Abstract
Stingless bees play a key role in natural environments and human economy because of their pollination services and high-value products. However, information on their nesting habitats and nest characteristics is scarce for almost all Ethiopian stingless bee species. To gain insights into the nest ecology and biology of stingless bees in the country, different habitats from 11 districts were assessed and general nest properties, such as entrance dimension, shape, entrance tunnel length, nest size, nesting substrate and internal nest architecture, were characterized. A total of 49 natural nests of two stingless bee species belonging to two genera: Meliponula and Liotrigona were found. The 47 nests of Meliponula baccarii (M. baccarii) (Gribodo) were found under the ground at an average depth of 50.50 ± 9.32 cm in three different soil types, while the two nests of Liotrigona bottegoi (L. bottegoi) were found in the cavities of Albizia shimperiana trunks at the heights of 2.8 and 3.0 m above the ground. Nest architecture and biology differed greatly between species but similar within each. Though the basic nest architecture and biology were similar within the species, they varied in size from nest to nest. The above characteristics therefore can be used for the management and conservation of the species. Finally, the data on nesting substrates, nest architecture and nest biology are of outmost importance for future designing suitable bee hives for keeping both species and also to conserve the natural nesting sites for their future utilization in pollination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stingless bees, belonging to the family Apidae and subfamily Meliponinae, are a group of eusocial, small to average sized honey producing bees, with vestigial stings. About 56 genera of stingless bees containing over 600 species are found throughout most of the tropical and subtropical regions of the globe (Roubik 1979; Eardley 2004; Cortopassi-Laurinoa et al. 2006). In Africa, only about 20 stingless bee species have been described (Eardley 2004). In Ethiopia, stingless bees are represented by about six species (Pauly and Hora 2013), and some of them have a high demand for their honey in traditional medicine; and all of them play a major role in pollinating native natural and cultivated crop plants, although their contribution has not been evaluated yet.
The richness and abundance of stingless bees vary among habitat types. Their most diverse habitat is natural forests, followed by secondary and utilized forests, and farm lands (Suriawanto et al. 2017; Njoya et al. 2018). In general, lower altitude environments have more diverse stingless bee species than higher altitudes (Salim et al. 2012). Although stingless bees are considered generalists in nesting site selection, they still exhibit a degree of plasticity in their nesting habitats and nesting substrates (Njoya et al. 2019). The majority of the species construct their nests in abandoned nests of other social insects, such as ants and termite nests, which are either above the ground or below ground environments (Roubik 1980). For instance, stingless bee species, such as M. baccarii, are known as forest species nesting in underground cavities, while M. bocandei (Spinola) is known as a forest species nesting either in underground cavities or in tree trunk cavities (Njoya et al. 2018). Many other species solely nest in the cavities of tree trunks or in underground cavities (Chemurot et al. 2021).
Stingless bee species inhabiting tropical and subtropical regions of the world considerably differ in colony size, body size and colour (Michener 2007). Typically, perennial colonies of stingless bees consist of a few dozens to more than 20,000 workers, single fertile queen and drones (Danaraddi et al. 1996; Viana et al. 2015; Njoya et al. 2018; Alves et al. 2019). Stingless bees also vary significantly in their nest architecture, with different characteristics of nest entrances and diverse designs in brood cells arrangements (Njoya et al. 2019). Brood cells are arranged either in horizontal or vertical cells with full combs or semi-combs, or in clustered cells (Kajobe 2007). The nest entrance of stingless bees also varies in shape, length and colour, which can be used for taxonomic studies (Suriawanto et al. 2017; Njoya et al. 2019). It has, indeed, been confirmed that stingless bees nest characteristics are useful in taxonomic studies, particularly in tropical African countries (Kajobe and Roubik 2006) like in Ethiopia, where a few have been studied. In Ethiopia, some studies like indigenous knowledge of stingless bee keeping (Jemberie et al. 2020; Kidane et al. 2021), stingless bee domestication (Bayeta and Hora 2021), stingless bee honey physicochemical characterization (Gela et al. 2021) and antibacterial activity (Ashenafi 1994) have been carried out. However, nesting habitats, nest biology and nest architecture of many stingless bee species have not been well described in Ethiopia in general and in Oromia in particular. Nevertheless, knowledge about the ecology and nest biology of stingless bees is vital for underpinning their adaptation to habitats and helps the development and implementation of proper conservation strategies for the species (Siqueira et al. 2012; Viana et al. 2015). Information on the ecology and nest biology of stingless bees is also important for developing and improving management practices in order to exploit the stingless bees both for pollination and acquisition of stingless bee products (Danaraddi et al. 1996). Hence, investigating the nesting habitats and nest biology of native stingless bee species in Ethiopia, where few studies have been conducted, is highly vital for the development of proper stingless bee management practices and conservation. Therefore, the aim of this work was to provide information on nesting ecology, nest architecture and nest biology of the stingless bees in the Oromia Regional State of Ethiopia through systematic investigation of wild stingless bee colonies. The habitats and natural nests made by these colonies are characterized, which may provide important information for sustainable colony management and species conservation.
Materials and methods
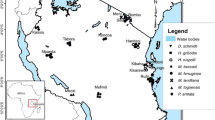
Stingless bees’ nests were sampled in 11 districts of six different zones of Oromia Regional State, Ethiopia. The six zones were Oromia Special Zone Around Finfine (OSZAF), West Shewa, Buno Bedele, Ilubabor, Jimma and East Guji (Fig. 1). The zones, districts and peasant associations were purposefully selected as sampling areas based on the presence of local stingless bees’ honey markets and potentials for diverse wild stingless bee nesting. After the purposive selection of sampling areas, different kinds of vegetation and crop fields within one kilometer of the vegetation in the sampling areas were assessed for the presence of stingless bee nests. The sampling sites included different nesting habitats, such as natural vegetation, plantations, protected forests, woody shrubs along crop fields, grass lands and even crop fields, because stingless bees can use those different habitats for their nesting. Nesting sites and natural stingless bee nests were found mainly based on information provided by local stingless bee hunters, farmers and beekeepers of the respective peasant associations. Once the nests were located, the coordinates for the locations were obtained using GPS (Global Positioning System). Then, nesting habitat type, soil type (for subterranean nesting stingless bees), tree species, position on the tree and height of the entrance above the ground (for tree trunk cavity nesting bees), and detailed external entrance characteristics were carefully recorded. The surroundings of each nest were cleaned and their entrance height above the ground and entrance tube diameter were measured using a digital caliper with a precision of 0.01 mm. For the subterranean nests, nest excavation was done following our previous method (Bayeta and Hora 2021). The lateral part of each nest was excavated to expose the nest. The dimensions (width and height) and length of the entrance tunnel above the nest to the ground level were measured using a measuring tape. The external entrance height above the ground, the diameter of the entrance and thickness of the tube were measured using the digital caliper.
After opening the layers of a nest involucrum, the number of brood combs per nest, the number of queen cells per nest, and the number of cells per 4 cm2 (an area of 20 mm × 20 mm) were counted. The presence or absence of a queen bee was visually checked by separating brood combs/clusters of brood cells. The shape of brood combs and storage pots, their arrangements and colours, as well as their placements in the nest, were carefully examined and recorded, particularly for the ground nesting one. The diameter of brood combs, comb thickness, pillar height, pillar thickness, worker cell depth and width were measured. To get the average width of a single cell, the widths of 10 cells were measured and the result was divided by 10. This close examination was conducted only for 14 colonies, as the method is destructive. Finally, stingless bees were taken from the nests and killed by drowning in soapy water in plastic bottles. Dead bee specimens were placed in labeled plastic sampling bottles containing 70% ethanol. Samples were identified by comparing morphological features such as colour, body size, scutum surface and head setae to reference specimens at Holeta Bee Research Center and using a key published in Pauly and Hora (2013).
Data analysis
All data on altitude, latitude, longitude, nesting habitat type, nesting substrate, nest architecture, and internal parameters measured, were entered into Microsoft Excel. Frequency analysis was used to obtain the proportion of nests across habitat types. A descriptive analysis was run to characterize internal and external nest sizes. Furthermore, Spearman’s rank correlation analyses were performed using IBM SPSS Statistics version 20 to see if there is a significant correlation between the number of brood combs and the number of queen cells counted per nest, at α = 0.05 level of significance.
Results
Nesting habitats
The altitudes at which the nests were sampled ranged from 1758 to 2543 m above sea level (m.a.s.l) with an average of 2256.56 ± 218.73 m.a.s.l (N = 49). During the survey, 49 nests of Meliponini bees were found in all the study areas, belonging to two genera (Meliponula and Liotrigona). The genera Meliponula and Liotrigona were each represented by one species. Meliponula baccarii represented the Meliponula and L. bottegoi represented the later genus. The bee species occupied two distinct nesting substrate types in which all 47 nests of M. baccarii were found in the ground in three different soil types, while the two nests of L. bottegoi were found in the cavity of an Albizia shimperiana trunk at the heights of 2.8 and 3.0 m above the ground (Table 1, Fig. S1a). Meliponula baccarii was the species most commonly found in all sampled sites and nesting habitat types, while the two nests of L. bottegoi were found in the cavities of the tree trunks only in the natural coffee forest. In contrast, the nesting habitat of M. baccarii greatly varied among the six nesting habitats recorded during sample collection. The highest number of M. baccarii nests was obtained from cultivated lands adjoining forest areas (17 nests), followed by protected forests (13 nests), and grass lands (5 nests) and woody shrubs along field edges (5 nests) (Fig. 2).
External characteristics of the nests
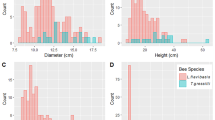
The entrance tube of L. bottegoi was horizontally arranged and faced eastwards. It was also surrounded by a dot-like rim of sticky material (Fig. S1b). In contrast, the nest entrance of M. baccarii was oriented upward, and the area closer to the tube was kept clean (Fig. S1c). Meliponula baccarii nests were hardly visible, with rounded shapes. The entrances and the openings of the nests did not differ in shape. The external entrance tubes were short and soft, built from dark cerumen, opening into a long and hard brittle internal tunnel. The external entrances of colonies were raised at 0.85 cm above the ground on average. The diameter of the entrance orifice, nest entrance tunnel thickness and number of guard bees on the internal part of the entrances are presented in Table 2. Unfortunately, it was difficult to measure the diameter and length of nest entrances and the number of guard bees at the entrance of L. bottegoi as the entrances were destroyed while cutting the tree trunks.
Internal characteristics of the nests
The nest depth below the ground to the bottom of the nest (Fig. S1d), the internal tunnel length above the nest to the ground level (Fig. 3a), and the width and height of the nest of M. baccarii are depicted in Table 2. The nests of the colonies were situated at different depths. The inner surface of the entrance tube was decorated with a black cerumen. All the nests were surrounded by dark brown to black brittle batumen layer, connected through short pillars protruding from the wall of the nests to the soil (Fig. 3b).
The nests contained food storage (honey and pollen pots) (Fig. 3c) and brood zones inside their batumen layers (Fig. 3d). The food pots were frequently located lateral to the brood nest, with honey pots at the periphery of a nest and pollen pots closer to the boundaries of brood nests. Next to the food pots, several brown layers of involucrum were arranged, separating the storage zone from the brood zone. Honey pots were oval in shape and built with thick dark to brown cerumen connected to the batumen layer through pillars. The honey pots were slightly darker than the pollen pots. The diameter and height of the honey pots ranged from 8–20 mm and 5.00–9.00 mm, with mean values of 13.79 ± 3.70 mm and 7.57 ± 1.22 mm, respectively. The volume of the honey pot varied from 12.00 to 30.00 ml with a mean value of 23.00 ± 6.12 ml.
Unlike that of M. baccarii, the internal entrance tube of L. bottegoi was inconspicuous. Like in M. baccarii, food pots were larger than brood cells; however, both food pots and brood cells were smaller than those of M. baccarii. The food pots of L. bottegoi were oval in shape, yellowish in colour and arranged in clusters. Honey pots were located at the periphery of a nest and some of them were intermixed with pollen pots.
Brood nest
The brood nest of M. baccarii was situated at the center of the nest and surrounded by several sheaths of involucrum. The brood nest contained horizontally arranged brood combs that were generally round in shape. The size and number of brood combs varied among nests from 38.36–90.40 mm in diameter and 5–12 in number, with mean values of 65.93 ± 13.38 and 8.91 ± 1.95, respectively. The average thickness of the brood comb was 4.52 ± 1.01 mm, with a range of 2.82–7.00 mm. Brood combs were arranged one over the other in all the nests assessed except in one colony, which was observed constructing combs united in a spiral shape. The average distance (pillar height) between two successive combs was 4.28 ± 0.77 mm. Combs were supported by many pillars, with thicknesses varying from 1–3 mm and an average of 1.59 ± 0.43 mm. Brood combs containing eggs and larvae were dark brown in colour (Fig. 4a), while combs with old pupae cells emerging into new adult bees were yellowish and creamy white (Fig. 4b), respectively. Unlike honeybees, M. baccarii cells were vertically constructed and the old comb from which young bees emerged was observed to be destructed by worker bees (Fig. 4b). Worker brood cells had an average depth of 6.72 ± 0.72 mm and an average width of 3.36 ± 0.24 mm. The number of cells per cm2 for M. baccarii varied from 9–10 cells between colonies, with a mean value of 9.60 ± 0.34 cells.
Brood nests of M. baccarii and L. bottegoi: young brood comb cells with stored larval food and eggs (a), old brood comb from which new worker bees started emerging at its center (upper left) (b), young and old brood combs with queen cells at the edge (c), amorphously clustered brood cells with queen and worker bees of L. bottegoi (d), queen bee of M. baccarii (e) and a nest of L. bottegoi in a trunk (f)
Large cells (queen cells) were detected at the periphery of brood combs in all the colonies of M. baccarii except in two colonies (Fig. 4c), and none in L. bottegoi. However, only a single queen per colony was found in all the assessed two L. bottegoi (Fig. 4d) and 14 M. baccarii (Fig. 4e) nests. The number of queen cells per colony ranged from 0–12 with an average of 7.92 ± 3.84. The queen cells were pale in colour and connected to brood combs with short pillars (Fig. 4d). The correlation between the number of queen cells and the number of brood combs was found to be significant (r = 0.51, p = 0.008).
Unlike in M. baccarii, the layer of involucrum around the brood was absent in L. bottegoi. Brood cells were oval in shape and creamy white in colour. The amorphously clustered brood cells were located at the center of the nest with some spaces, which enabled easy movement of the bees within the cluster of brood cells. The clustered brood cells were surrounded by pollen and honey pots (Fig. 4f).
Discussion
The diversity of stingless bees varies among different habitats and, usually, species diversity is the highest in natural forests (Nkoba et al. 2012; Suriawanto et al. 2017). In the present study, only two species of stingless bees were found in diverse habitats, but occupying two distinct nesting substrate types. The two stingless bee species (L. bottegoi and M. baccarii), were found in overlapping areas in the midland, whereas only M. baccarii occurred in the highland areas of Oromia. In general, highland ecosystems have fewer stingless bee species (Salim et al. 2012; Suriawanto et al. 2017). It should be noted that there are six stingless bee species existing in the county (Pauly and Hora 2013), however, the nests of only two species are found in the current study areas. Meliponula baccarii nest is the most widely distributed, occurring in all sampled districts, covering all habitat types, with three different soil types. The two nests of L. bottegoi were found in natural coffee forest, nesting in the cavities of Albizia shimperiana trunks, while previously reported nests were in hollow acacia trunks (Pauly and Hora 2013). The occurrence of two distinct stingless bee species in natural forests suggests that they provide different nesting substrates that serve as shelters for the bees (Siqueira et al. 2012). Natural forests, indeed, present various physiognomies that account for the stingless bee species diversity through providing suitable nesting sites, which are considered limiting factors for the diversity of stingless bees (Starr and Sakagami 1987). The occurrence of M. baccarii nests in soils with different physical properties suggests that selection of nesting cavity is not affected by soil type like in other subterranean stingless bee species, such as Geotrigona subterranean and Geotrigona nombuca (Barbosa et al. 2013).
Stingless bees have evolved a wide range of nesting and feeding behaviours, that allows them to adapt to various types of habitats for nesting (Roubik 2006). Stingless bees, like other animals, heavily rely on the quality of their environment to regulate nest population and nesting pattern (Nkoba et al. 2012). To this end, the variation in the number of nests among the six habitats explains why the highest number of M. baccarii nests were found in cultivated lands within one kilometer of forest areas. The abundance of stingless bee nests near forests and cropland edges is explained by habitat heterogeneity adjoining forests and croplands (Siqueira et al. 2012; Jemberie et al. 2020). Furthermore, stingless bees prefer to nest in open areas rather than dense vegetation. Open areas allow high light incidence, which directly affects the external activities of bees through initiating early morning foraging flights (Barbosa et al. 2013). These characteristics could explain the abundance of nests in croplands near forests, which provide both nesting sites and food resources. The nest entrances of stingless bees have distinct properties depending on the genus/species (Kajobe 2007; Kelly et al. 2014). Some species like M. ferruginea, M. lendliana and M.nebulata, construct circular entrances, while M. bocandei of the same genus known to construct V-shaped/funnel shaped entrances. Other species, such as H. gribodoi build the longest (1–5 cm) and most tubular and tapered nest entrances (Kajobe 2007), whereas the species Trigona thoracica (T. thoracica) construsts mount-shape entrances (Kelly et al. 2014). Nest entrance properties of stingless bees are affected by a variety of factors, including nest age, microclimate, foraging activities and defense (Roubik 2006). For instance, the eastward orientation of L. bottegoi’s nest entrance likely exposed the nest to the early morning sun light, resulting in a warm temperature and the initiation of foraging flight for this small bee species (Kajobe 2007). However, it is not possible to deduce that all L. bottegoi colonies prefer the eastward facing, since the information from only two colonies is not enough. The deposition of sticky resin around the entrance of this bee species may serve to guide foraging bees to the nest and helps to protect the colony from natural enemies as for other stingless bee species (Barbosa et al. 2013). The entrance to an insect nest is a critical location where the nest, with its resources, and the outside world, with its threats, communicate. Thus, the characteristics of the external nest entrance and its size play a fundamental role in the survival of the colony in the nest (Couvillon et al. 2008). Larger external nest entrance of M. baccarii aid high forager trafficking; small entrances facilitate colony defense; and the raised structure of the entrance provides a guiding and landing platform for the foragers (Biesmeijer et al. 2005; Viana et al. 2015). The current finding supports the previous report that nest entrance diameter is related to foraging activities and nest defense in M. baccarii (Viana et al. 2015).
The nests of eusocial insects are protected by guard bees. This behaviour helps to defend colony resources inside the nest (Grüter et al. 2011). In this context, several guard bees (0–13 bees) positioned at the inner part of the external entrance suggest the significance of guard bees in defending their wild colonies. The range of the number of guard bees keeping the entrance is almost similar to the previous reports for M. baccarii (Jemberie et al. 2020; Bayeta and Hora 2021). The variation in the number of guard bees may be related to several factors, including the time of the day, size of the colony, weather condition, size of the entrance, level of forager trafficking and presence or absence of natural enemies (Couvillon et al. 2008; Grüter et al. 2011; Jemberie et al. 2020; Bayeta and Hora 2021).
Meliponula baccarii constructed its nests at a great depth under the ground (34.00–74.00 cm) and connected them with a long entrance tunnel above the nest cavity to the ground level (14.00–43.00 cm). Such a long entrance tunnel was not observed in L. bottegoi, thereby indicating that different stingless bee species construct internal tunnels of different lengths. However, it is within the range of the entrance tunnel length of M. baccarii reported from Northern Ethiopia (23.4 to 35.4 cm) and Cameroon (27.5 to 34.5 cm) (Jemberie et al. 2020). The ground nesting bees appear to nest at a sufficient depth to maintain a stable nest environment even when outside temperatures are high; whereas stingless bees nesting in tree trunks maintained a stable microclimate due to the shade and insulation provided by the tree (Moritz and Crewe 1988). The current nest chamber width and length of M. baccarii are relatively smaller than those in the previous report from Northern Ethiopia (Jemberie et al. 2020), whereas it is larger than the nest size reported in Cameroon (Njoya et al. 2017). This is likely the result of restrictions imposed by the nesting cavity limitations and is often geographically and phylogenetically related (Roubik 2006; Gonzalez et al. 2018), which suggests further work on factors affecting the nest cavity of M. baccarii.
The brood chamber of M. baccarii is covered by layers of involucrum because the soil temperature at the average depth where subterranean bees like M. baccarii build their nests is lower than the brood nest (Barbosa et al. 2013). Hence, the layers of involucrum play a key role in the thermoregulation of the brood chamber, as stingless bees do not thermoregulate their nests as precisely as honeybees do (Alves et al. 2019). In this stingless bee, the involucrum retains thermal energy generated by adult bees and partly by the mass of brood in the brood area (Sung et al. 2008). Unlike in M. baccarii, involucrum was absent in the L. bottegoi colony that built its brood cells in a cluster. This result is in agreement with the finding that some species of stingless bees like T. fulviventris build no involucrum around their brood (Barbosa et al. 2013); thereby indicating that the involucrum may be an optional structure in stingless bee nests, and its construction is related to external temperature conditions.
In general, most stingless bees are cavity nesters and some of them arrange their brood cells in combs, others build brood cells in clusters (Michener 2007). In our results, the former type of cell arrangement was present in M. baccarii and the latter type of cell arrangement was found in L. bottegoi, suggesting that the two species adapted to different types of brood cell arrangement. Cluster cell arrangement is found in several groups of small to minute stingless bees (Michener 2007; Melo and Costa 2009), and it seems to be an adaptive feature of small bees nesting in small and irregular cavities (Gonzalez et al. 2018). In contrast, M. baccarii was observed to build vertical brood cells arranged in horizontal brood combs. The number of brood combs per colony of M. baccarii varies from 5–12, which is similar to what has been previously reported for nests of the same species from Northern Ethiopia (Jemberie et al. 2020). However, the diameter of the comb is slightly shorter than the range reported from the north, which may be influenced by geographical factors that can limit the size of nest cavities (Alves et al. 2019).
In the present study, a varying number of queen cells (0–12 cells/colony) were detected at the periphery of horizontal brood combs connected to the combs through short pillars in all colonies except in two colonies, suggesting that most M. baccarii colonies construct queen cells in wild colonies as reported in other stingless bee species (Alves et al. 2019). The significant positive correlation (p < 0.05) between the number of queen cells and brood combs indicates that a higher number of brood layers represents an increase in the number of queen cells, showing a high tendency for reproduction. This evidence suggests the existence of a high probability of swarming in wild colonies of M. baccarii, which could create a good opportunity for colony multiplication.
Conclusion
This study underlined that stingless bee nest population varies among different habitats and that usually, nest detection is highest in cultivated lands adjoining forest areas, indicating habitat type and its characteristics are important factors regulating nest population. Moreover, this study successfully described the external and internal nest characteristics and their nest biology. The results of nesting substrates and architecture for the studied species show a lot of similarities with the previous studies for the same stingless bee species. However, the variations in nest size within the species could be related to many factors, such as microclimate, predators and the foraging activities of the species. Thus, understanding the nesting habitat and substrate preferences, as well as nest architecture and nest biology of each species, might not only help to characterize the species but also helps the development and implementation of proper conservation strategies, thereby providing important information for sustainable colony management. Therefore, the information generated on nesting substrates, nest architectures and nest biology is an important starting point for designing suitable bee hives for keeping both species and also for conserving the natural nesting sites for their future utilization in pollination services. However, further research will be needed to generate sufficient data for unaddressed stingless bee species found at lower altitudes in the regional.
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Alves RMDO, Waldschmidt AM, Paixão JF, Santos DR, Carvalho CAL (2019) Geographic range and nest architecture of Cephalotrigona capitata smith, 1854 (Apidae: Meliponini) in the state of Bahia, Northeastern Brazil. J Apic Sci 63:49–60. https://doi.org/10.2478/JAS-2019-0006
Ashenafi M (1994) The in vitro antibacterial activity of “tazma mar” honey produced by the stingless bee (Apis mellipodae). Ethiop J Heal Dev 8:109–117. Available at: https://www.ajol.info/index.php/ejhd/article/view/216727
Barbosa FM, Alves RM, Souza BD, Carvalho CA (2013) Nest architecture of the stingless bee Geotrigona subterranea (Friese 1901 (Hymenoptera: Apidae: Meliponini). Biota Neotrop 13:147–152. https://doi.org/10.1590/S1676-06032013000100017
Bayeta AG, Hora ZA (2021) Evaluation of different hive designs for domestication and conservation of native stingless bee (Apidae: Meliponula beccarii) in Ethiopia. Int J Trop Insect Sci 41:1791–1798. https://doi.org/10.1007/s42690-020-00392-5
Biesmeijer JC, Giurfa M, Koedam D, Potts SG, Joel DM, Dafni A (2005) Convergent evolution: Floral guides, stingless bee nest entrances, and insectivorous pitchers. Naturwissenschaften 92:444–450. https://doi.org/10.1007/s00114-005-0017-6
Chemurot M, Otim AS, Namayanja D, Onen H, Angiro C, Mugume R, Kajobe R, Macharia J, Gikungu M, Abila PP, Kasangaki P (2021) Stingless beekeeping in uganda: an industry in its infancy. African Entomol 29:165–172. https://doi.org/10.4001/003.029.0165
Cortopassi-Laurinoa M, Imperatriz-Fonsecab VL, Roubikc DW, Dollind A, Hearde T, Aguilarf I, Venturieri GC, Eardley C, Nogueira-Neto P (2006) Global meliponiculture: challenge opportunities 37:275–292
Couvillon MJ, Wenseleers T, Imperatriz-Fonseca VL, Nogueira-Neto P, Ratnieks FLW (2008) Comparative study in stingless bees (Meliponini) demonstrates that nest entrance size predicts traffic and defensivity. J Evol Biol 21:194–201. https://doi.org/10.1111/j.1420-9101.2007.01457.x
Danaraddi CS, Shashidhar V, Basavanagoud K, Bhat AR (1996) Nesting habits and nest structure of stingless bee, Trigona iridipennis Smith at Dharwad, Karnataka. Karnataka J Agric Sci 22:310–313. Available at: https://www.cabdirect.org/cabdirect/abstract/20093330538
Eardley CD (2004) Taxonomic revision of the African stingless bees ( Apoidea : Apidae : Apinae : Meliponini ). African Plant Prot 10:63–96
Gela A, Hora ZA, Kebebe D, Gebresilassie A (2021) Physico-chemical characteristics of honey produced by stingless bees (Meliponula beccarii) from West Showa zone of Oromia Region, Ethiopia. Heliyon 7:e05875. https://doi.org/10.1016/j.heliyon.2020.e05875
Gonzalez VH, Amith JD, Stein TJ (2018) Nesting ecology and the cultural importance of stingless bees to speakers of Yoloxóchitl Mixtec, an endangered language in Guerrero, Mexico. Apidologie 49:625–636. https://doi.org/10.1007/s13592-018-0590-2
Grüter C, Kärcher MH, Ratnieks FLW (2011) The natural history of nest defence in a stingless bee, Tetragonisca angustula (Latreille) (Hymenoptera: Apidae), with two distinct types of entrance guards. Neotrop Entomol 40:55–61. https://doi.org/10.1590/S1519-566X2011000100008
Jemberie W, Negash W, Alemu K, Tarekegn A, Brhan M, Raja N (2020) Stingless bee Meliponula Cockerell (Hymenoptera: Apidae: Meliponini) ground nest architecture and traditional knowledge on the use of honey in the Amhara Region, Northwest Ethiopia. Isr J Entomol 50:14–17. https://doi.org/10.5281/zenodo.4588315
Kajobe R (2007) Nesting biology of equatorial Afrotropical stingless bees (Apidae; Meliponini) in Bwindi Impenetrable National Park. Uganda J Apic Res 46:245–255. https://doi.org/10.3896/ibra.1.46.4.07
Kajobe R, Roubik DW (2006) Honey-making bee colony abundance and predation by apes and humans in a Uganda forest reserve. Biotropica 38:210–218. https://doi.org/10.1111/j.1744-7429.2006.00126.x
Kelly N, Farisya MSN, Kumara TK, Marcela P (2014) Species diversity and external nest characteristics of stingless bees in meliponiculture. Pertanika J Trop Agric Sci 37:293–298
Kidane AA, Tegegne FM, Tack AJM (2021) Indigenous knowledge of ground-nesting stingless bees in southwestern Ethiopia. Int J Trop Insect Sci. https://doi.org/10.1007/s42690-021-00442-6
Melo GAR, Costa MA (2009) Uma nova espécie de Plebeia (Hymenoptera, Apidae) do leste do Brasil, com células de cria em cacho. Rev Bras Entomol 53:77–81. https://doi.org/10.1590/S0085-56262009000100019
Michener CD (2007) The bees of the world. The Johns Hopkins University Press, Baltimore
Moritz RFA, Crewe RM (1988) Air ventilation in nests of two African stingless bees Trigona denoiti and Trigona gribodoi. Experientia 44:1024–1027
Njoya MTM, Akwanjoh SR, Wittmann D (2019) Nest architecture and colony characteristics of Meliponula (Axestotrigona) ferruginea (Hymenoptera, Apidae, Meliponini) in Cameroon. J Biosci 44:13. https://doi.org/10.1007/s12038-018-9840-8
Njoya MTM, Seino RA, Wittmann D, Kenneth T (2018) Nest architecture and colony characteristics of Meliponula bocandei (Hymenoptera, (Hymenoptera, Apidae, Meliponini) in Cameroon. Int J Res Agric Sci 5:2348–3997. https://doi.org/10.1007/s12038-018-9840-8
Njoya MTM, Wittmann D, Balgah RA (2017) Subterranean Nest Architecture and Colony Characteristics of Meliponula (Meliplebeia) becarii (Hymenoptera, Apidae, Meliponini) in Cameroon. J Chem Bio Phy Sci 7:220–233
Nkoba K, Raina SK, Muli E, Mithofer K, Mueke J (2012) Species richness and nest dispersion of some tropical meliponine bees (Apidae: Meliponinae) in six habitat types in the Kakamega forest, western Kenya. Int J Trop Insect Sci 32:194–202. https://doi.org/10.1017/S1742758412000355
Pauly A, Hora AZ (2013) Apini and Meliponini from Ethiopia (Hymenoptera: Apoidea: Apidae: Apinae). Belgian J Entomol 16:1–36
Roubik D (1980) Foraging Behavior of Competing Africanized Honeybees and Stingless Bees. Ecology 61:836–845. https://doi.org/10.2307/1936754
Roubik DW (1979) Nest and colony characteristics of stingless bees from French Guiana (Hymenoptera: Apidae). J Kansas Entomol Soc 52:443–470
Roubik DW (2006) Stingless bee nesting biology. Apidologie 37:124–143
Salim HMW, Dzulkiply AD, Harrison RD, Fletcher C, Kassim AR, Potts MD (2012) Stingless bee (hymenoptera: Apidae: Meliponini) diversity in dipterocarp forest reserves in Peninsular Malaysia. Raffles Bull Zool 60:213–219
Siqueira ENL, Bartelli BF, Nascimento ART, Nogueira-Ferreira FH (2012) Diversity and nesting substrates of stingless bees (Hymenoptera, Meliponina) in a forest remnant. Psyche (london) 2012:1–9. https://doi.org/10.1155/2012/370895
Starr CK, Sakagami SF (1987) An extraordinary concentration of stingless bee colonies in the Philippines, with notes on nest structure (Hymenoptera: Apidae: Trigona spp.). Insectes Soc 34:96–107. https://doi.org/10.1007/BF02223828
Sung IH, Yamane S, Hozumi S (2008) Thermal characteristics of nests of the Taiwanese stingless bee Trigona ventralis hoozana (Hymenoptera: Apidae). Zool Stud 47:417–428
Suriawanto N, Atmowidi T, Kahono S (2017) Nesting sites characteristics of stingless bees (Hymenoptera: Apidae) in Central Sulawesi. Indonesia J Insect Biodivers 5:1–9. https://doi.org/10.12976/jib/2017.5.10
Viana JL, Sousa HD, Alves RM, Pereira DG, Silva Jr JC, Paixão JF, Waldschmidt AM (2015) Bionomics of Melipona mondury Smith 1863 (Hymenoptera: Apidae, Meliponini) in relation to its nesting behavior. Biota Neotrop 15:1–7.https://doi.org/10.1590/1676-06032015009714
Acknowledgements
The authors acknowledge the Oromia Agricultural Research Institute through the Holeta Bee Research Center for funding this research. We would also like to extend our special thanks to the technical staff of Holeta Bee Research Center, in particular Mr. Terefe Chimdi and Tesfaye Teshome, for their help during data collection. Finally, we owe our genuine appreciation to Getachew Haile, a GIS expert, who helped prepare the distribution map.
Author information
Authors and Affiliations
Contributions
Z.A.H and A.G.B conceived this research and designed the experiments. Z.A.H performed the experiment and data analysis. Both authors were involved in the interpretation of data and editing of the manuscript. T.N was involved in data collection.
Corresponding author
Ethics declarations
Conflicts of interest
The authors of this paper do hereby disclose that there are no conflicts of interest whatsoever in relation to this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hora, Z.A., Bayeta, A.G. & Negera, T. Nesting ecology and nest characteristics of stingless bees (Apidae: Meliponini) in Oromia Regional State, Ethiopia. Int J Trop Insect Sci 43, 409–417 (2023). https://doi.org/10.1007/s42690-023-00946-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-023-00946-3