Abstract
Worldwide application of synthetic insecticides as a main way of controlling aphids on various crops has resulted in diverse problems such as failures in pest control, negative public health and environmental impacts, and a build-up of resistance against insecticides by insect pests. Entomopathogenic fungi can be used as an alternative to insecticides since they offer the benefit of being environmentally friendly, without the risk of insect pests developing resistance. This study assessed 19 different indigenous fungi in the laboratory for their ability to control Aphis fabae Scopoli at various conidial concentrations (104, 105, 106 and 107 spores/ml) using the detached leaf method. A. fabae adults were reared on potted cowpea in cages. Both the 19 fungus types and their respective conidial concentrations used to treat A. fabae differed significantly (P < 0.001) in their ability to kill the pest. The A. fabae mortality rate increased in line with the increase in the conidial concentration of the fungus type, and Aspergillus flavus Link S18 and S19 performed better than the other fungus types evaluated. Aspergillus flavus S18 and S19 are recommended for further tests in the greenhouse to validate the laboratory results. The fact that the Aspergillus strains isolated and tested were all aflatoxin-producing strains calls for caution regarding their potential impacts on human and animal health. Further studies are recommended to conduct similar experiments using non-aflatoxin-producing strains of A. flavus, in order to determine whether they have similar effects on A. fabae.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aphids are generally serious pests in agricultural and horticultural crops all over the world, causing direct damage by sucking plant sap, and indirect damage through the transmission of more than 300 plant pathogenic viruses, and contamination through honeydew deposits on the surface of plant foliage, thus favoring the growth of sooty mold which reduces photosynthetic surface (Hogenhout et al. 2008; Van Emden and Harrington 2007). Both young (nymphs) and adult aphids feed on plant sap, attacking almost everywhere on plants; leaves, stem buds, flowers and fruits. In so doing, they cause curling or stunted yellow leaves, distorted or deformed flowers and formation of galls on roots and stem on the infested plant (Van Emden and Harrington 2007). Many horticultural crops are affected by aphids in East Africa, including cabbages (Oduor et al. 1996), French beans (Gogo et al. 2014), cucurbits (Nordey et al. 2020), and cowpea (Karungi et al. 1999). Aphis fabae Scopoli (Hemiptera: Aphididae), is particularly challenging in northern Tanzania because of its year-round pressure and its impact on export crops such as French beans and Kalanchoe (Domier et al. 2007). Aphids are reported by Nyambo (2009) and Mureithi et al. (2015) as being among the major pests on agricultural crops in Tanzania and Kenya. In the current study, cowpea, which is among the most preferred hosts by aphids (Mfeka et al. 2019) was used to culture the insects.
Worldwide application of synthetic insecticides as the main way of controlling aphids on various crops has resulted in diverse problems, such as failures in pest control, negative environmental impacts, and a build-up of insecticide resistance by aphids (Foster et al. 2007; Silva et al. 2012). Furthermore, the environmental and health impacts of synthetic pesticides are increasingly raising concerns, thus encouraging agricultural producers to search for and adopt effective alternative control methods such as biological control using natural enemies of pests (Inglis et al. 2001; Lacey et al. 2015).
Among the available biological control agents against insects, entomopathogenic fungi are the most significant pathogens where more than 16 species of fungi are known to naturally infect aphids (Pell et al. 2001; Chen et al. 2008). Entomopathogenic fungi are reported to be effective against all the developmental stages (eggs, larvae, intermediate stages and adults) of various insects (Shi and Feng 2004; Scholte et al. 2005). Since they are organisms that occur naturally, entomopathogenic fungi are regarded as the most promising alternative to the synthetic (chemical) pesticides and they are perceived as being friendlier to the environment than the synthetic pesticides (Butt et al. 2002; Sandhu et al. 2012). Moreover, the target insects are unlikely to develop resistance against them, since their mode of action appears to be less complex compared to their counterparts (Sandhu et al. 2012). Various works on entomopathogenic fungi (research, production, stabilization, formulation and application) have significantly contributed to the commercialization of more than 170 products of fungus-based biopesticides (de Faria and Wraight 2007). The most commonly used products are those based on Beauveria bassiana (Bals.-Criv.) Vuill., Metarhizium anisopliae (Metschnikoff) Sorokin, Isaria fumosorosea Wize, and Beauveria brongniartii (Sacc.) (de Faria and Wraight 2007). Studies have shown that the effectiveness of an entomopathogenic fungus depends on its ability to produce many spores and/or sticky spore surfaces or substances that enhance adhesion of spores to the host surface, its ability to germinate and penetrate the exoskeleton of the insect, its ability to survive digestion after being ingested by the insect (host) and ability to proliferate within the body of the host in order to collapse its immune system so that the insect subsequently dies (Vega et al. 2012; Sandhu et al. 2017). It has been reported that mortality of aphids due to treatment with entomopathogenic fungi increases with increase in spore concentration and the time of exposure (Asi et al. 2009; Sevim et al. 2013).
Although entomopathogenic fungi are increasingly used in Europe, the North America, and in Asia, their availability to growers in Africa is impeded by import restrictions, quality of products, and suitability with climatic conditions. Integrated pest management (IPM), the method which combines several techniques such as biological control, habitat modification, manipulation of cultural practices and use of crop resistant varieties are used to control pests by reducing the harmful effects of chemical pesticides (Dharam and Shankar 2012). IPM concept originated from the ideas about the ill effects of synthetic pesticides to non-targeted organisms including domestic animals and human, published by Carson (1962). Entomopathogenic fungi which are among the agents of biological control can well be included in IPM programs to control various arthropod pests (Allan et al. 2016; Maina et al. 2018).
The infection process of an entomopathogenic fungus starts with its entrance through the cuticle of the host insect (Sevim et al. 2015) occurring both physically and physiologically (enzymatically) (Clarkson and Chamley 1996). The sequence of events in this process occurs as follows: the fungus spores stick and settle on the cuticle of the insect, then the spores germinate and penetrate the cuticle to the haemocoel by forming appressoria, then hyphae develop in the hypodermis and they continue to multiply in the insect body and lymphatic cells and finally cause the death of the insect (Sani et al. 2020). According to Milner (1997), Roy et al. (2010), aphids are highly susceptible to entomopathogenic fungi especially the epizootic fungal diseases than any other arthropods.
This study was undertaken to assess the effectiveness/pathogenicity of 19 different entomopathogenic fungi (indigenous to Tanzania) against the black aphid, A. fabae, at different spore concentrations (104, 105, 106 and 107 conidia/ml) under laboratory conditions. The 19 entomopathogenic fungi were: S1, S2, S3, S4, S5, S6, S7, S8 (Aspergillus flavus), S9, S10, S11, S12, S13, S14, S15 (Aspergillus tamarii), S16, S17 (Aspergillus flavus), S18 (Aspergillus flavus) and S19 (Aspergillus flavus).
Materials and methods
The experiments were conducted from November 2018 to April 2019 in the entomology laboratory at the World Vegetable Center, Eastern and Southern Africa (WorldVeg-ESA) in Arusha, Tanzania (3.37406°S and 36.80560°E).
Plant material and aphid pure culture rearing
Cowpea, Vigna unguiculata (L.) Walp (Fabales: Fabaceae) which is among the hosts of A. fabae (Karungi et al. 1999) were cultured in one litter plastic pots (one plant per pot) in wooden cages, in a plastic film roofed screen house. The sides of the screen house were shielded with an insect-proof net of mesh size 0.6 mm whereas the sides and top of the cages (bottomed with wood) and the cage sleeves were shielded with a 0.3 mm mesh sized net to prevent untargeted insects from getting in as well as preventing the aphids (in the pure aphid culture – next paragraph) from getting out (Harmanto and Salokhe 2006). The cages (2 m long, 1 m wide and 0.8 m high) in the screen house were placed on tables at a height of 0.8 m from the ground.
Similar structures and cowpea potted plants were separately used to rear a pure culture of A. fabae. Black aphid nymphs were collected from cowpea plants in the field within and around World Vegetable Center compounds. They were introduced and reared on potted insect-free cowpea plants in cages in screen houses. The black aphid colonies were purified by shifting the newly born nymphs from the initial cage to new ones on plants that were fresh and free from aphids. From such lots, the experimental colonies were multiplied. When the plants were severely damaged by the aphid, new un-infested plants were placed in the cages and let the aphids shift themselves from the overcrowded plants to the new ones. Finally the dying plants due to aphid damage were removed from the cage and destroyed.
A planting medium of a mixture of loam forest soil and sand at 2:1 (v/v - loam forest soil: sand) was used for planting the cowpea in the pots to imitate the sandy loam soil which is preferred by cowpea (Makoko 2008; Mfeka et al. 2019). Plants were watered once to twice a week depending on the moisture of the soil detected by feel. Temperature and relative humidity (RH) in the cages were 25 ± 3 °C and 60 ± 5% respectively and a photoperiod of 12:12 h (L: D). Plants in one cage were used to raise A. fabae colony while those in other three cages were used as a source of young shoots on which the aphid (A. fabae) were fed during application of entomopathogenic fungi experiments in laboratory.
Fungus collection, isolation and purification
Several indigenous microbial propagules were randomly (informal visits) collected from farmers’ fields within Arumeru District in Arusha Region and within Moshi Rural District in Kilimanjaro Region for 4 days using a Microbiological air sampler (MicroBio-MB1 - Parrett Technical Developments, Bromley, England) pre-loaded with a sterile Petri dish containing Potato Dextrose Agar (PDA) fungal culturing medium. The collected spores/propagules were then incubated at 28 ± 1 °C, 75 ± 2% RH, until fungal growths were observed and matured.
The resulting 19 different fungal growths collected from Arusha and Kilimanjaro Regions (Table 1) were purified by carefully transferring spores using a sterile inoculation needle from the first Petri dish to new ones containing a similar culturing medium (PDA). The new Petri dishes containing PDA were initially sterilized by autoclaving at 121 °C for 15 to 20 min. They were then cultured in an incubator at 28 ± 1 °C and 75% ± 2% RH until fully grown colonies appeared, and the process was repeated until pure colonies were obtained.
After maturation and full sporulation, the conidia were harvested by gently scraping the surface of the cultures with a sterile inoculating loop to dislodge the conidia from the surface of the agar plates. The harvested conidia were stored at 4 °C and 0% RH. To purify the fungal types and determine the viability of the conidia, ten sterile Petri dishes with PDA medium were inoculated with 1 ml of conidial suspension of each type, then incubated for 5 days at 28 ± 1 °C and 75 ± 2% RH.
Assessing the effectiveness of the collected indigenous fungi against aphid
A factorial experiment under completely randomized design with two factors, i.e. type of fungus and concentration of conidial suspension, was used to compare the efficacy of the collected indigenous entomopathogenic fungi against adult A. fabae using the detached leaf method (Yokomi and Gottwald 1988). The experiments were divided into two with regard to the number of fungus types involved as indicated below.
Assessing the effectiveness of all the 19 fungus types collected against aphids
An initial experiment was conducted to compare the efficacy of the 19 isolated fungi (S1, S2, S3, S4, S5, S6, S7, S8 (Aspergillus flavus), S9, S10, S11, S12, S13, S14, S15 (Aspergillus tamarii), S16, S17 (Aspergillus flavus), S18 (Aspergillus flavus) and S19 (Aspergillus flavus) at different conidial concentrations (i.e., 105, 106 and 107 spores/ml) to a control (sterile distilled water), and each treatment (fungus type and conidial concentration) involved ten 3-day-old A. fabae adults and was replicated 10 times. Conidial concentrations were measured using a haemocytometer (Neubaeur chamber, Germany).
Assessing the effectiveness of the best five (out of the 19 collected) fungi against aphids
Based on the results of the first experiment which involved all the 19 fungi collected, five fungus types which were later identified as belonging to Aspergillus genus (A. tamarii Kita (S5), A. flavus Link (S8), A. flavus (S17), A. flavus (S18) and A. flavus (S19)), were then selected for further screening under laboratory conditions (at 28 ± 1 °C, 75 ± 2% RH). The efficacy of the five selected fungi was compared at four conidial concentrations (i.e., 104, 105, 106 and 107 spores/ml) to a control (sterile distilled water), and each treatment (fungus type and conidia concentration) was replicated 10 times. The whole experimental set-up was then repeated three times on different dates (i. e., experiments 1–3).
Young cowpea tip-shoots cultured in a greenhouse were rinsed in tap water for 15 min, washed three times with sterile distilled water, and air-dried under a sterile laminar flow hood. Working under the sterile hood, a blotting paper moistened with sterile distilled water was placed as a liner in the bottom of a sterile Petri dish to maintain a high relative humidity (~90%) throughout the duration of the experiment, to prevent the cowpea shoots from drying out before the end of the experiment. Under the sterile hood, the young cowpea tip-shoots were dipped into a suspension of fungal spores in accordance with the concentration level (treatment – see how they were prepared in the next paragraph) required. Each of these shoots were then placed in the Petri dishes previously lined with moist sterile bloating paper and covered with its lid.
Stock suspensions of fungal spores used as explained above were prepared by adding 20 ml of sterile distilled water to each Petri dish with a pure fungal colony, preceded by gentle scraping on the surface of the cultures, as explained above. The spore suspension was pipetted from the plate and filtered through three layers of cheesecloth. The spore concentration in a mother stock suspension was assessed using a haemocytometer (Neubaeur chamber, Germany) and stock suspension concentrations of 105, 106 and 107 spores/ml. were prepared.
Ten 3-day-old A. fabae adults previously reared on clean and healthy cowpea plants in pots placed in cages in screen house were placed on the leaves dipped in a fungal spore suspension in each formally prepared Petri dishes. As a control, same number of aphids of same age were placed on sterile cowpea leaves that had not been treated with any of the fungus types, but had just been dipped in sterile distilled water.
Data collection
Aphid mortality was recorded daily for up to seven days and the cause of death was confirmed by searching for fungal growths on the dead aphid body under a microscope. If a dead aphid did not show fungal outgrowths of similar characteristics to those of the one applied as the treatment, its death was considered as caused by another factor, or factors, and was therefore discarded.
Genetic identification of fungi and quantification of their aflatoxin production
Samples of the five most effective fungi against A. fabae, as observed under laboratory conditions, were subjected to molecular analysis using sequence data of the ITS region (ITS1 and ITS2 markers) of the nuclear ribosomal DNA (Henry et al. 2000), since it is frequently variable between different isolates of the same species (Gomes et al. 2002). A genetic analysis was conducted by an external laboratory with ISO/CEI 17025 certification (ADNId, Montferrier-sur-Lez, France). The procedure was followed by sequencing (Next Generation Sequencing – NGS) on a MiSeq (Illumina). Samples of the five selected strains were also sent to an external laboratory at Nelson Mandela African Institute of Science and Technology (NM-AIST) in Arusha, Tanzania, for analysis to quantify their production of aflatoxins (G1, G2, B1 and B2) using HPLC procedure.
Data analysis
Kruskal Wallis tests were used to assess the effect of the fungus types and their conidial concentrations on aphids as the data did not follow normal distribution. When significant differences were established, post-hoc analyses were carried out to compare fungus type efficiency for the same conidial concentration and to compare the efficiency of each fungus type at different conidial concentrations using Fisher’s least significant difference and the Holm method to adjust the P value. All statistical analyses were performed using R software with the agricolae package (de Mendiburu and de Mendiburu 2013). A statistical analysis of the genomic data was conducted using computer software and blasting into the on-line genomic databases.
Results and discussion
Nineteen different isolated fungus types collected from the surveyed areas of the Arusha and Kilimanjaro Regions are presented in Table 2. Almost all of the 19 fungal isolates showed a resemblance to the genus Aspergillus when the morphology of the colonies (colony structure) was observed in the cultures. This observation suggested that the fungal propagules collected in this study were possibly dominated by Aspergillus species which raised the need for molecular identification of the fungi that caused higher mortality on A. fabae to confirm or refute the initial results observed in the colony cultures.
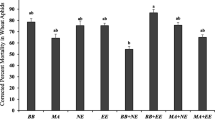
The fungal types and their respective conidial concentrations used to treat A. fabae differed significantly (P < 0.001) in their ability to kill A. fabae, both in the initial experiments with all the 19 fungus types collected (Table 2) and with the five (out of the 19 types collected) most efficient fungi re-tested separately thereafter (Table 3). Among the 19 fungus types collected and tested, strains S8, S15, S17, S18, and S19 were selected for further experiments (Table 3) since they were the most efficient to A. fabae with mortality percentages of more than 64, 71, and 78% at conidial concentrations of 105, 106, and 107 spores/ml, respectively (Table 2). S8, S17, S18 and S19 were all collected from Madira Farm in Arusha Region while S15 was collected from Kibosho in Kilimanjaro Region (Table 1). Molecular analysis revealed that out of the five selected fungi, four which originated from Madira Farm were found to be strains of the species A. flavus and one which originated from Kibosho belonged to the species A. tamarii.
To date, approximately 750 fungus species from about 90 genera have been documented as entomopathogenic, although only a few of these species are being developed as mycopesticides against insect pests (de Faria and Wraight 2007). Previously, Seye et al. (2014) reported the first strains of A. flavus and Aspergillus clavatus Long being pathogenic to aphids in laboratory bioassays, with a higher mortality of the pea aphid, Acyrthosiphon pisum (Harris) caused by A. flavus than that caused by A. clavatus and Metarhizium Anisopliae (Metschn.).
In the current study, the conidial concentration of 107 spores/ml gave higher A. fabae mortality than the rest of the concentrations (106, 105 and 104 spores/ml.) in all experiments.
Similar results were reported in Turkey by Sevim et al. (2013), where five conidial concentration levels (108, 107, 106, 105 and 104 spores/ml) of the entomopathogenic fungus B. bassiana against Corythucha ciliatawere (Say) (Hemiptera: Tingidae) showed that the higher the conidial concentration, the higher was the mortality of the insect. Aspergillus flavus S18 and S19 were found to be the most effective in controlling A. fabae, with mortality rates over 22, 46, 60, and 78% at 104, 105, 106, and 107 spores/ml, respectively, in all experiments in the current study.
Aspergillus flavus and A. tamarii are members of the Aspergillus section Flavi, which are generally regarded as toxin (aflatoxins and other mycotoxins) producers and are found mainly in humid climates (Marin et al. 2013; Frisvad et al. 2019; Norlia et al. 2019).
It was further confirmed in the current study that all the strains of A. flavus and the species A. tamarii identified produce aflatoxins in various amounts, i.e. from 8.4 to 135.2 μg.kg−1 (Table 4). Aflatoxins are toxic secondary metabolites produced by certain fungi belonging to the genus Aspergillus. The two most important aflatoxin producers are A. flavus and A. parasiticus (Gourama and Bullerman 1995), which are commonly found in soil and can contaminate agricultural crops, both in the field and after harvest as well as processed items originating from agricultural products (Visconti and Perrone 2008). Aflatoxins cause various ill effects in humans and animals, such as aflatoxicosis outbreaks and death (Lewis et al. 2005), chronic health risks such as cancer, immune suppression and child stunting (Guchi 2015; Gong et al. 2016). For these reasons, the findings in this study cannot be recommended for use as a technology to control A. fabae in agricultural production settings, but rather they may be useful for scientific settings. It is worth noting that there are A. flavus strains that do not produce aflatoxins (Ehrlich 2014). Interestingly, such strains have been found to outcompete the toxin-producing strains when they co-occur, hence they have been developed and used as a biological control agent against aflatoxin (the toxin-producing strains) and have proved to be highly effective (Cotty and Bhatnagar 1994). In Tanzania, a product called Aflasafe TZ01 developed by the International Institute of Tropical Agriculture (IITA) has been tested and registered for use as a bio-control agent against aflatoxin (Moral et al. 2020). This product can be tested to find out whether it is effective in causing mortality to aphids.
Conclusions
Out of 19 fungal isolates from fields in Tanzania, two strains of A. flavus were found to be the most effective in controlling A. fabae under laboratory conditions. Our study confirmed previous reports on the efficiency of Aspergillus in controlling different types of aphid. However, the results obtained in the current study are challenged by the fact that the five Aspergillus spp. isolated and tested were all aflatoxin-producing strains, so they cannot be recommended as a technology to be transferred to producers due to the toxicity of aflatoxin to human, animal and environment. It is therefore recommended that similar experiments be conducted using the non-aflatoxin-producing strains, in order to determine whether they have similar effects on A. fabae. However, it is believed that the results of the current study provide an indirect scientific contribution towards the worldwide efforts in searching for alternatives to synthetic pesticides to manage insect pests.
Data availability
The data and materials used in this study are available for references.
References
Allan M, Christian U, Nguya MK, Sunday E (2016) Integration of entomopathogenic fungi as biopesticide for the management of cowpea aphid (Aphis craccivora Koch). Afr J Hort Sci 9:14–31
Asi MR, Bashir MH, Afzali M, Imran S (2009) Effect of conidial concentration of entomopathogenic fungi on mortality of cabbage aphid, Brevicoryne brassicae L. Pak J Life Soc Sci 2:175–180
Butt TM, Jackson C, Magan N (2002) Fungi as biocontrol agents: progress, problems and potential. Plant Pathol 51:518–521
Carson R (1962) Silent Spring. Houghton Mifflin Company, Boston
Chen B, Li ZY, Feng MG (2008) Occurrence of entomopathogenic fungi in migratory alate aphids in Yunnan Province of China. BioControl 53:317–326. https://doi.org/10.1007/s10526-006-9063-z
Clarkson JM, Chamley AK (1996) New insights into the mechanisms of fungal pathogenesis in insects. Trends Microbiol 4(5):197–203
Cotty PJ, Bhatnagar D (1994) Variability among atoxigenic Aspergillus flavus strains in ability to prevent aflatoxin contamination and production of aflatoxin biosynthetic pathway enzymes. Appl Environ Microbiol 60:2248–2251
de Faria MR, Wraight SP (2007) Mycoinsecticides and mycoacaricides: a comprehensive list with worldwide coverage and international classification of formulation types. Biol Control 43:237–256
de Mendiburu F, de Mendiburu MF (2013) Agricolae: statistical procedures for agricultural research. R Package Version 1.1–4. http://CRAN.R-project.org/package=agricolae
Dharam PA, Shankar U (2012) History, overview and principles of ecologically-based Pest management. In: Dharam PA, Shankar U (eds) Integrated pest management: principles and practice. CAB International, Wallingford
Domier LL, Steinlage TA, Hobbs HA, Wang Y, Herrera-Rodriguez G, Haudenshield JS, McCoppin NK, Hartman GL (2007) Similarities in seed and aphid transmission among soybean mosaic virus isolates. Plant Dis 91:546–550
Ehrlich KC (2014) Non-aflatoxigenic Aspergillus flavus to prevent aflatoxin contamination in crops: advantages and limitations. Front Microbiol 5:50. https://doi.org/10.3389/fmicb.2014.00050
Foster SP, Devine G, Devonshire AL (2007) Insecticide resistance. In: Van Emden HF, Harrington R (eds) Aphids as crop pests. CAB International, London. 716 pp
Frisvad JC, Hubka V, Ezekiel CN, Hong S-B, Nováková A, Chen AJ, Arzanlou M, Larsen TO, Sklenár F, Mahakarnchanakul W, Samson RA, Houbraken J (2019) Taxonomy of Aspergillus section Flavi and their production of aflatoxins, ochratoxins and other mycotoxins. Stud Mycol 93:1–63. www.studiesinmycology.org
Gogo EO, Saidi M, Ochieng JM, Martin T, Baird V, Ngouajio M (2014) Microclimate modification and insect pest exclusion using Agronet improve pod yield and quality of French bean. Hortscience 49(10):1298–1304
Gomes EA, Kasuya MCM, de Barros EG, Borges AC, Araújo EF (2002) Polymorphism in the internal transcribed spacer (ITS) of the ribosomal DNA of 26 isolates of ectomycorrhizal fungi. Genet Mol Biol 25:477–483
Gong YY, Watson S, Routledge MN (2016) Aflatoxin exposure and associated human health effects, a review of epidemiological studies. Food Saf 4:14–27
Gourama H, Bullerman LB (1995) Aspergillus flavus and Aspergillus parasiticus: aflatoxigenic fungi of concern in foods and feeds: a review. J Food Protect 58(12):1395–1404
Guchi E (2015) Implication of aflatoxin contamination in agricultural products. Am J Food Nutr 3(1):12–20
Harmanto HJT, Salokhe VM (2006) Influence of insect screens with different mesh sizes on ventilation rate and microclimate of greenhouses in the humid tropics. Agricultural Engineering International: the CIGR Ejournal. Manuscript BC 05 017. Vol. VIII
Henry T, Iwen PC, Hinrichs SH (2000) Identification of Aspergillus species using internal transcribed spacer regions 1 and 2. J Clin Microbiol 38:1510–1515
Hogenhout SA, Ammar E-D, Whitefield AE, Redinbaugh MG (2008) Insect vector interactions with persistently transmitted viruses. Annu Rev Phytopathol 46:327–359
Inglis GD, Goettel MS, Butt TM, Strasser H (2001) Use of hyphomycetous fungi for managing insect pests. In: Butt TM, Jackson C, Magan N (eds) Fungi as biocontrol agents: progress, problems, and potential. CAB International, Wallingford, pp 23–69
Karungi J, Nampala MP, Adipala D, Kyamanywa S, Ogenga-Latigo MW (1999) Population dynamics of selected cowpea insect pests as influenced by different management practices in eastern Uganda. Afr Crop Sci J 7(4):487–495
Lacey L, Grzywacz D, Shapiro-Ilan D, Frutos R, Brownbridge M, Goettel M (2015) Insect pathogens as biological control agents: back to the future. J Invertebr Pathol 132:1–41
Lewis I, Onsongo M, Njapau H, Schurz-Rogers H, Luber G, Kieszak S, Nyamongo J, Backer L, Dahiye AM, Misore A, DeCock K, Rubin C (2005) Aflatoxin contamination of commercial maize products during an outbreak of acute aflatoxicosis in eastern and Central Kenya. Environ Health Perspect 113:1763–1767
Maina UM, Galadima IB, Gambo FM, Zakaria D (2018) A review on the use of entomopathogenic fungi in the management of insect pests of field crops. J Entomol Zool Stud 6(1):27–32
Makoko BR (2008) Assessment of cowpea, Vigna unguiculata (L) Walp. cultivars against Alectra vogelii (Benth) (Witchweed) collected from Dodoma, Tanzania. Dissertation, Sokoine University of Agriculture, Morogoro, Tanzania
Marin S, Ramos AJ, Cano-Sancho G, Sanchis V (2013) Mycotoxins: occurrence, toxicology, and exposure assessment. Food Chem Toxicol 60:218–237
Mfeka N, Mulidzi RA, Lewu FB (2019) Growth and yield parameters of three cowpea (Vigna unguiculata L. Walp) lines as affected by planting date and zinc application rate. S Afr J Sci 115(1/2), Art. #4474, 8 pages. https://doi.org/10.17159/sajs.2019/4474
Milner RJ (1997) Prospects for biopesticides for aphid control. Entomophaga 42:227–239
Moral J, Garcia-Lopez MT, Camiletti BX, Jaime R, Michailides TJ, Bandyopadhyay R, Ortega-Beltran A (2020) Present status and perspective on the future use of aflatoxin biocontrol products. Agronomy 10:491. https://doi.org/10.3390/agronomy10040491
Mureithi DM, Mworia JK, Meyhoefer R, Murungi LK, Losenge T, Akutse KS, Ekesi S, Fiaboe KKM (2015) Survey for pest and natural enemies of Amaranth and African nightshades in Kenya and Tanzania. Tropentag, September 16–18, 2015, Berlin, Germany. https://www.researchgate.net/publication/283730494_Survey_for_Pest_and_Natural_Enemies_of_Amaranth_and_African_Nightshades_in_Kenya_and_Tanzania
Nordey T, Ochieng J, Ernest Z, Mlowe N, Mosha I, Fernandes P (2020) Is vegetable cultivation under low tunnels a profitable alternative to pesticide use? The case of cabbage cultivation in northern Tanzania. Crop Prot 134:105–169
Norlia M, Jinap S, Nor-Khaizura MAR, Radu S, Samsudin NIP, Azri FA (2019) Aspergillus section Flavi and aflatoxins: occurrence, detection, and identification in raw peanuts and peanut-based products along the supply chain. Front Microbiol 10:2602. https://doi.org/10.3389/fmicb.2019.02602
Nyambo B (2009) Integrated pest management plan (IPMP). The United Republic of Tanzania, agricultural sector development program (ASDP). AFSP, Dar es Salaam, Tanzania. https://www.kilimo.go.tz/uploads/IPMP_Plan.pdf
Oduor GI, Lohr B, Seif AA (1996) Seasonality of major cabbage pests and incidence of their natural enemies in Central Kenya, pp 37–42. In: Sivapragasam A, Kole WH, Hassan AK, Lim GS (eds) The Management of diamondback moth and other crucifer pests: Proceedings of the 3rd International Workshop. 29th October–1st November 1996, Kuala Lumpur, Malaysia
Pell JK, Eilenberg J, Hajek AE, Steinkraus DC (2001) Biology, ecology and pest management potential of entomophthorales. In: Magan N, Butt TM, Jackson C (eds) Fungi as biocontrol agents. CAB International, Wallingford, pp 71–153
Roy HE, Brodie EL, Chandler D, Goettel MS, Pell JK, Wajnberg E, Vega F (2010) Deep space and hidden depths: understanding the evolution and ecology of fungal entomopathogens. Biol Control 55:1–6
Sandhu SS, Sharma AK, Beniwal V, Goel G, Batra P, Kumar A, Jaglan S, Sharma A, Malhotra S (2012) Myco-biocontrol of insect pests: factors involved, mechanism, and regulation. J Pathog Article ID 126819, 10 pages. https://doi.org/10.1155/2012/126819
Sandhu SS, Shukla H, Aharwal RP, Kumar S, Shukla S (2017) Efficacy of entomopathogenic fungi as green pesticides: current and future prospects. In: Panpatte D, Jhala Y, Vyas R, Shelat H (eds) Microorganisms for green revolution. Microorganisms for sustainability, vol 6. Springer, Singapore
Sani I, Ismail SI, Abdullah S, Jalinas J, Jamian S, Saad N (2020) A review of the biology and control of whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae), with special reference to biological control using entomopathogenic fungi. Insects 11:619. https://doi.org/10.3390/insects11090619
Scholte E-J, Ng'Habi K, Kihonda J, Takken W, Paaijmans K, Abdulla S, Killeen GF, Knols BG (2005) An entomopathogenic fungus for control of adult African malaria mosquitoes. Science 308:1641–1642
Sevim A, Demir I, Sönmlez E, Kocacevik S, Demirbag Z (2013) Evaluation of entomopathogenic fungi against the sycamore lace bug, Corythucha ciliata (say) (Hemiptera: Tingidae). Turk J Agric For 37:595–603
Sevim A, Sevim E, Demirbağ Z (2015) General biology of entomopathogenic fungi and their potential to control pest species in Turkey (Entomopatojenik fungusların genel biyolojileri ve Türkiye’de zararlı böceklerin mücadelesinde kullanılma potansiyelleri). Erzincan Üniversitesi Fen Bilimleri Enstitüsü Dergisi 8(1):115–147
Seye F, Bawin T, Boukraa S, Zimmer JY, Ndiaye M, Delvigne F, Francis F (2014) Effect of entomopathogenic Aspergillus strains against the pea aphid, Acyrthosiphon pisum (Hemiptera: Aphididae). Appl Entomol Zool 49:453–458
Shi WB, Feng MG (2004) Lethal effect of Beauveria bassiana, Metarhizium anisopliae, and Paecilomyces fumosoroseus on the eggs of Tetranychus cinnabarinus (Acari: Tetranychidae) with a description of a mite egg bioassay system. Biol Control 30:165–173
Silva AX, Jander G, Samaniego H, Ramsey JS, Figueroa CC (2012) Insecticide resistance mechanisms in the green peach aphid, Myzus persicae (Hemiptera: Aphididae) I: a transcriptomic survey. PLoS One 7(6):e36366. https://doi.org/10.1371/journal.pone.0036366
Van Emden HF, Harrington R (2007) Aphids as crop pests. CABI, Cromwell Press, Trowbridge. 717pp. ISBN-13: 978 0 85199 819 0. www.cabi.org
Vega FE, Meyling NV, Luangsa-ard JJ, Blackwell M (2012) Fungal entomopathogens. Insect Pathol 2:171–220
Visconti A, Perrone G (2008) The EU MycoGlobe project: global integration of mycotoxin and toxigenic fungal research for enhanced food safety. In: Leslie JF, Bandyopadhyay R, Visconti A (eds) Mycotoxins: detection methods, management, public health and agricultural trade. CAB International, Cromwell Press, Trowbridge, pp 3–9
Yokomi R, Gottwald T (1988) Virulence of Verticillium lecanii isolates in aphids determined by detached-leaf bioassay. J Invert Pathol 51:250–258
Funding
This research was financially supported by GIZ (German Corporation for International Cooperation) through a BMZ grant (12.1003.8-204.11) and the long-term strategic donors to the World Vegetable Center: Taiwan, the Foreign, Commonwealth & Development Office (FCDO) from the UK government, United States Agency for International Development (USAID), Australian Centre for International Agricultural Research (ACIAR), Germany, Thailand, Philippines, Korea, and Japan. Beneficiary companies: Kwekerij Lankhaar B.V. and Multiflower Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Code availability
Not applicable.
Ethics approval
Not applicable.
Consent to participate
All authors were contented to participate in this study.
Consent to publication
All authors are contented with publication of this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Boni, S.B., Mwashimaha, R.A., Mlowe, N. et al. Efficacy of indigenous entomopathogenic fungi against the black aphid, Aphis fabae Scopoli under controlled conditions in Tanzania. Int J Trop Insect Sci 41, 1643–1651 (2021). https://doi.org/10.1007/s42690-020-00365-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-020-00365-8