education Personnel (CAPES) for awarding scholarships to the first, second, fourth, and fifth authors. The sixth and seventh authors thank the CNPq for their productivity research fellowship (306812/2018-5 and 311218/2017-2, respectively).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interests. The funding agencies did not have any influence on the data acquisition, evaluation, and interpretation.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sena, P.T.S., do Nascimento, T.R., Lino, J. et al. Molecular, Physiological, and Symbiotic Characterization of Cowpea Rhizobia from Soils Under Different Agricultural Systems in the Semiarid Region of Brazil. J Soil Sci Plant Nutr 20, 1178–1192 (2020). https://doi.org/10.1007/s42729-020-00203-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-020-00203-3