Abstract
The appearance and rapid spread of the fall armyworm (Spodoptera frugiperda) (FAW) represents a serious threat to maize cultivation in India and elsewhere in South Asia. Chemical control illustrates one of the major means of reducing the infestation of FAW in maize-growing zones. However, existing information regarding the field-efficacy and non-target toxicity of different insecticides against this pest is not adequate and is also unable to capture the momentum change in the scenario of residual toxicity and insecticide resistance for redacting sustainable management. The present study was framed to establish the most suitable insecticidal schedule against FAW for maize producers. In the period from winter 2019–2020 to spring–summer 2020, seven treatment schedules against FAW were evaluated, and the efficacy was calculated according to the per cent maize plant damage (PD) by larvae, while safety was enumerated based on larval parasitization by Campoletis chlorideae and the abundance of coccinellid predators. In both seasons, the highest cumulative efficacy (9.88 and 10.19% PD) was confirmed for T3 (constituted with Barazide®, Delegate®, and Ampligo®) with a significantly higher yield (52.39 and 52.66 q ha−1), whereas T7 (Proclaim Fit®, Ampligo®, and Delegate®) and T6 (Fimecta®, Ampligo®, and Spintor®) exhibited a high cumulative efficacy (11.06 to 12.23% PD). T3 was found to be safe to coccinellid predators and the per cent larval parasitism by C. chlorideae was significantly higher in this module (2.57 and 2.75%) compared to T4 and T5 (1.00 to 1.67%). The residues of Barazide®, Delegate®, and Ampligo® were below detectable levels in maize plant, grain and soil samples. Therefore, the module could be recommended against FAW in the near future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The fall armyworm (FAW), Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) is one of the most destructive, highly polyphagous and migratory pests native to tropical and subtropical Americas (Hardke et al. 2011; Cruz et al. 2012; Blanco et al. 2016). It has a wide range of host plants and attacks several economically important field crops like maize, rice, millet, sorghum, wheat, cotton, peanut, cowpea, sugarcane, soybean and other fodder grasses in Argentina, Brazil, Canada, Chilli, Ethiopia, Kenya, and Nigeria etc. (Montezano et al. 2018). In 2016, severe outbreak of FAW was reported in African countries (Goergen et al. 2016), and the first report of the incursion of this pest into Asia was confirmed from Karnataka, India on maize during May 2018 (Sharanabasappa et al. 2018; Shylesha et al. 2018). Since then, FAW has spread to other states of India and several Asian countries, including Bangladesh, China, Sri Lanka, Myanmar, Vietnam, Laos, and Thailand (Deshmukh et al. 2020). The recent invasion of this invasive pest threatens the grain-maize production of India, as the Indian FAW population exhibits genetic similarity to the South African population (Nagoshi et al. 2019) and mostly fed on maize (Sharanabasappa et al. 2018). Insecticides have yielded satisfactory results in America and Africa (Gutierrez-Moreno et al. 2019; Sisay et al. 2019); hence, chemical control strategy is the first line of defense for controlling this voracious feeder in India. Some workers determined the individual toxicity and efficacy of various recommended and novel insecticides against FAW (Smith and Catchot, 2009; Hardke et al. 2011; Viteri et al. 2018; Sisay et al. 2019; Worku and Ebabuye 2019; Deshmukh et al. 2020), but a compact treatment schedule has not been reported to date. The cryptic feeding behavior of FAW larvae by remaining most of its life span inside the plant whorl reduces their direct contact with insecticides. Hence, at least 2–3 rounds of spray are required over a single cropping season of maize to control this pest (Deshmukh et al. 2020). However, multiple applications of the same molecule may lead to the rapid development of insecticide resistance (Gutierrez-Moreno et al. 2019) and may create adverse effects on beneficial arthropods in the field. Moreover, recent findings linking residual toxicity of insecticides profusely used to control FAW infestation in fodder maize, with cattle mortality in India (Deshpande 2019). Koli and Bhardwaj (2018) reported that unlike European countries, there are no authenticated information and registration guidelines for insecticide use concerning forage crops in India. Therefore, sustainable management of FAW through environmentally benign approaches has become necessary inexorable to break the plateau in maize production. In this context, the objective of this study is, therefore, to evaluate some proposed insecticidal schedules, based on government recommended molecules, against FAW in maize under open-field conditions. The toxicity of all the treatments to prevailing predators and parasitoid along with the plant and soil residues of the best management schedule were also evaluated to find the most compatible chemical module for successful control of FAW in India.
Materials and methods
Insecticides selected for field efficacy
The selection of commercial formulations of different molecules was purely based on recommendations from the Ministry of Agriculture, Government of India against FAW (POP 2019). The selected insecticide formulations were: chlorantraniliprole 18.5 SC (Coragen®, DuPont India Pvt. Ltd.), chlorantraniliprole 9.3% + lambda cyhalothrin 4.6% ZC (Ampligo®, Syngenta India), emamectin benzoate 5 SG (Proclaim®, Syngenta India), emamectin benzoate 1.5% + fipronil 3.5% SC (Fimecta®, Godrej Agrovet Ltd.), emamectin benzoate 5% + lufenuron 40% WG (Proclaim Fit®, Syngenta India), lambda cyhalothrin 9.5% + thiamethoxam 12.60% ZC (Alika®, Syngenta India), novaluron 5.25% + emamectin benzoate 0.9% SC (Barazide®, Adama India), spinosad 45 SC (Spintor®, Crop Science India), and spinetoram 11.7 SC (Delegate®, Dow Agrosciences India Pvt. Ltd.).
Field experiments
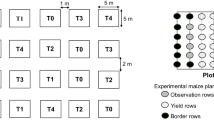
Layout
Supervised field experiments were carried out at Dhaanya Ganga Krishi Vigyan Kendra, Ramakrishna Mission Vivekananda Educational and Research Institute, Murshidabad, India, for two consecutive seasons. The winter season field trial was conducted from December 2019 to March 2020 and the spring–summer season from March 2020 to June 2020 with the maize variety “Rajkumar” in a randomized complete block design (RCBD) with seven treatments, including untreated control and replicated four times (Table 1). Maize was grown with 30 m × 20 m plots over a piece of 1.7 ha of land by following recommended agronomic practices except for plant protection activities. The land was left fallow for the last ten years and had no history of pesticide application over this period. Treatments were imposed using a battery-operated sprayer (V-Dyut Delux, ASPEE Sprayers and Farm Mechanized Equipment, Mumbai, India) fitted with a hollow-cone nozzle at 15-day intervals starting from the 30th and 26th day after sowing, when the FAW attained an economic threshold level with > 10% plant damage (Padhee and Prasanna 2019), during the first and second seasons, respectively, with 500-L spray volume ha−1. Three replicated plots (30 m × 20 m each) under each treatment were made for bioefficacy and non-target toxicity study, while the fourth plot was re-replicated thrice (10 m × 20 m each) and applied with double the recommended dosages of insecticides to perform the harvest time residue analysis.
Bioefficacy of treatment schedules against FAW
The number of plants with FAW infestation and the total number of plants in three randomly selected pairs of rows were recorded per plot, avoiding border rows before the first spray and 7 and 14 days after each spray and mean per cent plant damage was enumerated (Sudhanan et al. 2017). Data on grain yield of maize per plot was also recorded at the time of harvest and the average yield was expressed in q ha−1.
Safety of treatment schedules for natural enemies
The non-target toxicity of different treatment schedules in maize agroecosystem was evaluated for coccinellid predators and larval parasitization of FAW by Campoletis chlorideae. These two groups of populations were selected because of their abundance over other groups of natural enemies suitable for table data. Motile stages of coccinellids (grub and adult) were recorded on each replicated plot in twenty-five randomly selected plants 24 h before the first application of insecticides, and 14 days after each application. In addition, an abundance of C. chlorideae, through parasitization of FAW larvae, was enumerated by collecting at least hundred larvae of different instars on each plot from previously specified rows 24–48 h before the first spray, and 14 days after the third application (Gupta et al. 2004). The collected larvae were individually kept in a small glass vial to avoid the chance of cannibalism and separately placed under the laboratory condition according to different treatments. Larvae were observed after every 24 h up to 14 days for the emergence of adult parasitoid and data on per cent larval parasitism was recorded in all the treatments including untreated control. The selected rows, from which the FAW larvae were collected to study the larval parasitisation, were discarded while recording the bioefficacy data of insecticidal schedules for avoiding the physical control.
Residual study
Experiment on insecticidal residue and its recovery in the plant parts and soil was carried out with the insecticides, constituted the most effective schedule during two consecutive crop seasons, in July 2020.
Sampling
The recommended (applied) and double the recommended dosages of the commercial formulations of novaluron 5.25% + emamectin benzoate 0.9% SC, chlorantraniliprole 9.3% + lambda cyhalothrin 4.6% ZC, and spinetoram 11.7 SC were used in the harvest time residue study by providing the untreated control for comparison. A sampling of maize leaf and grain were done separately in an individual zip-lock bag at the time of harvest from the treated and untreated control plots, whereas soil samples were collected simultaneously using a handheld auger at a depth of 15 cm and a sub-sample (20 g soil) was taken for final analysis.
Chemicals and reagents
Commercial formulations of novaluron 5.25% + emamectin benzoate 0.9% SC, chlorantraniliprole 9.3% + lambda cyhalothrin 4.6% ZC, and spinetoram 11.7 SC were purchased from district pesticide dealer, while analytical grade novaluron (99.9% pure), emamectin benzoate (99% pure), chlorantraniliprole (96.5% pure), and lambda cyhalothrin (99.7% pure) were procured from Sigma-Aldrich and spinetoram (98.2% pure) were obtained from SPEX Europe. In addition, acetonitrile and hexane (HPLC grade, J.T. Baker, USA), millipore water (prepared from Milli-Q system, India), sodium chloride (NaCl), ethyl acetate, primary secondary amines (PSA: 40 μm, Bondesil, Agilent, USA) and anhydrous MgSO4 (ACS, Merck, India) were purchased from Sisco Research Laboratories Pvt. Ltd., Kolkata, India and used in residue analysis.
Preparation of standard solutions
Standard stock solutions of the individual insecticides (1000 μg ml−1) were prepared by dissolving 100 ml of technical grade insecticide in 100 ml of HPLC grade acetonitrile. Stock solutions (10 ml each) were diluted in 100 ml capacity volumetric flask, and volume was made up with 90 ml of acetonitrile to get a 100 μg ml−1 intermediate solution. Working standard solutions of 0.01, 0.02, 0.05, 0.10, 0.50 and 1.00 μg ml−1 were prepared by diluting 10 ml of intermediate solution serially with 90 ml of acetonitrile and were used for spiking and calibration.
Sample extraction
Modified QuEChERS method was followed to analyze the maize leaves and grains samples (Anastassiades et al. 2003). Representative homogenized leaf or ground grain sample (~ 10 g) was taken in 50 ml polypropylene centrifuge tubes with 20 ml acetonitrile, and shaken gently. Thereafter, 4 g of MgSO4 and 1 g of NaCl were added to this mixture, vortexed for a minute and was centrifuged for 10 min at 10,000 rpm. A volume of 6 ml from the upper clear supernatant was collected for further d-SPE clean up to remove impurities like carbohydrate, fatty acids, and pigments etc. Then the clear supernatant was transferred into a centrifuge tube (25 ml) containing MgSO4 (600 mg) and of PSA (100 mg). The tube was tightly sealed and vortexed for 1 min and then centrifuged at 5,000 rpm for 10 min using rotospin (Tarson) to separate solids from solution. Then the supernatant (4 ml) was transferred to a turbovap tube and was condensed up to dryness in turbovap evaporator. Finally, the volume of the tube was made with acetonitrile up to 1 ml and the absolute extract was stored for final determination.
Besides maize leaves and grains, 10 g of shade-dried and sieved soil sample was taken in 100 ml conical flask, gently mixed with 50 ml acetonitrile and shaken well through an electronic shaker for 1 h. The filtration followed by condensation of the solution was done and mixed with 10 ml of acetonitrile mixture (750 ml acetonitrile + 250 ml double distilled water + 0.8 ml triethanolamine) and again filtered through a membrane filter (0.2 μ) and was injected for analysis using High Performance Liquid Chromatography (HPLC) with a 15 min running time.
Instrumentation
The harvest-time residues of chlorantraniliprole, emamectin benzoate, lambda cyhalothrin, novaluron and spinetoram were estimated on reverse phase HPLC equipped with PDA detector (Shimadzu HPLC – 20 AT) in maize samples. The mobile phase comprised of Acetonitrile: water (60:40 v/v) with a fixed flow rate of 1.0 ml min−1. The chromatographic separation of spinetoram was redacted with RP C18 column (150 × 4.6 mm), while rests were performed with RP C18 column (250 × 4.0 mm). The injection volume was 20 μl and the retention time of chlorantraniliprole, emamectin benzoate, lambda cyhalothrin, novaluron and spinetoram were detected under those functional conditions at 6.48, 5.51, 7.33, 2.63 and 4.59 min, respectively (Fig. 1a). The control sample of maize did not exhibit any interfering peaks in the HPLC–MS chromatogram (Fig. 1b).
Recovery experiment
A recovery experiment was conducted before the sample analysis to access the extraction efficiency of the analytical procedure. The untreated samples were spiked at six different concentrations (0.01, 0.02, 0.05, 0.10, 0.50 and 1.00 μg g−1) and replicated thrice. Before extraction, the spiked samples were subjected to stand for 1 h to allow the spiked solution to insert the sample matrix and similarly obtained through the method of extraction and clean up, mentioned above. The residual quantity was determined through the comparison of the sample response of standard under similar functional conditions.
Statistical analysis and interpretation
Data on plant damage, non-target toxicity and grain yield of maize were subjected to statistical analysis with ANOVA using SPSS (version 18.0: Inc., Chicago, IL, USA) software after suitable transformation (sin−1 or √x + 0.5 transformation) whenever required. Mean values were separated by Duncan’s Multiple Range Test (DMRT) at p = 0.05 for interpretation of the results (Gomez and Gomez 1984). In residue analysis, the linearity (R2) of the method was checked from the calibration curve of each insecticide standard. The limit of quantification (LOQ) was 0.01 μg g−1, whereas the limit of detection (LOD) was 0.003 µg g−1 (SFCPB 2018). Accuracy of the method was tested through the recovery experiment by spiking the respective control matrix at the concentrations of 0.02, 0.05, 0.10, and 0.50 μg g−1 for novaluron and emamectin benzoate, 0.01, 0.05, 0.10, and 1.00 μg g−1 for spinetoram, and 0.01, 0.10, 0.50, and 1.00 μg g−1 for chlorantraniliprole and lambda cyhalothrin.
Results
Bioefficacy of different treatments against FAW
Before the imposition of the insecticidal treatments no significant difference of FAW damage was observed among different treatments schedules, while all the insecticides were found to be effective and significantly reduced the plant damage 7 days after first spray (DAFS) in winter maize (Table 2). The lowest per cent plant damage was observed in T4 and T6 followed by T2 and T7 at 7 DAFS (F7,14 = 10.22, P = 0.0012), but T3 followed by T6 registered the highest efficacy at 14 DAFS (F7,14 = 15.39, P = 0.0009). T3 exhibited the lowest per cent plant damage consistently in the field and found to be statistically at par with T7 (F7,14 = 7.55, P = 0.0016) and T6 (F7,14 = 14.19, P = 0.0008) at 7 and 14 days after second spray (DASS), respectively. All the treatment schedules significantly reduced the FAW damage after each insecticide application, whereas the untreated control (T1) showed the highest per cent plant damage irrespective of days. After third spray, T2 and T4 were statistically at par with each other on 7th day; however T3 and T7 exhibited their superiority at 7 (F7,14 = 19.48, P = 0.0019) and 14 (F7,14 = 11.61, P = 0.001) days after third spray (DATS), respectively (Fig. 2).
Almost similar trend of effectiveness of different treatment schedules against FAW in maize was encountered during the summer season (Table 3). Lowest per cent plant damage was recorded in T5 (F7,14 = 9.34, P = 0.0015) which was found to be statistically at par with T3 and T6 at 7 DAFS. Efficacy of T3 was comparable with T6 and T7 at 7 (F7,14 = 8.22, P = 0.0005) and 14 (F7,14 = 13.62, P = 0.0006) DASS, but it provided the significantly lowest per cent plant damage at 7 (F7,14 = 10.75, P = 0.0011) and 14 (F7,14 = 21.18, P = 0.0004) DATS with an excellent extrication. After three rounds of sprayings at 15 days interval, the order of efficacy based on the reduction of mean per cent plant damage over untreated control was T3 > T7 > T6 > T2 > T4 > T5.
Seed yield of maize
Mean seed yield of maize was higher in plots treated with various treatment schedules than untreated control (Table 4). In the winter season, the highest seed yield was obtained in T3 and found to be statistically at par with T6 and T7 (F7,14 = 18.26, P = 0.0003), while T3 registered significantly highest yield during the spring–summer season (F7,14 = 12.81, P = 0.0001) with 40.87% increase over control (T1).
Safety of different treatment schedules to natural enemies
The non-target toxicity of different treatment schedules was recorded on prevailing coccinellid predators in maize ecosystem and per cent larval parasitization by C. chlorideae. During the winter season, T4 consistently registered the lowest number of coccinellid fauna followed by T6 after first (SEm ± 0.23), second (SEm ± 0.37), and third (SEm ± 0.19) spray (Fig. 3). A similar trend of toxicity was also encountered in the spring–summer season maize where highest numbers of coccinellid predators were recorded in T1 followed by T3 (SEm ± 0.11), T5 (SEm ± 0.29), and T7 (SEm ± 0.42) after the consecutive three applications, respectively (Fig. 4). Figure 5 revealed that 2.72% FAW larvae were parasitized by C. chlorideae in T1 and found to be statistically at par with T3 and T7 (5.51 and 15.07% reduction over control, respectively) during the winter season. However, T4 and T5 registered the lowest per cent larval parasitization (SEm ± 0.03) with 63.23% and 58.54% reduction over control during the first and second season (Fig. 6), respectively.
Method validation of residue analysis and food-safety assessment
The validation of the analytical method was performed by examining linearity, recovery, precision and accuracy. Recovery studies were conducted to evaluate the validity of the present experiment by fortifying the maize samples with different concentrations of technical grade novaluron, emamectin benzoate, spinetoram, chlorantraniliprole and lambda cyhalothrin (Table 5). The average recoveries ranged between 86.6 and 92.9% for maize leaf, 89.0 and 92.9% for maize grain, and 86.6 and 91.5% for field soil. The terminal residues of the commercial formulations of selected insecticides, applied at recommended and its double dosages were analysed after harvest and the residues of the test insecticides were found to be below the detectable level (BDL) in all the collected substrates (Table 6).
Discussion
As is common with other crop pests, chemical insecticide is the prime option to control FAW in India (Tippannavar et al. 2019), America (Hardke et al. 2011), Mexico (Malo et al. 2004) and African countries (Assefa and Ayalew 2019). Several insecticide applications are required to kill the FAW larvae feeding inside the plant whorl; however proper treatment should be imposed at the vegetative stage to minimise the infestation load at the silking stage of maize (Foster 1989). In our two-season experiment, the insecticides currently recommended for control of FAW displayed a varied level of field-efficacy through specific treatment schedules. In both seasons, the initial efficacy of Barazide® and Proclaim Fit® was poorer than Fimecta®, but the significant superiority was shown by the former after two weeks. The delayed efficacy of these formulations was possibly due to the presence of insect growth regulators (IGRs) novaluron and lufenuron, which acted slowly on early larval instars of FAW for longer periods instead of quick knockdown (Ghosal and Chatterjee 2017; Kundu et al. 2018). However, higher efficacy of individual application of novaluron and emamectin benzoate against FAW was reported by Deshmukh et al. (2020). Satisfactory effect against FAW larvae was also exhibited by the insecticide Delegate® in T3 and T7, and the present results accord with those of Hardke et al. (2011), Belay et al. (2012), Viteri et al. (2018), and Sisay et al. (2019). But interestingly the per cent reduction of plant damage over control was comparatively higher in T3 when Delegate® was incorporated in the second spray schedule, than in the third (T7). It can be attributed that compared to the later instars, the early instars of FAW larvae are more susceptible to spinetoram (Roy and Biswas, 2020). In addition, a possible cross-resistance mechanism between spinosin and diamide classes of chemistry might play a role in this circumstance (Neto et al. 2016). On the other hand, the relative efficacy and field-consistency of Barazide® and Ampligo®, licensed for the management of FAW in India, was higher in the spring–summer season compared to the winter. Though the overall mean per cent reduction was higher during the first season, the reduction of plant damage after each spray of these molecules including Coragen® was remarkable. This might be attributable to the increased toxicity of synthetic pyrethroids (Jaleel et al. 2020), diamides (Li et al. 2020), and emamectin benzoate (Malik et al. 2018) with the rising atmospheric temperature, where temperature correlated positively with the efficacy of these ready-mix insecticides against lepidopteran caterpillars.
Quite similar numbers of coccinellid predators prevailed in T3, T7 and T1 (control) after the first spray, verified the safety of emamectin benzoate, novaluron and lufenuron usage in the field (El-Wakeil et al. 2013; Govindan et al. 2013). Moreover, selectivity and field-compatibility of chlorantraniliprole and spinosins with the ladybird beetles and lepidopteran parasitoids was conceded by Liu et al. (2017) and Parsaeyan et al. (2020), and fasten the present investigation. Significantly lower per cent larval parasitization by C. chlorideae was observed in T4, which might be due to the unsuitability of the host for egg-laying after the exposure to neonicotinoid insecticide combination in the third spray (Cloyd and Bethke 2011). It has been seen that irrespective of the treatments imposed, the overall coccinellid populations were reduced immediately after the spray (Sudhanan et al. 2017). In addition, there were a few chances that the natural enemies may move from the treated plots to the untreated control in search of food, as the treated plots harbour a lesser number of FAW larvae (Stanley 2004).
The results of insecticidal residues of Barazide® were in the line of Malhat et al. (2013, 2014), who substantiated that the half life of novaluron and emamectin benzoate in tomato were 2.08 and 2.50 days, respectively with 93 to 99% recoveries. In addition, the residue of lambda cyhalothrin was found to be decline very quickly in vegetables with half life period of 3.12 days (Malhat et al. 2016), whereas flubendiamide residues were at BDL in cardamom capsules and soil after the foliar application at 96 g a.i. ha−1 (Vinothkumar et al. 2008). Although many factors like physical and chemical characteristics of pesticides, method and site of application etc. govern the fate of pesticide in plants (Brady et al. 2006); spinetoram exhibited a smaller half life of 2.71 days in tomato with recoveries between 88.81 and 95.41% in the findings of Hafez et al. (2016), and corroborates the present investigation.
It can be concluded that the FAW attack in maize is expanding and, as such, require close monitoring and sustainable management. The risk of maize crop becoming infested with this invasive pest is also closely connected to the lack of potential natural control and the development of insecticide resistance within a shorter period. The results of our research showed the highest efficacy with negligible non-target toxicity through the application of Barazide®, Delegate®, and Ampligo® in a sequence and this module can be recommended for use in the conventional and integrated maize production zone from the point of plant protection, food safety and environmental health. Further investigations should be done to test the hypothesis that current resistance status of FAW to various insecticides may alter the dosages and usage pattern of the recommended molecules at a particular region. Indeed, the present data are crucial for making appropriate decisions about the application of the most effective insecticidal schedule against this invasive species across the globe.
References
(POP) Package of Practice for the management of fall army worm (2019) Directorate of Plant Protection, Quarantine and Storage, Government of India. http://ppqs.gov.in/sites/default/files/pop_sorghum.pdf. Accessed 20 Dec 2019
(SFCPB) Safety of the Food Chain Pesticides and Biocides (2018) European Commission, Directorate General for Health and Food Safety. https://ec.europa.eu/food/sites/food/files/plant/docs/pesticides_mrl_guidelines_wrkdoc_2017-11813.pdf2017-11813.pdf. Accessed 25 Aug 2020
Anastassiades M, Lehotay SJ, Stajnbaher D, Schenck FJ (2003) Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J AOAC Int 86(2):412–431. https://doi.org/10.1093/jaoac/86.2.412
Assefa F, Ayalew D (2019) Status and control measures of fall armyworm (Spodoptera frugiperda) infestations in maize fields in Ethiopia: A review. Cogent Food Agric 5(1):1641902. https://doi.org/10.1080/23311932.2019.1641902
Belay DK, Huckaba RM, Foster JE (2012) Susceptibility of the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), at Santa Isabel, Puerto Rico, to different insecticides. Fla Entomol 95(2):476–478. https://doi.org/10.1653/024.095.0232
Blanco CA, Chiaravalle W, Dalla-Rizza M, Farias J, Garcia-Degano M, Gastaminza G, Pieralisi B (2016) Current situation of pests targeted by Bt crops in Latin America. Curr Opinion Insect Sci 15:131–138. https://doi.org/10.1016/j.cois.2016.04.012
Brady JA, Wallender WW, Werner I, Fard BM, Zalom FG, Oliver MN, Wilson BW, Mata MM, Henderson JD, Deanovic LA, Upadhaya S (2006) Pesticide runoff from orchard floors in Davis, California, USA: a comparative analysis of diazinon and fenvalerate. Agric Ecosyst Environ 115(1–4):56–68. https://doi.org/10.1016/j.agee.2005.12.009
Cloyd RA, Bethke JA (2011) Impact of neonicotinoid insecticides on natural enemies in greenhouse and interiorscape environments. Pest Manag Sci 67(1):3–9. https://doi.org/10.1002/ps.2015
Cruz I, Figueiredo MDLC, Silva RBD, Silva IFD, Paula CD, Foster JE (2012) Using sex pheromone traps in the decision-making process for pesticide application against fall armyworm (Spodoptera frugiperda [Smith] [Lepidoptera: Noctuidae]) larvae in maize. Int J Pest Manag 58(1):83–90. https://doi.org/10.1080/09670874.2012.655702
Deshmukh S, Pavithra HB, Kalleshwaraswamy CM, Shivanna BK, Maruthi MS, Mota-Sanchez D (2020) Field efficacy of insecticides for management of invasive fall armyworm, Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) on maize in India. Fla Entomol 103(2):221–227. https://doi.org/10.1653/024.103.0211
Deshpande C (2019) Pesticide residue in armyworm-hit maize killing cattle? The Times of India. https://timesofindia.indiatimes.com/city/nagpur/pesticide-residue-in-armyworm-hit-maize-killing-cattle/articleshow/71140334.cms. Accessed 21 Aug 2020
El-Wakeil N, Gaafar N, Sallam A, Volkmar C (2013) Side effects of insecticides on natural enemies and possibility of their integration in plant protection strategies. In: Trdan S (ed) Insecticides – Development of safer and more effective technologies, IntechOpen. https://doi.org/10.5772/54199
Foster RE (1989) Strategies for protecting sweet corn ears from damage by fall armyworms (Lepidoptera: Noctuidae) in Southern Florida. Fla Entomol 72(1):146–151. https://doi.org/10.2307/3494981
Ghosal A, Chatterjee ML (2017) Novaluron 5.25% + Emamectin benzoate 0.9% SC against Spodoptera litura on cabbage. Pestis Res J 29(2):135–140
Goergen G, Kumar PL, Sankung SB, Togola A, Tamo M (2016) First report of outbreaks of the fall armyworm Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae), a new alien invasive pest in west and central Africa. PLoS One 11:e0165632
Gomez KA, Gomez AA (1984) Statistical procedures for agricultural research (2 ed.). John Wiley and sons, New York, 680p
Govindan K, Gunasekaran K, Kuttalam S (2013) Emamectin benzoate 5 SG: A safer insecticide to coccinellids predators in cotton ecosystem. African J Agril Res 8(21):2455–2460. https://doi.org/10.5897/AJAR2013.7167
Gupta RK, Raj D, Devi N (2004) Biological and impact assessment studies on Campoletis chlorideae Uchida: A promising solitary larval endoparasitoid of Helicoverpa armigera (Hubner). J Asia-Pacific Entomol 7(2):239–247. https://doi.org/10.1016/S1226-8615(08)60222-8
Gutierrez-Moreno R, Mota-Sanchez D, Blanco CA, Whalon M, Teran-Santofimio H, Rodriguez-Maciel JC, DiFonzo C (2019) Field-evolved resistance of the fall armyworm (Lepidoptera: Noctuidae) to synthetic insecticides in Puerto Rico and Mexico. J Econ Entomol 112(2):792–802. https://doi.org/10.1093/jee/toy372
Hafez AA, Halawa SM, Gameel SMM, Mahmoud MS (2016) Determination of spinetoram residues degradation in tomato fruits using high performance liquid chromatography (HPLC) and QuECHERS method. Egyptian Scientific J Pestis 2(3):8–12
Hardke JT, Temple JH, Leonard BR, Jackson RE (2011) Laboratory toxicity and field efficacy of selected insecticides against fall armyworm (Lepidoptera: Noctuidae). Fla Entomol 94(2):272–278. https://doi.org/10.1653/024.094.0221
Jaleel W, Saeed S, Naqqash MN, Sial MU, Ali M, Zaka SM, Sarwar ZM, Ishtiaq M, Qayyum MA, Aine QU, Anwar A, Sarmad M, Azad R, Latif M, Ahmed F, Islam W, Khan KA, Ghramh HA (2020) Effects of temperature on baseline susceptibility and stability of insecticides resistance against Plutella xyllostella (Lepidoptera: Plutellidae) in the absence of selection pressure. Saudi J Biol Sci 27(1):1–5. https://doi.org/10.1016/j.sjbs.2019.03.004
Koli P, Bhardwaj NR (2018) Status and use of pesticides in forage crops in India. J Pestic Sci 43(4):225–232. https://doi.org/10.1584/jpestics.D18-004
Kundu SS, Chettri D, Chatterjee S, Mukhopadhyay AK (2018) Evaluation of Novaluron 5.25% + Emamectin benzoate 0.9% SC against yellow stem borer (Scirpophaga incertulas) on rice. J Entomol Zool Stud 6(3):789–792
Li Y, Dou Y, An J, Tu X, Lv H, Pan W, Dang Z, Gao Z (2020) Temperature-dependent variations in toxicity of diamide insecticides against three lepidopteran insects. Ecotoxicol 29:607–612. https://doi.org/10.1007/s10646-020-02181-9
Liu Y, Gao Y, Liang G, Lu Y (2017) Chlorantraniliprole as a candidate pesticide used in combination with the attracticides for lepidopteran moths. PLoS One 12(6):e0180255
Malhat FM, Fayz AE, Loutfy NM, Ahmed MT (2013) Residues and dissipation of the pesticide emamectin benzoate under Egyptian field condition: a case study. Toxicol Environ Chem 95(7):1099–1107. https://doi.org/10.1080/02772248.2013.865908
Malhat FM, Loutfy NM, Ahmed MT (2014) Dissipation kinetics of novaluron in tomato, an arid ecosystem pilot study. Toxicol Environ Chem 96(1):41–47. https://doi.org/10.1080/02772248.2014.911538
Malhat FM, Loutfy NM, Ahmed MT (2016) Dissipation pattern and risk assessment of the synthetic pyrethroid lambda-cyhalothrin applied on tomatoes under dryland conditions, a case study. Int J Food Contam 3:8. https://doi.org/10.1186/s40550-016-0029-3
Malik G, Qadir A, Khan HAA (2018) Effect of temperature on the toxicity of biorational insecticides against Sitophilus oryzae (Linnaeus) in stored wheat. Pak J Zool 50(4):1569–1572. https://doi.org/10.17582/journal.pjz/2018.50.4.sc10
Malo EA, Bahena F, Miranda MA, Valle-Mora J (2004) Factors affecting the trapping of males of Spodoptera frugiperda (Lepidoptera: Noctuidae) with pheromones in Mexico. Fla Entomol 87:288–293. https://doi.org/10.1653/0015-4040(2004)087[0288:FATTOM]2.0.CO;2
Montezano DG, Specht A, Sosa-Gomez A, Roque-Specht DR, Sousa-Silva VF, Paula-Moraes JC, Peterson SV, Hunt TE (2018) Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. African Entomol 26(2):286–300. https://doi.org/10.4001/003.026.0286
Nagoshi RN, Dhanani I, Asokan R, Mahadevaswami HM, Kalleshwaraswami CM, Sharanabasappa MRL (2019) Genetic characterization of fall armyworm infesting South Africa and India indicate recent introduction from a common source population. PLoS One 14(5):e0217755. https://doi.org/10.1371/journal.pone.0217755
Neto JEL, Amaral MHP, Siqueira HAA, Barros R, Silva PAF (2016) Resistance monitoring of Plutella xylostella (L.) (Lepidoptera: Plutellidae) to risk-reduced insecticides and cross resistance to spinetoram. Phytoparasitica 44:631–640. https://doi.org/10.1007/s12600-016-0553-y
Padhee AK, Prasanna BM (2019) The emerging threat of fall armyworm in India. Indian Farming 69:51–54
Parsaeyan E, Saber M, Safavi SA, Poorjavad N, Biondi A (2020) Side effects of chlorantraniliprole, phosalone and spinosad on the egg parasitoid, Trichogramma brassicae. Ecotoxicol 29:1052–1061. https://doi.org/10.1007/s10646-020-02235-y
Roy D, Biswas S (2020) Identification, symptoms of damage and integrated management of invasive fall armyworm in maize. Agric Food: E-Newsl 2(7):216–219
Sharanabasappa D, Kalleshwaraswamy CM, Asokan R, Mahadevaswamy HM, Maruthi MS, Pavithro HB, Hegde K, Navi S, Prabhu ST, Goergin G (2018) First report of fall armyworm, Spodoptera frugiperda (J.E. smith) (Lepidoptera: Noctuidae) an alien invasive pest on maize in India. Pest Manag Hort Ecosyst 24:23–29
Shylesha AN, Jalali SK, Gupta A, Varshney R, Venkatesan T, Shetty P, Ojha R, Ganiger PC, Navik O, Subaharan K, Bakthavatsalam N, Ballal CR (2018) Studies on new invasive pest Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) and its natural enemies. J Biol Control 32(3):145–151. https://doi.org/10.18311/jbc/2018/21707
Sisay B, Tefera T, Wakgari M, Ayalew G, Mendesi E (2019) The efficacy of selected synthetic insecticides and botanicals against fall armyworm, Spodoptera frugiperda, in maize. Insects 10:45. https://doi.org/10.3390/insects10020045
Smith JF, Catchot AL (2009) Efficacy of selected insecticides against fall armyworm on corn, 2008. Arthropod Manag Tests 34(1):F18. https://doi.org/10.4182/amt.2009.F18
Stanley J (2004) Studies on baseline toxicity on emamectin and spinosad to Helicoverpa armigera (Hubner) and Spodoptera litura (Fab) and their bio-efficacy on brinjal fruit borers. M.Sc. thesis, Tamil Nadu Agricultural University, Coimbatore-3, India, 90pp
Sudhanan EM, Krishnamoorthy SV, Kuttalam S (2017) Bioefficacy, phytotoxicity, safety to natural enemies and residues of flubendiamide in sugarcane (Saccharum spp. L.) under field conditions. Crop Protec 100:21–28. https://doi.org/10.1016/j.cropro.2017.05.028
Tippannavar PS, Talekar SC, Mallapur CP, Kachapur RM, Salakinkop SR, Harlapur SI (2019) An outbreak of fall armyworm in Indian subcontinent: A new invasive pest on maize. Maydica electronic publication 64:M4
Vinothkumar B, Kumaran N, Boomathi N, Kuttalam S (2008) Determination of harvest time residues of flubendiamide insecticide in cardamom using HPLC. Green Farming 2(3):192–195
Viteri DM, Linares AM, Flores L, Irrizagi A (2018) Efficacy of biological agents and synthetic insecticides to control fall armyworm larvae. Agrotechnol 7:26. https://doi.org/10.4172/2168-9881-C1-031
Worku M, Ebabuye Y (2019) Evaluation of efficacy of insecticides against the fall armyworm Spodoptera frugiperda. Indian J Entomol 81(1):13–15. https://doi.org/10.5958/0974-8172.2019.00076.2
Acknowledgements
Authors would like to thank Project Director, Agriculture Technology Management Agency (ATMA), Murshidabad, and Deputy Director of Agriculture (Admin.), Department of Agriculture, Government of West Bengal, India for providing the required funds to carry out the research work (Grant no.: 237/1(7)/PD/ATMA/MSD/KVK-2019). Additionally authors are also grateful to American English editor for final editing of the manuscript.
Funding
The study was funded by Project Director, Agriculture Technology Management Agency (ATMA), Murshidabad, Department of Agriculture, Government of West Bengal, India (Grant No. 237/1(7)/PD/ATMA/MSD/KVK-2019).
Author information
Authors and Affiliations
Contributions
The work was carried out in collaboration with all authors. Author DR and SB conceived and designed the research work. DR, SB and SM conducted the laboratory and field experiments and collected data. DM and PKS analyzed data. DR and DM wrote the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors have declared that no conflict of interest exists.
Ethical approval
This article does not contain any studies with human or other animal subjects.
Rights and permissions
About this article
Cite this article
Roy, D., Biswas, S., Mondal, D. et al. Efficacy and safety-evaluation of insecticidal modules against Spodoptera frugiperda (Lepidoptera: Noctuidae) and the residues of the most effective schedule in maize. Int J Trop Insect Sci 41, 3155–3166 (2021). https://doi.org/10.1007/s42690-021-00511-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-021-00511-w