Abstract
Perennial trees especially fruit trees are considered to be the most competent biological system where atmospheric carbon dioxide is transformed into long-lived soil carbon despite their nutritional and export value. Higher carbon stock helps to sustain production and soil ecosystem services. Better crop nutrition promotes carbon stock. Feasibility of integrated nutrient management in carbon sequestration needs to be evaluated under a subtropical humid condition. An experiment was carried out in randomized block design to study the feasibility of integrated nutrient management for improving soil properties, nutrient availability, fruit yield, and carbon stock in a mango (Mangifera indica L.) (cv. Langra) orchard under a subtropical condition. Various combinations of integrated (farmyard manure, vermicompost, straw mulch, biofertilizers) nutrient management practices were evaluated in two consecutive years in a 30-year-old mango orchard. The results revealed that the organic mulching with straw and conjoint application of farmyard manure and vermicompost improve nutrient availability, microbial activeness (29–44%), and carbon stock (~ 40%) in soil at 0–60 cm soil depth which ultimately improves fruit yield (26–34%). Hence, adoption of integrated nutrient management practices through the application of farmyard manure, vermicompost, and organic mulching with straw would uphold the fruit yield and carbon stock in soil and also promote CO2 sequestration in soil and less greenhouse gas emission, which paved viable economic options to mitigate climate change.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The ‘4 per mille Soils for Food Security and Climate’ is the global issue of the most concern in the present decade. This issue was raised in the 21st Conference of the United Nations Framework Convention on Climate Change in Paris. This was the unique convention where voluntary initiative came into action to enhance world soil organic carbon (SOC) content at the rate of 0.4% per year of soils to a depth of 40 cm (Lal 2016). Soil organic carbon is designated as the key determinant of soil quality and plays a pivotal role in the soil’s ecosystem services (Stockmann et al. 2013; Adhikari and Hartemink 2016). Thus, its sequestration has been considered as the most plausible solution to enhance long-lived soil carbon stock by aspirating carbon dioxide (CO2) from the atmosphere. This phenomenon has some co-benefits like nutrient enrichment in soil, offsetting greenhouse gas emission, and obviously climate change mitigation (Minasny et al. 2017). Vegetation plays a crucial role in maintaining the quantity and vertical distribution of soil organic matter (SOM) by altering net primary productivity and diversity in plant allocation and assemblages (Jobb’agy and Jackson 2000). Thus, higher SOM formation and stabilization could be attained by increasing belowground carbon allocation through the selection of deep-rooted plant species (Jackson et al. 2016).
On the other hand, the sustainability of management practices and increase in total agricultural production are the urgent needs of the hour to uphold food and nutritional security. The challenges are especially daunting because of the changing and uncertain climate (Wilke and Morton 2015; Tubiello et al. 2007). There are several well-established low-cost strategies reported throughout the world to enhance the productivity of different agri-horticultural crops. Among them, the application of micronutrients, intercropping, adaptation of a high-density planting (HDP) system, drip irrigation, scientific training and pruning technique, and use of different plant growth regulators play a major role to enhance the productivity of different crops, particularly perennial crops (Thakur et al. 2018; Lipton 2012). But the major concern is the sustainability of all these techniques in the long term. Moreover, soil quality is a strong determinant of yield potentiality of any agricultural crops for a longer period of time. Without giving attention to soil health, it is not possible for any improved production technique to give sustainable yield year after year. Therefore, it is an urgent need to search for an alternative technique which will enhance the productivity and also improve the soil fertility status. Adoption of integrated nutrient management (INM) practices by means of biofertilizer, farmyard manure (FYM), vermicompost, and organic mulch application are the utilitarian option to enrich the soil fertility status by increasing soil organic matter (SOM) in the surface soil layer that ultimately will reduce soil erosion and improve the soil chemical and biological properties (Kheyrodin and Antoun 2011; Triplett and Dick 2008). Improvement of the soil chemical and biological properties will ultimately increase the yield potential of the crop. Now, among different crops, the perennial crop has great potentiality to retain the organic carbon in the soil as compared to annual crops and this organic carbon stock has great potentiality to improve crop productivity up to 400 kg ha−1 year−1(Srinivasarao et al. 2013). Mango (Mangifera indica L.) has an immense potential in carbon retention (Naik et al. 2017). Besides, among fruit crops grown in India, mango has the maximum area of coverage (34.71% of the total fruit orchard) (Anonymous 2018), with 40.38% of the total global mango production and 78 million US$ export earning per year (from fresh mango and mango pulp) (Directorate General of Commercial Intelligence and Statistics (DGCIS) 2018). Several studies depicted that INM through FYM, vermicompost, and biofertilizer in mango substantially improved the yield and quality of the produce, but their impact on carbon retention was not studied extensively especially under a subtropical humid climatic condition. Hence, the present study was conceived with the following objectives: (i) to assess the effect of INM on soil fertility and carbon stock and (ii) to study the impact of retained carbon on the yield potentiality of a mango orchard.
2 Materials and Methods
2.1 Experimental Site and Treatment Details
The study was conducted in the eastern part of India (N 85° 15′ 15″, E 87° 2′ 4″) under a subtropical climate with a hot and desiccating summer and a cold but frost-free winter. The average annual rainfall of the site is around 1170 mm, precipitating mainly in between the middle of June and the middle of October. The mean annual air temperature is 31.2 °C, with the maximum in the months of April and May (37.3 °C). The trial was laid out in a 30-year-old mango (Mangifera indica L.) cv. Langra orchard having a leveled surface with well-drained sandy loam soil (sand, 55.6%; silt, 24.9%; clay, 19.5%). The trial was continuing since 2009 with the following treatment combinations: T1, control [recommended dose of fertilizer (RDF), 1000:500:500 g N:P2O5:K2O]; T2, RDF + organic mulching (10 cm thick); T3, ½ RDF + 50 kg FYM + 250 g Trichoderma harzianum; T4, ½ RDF + 50 kg FYM + 250 g Azospirillum; T5, ½ RDF + 50 kg FYM + 250 g Azotobacter; T6, ½ RDF + 50 kg FYM + 5 kg vermicompost; and T7, ½ RDF + 50 kg FYM + 250 g Pseudomonas fluorescence. The experiment was laid out in randomized block design (RBD) with three replications. Hence, a total of 21 trees were utilized in this experiment. They were planted at 10 × 10 m spacing in a square planting system (Fig. 1).
Planting layout of mango (Mangifera indica L.) cv. Langra under INM practice. T1, control (RDF—1000:500:500 g N:P2O5:K2O); T2, RDF + organic mulching (10 cm thick); T3, ½ RDF + 50 kg FYM enriched with Trichoderma (250 g); T4, ½ RDF + 50 kg FYM + Azospirillum (250 g); T5, ½ RDF + 50 kg FYM + Azotobacter (250 g); T6, ½ RDF + 50 kg FYM + vermicompost (5 kg); T7, ½ RDF + 50 kg FYM + Pseudomonas fluorescence (250 g)
From the first year of the experiment, soil was evenly tilled 4–5 times during the growing season (February–August) using an 18-disc harrow (10 cm depth). A basin was prepared around the trunk of each and every mango plant with a radius of 2.5 m from the trunk. The treatments were applied in the tree basin in the 1st week of October. In the T2 treatment, paddy straw was used as organic mulch. Mulching was done on the entire basin of each plant at the rate of 10 cm thickness. Urea (46% N), diammonium phosphate (18% N, 46% P2O5), and muriate of potash (60% K2O) were used for inorganic fertilization and were applied in the basin. The plants were watered weekly in winter and at 2–3-day intervals in summer during first year of establishment. However, irrigation was given to the tree basin at the interval of 8–10 days during winter and at weekly intervals during the summer months from the second year onwards. Pruning was done in winter and all residues were removed. The entire experimental plots were maintained weed free during the entire course of the study and need-based plant protection measures were adopted.
2.2 Soil Sample Collection
Soil samples were collected in the month of September of 2017 and 2018 (before fertilizer application) from the border of the tree basin, i.e., around 2.5 m distance from the tree trunk as most of the feeder root of mangos is usually concentrated in that region. The samples were collected in triplicate from each experimental tree at four different depths, viz. 0–15 cm, 15–30 cm, 30–45 cm, and 45–60 cm using a soil core. Thereafter, three soil samples for each depth were mixed together to make one final sample. One part was sieved immediately through a 2-mm sieve and used for microbial biomass carbon estimation. The second part was used for bulk density estimation, and the third portions of the sampled soils were air-dried, passed through a 2-mm sieve, and kept at 4 °C for further analysis of the different physico-chemical properties of the soil and carbon fractions.
2.3 Measurement of Soil Physico-chemical Properties
The pH and electrical conductivity of the soil samples were measured with the help of a pH meter and a conductivity meter, respectively, using a soil to water ratio of 1:2.5 (Jackson 1967); however, the oxidizable organic carbon content was determined by the wet oxidation method as suggested by Walkley and Black (1934). The available nitrogen content was measured using the 0.32% potassium permanganate (KMnO4) oxidation method (Subbiah and Asija 1956). The available phosphorus content of the soil sample was extracted with 0.5 M NaHCO3 (pH 8.5) and measured by the method suggested by Olsen et al. (1954). The available potassium content in the soil sample was measured by using the flame photometric method (Jackson 1967) after extraction with neutral normal ammonium acetate solution.
2.4 Estimation of Bulk Density
In situ bulk density of the soil profile was determined by the core sampling method (Blake and Hartge 1986). For this method, undisturbed soil samples were taken out using the metallic cores of known internal volume (Vt) at 15-cm depth intervals (0–15, 15–30, 30–45, and 45–60 cm soil depth). The undisturbed soil cores were oven-dried at 105 °C to record the dry weight of soil (Ws). The bulk density (BD) was calculated using following formula and expressed as megagram per cubic meter.
2.5 Estimation of Microbial Biomass Carbon
The soil microbial biomass carbon was analyzed by the procedure given by Vance et al. (1987). For this procedure, two sets of 10 g fresh soil were taken in beakers; the first one was fumigated with chloroform for 24 h, while the second one was kept unfumigated. Both the fumigated and unfumigated soil samples were extracted using 0.5 M K2SO4. A small amount of extract was digested with potassium persulfate (K2S2O8) at 120 °C for 2 h in a digestion block. The carbon dioxide evolved during the digestion was trapped in 0.1 N NaOH taken in a vial kept over the constriction inside the digestion tube. A control was also run using 0.5 M K2SO4 in place of the extract. The unconsumed NaOH was determined by titration with standard 0.01 N H2SO4 using phenolphthalein indicator. The microbial biomass carbon (MBC) was calculated using the formula given below
where CF is carbon in fumigated soil, CUF is carbon in unfumigated soil, and KEC is efficiency of extraction (0.45).
2.6 Estimation of Total Organic Carbon, Soil Organic Carbon Pools, and Carbon Stock
Total soil carbon (TOC) was measured by dry combustion method using a CHNS Analyzer (model: Euro EA 3000). Complete combustion of carbon in the sample was ensured into the combustion reactor (at 980 °C), where the proper amount of oxygen and combustion catalysts combusted carbon. The TOC content was expressed in grams per kilogram soil basis.
Soil organic carbon pools were estimated through a modified Walkley and Black method as described by Chan et al. (2001) using 12.0 N, 18.0 N, and 24.0 N H2SO4. Comparing with total organic carbon allowed its separation into four different fractions: fraction I (very labile), the amount of organic carbon oxidized by 12.0 N H2SO4; fraction II (labile), the difference in the amount of carbon oxidized by 18.0 N and that by 12.0 N H2SO4; fraction III (less labile), the difference in the amount of carbon oxidized by 24.0 N and that by 18.0 N H2SO4; and fraction IV (non-labile), the difference in TOC and amount of carbon oxidized by 24.0 N H2SO4. Fractions I and II together represent the active pool, and fractions III and IV together represent the passive pool of organic carbon in soils (Chan et al. 2001).
The carbon stock in various soil depths was estimated on equivalent depth basis using the following formula as proposed by Lal et al. (1998):
where BD is the bulk density of the soil (Mg m−3) and D is the soil depth in meters.
2.7 Fruit Yield Estimation
The yield data was collected from the each mango plant for the year 2012–2013 to 2017–2018. As the Langra variety is an alternate bearer, therefore, from the 6-year yield data, only three alternate on years yield data was taken and pooled yield for different treatments were calculated to carry out the correlation study of the soil carbon pool with mango yield under the INM system.
3 Statistical Analysis
The observations were subjected to statistical analysis by using randomized block design (RBD) with three replications. The data were submitted to two-way ANOVA to assess the variability of integrated nutrient management practices and its spatial variability with depth. Data were analyzed using statistical analysis software (SAS 9.2; SAS Institute, Cary, NC, USA), and the means were compared using Tukey’s HSD mean separation test. Furthermore, a Pearson’s correlation study and principal components analysis (PCA) were performed with different soil physico-chemical parameters to estimate their relations with the yield pattern of the mango orchard under the INM system using SPSS software (SPSS version 16.0, Chicago, USA).
4 Results
4.1 pH and Electrical Conductivity
The results of the long-term response of different forms of biofertilizer, vermicompost, and organic mulch under the integrated nutrient management system of the mango orchard on soil pH indicate a significant variation among the treatments in the lower depth of 45–60 cm (Table 1). It indicates a higher pH in the control (7.25) and reduces significantly in T4 (6.68), T6 (6.70), and T3 (6.83) although Azospirillum application behaves quite differently in all soil depths. However, these treatments had no significant impact on the change of electrical conductivity (EC) of the soils but we could trace a minor change in EC at 30–45 cm soil depth.
4.2 Soil Organic Carbon Content
Addition of organic mulch (10 cm thick) was able to improve the SOC content at different depths of soil (9.48, 6.67, 8.09, and 4.51 g C kg−1 soil, respectively, at 0–15, 15–30, 30–45, and 45–60 cm soil depth) which was significantly higher than that of the control (Table 1). Besides organic mulching, conjoint application of FYM and vermicompost has a similar impact on SOC content throughout the depths (0–60 cm). Apart from these two, other treatments also had a significant effect on the improvement in soil organic carbon content as compared to control in all the depths although their magnitude was higher in surface soil and gradually decreased with the depth, irrespective of treatments. Treatments T2 and T6 resulted in 2.47 and 2.10 times more SOC content at 0–15 cm soil depth over the control (4.66 g kg−1 soil), respectively, and the same was reduced to 1.56 and 1.16 times over the control at 15–30 cm depth.
4.3 Available Nutrient Content
Available nitrogen content in soils showed a similar trend as that of the SOC content. Vermicompost and organic mulching significantly increased the available nitrogen content (Table 1). It was also evident that irrespective of treatment, the available nitrogen content increased gradually with the increase of soil depth up to 45 cm; however, with the further increase of soil depth, it started to decrease, resulting in a 12.95% and 19.78% increase of available nitrogen content in the soil at 15–30 and 30–45 cm soil depth, respectively, and 30.32% reduction at 45–60 cm depth as compared to 0–15 cm soil depth (256.5 kg ha−1) (average of all treatments). It is pertinent to mention that the available nitrogen content in the soil has been increased significantly in all the treatments as compared to the control particularly in the lower depth (below 30 cm). However, at all the depths, the available nitrogen content in the soil was recorded maximum in T2 (89.50%, 11.18%, 26.91%, and 18.85% higher than the respective control, respectively) (mean of all depths).
Irrespective of depth, it was recorded maximum in T6 (45.78 kg P2O5 ha−1 soil) with the minimum value in T3 (25.7 kg P2O5 ha−1 soil) (Table 1). Although the interaction between treatments with soil depth was non-significant, their increasing trends in available phosphorus content as compared to the control indicate the beneficial aspects of INM in mango orchard soil.
It was observed that the top soil in the orchard had the minimum available potassium content (82.4 kg K2O ha−1 soil) (mean of all treatments) which was increased significantly at subsequent lower depths that possess 107.4 kg K2O ha−1 soil in 15–30 cm depth, 107.3 kg K2O ha−1 soil in 30–45 cm depth, and 100.4 kg K2O ha−1 soil in 45–60 cm depth (Table 1). Regardless of depth, the available K content was higher in T2 (48.68% higher than the control). The impact of mulching and additional vermicompost application with FYM was clearly noted on the increased availability of potassium in the orchard soils.
4.4 Bulk Density
Upper-layer soil (0–15 cm soil depth) recorded a bulk density (BD) of 1.43 Mg m−3 which was reduced marginally in the subsequent two layers of soil, i.e., at 15–30 and 30–45 cm (1.42 and 1.41 Mg m−3 of soil, respectively); however, beyond 45 cm depth, a certain increase in BD was recorded (Table 1). Further, irrespective of soil depth, it was reduced marginally as compared to the control in T6 (1.41 Mg m−3 of soil). Mulches are able to reduce the bulk density at 0–15 cm depth. A similar pattern was observed for the soil depths of 30–45 and 45–60 cm with minimum bulk density (1.39 and 1.46 Mg m−3 of soil, respectively).
4.5 Microbial Biomass Carbon Content
Regardless of depth, there was higher microbial biomass carbon content in T6 (½ RDF + 50 kg FYM + 5 kg vermicompost) (1.35 times as compared to control) (Table 1). Irrespective of treatment, the microbial biomass carbon content was estimated maximum at 30–45 cm soil depth (197.1 μg C g−1 soil) (mean at each depth); however, it was reduced significantly with the further increase of soil depth (186.3 μg C g−1 soil). The perusal of data regarding the interaction between treatments with individual depth indicates that the microbial biomass carbon content attained a maximum in T6 in all the four different soil depths (29.37%, 44.11%, 33.61%, and 35.38% higher than the respective control, respectively).
4.6 Total Organic Carbon Content, Soil Organic Carbon Pools, and Carbon Stock
The data presented on Fig. 2 depicts the impact of integrated nutrient management (INM) on total organic carbon (TOC) content in the soil of the mango orchard, and significant variation was observed among the treatments at the depth from 0 to 60 cm. It was recorded maximum in T2 (13.43 g C kg−1 soil) and minimum in the control (7.99 g C kg−1 soil) at 0–15 cm depth. However, at 15–30 cm soil depth, it was recorded maximum in T6 (9.32 g C kg−1 soil) with par value in T2 (8.85 g C kg−1 soil) and minimum in T3 (6.95 g C kg−1 soil). A similar trend was observed in 30–45 and 45–60 cm soil depths with maximum TOC content in T6 (48.75% and 43.23% higher than the respective control, respectively) with par value in T2 (35.91% and 34.55% higher than the respective control, respectively). Amusingly, irrespective of treatment, an alternate pattern of increase and decrease in TOC content was found at 0–15 cm (9.96 g C kg−1 soil), 15–30 cm (7.74 g C kg−1 soil), 30–45 cm (10.15 g C kg−1 soil), and least at 45–60 cm depth (6.65 g C kg−1 soil).
Effect of integrated nutrient management system on total organic carbon (g kg−1) content in mango orchard. Bars indicate mean value of three replicates. Bars with different letters are significantly different (p ≤ 0.05) in Tukey’s HSD mean separation test. T1, control (RDF—1000:500:500 g N:P2O5:K2O); T2, RDF + organic mulching (10 cm thick); T3, ½ RDF + 50 kg FYM enriched with Trichoderma (250 g); T4, ½ RDF + 50 kg FYM + Azospirillum (250 g); T5, ½ RDF + 50 kg FYM + Azotobacter (250 g); T6, ½ RDF + 50 kg FYM + vermicompost (5 kg); T7, ½ RDF + 50 kg FYM + Pseudomonas fluorescence (250 g)
Soil organic carbon (SOC) pools depict the concentration and quality of carbon influenced by the nutrient management practices and soil depth. Application of organic inputs (straw mulch, FYM, vermicompost) and biofertilizers in culmination with inorganic fertilizer significantly influenced the soil carbon pools. The very labile pool of carbon was measured highest in T2 (2.09 to 4.38 g C kg−1) followed by T6 (2.51 to 3.63 g C kg−1) irrespective of soil depth (Table 2). The non-labile pool was significantly varied with the treatment in 30–45 cm soil depth with the maximum value in T6 (4.33 g C kg−1) with par result in T2 (3.87 g C kg−1) which was 81.93 and 62.61% higher than the control, respectively. However, a contrary behavior was found among the pools between the surface and subsurface soil. Labile carbon content (sum of very labile and labile carbon concentration) contributed larger proportion ranges from 49.3 to 57.6% and 45.9 to 55.5% at 0–15 cm and 15–30 cm soil depths, respectively. In contrast to the surface SOC pools, subsurface SOC pools were dominated by non-labile carbon content (sum of less labile and non-labile carbon concentrations) and ranged from 51.1 to 60.2% and 55.1 to 66.9% at 30–45 cm and 45–60 cm soil depths, respectively.
4.7 Total Organic Carbon Content
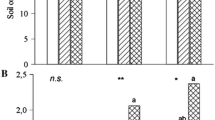
The depth equivalence of carbon stock was maximum in T6 and minimum in the control (64.52 Mg C ha−1) followed by T3, T4, T5, and T7 (Fig. 3). On the other hand, irrespective of treatment, it was measured maximum at 30–45 cm soil depth (21.53 Mg C ha−1); however, at the next layer of soil depth, i.e., at 45–60 cm, it was measured minimum among all the soil depths (14.61 Mg C ha−1). The peep of interaction of different treatments with the soil depth on carbon stock indicates significant variation of carbon stock retention at different depth with different treatments with the maximum in T2 and T6 followed by T3, T4, T5, and T7 and lowest in T1 (control) (carbon stock at 0–60 cm depth).
Effect of integrated nutrient management system on carbon stock (Mg C ha−1) of soil in mango orchard. Bars indicate mean value of three replicates. Bars with different letters are significantly different (p ≤ 0.05) in Tukey’s HSD mean separation test. T1, control (RDF—1000:500:500 g N:P2O5:K2O); T2, RDF + organic mulching (10 cm thick); T3, ½ RDF + 50 kg FYM enriched with Trichoderma (250 g); T4, ½ RDF + 50 kg FYM + Azospirillum (250 g); T5, ½ RDF + 50 kg FYM + Azotobacter (250 g); T6, ½ RDF + 50 kg FYM + vermicompost (5 kg); T7, ½ RDF + 50 kg FYM + Pseudomonas fluorescence (250 g)
4.8 Fruit Yield
It is envisaged from the data presented in Fig. 4 that fruit yield (the pooled yield of bearing year during 2011–2012 to 2016–2017) was obtained maximum in T6 and T2 (1.5 times that of control, respectively). Biofertilizer application also has a significant contribution in fruit yield. Treatments T3, T4, T5, and T7 recorded 34%, 31%, 28%, and 26% higher yield than the control, respectively (Fig. 4).
Pooled yield of mango cv. Langra orchard under INM system (error bars indicate standard deviation). T1, control (RDF—1000:500:500 g N:P2O5:K2O); T2, RDF + organic mulching (10 cm thick); T3, ½ RDF + 50 kg FYM enriched with Trichoderma (250 g); T4, ½ RDF + 50 kg FYM + Azospirillum (250 g); T5, ½ RDF + 50 kg FYM + Azotobacter (250 g); T6, ½ RDF + 50 kg FYM + vermicompost (5 kg); T7, ½ RDF + 50 kg FYM + Pseudomonas fluorescence (250 g)
4.9 Relationship of Yield with Soil Properties and Carbon Stock
The impact of INM on fruit yield in relation to different soil properties, nutrient availability, and carbon stock is presented in Table 3. It indicates that the soil pH, EC, available phosphorus, and potassium as well as the bulk density of the soil under long-term INM system of the mango orchard did not influence the yield of the mango orchard directly over the year (p > 0.05). However, the yield was positively associated with the available nitrogen content (r = 0.775, p < 0.05), MBC (r = 0.934, p < 0.01). The soil carbon fraction particularly the very labile (r = 0.820, p < 0.05), less labile (r = 0.810, p < 0.05), and non-labile carbon (r = 0.816, p < 0.05) fractions also influenced the fruit yield significantly. It is also envisaged from the data that the soil organic carbon content, TOC content, and carbon stock in the soil significantly enhanced the fruit yield over the period of 6 years. The PCA performed with the studied soil properties, soil carbon pools, and carbon stock with respect to fruit yield of the mango orchard (Fig. 5) showed 86% of the variability as explained by principal components (PC) 1 and 2. PC 1 explained about 73% variability in the system where fruit yield was attributed to available nitrogen content, MBC, labile and non-labile carbon pools, TOC, and carbon stock. PC 2 explained 13% variability related with soil pH, EC, and available phosphorus content in soil.
PCA factor scores of variations in soil properties and fruit yields in mango orchard considering different management practices and soil depth. pH, soil pH; EC, electrical conductivity; N, available nitrogen; P, available phosphorus; K, available potassium; OC, soil organic carbon; MBC, microbial biomass carbon; POOLI, very labile carbon; POOLII, labile carbon; POOLIII, less labile carbon; POOLIV, non-labile carbon; TOC, total organic carbon
5 Discussion
5.1 Soil pH and Electrical Conductivity
Treatment-induced variation in soil pH and electrical conductivity (EC) was quite inconsistent throughout the soil profile (0–60 cm depth). Little variation was found within the treatments and soil layers. Surface soil was attributed slight acidic behavior and subsequent increase in pH was found with depth. Surface-applied organic manure viz. FYM, vermicompost, and straw mulch, underwent decomposition and created acidity in the surface layer (0–15 cm). Translocation of decomposition-derived products and native bases with rain or irrigation water may cause higher pH and EC at lower depth than surface soil. But, Azospirillum (T4) behaves differently than other treatments. It could maintain an acidic pH still in lower depth of soil. Azospirillum is reported to be an endophyte living in the cortical region of the plant root (Alexander 1977). The feeder roots of mangos are mostly concentrated around 30–45 cm depth and may extend up to 60 cm (Majumder et al. 2001). Thus, Azospirillum-mediated nitrogen fixation which is a proton-donating biochemical reaction may cause acidic pH in soil. Electrical conductivity was little influenced by the integrated nutrient management practices despite their spatial variation with depth.
5.2 Nutrient Availability
Nutrient availability is imitative to soil function and supports ecosystem services. Orchards supplied with organic manure have uplifted the available nutrient content by supplying manure-derived nitrogen (N), phosphorus (P), and potassium (K), and this could also help to reduce the application of inorganic fertilizer (Montanaro et al. 2012). Mineralization of the applied organic manures has attributed to the increased availability of nutrients especially nitrate (NO3−). This could have a profound influence on nitrogen buildup in soil. Thus, application of vermicompost with FYM (T6) had shown the highest nitrogen availability in soil. Application of bio inoculants especially nitrogen fixers further broaden the magnitude of nitrogen buildup in soil that was a function of microbial activity and organic matter decomposition (Sánchez-Navarroa et al. 2019). Similarly, organic mulching with paddy straw could also offset the nutrient-supplying function of vermicompost and FYM as far as their limited availability is concerned. Phosphorus buildup was found highest in the treatment where vermicompost, FYM, and organic mulch were applied along with the reduced inorganic fertilization (i.e., half of the recommended dose of fertilizer). Application of these organics substantially increases the orthophosphate content in soil due to the release of P through microbial decomposition of organic inputs (Marinari et al. 2000; Arancon et al. 2006). Further, integrated nutrient management practices significantly improved the available potassium content in soil. The rice straw and vermicompost contain a higher amount of potassium; thus, enhanced potassium content in the treatment consists of RDF + organic mulching (10 cm thick) followed by ½ RDF + 50 kg FYM + vermicompost (5 kg). Mulching provides a favorable environment for microbial activity and nutrient release in soil (Adak et al. 2014a).
5.3 Bulk Density
The short-term nutrient management practices had a negligible impact on the soil bulk density, and we may expect significant changes in bulk density with the treatments over time. Application of organic mulching exhibited lesser bulk density. For better understanding of the bulk density and nutrient management practices, experiments need to be carried out at least for another 5 years.
5.4 Microbial Biomass Carbon
Microbial biomass carbon (MBC) plays a mechanistic role in mineralization, turnover, and stabilization of SOM. It behaves positively with organic matter input provided nutrient is hardly a limiting factor (Kirkby et al. 2014; Wardle 1992). Addition of organic matter linked with substrate abundance for microbial growth resulted in higher microbial biomass carbon. The result revealed that balanced nutrition and organic matter addition improved the microbial biomass carbon status in soil (Mandal et al. 2007). Thus, conjoint application of vermicompost and FYM had increased the microbial biomass carbon as compared to the organic mulch and FYM alone (Adak et al. 2014b). Moreover, maximum feeder root accumulation in mango is found around 30–45 cm depth, and thus, this zone was surplus with fresh root exudates which could aggravate the MBC pools in native soil.
5.5 Soil Organic Carbon, Carbon Pools, and Carbon Stock
The magnitude of carbon content in different carbon fraction is influenced by the availability of substrate (carbon source) added to the soil. Carbon inputs enhance the carbon content in the soil (Majumder et al. 2008; Seneviratne 2000). Straw mulch provides a favorable condition for microbial degradation of organic matter that enhances the carbon content with higher labile fraction over other treatments (Nayak et al. 2012). Increment in the carbon input causes subsequent upliftment of carbon content. Additional application of vermicompost improved the carbon content which was higher than the treatment with only FYM application. On the other hand, higher microbial activity also promotes the stabilization and polymerization of labile carbon into humus; thus, higher TOC content was found in the treatments T2 and T6. Higher TOC content subsequently increases the higher carbon stock. SOC native to lower depth are characterized with longer residence time. The labile to non-labile carbon ratio could mimic the residence time (Chan et al. 2001). A higher ratio value indicated more stabilized carbon content in the lower layer implying limited labile carbon substrate for microbial activity in subsoil (Heitkötter et al. 2017; Tian et al. 2016) (Table 2). Generally, feeder roots in mango are abundant around 30–45 cm which may extend to 60 cm in the case of high-yielding cultivars (Majumder et al. 2001). Straw mulching, FYM, and vermicompost application may promote deeper penetration of the feeder root by lowering the BD at the subsurface layer, and deep soil root production with limited availability of labile carbon would promote more stabilized carbon stock in soil (Pries et al. 2018). Thus, balanced application of nutrient along with organic matter not only improved the available nutrient contents in soil but also enhanced biological activeness and carbon retention in soil.
5.6 Correlation of Carbon Retention with Yield of Mango
The treatment comprising balanced nutrient along with organic matter (T6) as well as balanced nutrient with organic mulching (T2) significantly increased the soil organic carbon content, available N content, microbial biomass carbon content, labile carbon, total organic carbon content, and total carbon stock content in the soil which has direct correlation with the yield of the orchard. The correlation study also indicates that the total carbon content (r = 0.824, p < 0.05) and carbon stock (r = 0.826, p < 0.05) had positive relations with the labile carbon. Therefore, these two treatments have preferential advantage in improving the yield of the orchard very significantly as compared to other treatments as evidenced by the pooled yield data presented in Fig. 4.
6 Conclusion
From the present investigation, it may be concluded that the integrated nutrient management practices substantially influence the nutrient availability and carbon stock in the soil of the mango orchard. FYM along with vermicompost and organic mulch triggered their buildup in soil. Microbial inoculants also help in improving the nutritional status of the soil despite their limited contribution in soil carbon stock. This study indicates that the rejuvenation of the mango orchard with organic sources is indispensable especially in the subtropical climatic condition where most of the soils possess low organic matter and fertility level. Moreover, application of the organic manure and straw mulching promotes root production in deeper soil. This will promote more stabilized carbon stock in soil. Indirectly, it would promote more atmospheric CO2 sequestration in soil. Hence, adoption of integrated nutrient management practices with conjoint application of FYM and vermicompost and organic mulching with straw would not only uphold the fruit yield and carbon stock in soil but also promote CO2 sequestration in soil which paved viable economic options to mitigate climate change. In a country like India, where straw burning is a serious issue, organic mulching in the orchard crops could solve two alarming problems, greenhouse gas emission after straw burning and limited availability of FYM and vermicompost, and ultimately, climate-smart sustainable production practices may come into force.
References
Adak T, Kumar K, Singha A, Shukla SK, Singh VK (2014a) Assessing soil characteristics and guava orchard productivity as influenced by organic and inorganic substrates. J Anim Plant Sci 24(4):1157–1165
Adak T, Singha A, Kumar K, Shukla SK, Singh A, Singh VK (2014b) Soil organic carbon, dehydrogenase activity, nutrient availability and leaf nutrient content as affected by organic and inorganic source of nutrient in mango orchard soil. J Soil Sci Plant Nutr 14(2):394–406
Adhikari K, Hartemink AE (2016) Linking soils to ecosystem services — a global review. Geoderma 262:101–111
Alexander MA (1977) Introduction to Soil Microbiology. John Wiley & Sons, New York, USA
Anonymous (2018) Horticultural statistics at a glance. Horticulture Statistics Division, Department of Agriculture, Cooperation and Farmers’ Welfare, Ministry of Agriculture and Farmers’ Welfare, Government of India. India Offset Press, New Delhi.
Arancon NQ, Edwars CA, Bierman P (2006) Influences of vermicomposts on field strawberries: 2. Effects on soil microbiological and chemical properties. Bioresour Technol 97:831–840
Blake GR, Hartge KH (1986) Bulk density. In: Klute A (ed) Methods of soil analysis. Part 1. Physical and mineralogical methods, 2nd edn, Madison
Chan KY, Bowman A, Oates A (2001) Oxidizible organic carbon fractions and soil quality changes in an oxicpaleustalf under different pasture leys. Soil Sci 166:61–67
Directorate General of Commercial Intelligence and Statistics (DGCIS) (2018) http://www.dgciskol.gov.in. Accessed 10 May 2019
Heitkötter J, Heinze S, Marschner B (2017) Relevance of substrate quality and nutrients for microbial C-turnover in top- and subsoil of a Dystric Cambisol. Geoderma 302:89–99
Jackson ML (1967) Soil chemical analysis. Prentice Hall of India Pvt Ltd, New Delhi
Jackson RB, Lajtha K, Crow SE, Hugelius G, Kramer MG, Pin˜eiro G (2016) The ecology of soil carbon: pools, vulnerabilities, and biotic and abiotic controls. Annu Rev Ecol Evol Syst 48:419–445
Jobb’agy EG, Jackson RB (2000) The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol Appl 10(2):423–436
Kheyrodin H, Antoun H (2011) Tillage and manure effect on soil physical and chemical properties and on carbon and nitrogen mineralization potentials. Afr J Biotechnol 10(48):9824–9830
Kirkby CA, Richardson AE, Wade LJ, Passioura JB, Batten GD, Blanchard C, Kirkegaard JA (2014) Nutrient availability limits carbon sequestration in arable soils. Soil Biol Biochem 68:402–409
Lal R (2016) Beyond COP21: potential and challenges of the “4 per thousand” initiative. J Soil Water Conserv 71(1):20–25
Lal R, Kimble JM, Follett RF, Cole CV (1998) The potential of US crop land to sequester carbon and mitigate the greenhouse effect. Ann Arbor Press, Chelsea
Lipton M (2012) Learning from others: increasing agricultural productivity for human development in sub-Saharan Africa. WP 2012-007. UNDP, Addis Ababa
Majumder PK, Sharma DK, Sanyal D (2001) Mango. In: Bose TK, Mitra SK, Sanyal D (eds) Fruits: tropical and subtropical, v1, 3rd edn. Naya Udyog, Kolkata, pp 1–108
Majumder B, Mandal B, Bandyopadhyay PK, Gangopadhyay A, Mani PK, Kundu AL (2008) Organic amendments influence soil organic carbon pools and crop productivity in a 19 years old rice-wheat agroecosystem. Soil Sci Soc Am J 72:775–785
Mandal A, Patra AK, Singh D, Swarup A, Masto RE (2007) Effect of long-term application of manure and fertilizer on biological and biochemical activities in soil during crop development stages. BioresourceTechnol 98:3585–3592
Marinari S, Masciandaro G, Ceccanti B, Grego S (2000) Influence of organic and mineral fertilizers on soil biological and physical properties. Bioresour Technol 72:9–17
Minasny B, Malone BP, McBratney AB, Angers DA, Arrouays D, Chambers A, Chaplot V, Chen Z, Chengg K, Das BS, Fielda DJ, Gimona A, Hedley CB, Hong SY, Mandal B, Marchant BP, Martin M, McConkey BG, Mulder VL, O'Rourke S, Richer-de-Forges AC, Odeh I, Padarian J, Paustian K, Pan G, Poggio L, Savin I, Stolbovoy V, Stockmann U, Sulaeman Y, Tsui C, Vågen T, Wesemael B, Winowiecki L (2017) Soil carbon 4 per mille. Geoderma 292:59–86
Montanaro G, Dichioa B, Bati CB, Xiloyannisa C (2012) Soil management affects carbon dynamics and yield in a Mediterranean peach orchard. Agr Ecosyst Environ 161:46–54
Naik SK, Maurya S, Bhatt BP (2017) Soil organic carbon stocks and fractions in different orchards of eastern plateau and hill region of India. Agrofor Syst 91(3):541–552
Nayak AK, Gangwar B, Shukla AK, Mazumdar PS, Kumar A, Raja R, Kumar A, Kumar V, Rai PK, Mohan U (2012) Long-term effect of different integrated nutrient management on soil organic carbon and its fractions and sustainability of rice-wheat system in indo Gangetic Plains of India. Field Crop Res 127:129–139
Olsen SR, Cole CV, Watanabe FS, Dean LA (1954) Estimation of available phosphorus in soil by extraction with sodium bicarbonate. Cricular 939:19
Pries CEH, Sulman BN, West C, O'Neill C, Poppleton E, Porras RC, Castanha C, Zhu B, Wiedemeier DB, Torn MS (2018) Root litter decomposition slows with soil depth. Soil Biol Biochem 125:103–114
Sánchez-Navarroa V, Zornoza R, Faz Á, Fernández JA (2019) Comparing legumes for use in multiple cropping to enhance soil organic carbon, soil fertility, aggregates stability and vegetables yields under semiarid conditions. Sci Hortic 246:835–841
Seneviratne G (2000) Litter quality and nitrogen release in tropical agriculture: a synthesis. Biol Fertil Soils 31:60–64
Srinivasarao CH, Venkateswarlu B, Lal R, Singh AK, Kundu S (2013) Sustainable management of soils of dry land ecosystems of India for enhancing agronomic productivity and sequestering carbon. In: Sparks DL (ed) . Adv.Agron. Academic Press, Burlington, pp 253–329
Stockmann U, Adams MA, Crawford JW, Field DJ, Henakaarchchi N, Jenkins M, Minasny B, McBratney AB, de Courcelles V d R, Singh K, Wheeler I, Abbott L, Angers DA, Baldock J, Bird M, Brookes PC, Chenu C, Jastrow JD, Lal R, Lehmann J, O’Donnell AG, Parton WJ, Whitehead D, Zimmerman MJ (2013) The knowns, known unknowns, and unknowns of sequestration of soil organic carbon. Agr Ecosyst Environ 164:80–99
Subbiah BV, Asija GL (1956) A rapid procedure for the determination of available nitrogen in soils. Curr Sci India 25:259–260
Thakur O, Kumar V, Singh J (2018) A review on advances in pruning to vegetable crops. Int J Curr Microbiol App Sci 7(2):3556–3565
Tian Q, Yang X, Wang X, Liao C, Li Q, Wang M, Wu Y, Liu F (2016) Microbial community mediated response of organic carbon mineralization to labile carbon and nitrogen addition in topsoil and subsoil. Biogeochem 128:125–139
Triplett GB, Dick WA (2008) No-tillage crop production: a revolution in agriculture. Agron J 100(3):S153–S165
Tubiello FN, Soussana JF, Howden SM (2007) Crop and pasture response to climate change. P Natl Acad Sci USA 104(50):19686–19690
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter and a proposed modification of chromic acid titration method. Soil Sci 37(1):29–38
Wardle DA (1992) A comparative assessment of factors which influence microbial biomass carbon and nitrogen levels in soil. Biol Rev 67:321–358
Wilke AK, Morton LW (2015) Climatologists' patterns of conveying climate science to the agricultural community. Agric Hum Values 32(1):99–110
Acknowledgments
The authors are thankful to the Vice Chancellor, Bihar Agricultural University (BAU), Bhagalpur, Bihar, India, for providing the necessary facilities and the Director of Research, BAU, for his support and critical suggestions. Special thanks to the scientists associated with AICRP on Tropical and Subtropical Fruits, Sabour unit. The financial support from AICRP on Tropical and Subtropical Fruits (ICAR) and Post Graduate Research Contingency, Bihar Agricultural College, BAU, Sabour, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumari, R., Kundu, M., Das, A. et al. Long-Term Integrated Nutrient Management Improves Carbon Stock and Fruit Yield in a Subtropical Mango (Mangifera indica L.) Orchard. J Soil Sci Plant Nutr 20, 725–737 (2020). https://doi.org/10.1007/s42729-019-00160-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-019-00160-6