Abstract
Aphids (Homoptera: Aphididae) are sap-sucking insect pests that feed on several plants of agronomical importance. Entomopathogenic fungi are valuable tools for potential aphid control. As part of a selection process, laboratory bioassays were carried with five different concentrations of Aspergillus clavatus (Desmazières), Aspergillus flavus (Link) and Metarhizium anisopliae ((Metschnikoff) Sorokin) spores against the pea aphid, Acyrthosiphon pisum (Harris). Aspergillus isolates induced higher mortalities than M. anisopliae, which is a well-known entomopathogen in the literature. Lethal concentrations (LC50 and LC90) were 1.23 × 103 and 1.34 × 107 spores/ml for A. flavus, 4.95 × 102 and 5.65 × 107 spores/ml for A. clavatus, and 3.67 × 103 and 9.71 × 107 spores/ml for M. anisopliae 5 days after treatment. Mycelia development and sporulation on adult cadavers were observed 48 h after incubation. The intrinsic growth rate of A. pisum decreased with increased spore concentration for all fungal strains, suggesting an increase in pathogen fitness related to a consumption of host resources. In conclusion, Aspergillus species could be useful in aphid control as pest control agents despite their saprophytic lifestyle. This is also to our knowledge the first report of A. clavatus and A. flavus strains pathogenic to aphids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Acyrthosiphon pisum (Harris) aphid (Homoptera: Aphididae), also called pea aphid, is a pest of many cultivated and wild plants such as the worldwide crop Vicia faba (L.), limiting their growth. These aphids have also been reported to be a vector of some viral diseases (Brault et al. 2010; Emden and Harrington 2007). Different strategies have been employed to control these pests. The most widely used methods are physical, chemical and more recently an integrated approach that includes biological control using many kinds of organisms as parasitoids and pathogens. Entomopathogenic fungi are well known to be effective against insects. Their pathogenicity depends on the fungal species and strain virulence. In the latter case, substrate and culture methods are of primary importance for spore effectiveness by increasing or decreasing the secretion of insecticidal compounds recognized as virulence factors (Shah et al. 2005). In this context, agricultural by-products could be used as inexpensive media with high spore yield production (Sahayaraj and Namasivayam 2008). Various entomopathogenic fungi such as Lecanicillium lecanii (Zare and Gams) (Jung et al. 2006), Beauveria bassiana ((Balsamo) Vuillemin) (Sivasundaram et al. 2007) and Metarhizium anisopliae ((Metschnikoff) Sorokin) (Dong et al. 2007) have been effectively used to control aphids, lepidopteran larvae and other pests. Vu et al. (2007) also demonstrated the pathogenicity of many entomopathogenic fungi such as M. anisopliae against aphids. Many other studies have shown the pathogenicity of fungi against aphids (Hall 1979, 1980; Khan et al. 1990), but by direct application on the cuticle or by leaf treatment. However, because of the high growth rates of aphid populations, it is unlikely that they can be fully controlled in all cases. Due to emerging insecticide resistances, the research into biological control of aphids still continues. In this present work, we showed the effectiveness of three fungal isolates, M. anisopliae, Aspergillus flavus (Link) and A. clavatus (Desmazières), regarding the adult mortality and population growth rate by directly treating V. faba plants infected with A. pisum aphids in laboratory conditions.
Materials and methods
Fungal cultures
All the fungal strains were identified by the Mycothèque de l’Université catholique de Louvain (MUCL, Belgium). M. anisopliae (Seye et al. 2012, 2013) and A. clavatus (Seye and Ndiaye 2008; Seye et al. 2009; accession no. MUCL 55275) were recently isolated from Oedaleus senegalensis (Krauss) (Orthoptera: Acrididae). A. flavus (accession no. MUCL 55276) was isolated from Agriotes lineatus (L.) larvae (Coleoptera: Elateridae). All these isolates were cultured in 250-ml Erlenmeyer flasks on a solid-state substrate constituted by 5 g wheat bran supplemented with 20 ml of a nutrient solution (1 % peptone, 1 % yeast extract, 0.005 % chloramphenicol) and previously sterilized at 121 °C for 20 min. Seven days after, fungal masses produced on the media were washed with 150 ml distilled water containing 0.05 % Tween 80 on a rotary shaker (150 rpm) for 2 h. These solutions were then filtered and centrifuged (8000 rpm, 15 min) to remove the conidia. For each strain, the conidia were diluted in sterilized water containing 0.05 % Tween 80, and final suspensions were adjusted to 103, 104, 105, 106 and 107 spores per ml using a hemocytometer (Thoma®).
Aphid rearing
A. pisum aphids were isolated on Phaeolus vulgaris L. (France, 2011) and reared in laboratory conditions on ordinary broad bean (V. faba) plants growing in perlite/vermiculite mix pots. Rearing conditions were a 16L:8D photoperiod, 75 % relative humidity and 25 ± 2 °C temperature.
Bioassays
For each conidial suspension, 20 adult female aphids were dropped on a young plant in a pot (10 cm diameter) surrounded by a transparent plastic sleeve closed at the ends with muslin and directly treated. Each fungal strain was applied (1.6 ml of conidial suspension) with a dedicated sprayer (Di Martino spa—Conico 1000) to 20 cm with the top of the plant. This volume was sufficient to cover the entire system without impacting aphid viability. The control aphids were treated only with distilled water containing 0.05 % Tween 80. Bioassay conditions were a 16L:8D photoperiod, 75 % relative humidity and 25 ± 2 °C temperature. Aphid mortality and nymphs produced were recorded daily for 5 days after inoculation. All newborn nymphs and dead adults were removed. The cadavers were transferred to plates with moist filter paper in order to allow fungal filament emergence and placed in room conditions as previously described. Each treatment was made in four replicates of 20 adult female aphids with a new conidial suspension in different time periods.
Statistical analysis
The lethal concentrations (LC50 and LC90) were calculated by regression analysis. Aphid mortalities were corrected using Abbott’s formula (Abbott 1925), and corrected mortality proportions were linearized using logit transformation (Dagnelie 1970): \( \log {\text{it}} \left( P \right) = \ln (P/(1 - P)) \). A single linear regression was used for modeling the relationship between logit-transformed mortality and logarithm-transformed values of fungal concentrations as explanatory variable: \( \log {\text{it(}}P )= {\text{slope}} \times \ln ({\text{concentration}}) + \text{int} {\text{ercept}} \). The relationship between aphid mortality and spore concentrations was assessed considering Snedecor-F distribution and p values. Reliability of the regression coefficients was assessed using Student’s t test.
The lethal times (LT50) were calculated by Kaplan-Meier analysis, and the log-rank test (providing the chi-square and p value) was carried out to check for significant differences between treated and control aphids.
The number of nymphs produced daily per female aphid was expressed as intrinsic growth rate (Wyatt and White 1977) and subjected to the ANOVA-1 test to check for significant differences between treated and untreated aphids for each time interval.
All analyses were performed with Statistica 9 software. In all the cases, results were considered statistically significant when the p value of the analysis was lower than 5 % (p < 0.05).
Results and discussion
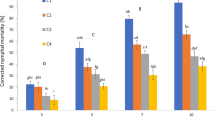
Aphid mortality was monitored daily up to 5 days (four replicates). Control mortalities ranged from 3.75 (day 1) to 17.50 % (day 5) for A. flavus, from 2.5 to 12.5 % for A. clavatus and from 3.75 to 10.00 % for M. anisopliae. Corrected percent mortality increased with time interval (day 1 to day 5) and spore concentration (103 to 107 spores/ml). At day 5, mortality of aphids ranged between 49.90–90.90 % for A. flavus, 51.47–84.06 % for A. clavatus and 30.37–78.05 % for M. anisopliae depending on the spore concentration (Fig. 1). Corresponding lethal concentrations (LC50 and LC90) were, respectively, 1.23 × 103 and 1.34 × 107 (F (1,17) = 59.17; p < 0.001), 4.95 × 102 and 5.65 × 107 (F (1;17) = 20.44; p < 0.001) and 3.67 × 103 and 9.71 × 107 (F (1,18) = 26.92; p < 0.001) spores/ml (Table 1). At the highest concentration of 107 spores/ml, lethal times (LT50) were reached, respectively, with A. flavus at day 2.50 (\( \chi_{(0.95,1)}^{2} \) = 99.68; p < 0.001), with A. clavatus at day 2.85 (\( \chi_{(0.95,1)}^{2} \) = 92.70; p < 0.001) and with M. anisopliae at day 3.26 (\( \chi_{(0.95,1)}^{2} \) = 80.34; p < 0.001). It was demonstrated that there were rapid declines in aphid populations for 5 days. Regarding the lethal times and concentrations, fungal strains would not have the same pathogenicity. Aspergillus isolates induced higher mortalities than M. anisopliae, which is a well-known entomopathogen in the literature. These variations might be due to the differences in the virulence of the fungal isolates, the host species or the method used for fungal application. For example, differences in the virulence of four M. anisopliae isolates was shown against Aphis craccivora (Koch) with LC50 ranging from 3.1 × 105 to 7.4 × 106 spores/ml (Ekesi et al. 2000). Similarly, Loureiro and Moino (2006) showed susceptibility differences in Myzus persicae (Sulzer) and Aphis gossypii (Glover) treated with 106 spores/ml of the same M. anisopliae isolate with LT50 of 2.91 and 3.9 days after treatment. Regarding other studies, our results are similar to those of Saranya et al. (2010) against A. craccivora adults for the same treatment time. This was not the case for Yokomi and Gottwald (1988) who reported 100 % mortality of three aphid species [M. persicae, A. gossypii and Aphis citricola (Van Der Goot)] with 106–107 concentrations of five isolates of L. lecanii and Hirsutella thompsonii (Fisher) after 4-day treatment.
Postmortem observations revealed mycelia development (corresponding to the inoculated strain) around the dead treated aphids 48 h after incubation in petri dishes for each fungal isolate (Fig. 2). No mycelia appeared from dead control aphids. This suggested that conidia adhered to aphid’s cuticle when sprayed and led to their death. Even though Aspergillus species such as A. clavatus and A. flavus are saprophytic fungi, the isolates used in this study might have completed an infectious cycle on aphids as previously reported for other insects. Previously, microscopic observations revealed that A. clavatus adhered to and penetrated the cuticle of mosquito larvae (Diptera: Culicidae). Surviving Culex quinquefasciatus (Say) larvae that reached the pupal stage produced infected adults (Seye et al. 2009). Similary, surface-inoculated A. flavus completed its life cycle on Bombyx mori (L.) larvae in 6 to 7 days including hemocoele invasion (Kumar et al. 2004). Moreover, mycelia development and sporulation observed on dead adults could promote fungal persistence in the environment. Aphid reproduction is very fast, and densities could increase rapidly. The autodissemination of spores between adults and their nymphs could be raised as an objective study since the high densities of aphids could increase the contact possibilities among them and consequently horizontal transmission.
Non-treated Acyrthosiphon pisum (a) and treated with Aspergillus clavatus (b), Metarhizium anisopliae (c) and Aspergillus flavus (d) 48 h after incubation on wet paper. Filaments (arrows) appeared around the aphid cuticle. Compared to the control (a), the treated adults were attacked by fungi. The fungal development was more effective with A. clavatus (b) than M. anisopliae (c) and less with A. flavus (d). h head, th thorax, ab abdomen
Untreated adults produced more nymphs than treated adults. The production of A. pisum nymphs decreased with increased conidial concentration for all fungal strains (Fig. 3). The ANOVA-1 test showed that the intrinsic growth rates varied significantly from untreated adults on day 5 (p = 0.016) for A. flavus, on days 4 (p = 0.003) and 5 (p < 0.001) for A. clavatus and on days 4 (p = 0.005) and 5 (p = 0.011) for M. anisopliae. Many studies showed that entomopathogenic fungi could affect the fecundity of insect species (Castillo et al. 2000; Mulock and Chandler 2001; Quesada-Moraga et al. 2004; Scholte et al. 2006). Our results are consistent with Baverstock et al. (2006), which also revealed that Pandora neoaphidis [(Remaudière and Hennebert) Humber] and B. bassiana infection appears to have direct effects on adult A. pisum reproduction, but no indirect effects on the fitness of their progeny. By contrast, Wang and Knudsen (1993) did not find similar effects with B. bassiana against the Russian wheat aphid [Diuraphis noxia (Kurdyumov)]. Our results are consistent with the hypothesis that a reduction in aphid fecundity may be related to an increase in pathogen fitness as aphid resources are used for mycelia development and conidia production (Baverstock et al. 2006).
Aspergillus species display a wide diversity of lifestyles including in clinical, industrial and agricultural environments; some of them may be opportunistic pathogens of a wide range of organisms including agricultural pests (Gibbons and Rokas 2012). To our knowledge, A. clavatus and A. flavus were reported here for the first time to be pathogenic against aphids. Toxicity tests and postmortem observations suggested that Aspergillus species could be useful in aphid control as pest control agents. However, these saprophytic fungi not target only insects, but can also affect immunodepressed humans, mammals and birds (Tell 2005). Non-aflatoxin-producing and non-toxigenic A. flavus strains are currently studied in biological control to reduce preharvest contamination of crops with aflatoxin (Ehrlich 2014). Their effects on non-targeted organisms including human health should be investigated.
In conclusion, the adult A. pisum aphid was susceptible to the entomopathogenic fungi A. clavatus, A. flavus and M. anisopliae. Adult mortality increased with time interval and concentration. The reproductive potential of these aphids also decreased with increased concentration. A. clavatus and A. flavus were here reported for the first time to be pathogenic against aphids. These results suggest that these fungi may be candidates for aphid control. However, toxin extraction, identification and investigation on non-targeted organisms should be performed before use in biological control.
References
Abbott WS (1925) A method of computing effectiveness of insecticides. J Econ Entomol 18:265–267
Baverstock J, Roy HE, Clark SJ, Alderson PG, Pell JK (2006) Effect of fungal infection on the reproductive potential of aphids and their progeny. J Invertebr Pathol 91:136–139
Brault V, Uzest M, Monsion B, Jacquot E, Blanc S (2010) Aphids as transport devices for plant viruses. C R Biol 333:524–538
Castillo MA, Moya P, Hernández E, Primo-Yúfera E (2000) Susceptibility of Ceratitis capitata Wiedemann (Diptera: Tephritidae) to entomopathogenic fungi and their extracts. Biol Control 19:274–282
Dagnelie P (1970) Théorie et méthodes statistiques: applications agronomiques. Les méthodes d’inférence statistique, vol 2. Duculot, Gembloux
Dong C, Zhang J, Chen W, Huang H, Hu Y (2007) Characterization of a newly discovered China variety of M. anisopliae (M. anisopliae var. dcjhyium) for virulence to termites, isoenzyme and phylogenic analysis. Microbiol Res 162:53–61
Ehrlich KC (2014) Non-aflatoxigenic Aspergillus flavus to prevent aflatoxin contamination in crops: advantages and limitations. Front Microbiol 5:50. doi:10.3389/fmicb.2014.00050
Ekesi S, Akpa AD, Onu I, Ogunlana O (2000) Entomopathogenicity of Beauveria bassiana and Metarhizium anisopliae to the cowpea aphid, Aphis craccivora Koch (Homoptera: Aphididae). Arch Phytopath Pflanz 33:171–180
Emden VHF, Harrington R (2007) Aphids as crop pest. CABI Publishing, London
Gibbons JG, Rokas A (2012) The function and evolution of the Aspergillus genome. Trends Microbiol 21:1. doi:http://dx.doi.org/10.1016/j.tim.2012.09.005
Hall RA (1979) Pathogenicity of Verticillium lecanii (Zimm.) conidia and blastospores against the aphid. Entomophaga 24:191–198
Hall RA (1980) Control of aphid in glass house with fungus Verticillium lecanii (Zimm.): effect of spore concentration. Entomol Exp Appl 27:1–5
Jung HS, Lee HB, Kim K, Lee EY (2006) Selection of Lecancillium strains for aphid control. Korean J Mycol 34:112–118
Khan M, Khalil SK, Karimullah (1990) Biological control of aphid with a entomopathogenic fungus. Pak J Agric Res 11:174–177
Kumar V, Singh GP, Babu AM (2004) Surface ultrastructural studies on the germination, penetration and conidial development of Aspergillus flavus Link:Fries infecting silkworm, Bombyx mori Linn. Mycopathologia 157:127–135
Loureiro EDS, Moino JA (2006) Pathogenicity of hyphomycete fungi to aphids Aphis gossypii Glover and Myzus persicae (Sulzer) (Hemiptera: Aphididae). Neotrop Entomol 35:660–665
Mulock BS, Chandler LD (2001) Effect of Beauveria bassiana on the fecundity of Western Corn Rootworm, Diabrotica virgifera virgifera (Coleoptera: Chrysomelidae). Biol Control 22:16–21
Quesada-Moraga E, Santos-Quirós R, Valverde-García P, Santiago-Álvarez C (2004) Virulence, horizontal transmission, and sublethal reproductive effects of Metarhizium anisopliae (Anamorphic fungi) on the German cockroach (Blattodea: Blattellidae). J Invertebr Pathol 87:51–58
Sahayaraj K, Namasivayam SKR (2008) Mass production of entomopathogenic fungi using agricultural products and by products. Afr J Biotechnol 7:1907–1910
Saranya S, Ushakumari R, Sosamma J, Babu MP (2010) Efficacy of different entomopathogenic fungi against cowpea aphid, Aphis craccivora (Koch). J Biopest 3:138–142
Scholte E-J, Knols B, Takken W (2006) Infection of the malaria mosquito Anopheles gambiae with the entomopathogenic fungus Metarhizium anisopliae reduces blood feeding and fecundity. J Invertebr Pathol 91:43–49
Seye F, Ndiaye M (2008) Compatibilité entre Aspergillus clavatus (Hyphomycetes) et l’huile de neem (Azadirachta indica) contre le moustique vecteur de filarioses Culex quinquefasciatus (Say, 1823) (Diptera: Culicidae). Bacteriol Virusol Parazitol Epidemiol 53(1):43–48
Seye F, Faye O, Ndiaye M, Njie E, Afoutou JM (2009) Pathogenicity of the fungus, Aspergillus clavatus, isolated from the locust, Oedaleus senegalensis, against larvae of the mosquitoes Aedes aegypti, Anopheles gambiae s.l. and Culex quinquefasciatus. J Insect Sci 9:1–7
Seye F, Ndiaye M, Faye O, Afoutou JM (2012) Evaluation of entomopathogenic fungus Metarhizium anisopliae formulated with suneem (neem oil) against Anopheles gambiae s.l. and Culex quinquefasciatus adults. Malar Chemo Contr Elim 1:6. doi:10.4303/mcce/235494
Seye F, Ndione RD, Touré M, Ndiaye M, Boukraa S, Bawin T, Zimmer J-Y, Francis F (2013) Laboratory and semi-field environment tests for the control efficacy of Metarhizium anisopliae formulated with neem oil (suneem) against Anopheles gambiae s.l. adult emergence. Acad. J Biotechnol 1(1):46–52
Shah FA, Wang CS, Butt TM (2005) Nutrition influences growth and virulence of the insect-pathogenic fungus Metarhizium anisopliae. FEMS Microbiol Lett 251:259–266
Sivasundaram V, Rajendran L, Muthumeena K, Suresh S, Raguchander T, Samiyappan R (2007) Effect of talc-formulated entomopathogenic fungus Beauveria against leaf folder (Cnaphalocrosis medinalis) in rice. World J Microbiol Biotechnol 24:1123–1132
Tell LA (2005) Aspergillosis in mammals and birds: impact on veterinary medicine. Med Mycol 43:S71–S73
Vu VH, Hong SI, Kim K (2007) Selection of entomopathogenic fungi for aphid control. J Biosci Bioeng 104:498–505
Wang ZG, Knudsen GR (1993) Effect of Beauveria bassiana (Fungi: Hyphomycetes) on fecundity of the Russian wheat aphid (Homoptera: Aphididae). Environ Entomol 22:874–878
Wyatt IJ, White PF (1977) Simple estimation of intrinsic rates from aphids and tertanyxhid mites. J Appl Ecol 14:757–766
Yokomi RK, Gottwald TR (1988) Virulence of Verticillium lecanii isolates in aphids determined by bioassay. J Invertebr Pathol 51:250–258
Acknowledgments
The authors thank the Islamic Development Bank for the financial support of a Post-Doctoral scholarship on high technologies to Fawrou Seye.
Author information
Authors and Affiliations
Corresponding author
Additional information
F. Seye and T. Bawin contributed equally to the manuscript.
Rights and permissions
About this article
Cite this article
Seye, F., Bawin, T., Boukraa, S. et al. Effect of entomopathogenic Aspergillus strains against the pea aphid, Acyrthosiphon pisum (Hemiptera: Aphididae). Appl Entomol Zool 49, 453–458 (2014). https://doi.org/10.1007/s13355-014-0273-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13355-014-0273-z