Abstract
Spodoptera litura is one of the major polyphagous pests of agro-economically important crops in Asia, Africa and Europe, causing up to 100% loss in crop production. Recent research in eco-friendly plant based insecticides has attracted the attention of researchers. In this study, protease inhibitors (PIs) from non-host plant Cassia glauca were partially purified, with 73% trypsin inhibitory activity, to assess its anti-insect potential. Larval growth and development parameters were assessed by supplementing this PI in artificial diet at various concentrations (25–800 μg/ml). Bioassay studies revealed the inhibitory potential of this PI. Increasing concentrations of PI produced a decrease in larval duration, larval weight, number of pupae formed from treated larvae, pupal weight, percentage female emergence, fecundity and percentage hatching. Food utilization experiments showed an anti-nutritional effect of PI as relative growth rate, efficiency of conversion of ingested and digested food declined in a dose response manner whereas relative consumption rate, approximate digestibility and metabolic cost increased in larvae reared on PI supplemented diet. Experiments with digestive proteases in vitro showed an inhibition in trypsin and chymotrypsin activity in the insect lumen. In conclusion, these PI proteins might be suitable for insect pest management of food crops.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several direct plant-herbivore interactions in tropical forests laid the foundation for the theory of phytochemical co-evolution (Labandeira 2007). These interactions favouring either the plant or insect formed the basis for the production of various toxins in plants and evolution of adaptive mechanisms in herbivores. Among the most important protective mechanisms in plants, proteinaceous compounds like protease inhibitors (PIs) are widely distributed in plant tissues (Murdock and Shade 2005). PIs are recognized as an effective natural defensive system of plants and their evolutionary association with insect herbivores has also been well-documented (Zhao et al. 2009; Karban 2011). These low molecular weight proteins form an essential part of plant defense against herbivores by inhibiting proteinases of insect pests and phytopathogens (Christeller 2005; Green and Ryan 1972). PIs are not directly toxic to insects but their ingestion lead to interference in digestive physiology, which in turn affects insect growth and development.
PIs are primary gene products and are therefore promising candidates for conferring pest resistance in plants (Boulter 1993). This was first confirmed by Hilder et al. (1987) who transferred the Vigna unguiculata trypsin inhibitor gene to tobacco, which conferred resistance to wide range of insect pests including Lepidopterans, Coleopterans, and Orthoptera. Subsequent reports on the success of genetically engineered resistance in plants has led to numerous studies on PIs from different plant sources. Non-host plants may be an effective source of PIs for the management of insect pests as the insects are not pre-exposed to these PIs (Tamhane et al. 2005). Thus, non-host plant PIs are of dual advantage as they act against the insect gut proteases and protect host plant defence proteins from proteolysis. However, before considering any kind of insect control strategy, firstly, it is important to understand molecular targets present in the insect gut, and secondly, in vitro and in vivo bioassays using plant PIs need to be carried out to ascertain their effectiveness against the insect pest (Silva et al. 2006). The present study aimed to evaluate the biopotency of PIs isolated from non-host plant Cassia glauca on growth and development of Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae). Cassia glauca is a glabrous tree (Family: Caesalpiniacea) found throughout India, tropical Asia and Australia. The bark and leaves of the plant are used for the treatment of diabetes and gonorrhea in traditional medicine. S. litura is a polyphagous pest, causing economic losses to many agro- economically important crops such as cauliflower, jute, tobacco, tomato, rice, cabbage, onion and peanut in India and other countries (Kumar and Chapman 2006). Irrational use of insecticides for the management of this pest causes harm to non-target and beneficial insects, outbreaks of secondary pests and bioaccumulation of pesticide residues in food chain. Due to the growing concern regarding the harmful side effects of these synthetic chemicals, eco-friendly strategies for pest control are increasingly being explored. In the present study, we evaluated the potential of partial purified PIs from mature dry seeds of C. glauca to control the insect pest S. litura through bioassays, nutritional assay and biochemical analysis.
Materials and methods
Plant materials
Mature dry seeds of C. glauca were collected from the Botanical Garden of Guru Nanak Dev University, Amritsar, Punjab, India.
Insect culture
The egg batches of S. litura were collected from castor (Ricinus communis) leaves around Guru Nanak Dev University. The larvae were reared in laboratory on castor leaves in jars (15 × 10 cm) at 25 ± 2 °C and 60–70% relative humidity. The fresh castor leaves were washed with 1% sodium hypochlorite solution and changed daily till pupation and jars were regularly cleaned to avoid any type of infection. After pupation, the pupae were transferred to pupation jars with a 2–3 cm layer of moist sterilized sand covered with filter paper. Emerging adult moths were then transferred to oviposition jars at a ratio of 1 male: 2 females. The jars were lined with rough filter paper to assist egg laying and contained a cotton swab dipped in 10% sugar solution as a food source. The jars were covered with muslin cloth. The eggs were then transferred to plastic Petri dishes containing a moist cotton swab.
Extraction of PIs from seeds
Dry seeds of C. glauca were ground to fine powder and extracted with 100 ml of distilled water (1:10 w/v) for 2 h at room temperature. The extract was centrifuged at 6000 g for 20 min. Sediment was collected and further extracted with 5 volumes of distilled water at room temperature for 30 min. This crude extract was stored at 55 °C for 10–15 min and then was centrifuged at 6000 xg for 20 min. The supernatant was collected and adjusted to different ammonium sulphate precipitation percentage to obtain maximum protein with continuous stirring for 3–4 h at 4 °C. Various fractionation range of ammonium sulphate percentages used were 0–20%, 0–40%, 0–60%, 0–80%, 0–100% and combined fractions like 20–40%, 40–60%, 60–80%, 80–100% for extracting maximum protein with high inhibitory activity. After this, the adjusted supernatant was stored at 4 °C overnight. Next day, precipitates were obtained by centrifuging the contents at 10,000 xg for 20 min. Precipitates were dissolved in minimum amount of 100 mM Tris-HCl (pH 8.0) containing 20 mM CaCl2. The sample then was treated as partially purified PI which was then subjected to further analysis of protein content and inhibitory activity.
Trypsin inhibition assay
Trypsin inhibitory activity was determined using BApNA (N-benzoyl-DL-arginine p-nitroanilide) as substrate according to the methodology of Paulino da Silva et al. (2001). The bovine trypsin was pre-incubated with the partially purified inhibitor in 50 mM Tris HCl buffer (pH 8.2) for 10 min at room temperature. The assayed residual activities were followed by the hydrolysis of 1 mM BApNA and the liberation of p-nitroaniline was measured at 410 nm. One unit of inhibitor activity corresponded to its amount that produced a decrease in absorption at 410 nm by 0.01 units. The inhibitory activity was calculated as the difference between proteolytic activity with and without inhibitor.
Protein estimation
Protein estimation was done for each extraction step by the method of Lowry et al. (1951). Bovine Serum Albumin (BSA) (Loba, India) at concentration of 1 mg/ml was used as a protein standard as well as for preparing various test concentrations (25, 50, 100, 200, 400, 800 μg/ml) for bioassay, nutritional and biochemical assay studies.
Bioassays
Bioassays were carried out by the method of Vasudev and Sohal (2013). To evaluate the effect of partially purified PIs from C. glauca on growth and development of S. litura, the artificial diet was supplemented with five concentrations of the inhibitor (25, 50, 100, 200, 400 and 800 μg/ml). Then, second instar larvae were reared on amended and unamended diets at 25 °C and 70% relative humidity (RH). Each experiment was replicated six times with five larvae/replication. The larvae were kept individually in plastic containers (4 × 6 cm) and the diet was changed regularly. Observations were made on larval duration, pupal duration and number of pupae formed, male and female emergence, development, adult longevity, fecundity and egg viability.
Nutritional analysis
A gravimetric technique was used to determine weight gain, food consumption and faeces production in second instar S. litura larvae when given partially purified C. glauca PIs in a supplemented diet. All weights were measured using a Citizen Electronic Balance with an accuracy of 0.1 mg. In these experiments, larvae were provided with artificial diet supplemented with 25, 50, 100, 200, 400 and 800 μg/ml of PIs partially purified from C. glauca. The larvae starved for 3–4 h were weighed individually and released in plastic containers containing known amount of control and treated diets. The larvae were allowed to feed for 3 days at 25 ± 2 °C. After 3 days, observations were made on larval weight, residual diet and faecal matter. The dry weight of the larvae was determined by incubating at 60 °C for 72 h. Similarly, dry weights of diet and faecal matter were recorded to determine the loss of water under experimental conditions. The data obtained were used to determine the nutritional indices on dry weight basis as proposed by Waldbauer (1968). Relative growth rate (RGR) and relative consumption rate (RCR) were calculated on dry weight basis after 3 days of feeding as G/I (G = change in larval dry weight/ day and I = starting larval dry weight) and C/I (C = change in diet dry weight/day and I = starting larval dry weight), respectively. The index of food conversion efficiency (ECI) was calculated as 100 × G/C, where G = dry weight gain of the insect and C = dry weight of food consumed, efficiency of conversion of digested food (ECD) as (weight gained/(food ingested – frass weight) × 100) and approximate digestibility (AD) as (food ingested - frass weight)/food ingested × 100).
Preparation of S. litura gut extracts and in vitro enzyme assay
The potential of partially purified PIs from C. glauca in reducing the activity of digestive proteases was evaluated by estimating the larval gut and luminal trypsin and chymotrypsin enzymes. The activities of both digestive enzymes were monitored for three consecutive time intervals (24, 48 and 72 h) after feeding the second-instar larvae of S. litura on artificial diet incorporated with four concentrations (namely 100, 200, 400 and 800 μg/ml) of partially purified PIs of C. glauca. The midguts of the larvae from both treatment and control were carefully dissected in 0.15 M NaCl on ice after decapitation. After dissection, midguts were split lengthwise and the walls were washed with chilled 0.15 M NaCl, the lumen content and gut wall were homogenized separately (10% w/v). Dissected midguts were crushed with a mortar and pestle and the resulting homogenates were centrifuged at 14,000 xg for 20 min at 4 °C. The supernatants were used as crude enzyme preparations for evaluation of trypsin and chymotrypsin-like activity. The enzyme activity was measured in lumen contents (including peritrophic membrane) and tissue (gut wall) homogenates.
Results and discussion
Figure 1 documents the protein precipitation on the basis of different fractionation range of ammonium sulphate. It was found that ammonium sulphate precipitation gave maximum protein content as well as bovine trypsin inhibitory activity in the 0–60% fraction with 8.02 mg/ml and 73.29% respectively compared to the no inhibitor control (Fig. 1a). The 40–60% combined fractions gave 5.92 mg/ml protein and 65% trypsin inhibitory activity, which was low than the isolated fractions (Fig. 1b). Protease inhibitors partially purified from C. glauca exhibited 72.56% inhibitory activity against bovine trypsin with 3.6 purification fold in comparison to crude extract (Table 1).
Bioassay studies
The detrimental effect of C. glauca PI in its partially purified form on survival and developmental physiology of S. litura is evident from the bioassay studies. Significant reduction in larval duration was recorded at all concentrations from 25 to 800 μg/ml in comparison to control. At higher treatments (400 and 800 μg/ml) it decreased by 2.48 and 3.31 days respectively when compared with control group (Table 2). The larvae fed on PI supplemented diet showed a significant reduction in mean larval weight to 375.2 mg as compared to control (857.2 mg) at 800 μg/ml (Table 2). The weight loss was increased as concentration increased. Food assimilated with respect to control was found to decrease with increase in concentration in artificial diet (Fig. 2a). Partially purified PIs from C. glauca when supplemented in the diet given to S. litura larvae significantly decreased the percentage pupation in all treatments compared to control. The effect was more pronounced at highest concentration (800 μg/ml) where the percentage pupation was 50.06% compared to 91.30% in control (Table 2). Pupal weight showed a consistent decline in all treatments compared to the control with maximum decrease at highest concentration, i.e. 800 μg/ml, which gave a pupal weight of 234.5 mg compared to control (487.4 mg) (Table 2). Pupal period prolonged significantly as concentration increased.
The fall in larval weight may be attributed to the non-utilization of amino acids necessary for insect growth and development. Our findings are in agreement with the outcome of our earlier work (Vasudev and Sohal 2013), where the mean weight of S. litura larvae fed on diet containing partially purified cauliflower PI was significantly lower than the mean weight of larvae fed on control diet. The gut is the main site of digestive activities and serine proteinases are predominant in the gut of lepidopteran insects (Pauchet et al. 2008). The inhibitory action of PIs suggests that they act to significantly decrease protein digestion. Following a similar mode of action, the ingestion of PIs may disturb the transport of enzymes to S. litura midgut and in combination with limited proteolysis, may restrict the availability of amino acids consequently leading to poor larval growth and development.
PIs from C. glauca also affected the survival and performance of adults emerged from treated larvae at all concentrations in comparison to control. Percentage female emergence declined in a dose dependent manner. At higher concentrations (400 and 800 μg/ml), 37.00% and 35.08% females emerged whereas in control 55.27% female emergence was observed (Table 3). However, as female emergence decreased, male emergence increased, i.e. adults emerged were male rather than female. The egg laying capacity of emerged females was drastically affected. A detrimental effect on fecundity and fertility has previously been reported on S. litura with partially purified PIs from Brassica oleracea (Vasudev and Sohal 2013). In lepidopteran insects, the larva is an actively feeding stage, accumulating most of the proteins required for its growth and vitellogenesis. Any disturbance in the protein metabolism at larval stage subsequently affects the egg laying capacity of female moths. Less fecundity as well as fewer female adults in the present study may be the result of reduced bioavailability of protein or amino acids. This can also be correlated with decrease in larval and pupal mass. The decrease in the weight of pupae formed from larvae of S. litura fed on non-host PI amended diet seems to have adversely affected the reproductive capacity of emerged females. Inhibitory rate, which was calculated from adults emerged from the larvae reared on different concentrations of PIs amended diet, increased with increase in concentration (Fig. 2b). Percent hatching was also affected as at highest concentration of 800 μg/ml: only 34.67% eggs were viable to hatch when compared to control (Table 3).
The ingestion of PIs from C. glauca adversely affected the protein uptake at the larval stage, which was responsible for developmental abnormalities including reduction in the fertility and fecundity of the adults. PIs extracted from nonhost source such as bitter gourd too had detrimental effect on fertility and fecundity of S. litura and H. armigera larvae (Telang et al. 2003).
Nutritional indices
Nutritional analysis revealed that PIs from C. glauca had a growth inhibitory effect when ingested by second instar larvae of S. litura. Reduction in RGR, ECI and ECD and increase in RCR and AD was observed at all treatments when compared with control. Amended diet at 50–800 μg/ml concentrations resulted in 4.34–67.70% reduction in RGR compared to control. However, RCR increased in a dose response manner with maximum increase of 23.37% at highest concentration of 800 μg/ml when compared with control. The concentrations of 400 and 800 μg/ml caused a reduction in ECI (34.57%) and ECD (31.33%) which was significantly different from control (52.84%, 55.30% respectively). Approximate digestibility increased as concentration increased and was found to be maximum at highest concentration (91.13%). Metabolic cost in S. litura larvae increased from 50 to 800 μg/ml with maximum increase of 68.67% at highest PI concentration (Table 4). These results suggest that the digestive physiology of the insect was affected with treatment.
Reduction in growth rate and faecal production of S. litura larvae suggest that PIs of C. glauca acts on intestinal tract and/or interferes with digestive capabilities of the insect. Biologically, relative growth rate is the result of relative consumption rate and the efficiency in converting ingested food, which additionally depends on the efficiency of utilization of digested food. We can conclude that PIs partially purified from C. glauca can directly affect S. litura growth by increasing food intake. However, the decrease in ECI values is associated with energy consumption during physiological activities such as moulting and the approach of maturity (Carne 1966). This could be because of less digestibility of food and availability of digestible portion of food which is required for body substance and energy metabolism to maintain life.
The decrease in dietary utilization indicated that reduced growth and decrease in faecal production resulted from physiological effects (post ingestion) probably caused by the retention of the food in the gut for longer durations to maximize AD. The approximate digestibility in insects, based on differences between the weight of food ingested and the weight of the faeces, actually represents the food which is stored or metabolized. A greater AD would help to meet the increased requirement for nutrients (Koul et al. 2003) and balance for the deficiencies in food conversion caused by decreased ECI and ECD, possibly by diverting energy from biomass production into detoxification (Wheeler and Isman 2001). ECD is an overall measure of the ability of larvae to utilize digested food for growth. ECD decreases as the amount of digested food metabolized for energy increases (Carlini and Grossi-de-Sa 2002). The lower values of ECI and ECD observed suggest chronic toxicity and the reduction in ECD likely resulted from an increase in the proportion of assimilated energy diverted from growth to cover metabolic cost (MC) associated with detoxification and excretion of the PI.
Effect on digestive proteases
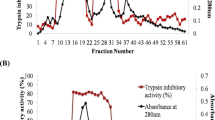
Trypsin activity in lumen was increased after 24 and 48 h of treatment whereas at 72 h treatment interval it decreased at 100, 200, 400 and 800μg/ml concentrations but at the highest concentration of 800 μg/ml, the decrease in enzyme activity observed was more in comparison to other treatments (Fig. 3a). However, tryspin activity in gut tissue increased at 24 h interval at all concentrations. With increase in concentration, activity decreased with maximum inhibition at 800 μg/ml (Fig. 3b). Also in faecal matter, trypsin activity increased at 400 and 800 μg/ml in comparison to other treatments (Fig. 3c).
Trypsin activity as a percentage of control in a lumen, b gut wall and c faeces of second instar of S. litura larvae after feeding on artificial diet amended with different concentrations (100, 200, 400 and 800 μg/ml) of PIs partially purified from C. glauca seeds for three time intervals (24 h, 48 h and 72 h)
The observations made on chymotrypsin activity in the lumen of larvae revealed significant decrease in enzyme activity with prolonged feeding on PI incorporated diet in all the treatments (Fig. 4a). However, in the gut wall tissue a significant decrease in chymotrypsin activity was observed at 100 μg/ml concentration (Fig. 4b). Subsequent analysis of faeces revealed an increase in chymotrypsin activity in all the treatments at all concentrations (Fig. 4c).
Chymotrypsin activity (percentage of control) in a lumen, b gut wall and c faeces of second instar S. litura larvae after feeding on artificial diet amended with different concentrations (100, 200, 400 and 800 μg/ml) of PIs partially purified from C. glauca seeds for three time intervals (24 h, 48 h and 72 h)
Larval diet containing PIs of C. glauca showed increased trypsin and less chymotrypsin activities in lumen content. The inhibitor slows down larval midgut proteinase activity by blocking the enzymes engaged in the process of digestion. An increased release of digestive enzymes in many insects is related to feeding. For insects that feed continuously (including Lepidoptera larvae), enzyme activity is minimal at the time of moulting and increases with the intensity of feeding (Lehane and Billingsley 1996). Our results revealed elevation in the digestive enzyme activity in the midgut of second instar larvae of S. litura after giving PI in the diet, which was associated with an increased consumption rate. The efficiency of conversion of ingested food into biomass depends, among other factors, on the activity of digestive enzymes. Increased trypsin and chymotrypsin activity in lumen and faecal matter of S. litura clearly indicated that there might be secretion of other isoforms of proteases to complete the digestion.
Conclusion
The results from both in vivo and in vitro studies clearly showed that the protease inhibitors isolated and partially purified from the mature dry seeds of C. glauca have potential to inhibit the growth and development of S. litura. Further, these inhibitor proteins could be exploited in insect-pest management of food crops.
References
Boulter D (1993) Insect pest control by copying nature using genetically engineered crops. Biochemistry 34:1453–1466
Carlini CR, Grossi-de-Sa MF (2002) Plant toxic proteins with insecticidal properties. A review on their potentialities as bioinsecticides. Toxicology 40:1515–1539
Carne PB (1966) Growth and food consumption during the larval stages of Paropsis atomaria (Coleoptera: Chrysomelidae). Entomologia Experimetalis et Applicata 9:105–112
Christeller JT (2005) Evolutionary mechanisms acting on proteinase inhibitor variability. FEBS J 272:5710–5722
Green TR, Ryan CA (1972) Wound-induced proteinase inhibitor in plant leaves: a possible defense mechanism against insects. Science 175:776–777
Hilder VA, Gatehouse AMR, Sherman SE, Barker RF, Boulter D (1987) A novel mechanism of insect resistance engineered into tobacco. Nature 300:160–163
Karban R (2011) The ecology and evolution of induced resistance against herbivores. Funct Ecol 25:339–347
Koul O, Multani JS, Singh G, Daniewski WM, Berlozecki S (2003) 6b-Hydroxygedunin from Azadirachta indica, its potentiation effects with some non-azadirachtin limonoids in neem against lepidopteran larvae. J Agric Food Chem 51:2937–2942
Kumar K, Chapman RB (2006) Sublethal effects of insecticides on the diamondback moth Plutella xylostella (L.). Pestic Sci 15:344–352
Labandeira C (2007) The origin of herbivory on land: initial patterns of plant tissue consumption by arthropods. Insect Sci 14:259–275
Lehane MJ, Billingsley PF (1996) Biology of the insect midgut. Chapman and Hall, London, p 486
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Murdock LL, Shade RE (2005) Lectins and protease inhibitors as plant defense against insects. J Agric Food Chem 50:6605–6611
Pauchet Y, Muck A, Svatos A, Heckel DG, Prelss S (2008) Mapping the larval midgut lumen proteome of Helicoverpa armigera, a generalist herbivorous insect. J Proteome Res 7:1629–1639
Paulino da Silva L, Leite JRSA, Bloch C Jr, Maria de Freitas S (2001) Stability of a black eyed pea trypsin/chymotrypsin inhibitor (BTCI). Protein Pept Lett 8:33–38
Silva CBLF, Alcazar AA, Macedo LLP, Oliveira AS, Macedo FP, Abreu LRD, Santos EA, Sales MP (2006) Digestive enzymes during development of Ceratitis capitata (Diptera: Tephritidae) and effects of SBTI on its digestive serine proteinase targets. Insect Biochem Mol Biol 36:561–569
Tamhane VA, Chougule NP, Giri AP, Dixit AR, Sainani MN, Gupta VS (2005) In vitro and in vivo effects of Capsicum annum proteinase inhibitors on Helicoverpa armigera gut proteinases. Biochim Biophys Acta 1722:156–167
Telang M, Srinivasan A, Patankar A, Harsulkar A, Joshi V, Damle A, Deshpande V, Sainani M, Ranjekar PK, Gupta G, Birah A, Rani S, Kachole M, Giri AP, Gupta V (2003) Bitter gourd proteinase inhibitors: potential growth inhibitors of Helicoverpa armigera and Spodoptera litura. Phytochemistry 63:643–652
Vasudev A, Sohal SK (2013) Bioinsecticidal potential of partially purified proteinase inhibitors from Brassica oleracea (L.) against Spodoptera litura (Fab.) (Lepidoptera: Noctuidae). Efflatounia 13:1–7
Waldbauer GP (1968) The consumption and utilization of food by insects. Adv Insect Physiol 5:229–288
Wheeler DA, Isman MB (2001) Antifeedant and toxic activity of Trichilia americana extract against the larvae of Spodoptera litura. Entomologia Experimentalis et Applicata 98:9–16
Zhao LY, Chen JL, Cheng DF, Sun JR, Liu Y, Tian Z (2009) Biochemical and molecular characterizations of Sitobion avenae-induced wheat defense responses. Crop Prot 28:435–442
Acknowledgements
The authors acknowledge the Department Of Botanical and Environmental Sciences for identifying the seeds. The first author is the recipient of University with Potential for Excellence research fellowship, University Grants Commission under the PhD programme of Guru Nanak Dev University, Amritsar.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
Authors declare that there is no conflict of interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vasudev, A., Sohal, S.K. Negative effect of non-host protease inhibitors partially purified from Cassia glauca seeds on developmental physiology of Spodoptera litura. Int J Trop Insect Sci 39, 1–8 (2019). https://doi.org/10.1007/s42690-019-00001-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-019-00001-0