Abstract
Over 50 % of soil carbon (C) is stored in subsoil (below 30 cm). Inputs of labile C and nutrients can stimulate soil organic carbon (SOC) mineralization priming effect (PE) and subsequently affect subsoil C dynamics. However, little is known about the magnitude and mechanism of the PE occurring in subsoil, which complicates the prediction of subsoil C dynamics. Using a lab incubation experiment, the effects of glucose and nitrogen (N) addition on SOC mineralization were studied for three soil layers (0–10, 10–30, and 30–60 cm) from a subtropical forest. Five glucose (5.16 atom % 13C) levels were applied according to soil microbial biomass in each soil layer. Meanwhile, community-level physiological profiling was conducted to reflect microbial functional diversity and activity. We found positive PE for all soil layers. The PE magnitude in subsoil was about two times higher than that in topsoil with stronger increase in the microbial activity in mining components of SOC. N addition led to a reduction of 45 % in the PE magnitude in topsoil with relatively lower microbial activity in mining N-containing substrates (amino acids and amines) but caused an increase of 18 % in subsoil PE. Soil C and N availability were associated with microbial functional activity, the shifts of which then mediated the SOC mineralization in the presence of labile C and nutrient. These results suggested that mineralization of subsoil C was more sensitive to labile C and N addition. Any future changes in environmental conditions that affect the input and distribution of labile C and N in soil profiles could affect C dynamics in deep soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over 50 % of soil organic carbon (SOC) is stored in subsurface soil horizons (below 30 cm) (Rumpel and Kögel-Knabner 2011). Although the mineralization rate of subsoil organic carbon (C) is much lower than that in topsoil, the amount of CO2 released in deeper soil layers can be substantial because of its large stock size. Thus, small changes in subsoil C cycling have significant effects on the global C budget. Global change factors, such as climate warming, nitrogen (N) deposition, and precipitation pattern change, can alter terrestrial ecosystem productivity, species composition, and nutrient cycling (Nemani et al. 2003; Sala et al. 2000). However, these factors do not directly act on subsoil because of the barrier and buffer effect of topsoil. Instead, they alter the labile C and nutrient input to subsoil through deep root system and flow path (Chabbi et al. 2009; Kaiser and Guggenberger 2005; Marin-Spiotta et al. 2011), which subsequently affect subsoil organic C mineralization and immobilization. Knowledge on how subsoil organic C responds to labile C and nutrient supply is essential to better understand how subsoil C pool may respond to global change.
Addition of labile C or N to soil can increase SOC-derived CO2 release, which is refered as the priming effect (PE) (Blagodatskaya and Kuzyakov 2008; Kuzyakov 2010). Although PE was well studied in topsoil, the magnitude and direction of PE occurring in subsoil are less known, especially in forest soils. Earlier studies have indicated that the magnitude and direction of PE are mainly affected by microbial community, SOC stability, and nutrient availability (Chowdhury et al. 2014; Fontaine et al. 2011; Sullivan and Hart 2013), which all vary with soil layers (Goberna et al. 2006; Kramer et al. 2013; Vancampenhout et al. 2012). How the differences of these factors between topsoil and subsoil may influence PE remains unclear. The few previous comparative PE studies between topsoil and subsoil reported inconsistent results and showed that PE in subsoil is either higher (Hamer and Marschner 2005; Wang et al. 2014b) or lower (Paterson and Sim 2013; Salomé et al. 2010) than topsoil. More studies are needed to examine the causes and consequences of this inconsistency, which will improve our understanding of how subsoil may respond to future changes and of the underlying ecological processes and regulating mechanisms.
The types and levels of added C substrate can influence the responses of SOC mineralization. For example, glucose or fructose has similar effects on soil microbial growth, whereas cellulose and fresh litter can stimulate fungi growth, which then causes stronger SOC decomposition by hyphae (Fontaine et al. 2011). Here we choose the widely used glucose as the labile C substrate to add to soils. In addition to substrate types, the amount of substrate addition also affects the PE magnitude, and this response is shown to be soil-specific (Blagodatskaya and Kuzyakov 2008; Guenet et al. 2010b; Paterson and Sim 2013). The amount of added substrate is suggested to be based on the microbial biomass C (MBC) (Blagodatskaya and Kuzyakov 2008). Soils from different layers have different MBC amounts, so we selected a range of C addition levels based on their respective MBC values.
Microbial community in soil and their activity control SOC turnover and dynamics. Research through δ13C differences of phospholipid fatty acid indicated that microorganisms in deeper soil tend to use decade-old SOC, and topsoil microorganisms tend to use newly plant-derived C (Kramer and Gleixner 2008). Because of the low input of root residues and exudates in deep layer, subsoil SOC mineralization is often constrained by the lack of C source and energy (Fierer et al. 2003; Fontaine et al. 2007). Thus, we hypothesized that once labile C as source of energy is added to soil, degradation of these complex components will be stimulated by the increasing activity of old SOC-preferring microorganisms, which will probably cause stronger SOC mineralization in subsoil than in topsoil.
The degradation of native SOC is modulated by N availability. In general, the magnitude of positive PE will decrease if the added N is available for microorganisms (Chowdhury et al. 2014; Fontaine et al. 2011; Wang et al. 2014a). This negative effect of added N on native SOC decomposition supports “microbial N mining” theory. The theory assumes that decomposers utilize recalcitrant soil C to mine N to meet their growth needs (Craine et al. 2007). Given that added N is easily available, the decomposition of recalcitrant soil C will decline. However, in N poor soils, some studies found that N addition enhances SOC mineralization through increased oxidase enzyme activity (Nottingham et al. 2012; Sistla et al. 2012; Waldrop and Firestone 2004). This positive effect on native SOC decomposition supports the “stoichiometric decomposition” hypothesis (Chen et al. 2014), which assumes that optimal C and N stoichiometry can match microbial demand and lead to maximal microbial activity and decomposition rate. Thus, we hypothesized that N addition will increase PE in N-poor subsoil and decrease PE in topsoil because of “stoichiometric decomposition” and “microbial N mining” theories, respectively.
Previous studies on the role of soil microbial community in priming mainly focused on which microbial groups governed the magnitude and direction of priming, and results showed that either fungi or Gram-positive bacteria, or no specific microbial group responded to PE (Bird et al. 2011; Fontaine et al. 2011; Nottingham et al. 2012; Sullivan and Hart 2013). This approach of detecting taxonomic microbial community may not reflect their actual functional activity. Thus, functional rather than taxonomic information can provide greater insight into the microbial roles in SOC mineralization (Pignataro et al. 2012; Zak et al. 1994). Derrien et al. (2014) found the substrate utilization pattern of microorganisms changed after labile C addition. Therefore, in this study, we used soil microbial community-level physiological profiles to trace the shifts of functional diversity and activity of microbial communities with the addition of labile C and N.
In this study, three soil layers (0–10, 10–30, and 30–60 cm) were incubated with addition of 13C-labeled glucose and NH4NO3. Efflux rates of CO2 and their corresponding δ13C were measured to test whether the addition of labile C and N stimulates SOC mineralization. The objectives of this study were: (1) to compare the magnitude and direction of PE in topsoil and subsoil; (2) to examine the effects of N addition on PE in topsoil and subsoil; and (3) to understand how microbial community is associated with the observed SOC mineralization patterns and changes.
Materials and methods
Soil collection
Soils were collected from the Badagongshan National Nature Reserve, Hunan Province (29°46.04′N, 110°5.24′E), in north of the Wuling Mountain in the mid-subtropical zone in China. The climate is subtropical mountain humid monsoon with an average annual rainfall of 2100 mm. The mean temperature ranges from 0.1 °C in January to 22.8 °C in July, with an annual mean of 11.5 °C. Topography is characterized by deep valleys, steep slopes, and flat tops. The vegetation is dominated by evergreen and deciduous broad-leaved mixed forests. Dominant trees include Fagus lucida, Carpinus fargesii, Schima parviflora, Sassafras tzumu, Castanea seguinii, Cyclobalanopsis multinervis, and Cyclobalanopsis gracilis.
The study soil was collected at elevation of 1400 m. The soil is classified as Hapludalfs, consisting of a thin mineral A horizon (about 10 cm) and a Bts horion (about 60 cm). Leaching, clay accumulation, and litter deposition are the main soil genesis processes caused by humid and warm climate conditions. To collect soil from different layers, a trench was dug, and soil was collected with a shovel from three depth intervals: 0–10 (topsoil), 10–30 (midsoil), and 30–60 cm (subsoil). The three soil samples were immediately brought to the laboratory and passed through a 4 mm sieve. Roots and visible residues were removed manually. Soils were stored in a refrigerator (<4 °C) until further incubation.
Soil incubation
Two incubation experiments were designed to examine soil responses to labile C and N addition. The first experiment aimed to examine the effects of glucose addition levels and soil layers on SOC mineralization. The second experiment was carried out to test the effects of N addition on the PE of topsoil and subsoil (with or without labile C addition).
Experiment 1: Glucose addition
This experiment included five glucose addition levels according to the MBC (results from soils after 2 weeks of pre-incubation) percentage in each soil: control, 10 %, 50 %, 100 %, and 200 % of MBC with six replicates. For each replicate, about 30 g of dry soil was weighed into individual 250 ml triangular flasks. Before incubation, soil moisture was adjusted to 65 % water-holding capacity by adding deionized H2O. All samples were pre-incubated at 20 °C for 2 weeks. After pre-incubation, 3 ml of the appropriate glucose solutions (unit labeled, 5.16 atom % 13C) was added to each sample. Meanwhile, 3 ml of deionized H2O was added to the control soil samples. CO2 efflux rates from soil were measured at 1 and 12 h after treatment additions and on days 2, 3, 4, 5, 7, 10, 15, 22, and 30.
At each time period, three replicates from each treatment were used to measure CO2 efflux rates from soils and 13C composition in CO2. The three other replicates were under incubation before they were used to measure microbial biomass on the seventh day after glucose addition. When measuring CO2 efflux rates, incubation flasks were flushed with CO2-free air for 5–10 min (air through a soda-lime column), during which the outflow was connected to an infrared gas analyzer (IRGA; EGM-4, PP Systems, Amesbury, MA, USA). The flushing reduced CO2 concentration to less than 5 μmol mol−1. The ports were then closed, and flasks were returned to the 20 °C incubator for 0.5–3 h (depending on the CO2 efflux rate to make sure CO2 concentration reaches at least 50 μmol mol−1). Approximately 10 ml of headspace was extracted from each flask and injected into the IRGA to determine the CO2 concentration (to calculate CO2 efflux rates). Another gas sample was collected in an evacuated gas vial for 13C composition (Carbon Isotope Analyzer, 912-0003, LGR, USA) to partition CO2 between sources.
Experiment 2: Effects of N addition on PE
This experiment included four treatments with six replicates: control, soil with mineral N (N), soil with glucose (Glu), and soil with glucose and mineral N (Glu + N). Based on the results of the first experiment, we used the glucose addition level of 200 % MBC. Mineral N was applied at 200 μg N g−1 soil to increase soil N availability. Soil samples were prepared as the same as the first experiment. CO2 efflux rates and 13C composition of CO2 were measured over a seven-day period. At the end of the seventh day of incubation, the microbial biomass and community level physiological profiles of each treatment were determined.
Laboratory analyses
Soil samples were tested for the presence of inorganic soil carbon and no inorganic carbon was found. SOC, total N, and δ13 C of SOC were measured with an elemental analyzer (Thermo Fisher Flash 2000, USA) interfaced with a Delta Plus Advantage mass spectrometer (Thermo Finigan, Bremen, Germany). Dissolved organic C was extracted on a paste of 1:5 (weight: volume) of air-dried soil and deionized water. The mixed paste was shaken for 0.5 h at 250 rpm at 25 °C and then centrifuged for 10 min at 4000 rpm. Subsequently, the supernatant liquid was filtered through a 0.45 mm filterable membrane. Dissolved organic C and N in extracts were measured by a TOC Analyzer (Vario TOC, Elemental, Germany). The light and heavy fractions of SOC were separated using NaI solution (von Lützow et al. 2007), with a density of 1.7 g cm−3. Each fraction was analyzed for the C and N contents as described above. Meanwhile, soil texture was determined by laser particle size analysis. Soil available phosphorous was extracted with 0.03 mol l−1 NH4F, and the absorbance was detected using a spectrophotometer at 700 nm. Soil pH was measured with a calomel electrode on a paste of 1:2.5 (weight: volume) of air-dried soil and deionized water.
Microbial biomass was measured by substrate-induced respiration technique (Anderson and Domsch 1978). First, glucose solution (60 g L−1) was added to each soil sample using a 5 mL syringe with a needle tip. In this study, 6–10 mg of glucose g−1 soil (depending on SOC content) was used to ensure excess carbon for the use by microorganisms. CO2 efflux rate was then measured at 25 °C within 2 h after glucose addition when CO2 efflux rate remained nearly constant during the initial 4 h period before any notable microbial growth (Lin and Brookes 1999). Glucose solution was evenly applied to avoid saturating the soil with liquid, which would restrict CO2 evolution from the sample.
Community level physiological profiles (CLPPs) of microbial communities were assessed using the BIOLOG Eco-Plates TM system (Biolog, Hayward, CA, USA). BIOLOG ECO-plates have been used in ecological studies to estimate metabolic potential of microbial communities, thus develop a fingerprint of the microbial community’s substrate use (Derrien et al. 2014). This technique is based on tetrazolium dye reduction as an indicator of sole-C-source utilization. Utilization of 31 widely used C sources is reflected by the color developments after application of soil solution. About 5 g of fresh soil was suspended in 50 ml of 0.85 % sterile NaCl solution and shaken for 30 min on a reciprocal shaker. 1 ml of soil solution was taken out immediately after soil shaking. The soil solution was cleaned and purified for three times by vortex and centrifugation for 20 min at 10,000 rmp. Sample suspension was then centrifuged for 1 min at 2000 rmp, and decanted and diluted to 1/10 with a NaCl solution (0.85 %). Each well of the Eco-plates was inoculated with 150 μl of the sample supernatant, and then incubated at 20 °C in the dark. Color developments were measured as the absorbance readings at 590 and 750 nm after inoculation and at 12 h intervals for 204 h using a microplate reader (Multilabel Plate Readers, M200 PRO, Tecan, Austria). The absorbance measurements with BIOLOG Eco-Plates for individual substrates were corrected against the control well containing water only. The absorbance reading at 590 nm was subtracted by the reading at 750 nm to eliminate turbidity. The average well color developments (AWCD) were calculated according to Garland (1996) to represent soil microbial activity. To obtain information about the substrate utilization pattern, the 31 substrates were assigned into six groups according to their chemical nature: amines, amino acids, carbohydrates, carboxylic acids, miscellaneous, and polymers (Derrien et al. 2014).
Calculations and statistical analyses
The amount of CO2–C derived from SOC was calculated as follows
where C SOC is the soil CO2 efflux rate derived from SOC, C measured is the overall measured CO2 efflux rate, A 13 C measured is the isotopic abundance (13C atom %) of the released CO2, A 13 C SOC is the isotopic abundance of the decomposed SOC and is equal to the isotopic abundance of the released CO2 in control, and A 13 C glucose is the isotopic abundance of the glucose C added to the samples (13C atom % = 5.16 %). The isotopic signatures of glucose and SOC were assumed to be homogeneous, and the isotopic fractionations were not considered.
The PE induced by glucose was calculated by the percentage change in SOC mineralization compared with the control
where C SOC(treatment) is the soil CO2 efflux rate derived from SOC with glucose addition, and C SOC(control) is the SOC mineralization rate in control.
The effect of glucose addition on SOC balance was calculated as follows:
where C input is the glucose-C immobilized into soil at the end of the incubation period, and C output is the amount of stimulated SOC mineralization by PE at the end of the incubation period. A ratio higher than one means a net C sequestration.
Microbial biomass carbon was calculated using substrate induced respiration according to (Anderson and Domsch 1978) and adjusted to account for use at 25 °C (West and Sparling 1986):
where MBC is microbial biomass carbon (μg C g−1 soil), R Gl+ the soil substrate induced respiration rate at 25 °C (μL CO2 g−1 soil h−1).
Microbial diversity was evaluated by calculating Shannon’s index:
where p i is the ratio of optimal density reading on the ith substrate to the sum of absorbance on the all substrates. At an incubation time of 120 h, the highest rate of microbial growth was observed, thus diversity indexes were based on the Eco-plates readings at 120 h.
Principal component analysis (PCA) was carried out to analyze substrate utilization pattern of soil microbial community. The color development readings of the wells in the ECO-Plates from 72 h (microorganisms began to grow) to 204 h were used to record the successions of substrate utilization patterns during BIOLOG ECO-Plates incubation. To standardize the data, the readings of each well were relativized by dividing the average absorbency for the whole plate. The PCA analysis was conducted using PC-ORD v.6 for Windows.
The differences in soil characteristics and base respiration were compared using one-way ANOVA. In the first experiment, two-way ANOVA was used to test the effects of soil layers and glucose levels on PE and SOC-derived CO2 efflux rate. Significant differences in PE and SOC-derived CO2 efflux rate, along the incubation time, were determined by repeated ANOVA measurements. For the second experiment, the effects of N addition and soil layers on PE were also analyzed by two-way ANOVA. The effects of N addition, glucose addition and soil layers on soil microbial activity (AWCD) and diversity (Shannon index) were analyzed by three-way ANOVA. Tukey’s post hoc test was used to identify significant differences among the treatments at P < 0.05. Statistical analyses were performed using SPSS version 13.0 for Windows.
Results
Soil properties
SOC and total N, dissolved organic C and N, light fraction C, and available phosphorous decreased significantly (P < 0.001) with soil layers (Table 1). The δ13C value of SOC in topsoil was significantly lower (P < 0.01) than that in subsoil. C/N showed no difference among the three soil layers. Topsoil had significantly higher (P < 0.05) clay and sand particle fractions than subsoil but lower silt fraction. After 2 weeks of pre-incubation, soil respiration reached steady rates, which remained constant throughout the experimental period. The soil respiration rate and microbial metabolic quotient (represented as respiration rates per MBC) declined significantly (P < 0.001) with soil layers. Although SOC decreased with soil layers, the SOC-specific respiration rate in subsoil was significantly lower (P < 0.001) than that in topsoil.
Effects of glucose addition on SOC mineralization
Following glucose addition to the soils, the 13C atom % of the total released CO2 was used to partition CO2 efflux into SOC-derived and glucose-derived components (Eq. 1). SOC-derived CO2 efflux rate to glucose addition strongly depended on the glucose addition levels (P < 0.001) and soil layers (P < 0.001) (Fig. 1). SOC-derived CO2 efflux rate in topsoil was significantly (P < 0.001) higher than subsoil for all glucose addition levels. The SOC-derives CO2 efflux rate increased with increasing glucose addition levels for all soil layers. At 10 % of MBC addition level, the SOC-derived CO2 efflux rate reached the maximum at 12 h. At higher addition levels, the rate reached its maximum at 24 h. However, for 200 % of MBC addition level, the maximal rate appeared at 48 h. After reaching the maximum, the rate declined sharply in 1 or 2 days and then declined slowly over the later period.
The magnitude of PE was significantly (P < 0.001) influenced by the levels of glucose addition and soil layers (Fig. 1). The PE magnitude increased with increasing glucose addition levels for all soil layers. The maximal PE appeared earlier for lower glucose addition levels and later for higher glucose addition levels. After the maximal magnitude, the PE quickly decreased in 2–4 days to a much lower level and was relatively constant over the remaining incubation period.
The PE magnitude showed no difference among the three soil layers at 10 % glucose addition level. At 50 % and 100 % of MBC addition levels, the PE in subsoil was significantly higher (P < 0.001) than that in topsoil and midsoil. At 200 % of MBC level, the PE increased significantly (P < 0.001) with the soil layers. These response ranks were consistent during incubation. The results of the seventh day were shown in Fig. 2.
Glucose-C mineralization and sequestration into soil
Glucose-derived CO2 efflux was significantly (P < 0.001) affected by glucose addition levels and soil layers (Online Resource 1). At the end of incubation, the accumulated percent of mineralized glucose-C ranged from 35 to 48 %, 25–42 %, and 21–41 % of the glucose added in topsoil, midsoil, and subsoil, respectively. The remaining glucose-C in soil was thought to be immobilized into soil.
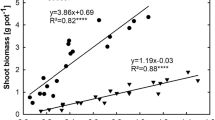
The ratio of immobilized glucose–C and stimulated SOC mineralization reflected the net sequestration of C into soil or net SOC release. The ratio was higher than one (positive C sequestration) in all treatments, except in subsoil at the lowest glucose addition level (Fig. 3). The ratio was significantly (P < 0.001) affected by soil layers. Glucose addition levels showed no effect (P = 0.237) on the ratio. However, significant (P < 0.001) interaction effect between glucose addition levels and soil layers was found for the ratio. Positive C sequestration in subsoil was significantly (P < 0.001) lower than that in topsoil and midsoil at the same glucose addition level. The magnitude of positive C sequestration in topsoil and midsoil showed little variation with increasing glucose addition levels. In subsoil, the magnitude of positive C sequestration increased (P < 0.01) with increasing addition levels.
Effects of N addition on PE
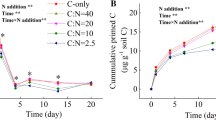
N addition showed significant (P < 0.05) interaction effects with glucose addition and soil layers to SOC-derived CO2 efflux rate. When N was added alone, SOC-derived CO2 efflux rate in topsoil showed no difference with the control, whereas the rates in midsoil and subsoil were 20 % and 37 % higher (P < 0.01) than the control, respectively (Fig. 4a). When N was added with 200 % of MBC glucose, N significantly decreased (P < 0.01) SOC-CO2 efflux rate in topsoil, whereas no effect was shown in the other two soil layers on the seventh day of incubation. The effect of N addition on the PE induced by glucose depended on soil layers (Fig. 4b). The PE decreased significantly (P < 0.05) in topsoil with N addition. By contrast, the PE increased in midsoil (P < 0.05) and subsoil (P < 0.01) with N addition.
SOC-derived CO2 efflux rates (a) and priming effect (PE) expressed as the percentage increase in SOC mineralization (b) with 200 % of MBC glucose and N addition for the different soil layers on the seventh day of incubation. Results are means (n = 3) ±SD. Bars with different letters indicate a significant difference within the same soil layer
Effects of C and N addition on soil microorganisms
Soil MBC were significantly affected by glucose addition levels (P < 0.001) and soil layers (P < 0.001) (Table 2). Soil MBC in topsoil was significantly (P < 0.001) higher than subsoil for all glucose addition levels. Soil MBC increased (P < 0.001) with increasing glucose addition levels for all soil layers. This increase was higher in topsoil than that in deeper soils. At the 200 % of MBC glucose addition level, soil MBC was 135 %, 69 %, and 60 % higher than the control from topsoil to subsoil. The effects of N addition on soil MBC were depended on glucose addition and soil layers (Table 3). N addition alone decreased soil MBC for all three soil layers, especially in subsoil. When 200 % of MBC glucose was added with N, soil MBC decreased in topsoil, but no difference was observed in the two deeper soils.
Glucose addition and soil layers showed significant effects (P < 0.001) on overall substrate utilization (AWCD) and diversity index (Table 3). Total AWCD was significantly higher (P < 0.001) in topsoil than that in deeper soils (Table 3). Glucose addition increased the total AWCD and diversity index (P < 0.001) for all soil layers, and the increase was more pronounced in topsoil. N addition alone showed no effect on AWCD for all soil layers and no effect on diversity index in topsoil, but increased diversity index (P < 0.05) in deeper soils. In general, addition of N with glucose significantly increased MBC, AWCD and diversity index for all soil layers compared with control and N addition alone. However, addition of N with glucose only significantly decreased MBC in topsoil compared with addition of glucose alone, but no effects on MBC, AWCD and diversity for other layers.
PCA ordination of the substrate utilization pattern revealed that subsoil microbial community had a greater relative utilization of amino acids and miscellaneous, and topsoil used relatively more carboxylic acids, carbohydrates, and polymers (Fig. 5). In topsoil, glucose addition alone mainly increased the relative utilization of simple substrates, such as carboxylic acids (Fig. 6). In subsoil, glucose addition increased the relative utilization of simple carboxylic acids and carbohydrates, as well as complex polymers. The relative utilization of amino acids and amines decreased in both topsoil and subsoil after glucose addition (Fig. 6; Online Resource 2). N addition alone had a slight effect on substrate utilization pattern and decreased the relative utilization of carbohydrates for all soil layers. Similar relative substrate utilization patterns were found between glucose addition and glucose plus N addition; nevertheless, slight differences were observed for the three soil layers. Compared with glucose addition alone, addition of N with glucose decreased the relative utilization of amino acids and amines in topsoil and midsoil. However, in subsoil, the substrate utilization pattern shifted toward higher amino acid and amine utilization.
PCA of microbial substrate utilization pattern (using normalized average well color development results from BIOLOG Eco-Plates) for the control soils from the different layers. The succession lines from head to end are the data from 72 to 204 h. A total of 31 substrates were separated into six groups as follows: CH carbohydrates, CA carboxylic acids, AA amino acids, P polymers, AM amines, M miscellaneous
PCA of microbial substrate utilization pattern (using relativized average well color development results from BIOLOG Eco-Plates) with 200 % of MBC glucose and N addition for the different soil layers of (a) topsoil, (b) midsoil, and (c) subsoil on the seventh day of incubation. The symbols in succession lines from head to end are the data from 72 to 204 h. A total of 31 substrates were separated into six groups as follows: CH carbohydrates, CA carboxylic acids, AA amino acids, P polymers, AM amines, M miscellaneous
Discussion
SOC mineralization in topsoil versus subsoil
We found significant differences in soil properties among the three soil layers. Compared with topsoil, subsoil had a much lower organic C density. The relatively smaller portion of active C (dissolved organic C and light fraction C, Table 1) in subsoil indicated that subsoil contained more recalcitrant C (carbon pool with a slow turnover rate) than topsoil. The lower δ13C value of SOC, also indicated the organic matter in subsoil was more processed and humified through longer period of microbial action (Rumpel and Kögel-Knabner 2011). Meanwhile, soil total N and available phosphorous were also much lower in subsoil, thereby showing a nutrient-limiting condition in subsoil compared with topsoil.
The SOC mineralization rate was significantly higher in topsoil than that in subsoil, thereby suggesting that decomposability decreased with depth, which was consistent with other studies (Rumpel and Kögel-Knabner 2011). Generally, SOC mineralization is affected by several factors, including substrate quality and quantity, nutrient and O2 availability, microbial activity, and physical protection (Fierer et al. 2003; Salomé et al. 2010). In this study, as soil structure was completely destroyed by sieving (4 mm), physical protection of SOC was not considered. Our δ13C data and light fraction C data suggested that subsoil had a more recalcitrant SOC pool than topsoil. The observed differences in the SOC mineralization rates between topsoil and subsoil were likely caused by the differences in substrate quality and quantity, and the associated differences in microbial activities.
Microorganisms are the key drivers of SOC mineralization. Compared with topsoil, subsoil not only had a much lower MBC, but also had significantly lower microbial metabolic quotient (Table 1), which indicated that a higher proportion of microbial decomposed SOC was used in micro-synthesis than their own maintenance activity. This result was probably caused by two reasons: (1) subsoil had a higher proportion of recalcitrant C, which was hardly utilized by microbial community; this finding was supported by our dissolved organic C and light fraction C data (Table 1); (2) the microbial community in subsoil had a large percentage of dormant microbes which was supported by our microbial biomass and microbial activity (AWCD value) data, and the lower ratio of microbial activity/microbial biomass in subsoil (Table 3). Our observed microbial metabolic quotient trend was consistent with Moritz et al. (2009), who also found that the contribution of microbial-derived C increases with increasing soil layers.
Labile C addition induced PE
Labile C addition increased native SOC mineralization for all three soil layers, causing a positive PE. The PE magnitude increased with increasing glucose addition levels (Fig. 2). In general, the PE curve increases at low C addition level, peaks when C addition level approaches the decomposing capacity of the whole microbial community, and finally decreases at higher C addition level when most microorganisms utilize only labile C instead of SOC (Blagodatskaya and Kuzyakov 2008; Paterson and Sim 2013). However, no decrease or peak value was observed for PE, even at 200 % of MBC glucose addition level. A probable reason for the continued PE increase was that the amount of added glucose was not too high to inhibit SOC utilization by the microorganisms in this experiment (Blagodatskaya and Kuzyakov 2008; Paterson and Sim 2013).
An important finding of our study was the strong difference of PE with soil layers. Negative PE has been found in soil pre-treated with black C and soil free of vegetation for 80 years where SOC was supposed to be stable (Chowdhury et al. 2014; Guenet et al. 2010a; Zimmerman et al. 2011). Therefore, SOC with lower decomposability would be less susceptible to priming (Jenkinso 1971). However, we observed the opposite trend, in which PE was most pronounced in subsoil horizons where SOC decomposability was much lower. Thus, in addition to SOC stability, other factors (such as microbial functional community and nutrient availability) might affect PE more strongly.
Microorganisms mediated PE in topsoil and subsoil
Compared with topsoil, subsoil microbial community had a lower biomass and lower activity. When labile C was added, the formation of microbial biomass was lower in subsoil than that in topsoil (Table 2). However, the increase in microbial activity after glucose addition in subsoil was stronger than that in topsoil (Table 3), which indicated a trade-off between the formation of microbial biomass and the maintenance of microbial activity. More SOC-degrading enzymes were possibly released in deeper soil and led to an increase in SOC mineralization.
In addition to the changes in microbial activity, we also found distinct soil microbial substrate utilization patterns in different soil layers (Fig. 6). In topsoil, glucose addition mainly increased the relative utilization in carboxylic acids, which are simple and more easily decomposable. In subsoil, glucose addition increased not only the relative utilization in carboxylic acids, but also polymers, which are regarded as more complex and recalcitrant. These results indicated that glucose addition in subsoil increased the microbial activity in degrading recalcitrant substrates. Chemical composition studies demonstrated that complex components, such as long-chain aliphatics and cellulose-derived anhydrosugars, accumulated in deep soil (Vancampenhout et al. 2012). Thus, these complex components in deep soil can be easily degraded when glucose is added, thereby inducing stronger SOC mineralization than topsoil.
It’s notable that soils in this study were taken out from soil profile and incubated in the laboratory. The changed environment and destruction of soil physical structure might cause a different SOC mineralization patterns and dynamics than their in situ conditions (Salomé et al. 2010). The destruction of physical structure and improved air condition in subsoil are likely to increase base SOC mineralization, and thus potentially cause an underestimation of the effect of labile C addition on SOC mineralization. However, field experiments of such is impossible given difficulties in treatments and monitoring.
The effect of N addition on PE
N addition was shown to affect the SOC mineralization rates (Janssens et al. 2010) and the priming response to labile C (Chen et al. 2014; Hartley et al. 2010; Kuzyakov 2010). The negative effect of N addition on PE in topsoil was consistent with previous findings (Garcia-Pausas and Paterson 2011; Hartley et al. 2010). Microbial N mining theory assumes that microorganisms tend to decompose recalcitrant SOC to acquire N (Moorhead and Sinsabaugh 2006). In this experiment, when N was added concurrently with glucose, the relative utilization in N-containing substrates (amino acids and amines) decreased in topsoil (Fig. 6), indicating that an increase in N availability reduced microbial activity in mining SOC (N-containing substrates in soil) to meet N requirement and reduced the magnitude of PE. Moreover, N salt addition could also cause a cytotoxic effect on microbial communities, thereby reducing their activity for SOC decomposition (Enrique et al. 2008; Geisseler and Scow 2014). The lower microbial biomass and activity (Tables 2, 3) after N addition at glucose treatment indicated that the latter effect might also contribute to the decrease of PE in topsoil.
Conversely, this situation changed in deep soil. Although subsoil microbial biomass declined after N addition at glucose treatment, which was the same as topsoil, the activity showed no change, and the relative utilization in N-containing substrates relatively increased. This result cannot be explained by “microbial N mining” theory, and “stoichiometric decomposition” theory seemed to be reasonable to explain this result (Chen et al. 2014; Janssens et al. 2010). Previous reports have indicated that nutrient supply in nutrient-poor soils can increase microbial activity (Kirkby et al. 2013; Waldrop et al. 2004). The stimulation of organic C mineralization after single N addition in our subsoil (Fig. 4) suggested that N was a major limiting factor on subsoil C dynamics (Fontaine et al. 2007). Subsoil microbial communities were also more efficient in decomposing N-containing substrates, such as amino acids (Fig. 5), which further indicated that subsoil was more N-limited. Thus, addition of N with glucose could further stimulate microorganisms in this N-poor soil (Chowdhury et al. 2014). This positive effect on PE even suppressed the negative effect caused by the decreasing microbial biomass with N addition. In contrast to our results, in a coniferous forest deep soil, N addition negatively affects PE induced by fresh litters (Wang et al. 2014b). This result might be due to the fact that the soil in the latter study was not nutrient-poor, because N addition alone showed no effect on CO2 production.
Based on our findings and previous studies, we proposed a conceptual model that soil C and N availability were associated with microbial functional activity, the shifts of which then mediated PE in different soil layers in the presence of labile C and N (Fig. 7). When labile C was added, the changes in soil microorganism’s functional diversity and activity were much stronger in subsoil than in topsoil, which then led to higher PE. Simultaneously, the shifts of microbial functional activity in further N input were governed by soil natural N availability. In N-normal soil at surface layer, the lower relative microbial activity in mining N-containing substrates (recalcitrant SOC holding N in soil) after N addition decreased the positive PE, which was supported by “microbial N mining” theory. However, in N-poor soil at deeper layer, the stronger microbial activity in mining N-containing substrates (recalcitrant SOC holding N in soil) increased the positive PE, which was supported by “stoichiometric decomposition” theory.
Conceptual model showing how the addition of labile C and N may affect microbial functional activities, and soil C dynamics in soils of different depths. The blue lines show the change in substrate utilization patterns in topsoil after labile organic C and N addition, and the red lines show that in subsoil. The solid lines show the processes caused by C addition alone, and the dashed lines show the processes caused by labile C and N addition. The number of plus signs before “PE” shows the magnitude of priming effect (PE). (Color figure online)
C sequestration after substrate addition
To evaluate the impacts of the observed PE on soil C pool, C balance was calculated. The PE was generally neglected in soil C balance calculation in previous studies because C balance was generally positive, even in treatments with positive PE (Fontaine et al. 2011; Wang et al. 2014b). However, a recent study indicated that labile C input can destabilize SOC because the newly incorporated substrate-C is more decomposable than the old stimulated mineralized SOC (Derrien et al. 2014). More importantly, we found a negative balance with the remaining glucose-C lower than the stimulated mineralized SOC at the deepest soil, with the lowest C addition level. Therefore, the quantity of labile C input might determine the direction of soil C storage at deep soil. In situ, organic C input into subsoil mainly occurred as dissolved organic C from the upper soil layers, root litter and exudates, and bioturbation (Rumpel and Kögel-Knabner 2011). Considering the pulse input of these labile C sources, the amount of input substrates in single incidents in deep soil would be tiny, which then probably impact soil C storage negatively. This result concurred with the recent report that greater C input in soil during reforestation increases topsoil C storage but decreases the amount of subsoil C pool (Mobley et al. 2015).
Conclusions
Deep soil was both energy and N limited, and either glucose or N addition could stimulate native SOC mineralization. We found a higher PE in subsoil than topsoil at the same glucose addition level (%MBC). The effects of N addition on the PE magnitude varied with soil natural N availability: (1) increasing the PE magnitude in N-poor soil at deeper layer, (2) reducing the PE magnitude in N-normal soil at surface layer. Soil C and N availability were associated with microbial functional activity, the shifts of which then mediated the SOC mineralization in the presence of labile C and nutrient. The deep soil organic C pool is more vulnerable to fresh C and N input, which then probably impacts soil C storage. Any changes in environmental conditions that influence distribution of labile C and N in soil profiles could affect C dynamics in deep soil, which could have significant implications on terrestrial C cycling under global change.
It’s notable that the quantity and quality of C input in the field is more complex than laboratory conditions, and the soil responses to the C input may be different from our laboratory results. In future researches, we should give more attention to conditions of how the soils are typically exposed to in the field.
References
Anderson JPE, Domsch KH (1978) Physiological method for quantitative measurement of microbial biomass in soils. Soil Biol Biochem 10:215–221. doi:10.1016/0038-0717(78)90099-8
Bird JA, Herman DJ, Firestone MK (2011) Rhizosphere priming of soil organic matter by bacterial groups in a grassland soil. Soil Biol Biochem 43:718–725. doi:10.1016/j.soilbio.2010.08.010
Blagodatskaya E, Kuzyakov Y (2008) Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: critical review. Biol Fertil Soils 45:115–131. doi:10.1007/s00374-008-0334-y
Chabbi A, Kögel-Knabner I, Rumpel C (2009) Stabilised carbon in subsoil horizons is located in spatially distinct parts of the soil profile. Soil Biol Biochem 41:256–261. doi:10.1016/j.soilbio.2008.10.033
Chen RR, Senbayram M, Blagodatsky S et al (2014) Soil C and N availability determine the priming effect: microbial N mining and stoichiometric decomposition theories. Glob Change Biol 20:2356–2367. doi:10.1111/gcb.12475
Chowdhury S, Farrell M, Bolan N (2014) Priming of soil organic carbon by malic acid addition is differentially affected by nutrient availability. Soil Biol Biochem 77:158–169. doi:10.1016/j.soilbio.2014.06.027
Craine JM, Morrow C, Fierer N (2007) Microbial nitrogen limitation increases decomposition. Ecology 88:2105–2113. doi:10.1890/06-1847.1
Derrien D, Plain C, Courty PE et al (2014) Does the addition of labile substrate destabilise old soil organic matter? Soil Biol Biochem 76:149–160. doi:10.1016/j.soilbio.2014.04.030
Enrique A-G, Bruno C, Christopher A, Virgile C, Steven C (2008) Effects of nitrogen availability on microbial activities, densities and functional diversities involved in the degradation of a mediterranean evergreen oak litter (Quercus ilex L.). Soil Biol Biochem 40:1654–1661. doi:10.1016/j.soilbio.2008.01.020
Fierer N, Allen AS, Schimel JP, Holden PA (2003) Controls on microbial CO2 production: a comparison of surface and subsurface soil horizons. Glob Change Biol 9:1322–1332. doi:10.1046/j.1365-2486.2003.00663.x
Fontaine S, Barot S, Barré P, Bdioui N, Mary B, Rumpel C (2007) Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 450:277–280. doi:10.1038/nature06275
Fontaine S, Henault C, Aamor A et al (2011) Fungi mediate long term sequestration of carbon and nitrogen in soil through their priming effect. Soil Biol Biochem 43:86–96. doi:10.1016/j.soilbio.2010.09.017
Garcia-Pausas J, Paterson E (2011) Microbial community abundance and structure are determinants of soil organic matter mineralisation in the presence of labile carbon. Soil Biol Biochem 43:1705–1713. doi:10.1016/j.soilbio.2011.04.016
Garland JL (1996) Analytical approaches to the characterization of samples of microbial communities using patterns of potential C source utilization. Soil Biol Biochem 28:213–221. doi:10.1016/0038-0717(95)00112-3
Geisseler D, Scow KM (2014) Long-term effects of mineral fertilizers on soil microorganisms: a review. Soil Biol Biochem 75:54–63. doi:10.1016/j.soilbio.2014.03.023
Goberna M, Sánchez J, Pascual JA, García C (2006) Surface and subsurface organic carbon, microbial biomass and activity in a forest soil sequence. Soil Biol Biochem 38:2233–2243. doi:10.1016/j.soilbio.2006.02.003
Guenet B, Leloup J, Raynaud X, Bardoux G, Abbadie L (2010a) Negative priming effect on mineralization in a soil free of vegetation for 80 years. Eur J Soil Sci 61:384–391. doi:10.1111/j.1365-2389.2010.01234.x
Guenet B, Neill C, Bardoux G, Abbadie L (2010b) Is there a linear relationship between priming effect intensity and the amount of organic matter input? Appl Soil Ecol 46:436–442. doi:10.1016/j.apsoil.2010.09.006
Hamer U, Marschner B (2005) Priming effects in different soil types induced by fructose, alanine, oxalic acid and catechol additions. Soil Biol Biochem 37:445–454. doi:10.1016/j.soilbio.2004.07.037
Hartley IP, Hopkins DW, Sommerkorn M, Wookey PA (2010) The response of organic matter mineralisation to nutrient and substrate additions in sub-arctic soils. Soil Biol Biochem 42:92–100. doi:10.1016/j.soilbio.2009.10.004
Janssens IA, Dieleman W, Luyssaert S et al (2010) Reduction of forest soil respiration in response to nitrogen deposition. Nat Geosci 3:315–322. doi:10.1038/ngeo844
Jenkinso DS (1971) Studing on decomposition of C14 labelled organic matter in soil. Soil Sci 111:64. doi:10.1097/00010694-197101000-00008
Kaiser K, Guggenberger G (2005) Storm flow flushing in a structured soil changes the composition of dissolved organic matter leached into the subsoil. Geoderma 127:177–187. doi:10.1016/j.geoderma.2004.12.009
Kirkby CA, Richardson AE, Wade LJ, BattenB GD, Blanchard C, Kirkegaard JA (2013) Carbon-nutrient stoichiometry to increase soil carbon sequestration. Soil Biol Biochem 60:77–86. doi:10.1016/j.soilbio.2013.01.011
Kramer C, Gleixner G (2008) Soil organic matter in soil depth profiles: distinct carbon preferences of microbial groups during carbon transformation. Soil Biol Biochem 40:425–433. doi:10.1016/j.soilbio.2007.09.016
Kramer S, Marhan S, Haslwimmer H, Ruess L, Kandeler E (2013) Temporal variation in surface and subsoil abundance and function of the soil microbial community in an arable soil. Soil Biol Biochem 61:76–85. doi:10.1016/j.soilbio.2013.02.006
Kuzyakov Y (2010) Priming effects: interactions between living and dead organic matter. Soil Biol Biochem 42:1363–1371. doi:10.1016/j.soilbio.2010.04.003
Lin Q, Brookes PC (1999) An evaluation of the substrate-induced respiration method. Soil Biol Biochem 31:1969–1983. doi:10.1016/s0038-0717(99)00120-0
Marin-Spiotta E, Chadwick OA, Kramer M, Carbone MS (2011) Carbon delivery to deep mineral horizons in Hawaiian rain forest soils. J Geophys Res 116:G03011. doi:10.1029/2010jg001587
Mobley ML, Lajtha K, Kramer MG, Bacon AR, Heine PR, Richter DD (2015) Surficial gains and subsoil losses of soil carbon and nitrogen during secondary forest development. Glob Change Biol 21:986–996. doi:10.1111/gcb.12715
Moorhead DL, Sinsabaugh RL (2006) A theoretical model of litter decay and microbial interaction. Ecol Monogr 76:151–174. doi:10.1890/0012-9615(2006)076[0151:ATMOLD]2.0.CO;2
Moritz LK, Liang C, Wagai R, Kitayama K, Balser TC (2009) Vertical distribution and pools of microbial residues in tropical forest soils formed from distinct parent materials. Biogeochemistry 92:83–94. doi:10.1007/s10533-008-9264-x
Nemani RR, Keeling CD, Hashimoto H et al (2003) Climate-driven increases in global terrestrial net primary production from 1982 to 1999. Science 300:1560–1563. doi:10.1126/science.1082750
Nottingham AT, Turner BL, Chamberlain PM, Stott AW, Tanner EVJ (2012) Priming and microbial nutrient limitation in lowland tropical forest soils of contrasting fertility. Biogeochemistry 111:219–237. doi:10.1007/s10533-011-9637-4
Paterson E, Sim A (2013) Soil-specific response functions of organic matter mineralization to the availability of labile carbon. Glob Change Biol 19:1562–1571. doi:10.1111/gcb.12140
Pignataro A, Moscatelli MC, Mocali S, Grego S, Benedetti A (2012) Assessment of soil microbial functional diversity in a coppiced forest system. Appl Soil Ecol 62:115–123. doi:10.1016/j.apsoil.2012.07.007
Rumpel C, Kögel-Knabner I (2011) Deep soil organic matter-a key but poorly understood component of terrestrial C cycle. Plant Soil 338:143–158. doi:10.1007/s11104-010-0391-5
Sala OE, Chapin FS, Armesto JJ et al (2000) Biodiversity - Global biodiversity scenarios for the year 2100. Science 287:1770–1774. doi:10.1126/science.287.5459.1770
Salomé C, Nunan N, Pouteau V, Lerch TZ, Chenu C (2010) Carbon dynamics in topsoil and in subsoil may be controlled by different regulatory mechanisms. Glob Change Biol 16:416–426. doi:10.1111/j.1365-2486.2009.01884.x
Sistla SA, Asao S, Schimel JP (2012) Detecting microbial N-limitation in tussock tundra soil: implications for Arctic soil organic carbon cycling. Soil Biol Biochem 55:78–84. doi:10.1016/j.soilbio.2012.06.010
Sullivan BW, Hart SC (2013) Evaluation of mechanisms controlling the priming of soil carbon along a substrate age gradient. Soil Biol Biochem 58:293–301. doi:10.1016/j.soilbio.2012.12.007
Vancampenhout K, De Vos B, Wouters K, Swennen R, Buurman P, Deckers J (2012) Organic matter of subsoil horizons under broadleaved forest: highly processed or labile and plant-derived? Soil Biol Biochem 50:40–46. doi:10.1016/j.soilbio.2012.03.005
von Lützow M, Kögel-Knabner I, Ekschmitt K, Flessa H, Guggenberger G, Matzner E, Marschner B (2007) SOM fractionation methods: relevance to functional pools and to stabilization mechanisms. Soil Biol Biochem 39:2183–2207. doi:10.1016/j.soilbio.2007.03.007
Waldrop MP, Firestone MK (2004) Altered utilization patterns of young and old soil C by microorganisms caused by temperature shifts and N additions. Biogeochemistry 67:235–248
Waldrop MP, Zak DR, Sinsabaugh RL (2004) Microbial community response to nitrogen deposition in northern forest ecosystems. Soil Biol Biochem 36:1443–1451. doi:10.1016/j.soilbio.2004.04.023
Wang QK, Wang SL, He TX, Liu L, Wu JB (2014a) Response of organic carbon mineralization and microbial community to leaf litter and nutrient additions in subtropical forest soils. Soil Biol Biochem 71:13–20. doi:10.1016/j.soilbio.2014.01.004
Wang QK, Wang YP, Wang SL, He TX, Liu L (2014b) Fresh carbon and nitrogen inputs alter organic carbon mineralization and microbial community in forest deep soil layers. Soil Biol Biochem 72:145–151. doi:10.1016/j.soilbio.2014.01.020
West AW, Sparling GP (1986) Modifications to the substrate-induced respiraton method to permit measurement of microbial biomass in soils of differing water contents. J Microbiol Methods 5:177–189. doi:10.1016/0167-7012(86)90012-6
Zak JC, Willig MR, Moorhead DL, Wildman HG (1994) Functional diversity of microbial communities: a quantitative approach. Soil Biol Biochem 26:1101–1108. doi:10.1016/0038-0717(94)90131-7
Zimmerman AR, Gao B, Ahn M (2011) Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol Biochem 43:1169–1179. doi:10.1016/j.soilbio.2011.02.005
Acknowledgments
This study was funded by the Natural Science Foundation of China (31270515), the Chinese National Key Development Program for Basic Research (2014CB954003), the China Postdoctoral Science Foundation funded project (2015M570673), and the Natural Science Foundation of China (31400463).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no potential conflicts of interest.
Additional information
Responsible Editor: Kate Lajtha.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10533_2016_198_MOESM1_ESM.docx
Online Resource 1: This data showed the cumulative CO2–C derived from glucose (% added glucose) after glucose addition for the different soil layers during the experimental period (30 days). Supplementary material 1 (DOCX 444 kb)
10533_2016_198_MOESM2_ESM.docx
Online Resource 2: This data showed the utilization of C substrates by microbial communities in BIOLOG Eco-Plates after 200 %MBC glucose and N addition for soil from different layers. The utilization ability was expressed as average well color development (AWCD) value by six groups (carbohydrates, carboxylic acids, amino acids, polymers, amines and miscellaneous). Supplementary material 2 (DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Tian, Q., Yang, X., Wang, X. et al. Microbial community mediated response of organic carbon mineralization to labile carbon and nitrogen addition in topsoil and subsoil. Biogeochemistry 128, 125–139 (2016). https://doi.org/10.1007/s10533-016-0198-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-016-0198-4