Abstract
Medicinal plants contain chemical substances that can modulate biological processes similar to synthetic drugs and also demonstrate certain degree of toxicity. The current study investigated the safety of aqueous and ethanol extracts of Triumfetta rhomboidea leaves in normal male albino rats. Leaves of T. rhomboidea were collected and prepared to obtain aqueous (AETR) and ethanol (EETR) extracts of T. rhomboidea. Acute toxicity testing followed standard procedure. In sub-chronic testing, animals were allotted into 6 groups containing 5 animals each: Animals in group 1 (control) were given distilled water while groups 2–6 were respectively administered 100, 500, 1000, 3000 and 5000 mg extract/kg body weight daily in single dose using oral gavage. After 28th days of extracts dosing, rats were sacrificed and samples were collected for biochemical analysis. The results of LD50 revealed toxicity level above 5000 mg extract/kg for AETR and EETR in acute exposure. Sub-chronic administration of AETR and EETR caused significant (P < 0.05) increase in rat body weight. Doses of AETR and EETR demonstrated significant reduction in AST, ALP, GGT, creatinine while only high doses above 3000 mg AETR/kg significantly (P < 0.05) elevated urea. Despite cholesterol was significantly elevated in AETR and EETR treated animals, the concentration of HDL-C also increased significantly. Conclusively, this study has experimentally demonstrated the safety of aqueous and ethanol extracts of Triumfetta rhomboidea, but caution should be observed when extrapolating these results in humans because continuous dosing could alter organ structures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Medicinal plants have been exploited time immemorial for the management/treatment of diseases. This orthodox practice is still maintained globally today owing to its low cost and cultural acceptability of the safety. Approximately 80% of global population depends on medicinal plant with Africa occupying 17% of the worldwide distribution (Chung-Hung et al. 2012; Mahomoodally 2013). Despite medicinal plants confers excellent healing values due to multiplicity of its active compounds, associated toxicities in laboratory and field observations have been reported. Adverse effects of these toxic responses are associated with physiological changes in cellular biomarkers, architecture and overall functions of body organs (Mounanga et al. 2015; Akintimehin et al. 2021).
Triumfetta rhomboidea (Tiliaceae) is a pantropical plant that is widely distributed in the tropical and subtropical regions. It is locally used for the treatment diarrhea, tumor, gonorrhea and diabetes mellitus (Bosch 2011). Previous phytochemical examination on T. rhomboidea leaf extracts revealed the presence of saponins, tannin, phenols, flavonoid and steroids (Akintimehin et al. 2022). The leaf has high energy value and is commonly considered as appetizer, occasionally mixed with baby food and young toddler that are yet to eat coarse starchy foods. The soup is often the first dish given to women who have delivered a child (Neuwinger 2000).
The wide application of T. rhomboidea leaf in the treatment of diseases is probably due to the presence of active constituents and assumption that it is of relatively low toxicity. However, certain circumstance such as long–term administration of high dose (up to 5000 mg/kg) may be lethal to vulnerable population. In bid to establish the safety of medicinal plants for pharmaceutical purpose, cosmetics and food processing for human consumption, toxicological reports on the lethal dose of medicinal plants or their derivatives from scientifically controlled studies are significant. This study is therefore designed to assess the effect of acute and sub-chronic administration of Triumfetta rhomboidea leave extracts in healthy male albino rats.
Methods
Plant collection and preparation
Triumfetta rhomboidea leaves were harvested between July and August, 2021 in Igbokoda (Latitude: 6 21’ 00’’ Longitude: 4 48’ 00’’), Ondo State, Nigeria. Specimen was identified and authenticated in Plant Biology and Biotechnology Department, University of Benin with voucher number: UBHT–403. The leaves were thoroughly cleaned, air-dried for two (2) weeks and pulverized using electrical blender. Aqueous and ethanol extracts of T. rhomboidea leave (AETR and EETR) were prepared following the method of Akintimehin et al. (2022).
Experimental animals
Male albino rats (100–150 g) were obtained from the Animal House, Department of Biochemistry, University of Benin. Animals were acclimatized for 14 days in a well-ventilated room, gave free access to water and rat feed. Handling of experimental animals followed manual guidelines of laboratory animal care of National research council (NRC, 1997) as approved by the Institution ethical committee.
Toxicology protocol
Phase I and phase II acute toxicity testing was carried out according to Lorke (1983). In phase I, a total of nine (9) albino rats for each AETR and EETR were randomly allotted into 3 groups comprising 3 rats each. Groups I, II and III were administered 10, 100 and 1000 mg/kg body weight (b.w) of rats using an oral gavage in a single dose. In phase II, rats were divided into 3 groups, containing single rats. Animals in group I, II and III were respectively administered 1500, 2900 and 5000 mg extract/kg. Animals were initially monitored for manifestation of toxicity and mortality for 24 h and an extended period of 72 h. In sub-chronic toxicity, a total of fifty-five (55) rats were randomly divided into 6 groups of 5 rats each. Animals in group 1 (control) were administered 1 mL of distilled water. Groups 2, 3, 4, 5, 6 were respectively administered 100, 500, 1000, 3000 and 5000 mg/kg of AETR and EETR daily for 28 days using gavage. The weights of rats were taken at interval of 7 days.
Sample collections and preparations
After 28 days of administration, animals were deprived of food overnight and sacrifice thereafter to collect samples for analyses. Blood samples were withdrawn from the aortic valve using 2 mL syringe into plain sterile bottles. The blood samples were left for 30 min to clot and further centrifuged at 3000 x g for 10 min using electric centrifuge (Model: 80–2, Finlab) to collect clear sera. Organs were extracted, cleaned between layers of Whatman filter paper and weighed. Portion of the organs were removed and stored in 10% formaldehyde for histology.
Relative body organ weights ratio
Relative organ/body weight ratio was estimated by dividing the weight of organ(s) by the final body weight of rats.
Biochemical analysis
Biochemical assays were performed using Randox diagnostic kits (Randox Laboratories Limited, UK). Total serum protein (TP), alanine and aspartate aminotransferases (ALT and AST), alkaline phosphatase (ALP), gamma glutamyl transferase (GGT), urea, creatinine (CRE), total triglyceride (TTG), total cholesterol (TC), high density lipoprotein-cholesterol (HDL-C) and low-density lipoprotein–cholesterol (LDL-C) were carried out followed standard protocols in the manufacturer’s manual. Reaction mixtures of analyses were quantified using spectrophotometer (T70 + UV/VIS, PG Instrument Limited, England).
Histology
Sections of liver and kidneys were processed for histopathological examination using automatic tissue processor (Leica TP 1020). Samples were dehydrated using graded percentage of absolute alcohol and diluted alcohol, then dewax for 15 min in xylene solution and thereafter stained with eosin and hematoxylin. After staining, samples were placed on glass slides and microscopically viewed at different magnifications (Avwioro 2010).
Data analysis
Results were expressed as mean ± SEM. Data were evaluated statistically with statistical package for social sciences (SPSS17). Hypothesis testing was done using one-way analysis of variance (ANOVA) while post hoc test was done using least significant difference (LSD) and level of significance were considered when p < 0.05.
Results
Oral acute toxicity
The result obtained from the acute toxicity study is presented in Table 1. The LD50 of AETR and EETR is above 5000 mg/kg b.w without any observed toxicity signs after 24 h of scrutiny.
Body weight changes and mean organ/body weight ratio
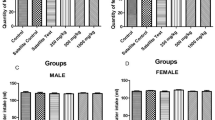
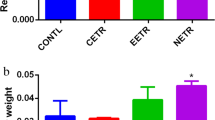
The relative difference in weekly body weight of rats administered doses of AETR and EETR is presented in Table 2. Only animals that were administered AETR and EETR at 3000 mg/kg and below demonstrated significant increase in weekly body weight. The mean organ/body weight ratio of AETR administered rats was insignificant relative to the untreated groups. Only 1000 and 5000 mg/kg doses of EETR respectively caused significant (p < 0.05) changes in liver and kidney (Fig. 1).
The effects of oral administration of AETR and EETR on mean organ/body weight ratio
Values are mean organ/body weight ratio of rats administered with extracts of T. rhomboidea leaves and is mean ± standard error of mean of at least four (4) independent experiments. Asterisk (*) on any bar is significantly different (p < 0.05) relative to the control
Magnified views of pancrease micromorphological section as revealed by Haematoxylin and Eosin staining at magnification x 400. The pancreatic parenchyma consisting of serous acinar and zymogenic cells, interlobular duct duct (black), islet of langerhans as well as pancreatic vessels (white) are all conspicous across the various groups
Results of biochemical studies in blood samples
The results of TP and organ function markers of animals administered AETR and EETR are shown in Table 3. There was a significant (p < 0.05) increase in TP of animals that received only 5000 mg/kg for both extracts relative to the control. Only rats that received 3000 mg AETR/kg showed obvious increase in ALT activity. All doses of AETR caused insignificant changes in AST activity while doses of EETR significantly reduced AST relative to the control. The activity of ALP was significantly elevated only in group that received 100 mg EETR/kg while the activity reduced in other treated groups. The level of GGT also reduced in animals that were administered both AETR and EETR. Only high doses (3000 mg/kg above) of AETR caused significant increase (p < 0.05) in urea. Doses of AETR and EETR caused significant decline in creatinine compared to the control.
Both AETR and EETR lowered TTG concentrations compared to the untreated groups. Doses of AETR up to 3000 mg/kg caused significant increase in TC while noticeable increase was only observed in 100 and 500 mg EETR/kg treated rats. Dose of AETR at 500 mg/kg show low HDL-C while doses at 100, 1000 and 3000 mg/kg was significantly elevated. Low dose of AETR significantly raise LDL-C while the concentration reduced at higher dose. Only group that received the highest dose of EETR caused significant decrease (p < 0.05) in LDL-C concentration when compared to the control (Table 4).
Histopathology results
The liver of rats in control group showed no pathological lesion as the central venules, hepatocytes morphology and sinusoids appeared normal (Fig. 2). Groups administered with graded dose of AETR and EETR shows similar hepatocyte morphology and sinusiod relative to control except the group that received 100 mg/kg of AETR that showed severe steatosis (blue arrow) and infiltrated cytoplasm by fat droplets. Sinusoid of group that received 1000 mg/kg also showed scanty infiltration. Only group that were administered 100 and 500 mg AETR/kg showed mild infiltration and congested central venules. Animals administered 100 mg EETR/kg also showed mildly congested central venules (white arrow) with group that receive only 5000 EETR/kg showing hemorrhage in focal area (white).
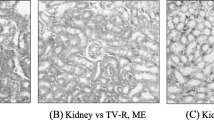
Rats in control group showed normal glomeruli, mesangial cells and capsular spaces (white arrow), renal tubules (blue arrow) and interstitial spaces. Most treated groups showed similar renal tubules and interstitial space relative to control while rat that were administered only 500 mg/kg AETR showed mild vascular congestion in interstitial space and few sclerotic glomeruli. Group that received 3000 and 5000 mg/kg of extracts showed thickened membrane with wide capsular space. Except for groups that received EETR between 100 and 1000 mg/kg that has normal renal cortex (white), other groups showed fluid accumulation and sclerotic glomeruli in the renal cortex (Fig. 3).
Animal in control showed normal architecture with the parenchyma of the pancreas showing normal serous acinar and zymogenic cells (black arrow), normal interlobular connective tissues (blue arrow) and islets of Langerhans (white arrow) consisting of round to oval collections of endocrine cells. Rats that received 100 mg/kg and 500 mg/kg of AETR has highly fibrotic interlobular connective tissues (blue arrow) and slightly diffuse islet of Langerhans (white arrow). Mild vascular congestion (black arrow) and few islets of Langerhans with vacuolation (white arrow) were also observed in groups that received 3000 mg AETR/kg. Similar to other treated groups, animals that were administered 5000 mg AETR/kg shows moderate thickening of vessel and possess islets of Langerhans (white arrow) with vacuolation and spaces. Rats that were administered 100 mg/kg EETR shows atrophic islets of Langerhans (white arrow). Group that was administered 500 mg/kg EETR demonstrated highly fibrotic interlobular connective tissues with heavy deposition of connective tissue. Except for groups that received 5000 mg EETR/kg that demonstrated mild fluid accumulation in the islets of Langerhans, animals that received between 1000 and 5000 mg EETR/kg doses showed moderate architecture as with diffused islets of Langerhans (Fig. 4).
Discussion
The development of tolerance to substances is frequently examined by an acute exposure study. In this study, absence of toxicity signs and mortality after 24 h of acute administration of AETR and EETR could signify that T. rhomboidea leaf is acceptable for consumption and unharmful. Findings from this study reveal progressive increase in body weight during 28 days of extract administration. The significant rise in body weight could be attributed to the high calorific value of T. rhomboidea leave, possibly enhancement of physiological conditions (like food and water intake) and metabolic processes in the experimental animals (Bosch 2011; Alkali et al. 2018). The increase in animal’s body weight is in agreement with the findings of Suresh et al. (2015), who also observed significant increase in body weight of rats after oral administration of petroleum ether extract of T. rhomboidea. The non-significant changes obtained in organ weight of animals that received AETR could connote non-injurious effects of AETR on the organs. The significant reduction that was observed in liver of rats that received 1000 mg EETR/kg is quite puzzling since animals that received above 1000 mg/kg demonstrated insignificant changes compared to the control. Nonetheless, factors such as body makeup of animals and toxic response owing to daily dosing might have elicited the changes (Arsad et al. 2013).
From this study, the non–significant increase in TP of animal that were administered graded dose between 100 and 3000 mg/kg of AETR and EETR could suggest non-harmful effects of extracts on protein synthesis and maintenance of normal protein function in circulation. While most cells need proteins for proper metabolisms and survival, impairment in protein synthesis due to toxic substance can also adversely influence their physiological roles (Kifayatullah et al. 2015). Cellular damage due to exposure to drugs, toxicants and diseases often result to surge in organ function indices in the extracellular fluid. The non-significant changes of hepatic markers in this study revealed that AETR and EETR does not produce severe toxicity in the liver. The significant rise in ALT and ALP of animals that were respectively administered 3000 mg AETR/kg and 100 mg EETR/kg might not necessarily portray hepatic injury since other tested doses of AETR and EETR were not affected. The unexpected rise in ALT and ALP in this study could probably be attributed to internal conditions such as increase hepatocellular production or normal physiological release from extra-hepatic sources and obstruction of bile flow in the biliary tract (Akindele et al. 2018). To further justify that the elevated ALP might not directly link to toxic response, a specific and sensitive hepatic biomarker (GGT) for detecting the source of indistinct ALP surge was not altered (Akpovona et al. 2016).
In renal dysfunction, creatinine and urea accumulate in the plasma and are commonly used as indices of nephrotoxicity (Oso et al. 2019). The elevated urea in this study may be connected to rise in protein metabolism or toxic response from plant bioactive constituents. High glomerular filtration owning to continuous administration of extracts, high dietary protein and increased protein degradation have been reported to elevated urea in the blood (Muhammad et al. 2011; Olaniyan et al. 2016). The low creatinine concentration in this study could suggest the absence or less severity of nephrotoxicity by extracts, protection against muscle wastage and maintenance of muscle bulk in experimental animals (Hounkpatin et al. 2019).
From this study, the non-significant reduction in triglyceride of animals that received AETR and EETR could presumably be due to dietary fiber contents in the leave and antilipase activities of bioactive compounds in the extracts (Han et al. 2002). The rise in cholesterol level in this study could suggest poor anti-hypercholesterolemia effects or extract participation in endogenous synthesis of cholesterol. Aside from diet, cholesterol is also produced endogenously for the production of steroids like hormones, bile acids and vitamins. Previous report has revealed that leave decoction of T. rhomboidea is commonly given to pregnant women in some African countries to ease and hasten childbirth (Bosch 2011). The result of HDL and LDL revealed that the extracts could however assist in the transport of cholesterol from peripheral tissue to the liver. The ratio of HDL/LDL is a common parameter for accessing the risk of arteriosclerosis and related cardiovascular disorders (Wang et al. 2010).
The mild fatty congestion and scanty infiltration that were observed in the liver could be linked to the elevated cholesterol that was observed in this study. Observed hemorrhage in the liver focal area of animals that received high dose of extracts could be associated with liver responses to the activities of the extracts. Mild lesion due to moderate necrosis in hepatic acinus specific zones have been reported to commonly occur during biotransformation of xenobiotics (Roberts et al. 2003). Despite this condition, liver can also display distinguish feature of cellular regeneration whereby necrotic cell are committed to apoptosis for the generation of new cell to maintain the normal hepatic function (Roberts et al. 2003). The few sclerotic glomeruli, thickened membrane and wide capsular space that was observed in the kidney of AETR and EETR treated rats could suggest precautionary measure when considering the plant extract for the treatment of disease. Possible reasons for the fair kidney architecture in treated animals could be daily dosing, overburdened elimination process and activity of extracts metabolites. Several active constituents in plants such as glycosides, tannin, oxalates have been reported to occasionally impair the activity of kidney (Kifayatullah et al. 2015). The observed fibrotic connective tissues in this study could be due to deposition of extracellular matrix and collagen as a result of repeated apoptosis, necrosis or repair of damaged pancreatic tissues. The slightly diffused islet of Langerhans with vacuolation that was seen in selected groups could suggest degenerative changes, autophagic vacuolation to remove garbage into intestine and vacuole formation as a result of mild congestion of fat droplets (Jones et al. 2010; Longnecker 2014).
Conclusion
This study has demonstrated the safety of T. rhomboiea leave in both acute and daily administration for 28 days. Despite no observed adverse effects on organ indices, histology studies revealed that the extracts might contain active constituents that could modulate the functionalities and architecture of the organs. The elevated cholesterol observed in this research is worrisome and demands further study to ascertain possible correlation between the T. rhomboidea extracts and cholesterol metabolism.
References
Akindele AJ, Oladimeji-Salami JA, Oyetola RA, Osiagwu DD (2018) Sub-chronic toxicity of the Hydroethanolic Leaf Extract of Telfairia occidentalis Hook. f. (Cucurbitaceae) in male rats. Medicines 5:4. https://doi.org/10.3390/medicines5010004
Akintimehin ES, Karigidi KO, Omogunwa TS, Adetuyi FO (2021) Safety assessment of oral administration of ethanol extract of Justicia carnea leaf in healthy wistar rats: hematology, antioxidative and histology studies. Clin Phytosci 7:2. https://doi.org/10.1186/s40816-020-00234-4
Akintimehin E, Onoagbe I, Abu O (2022) Proximate composition, qualitative phytochemicals screening and In-vitro antioxidant potential of Triumfetta rhomboidea leaves extracts. Trends in Sciences 19(24):3033. https://doi.org/10.48048/tis.2022.3033
Akpovona AE, Onoagbe IO, Eze GI, Omonkhua AA (2016) Acute and sub-chronic toxicity studies of ethanol extract of terminalia macroptera stem bark in wistar albino rats. JMBR: a peer-review. J Biomed Sci 15(1):62–73
Alkali YI, Jimoh AO, Muhammad U (2018) Acute and sub-chronic toxicity studies of methanol Leaf Extract of Cassia singueana F. (Fresen) in Wistar rats. Herb Med 4(2):6
Arsad SS, Mohd EN, Hamzah H, Othman F (2013) Evaluation of acute, subacute and subchronic oral toxicity of Rhaphidophora decursiva (Roxb.) Schott extract in male Sprague Dawley rats. J Med Plant res 7:3030–3040
Avwioro OG (2010) Histochemistry and tissue pathology, principle and techniques. Claverianum press, Ibadan, Nigeria
Bosch CH (2011) Triumfetta rhomboidea Jacq. [Internet] Record from PROTA4U. Brink, M. & Achigan-Dako, E.G. (Editors). PROTA (Plant Resources of Tropical Africa /Ressources végétales de l’Afrique tropicale), Wageningen, Netherlands. http://www.prota4u.org/search.asp
Chung-Hung C, Gek-Cheng N, Yusoff R (2012) A brief review on antidiabetic plants: global distribution, active ingredients, extraction techniques and acting mechanisms. Pharmacogn Rev 6(11):22–28
Han LK, Zheng YN, Xu BJ, Okuda H, Kimura Y (2002) Saponins from platycodi radix ameliorate high fat diet induced obesity in mice. J Nutri 132(8):2241–2245
Hounkpatin HO, Fraser SDS, Glidewell L, Blakeman T, Lewington A, Roderick PJ (2019) Predicting Risk of recurrent Acute kidney Injury: a systematic review. Nephron 142(2):83–90
Jones HB, Nugent D, Jenkins R (2010) Variation in characteristics of islets of Langerhans in insulin-resistant, diabetic and non-diabetic-rat strains. Int J Exp Path 91:288–301. https://doi.org/10.1111/j.1365-2613.2010.00713.x
Kifayatullah M, Mustafa MS, Sengupta P et al (2015) Evaluation of the acute and sub-acute toxicity of the ethanolic extract of Pericampylus glaucus (Lam.) Merr. In BALB/c mice. J Acute Disease 4(4):309–315. https://doi.org/10.1016/j.joad.2015.06.010
Longnecker D (2014) Anatomy and histology of the pancreas. Exocrine Pancreas Knowledge Base, Pancreapedia. https://doi.org/10.3998/panc.2014.3
Lorke D (1983) A new approach to practical acute toxicity testing. Archive of Toxicology 53:275–289
Mahomoodally MF (2013) Traditional medicines in Africa: An appraisal of ten potent African medicinal plants. Evidence-based Complementary and Alternative Medicine. 2013; 2013:617459
Mounanga MB, Mewonob L, Angone SA (2015) Toxicity studies of medicinal plants used in sub-saharan Africa. J Ethnopharmacol 174:618–627. https://doi.org/10.1016/j.jep.2015.06.005
Muhammad S, Hassan LG, Dangoggo SM, Hassan SW, Umar KJ, Aliyu RU (2011) Acute and subchronic toxicity studies of kernel extract of Sclerocarya birrea in rats. Sci World J 6(3):11–14
National Research Council (1997) Occupational Health and Safety in the Care and Use of Research animals. The National Academies Press, Washington, DC. https://doi.org/10.17226/4988
Neuwinger HD (2000) African traditional medicine: a dictionary of plant use and applications. Medpharm Scientific, Stuttgart, Germany. 589 pp
Olaniyan JM, Muhammad HL, Makun HA, Busari MB, Abdullah AS (2016) Acute and sub-acute toxicity studies of aqueous and methanol extracts of Nelsonia Campestris in rats. J Acute Disease 5(1):62–70. https://doi.org/10.1016/j.joad.2015.08.006
Oso BJ, Oyewo EB, Oladiji AT (2019) Influence of ethanolic extracts of dried fruit of Xylopia aethiopica (Dunal) A. Rich on haematological and biochemical parameters in healthy Wistar rats. Clin Phytosci 5:9. https://doi.org/10.1186/s40816-019-0104-4
Roberts S, James RC, Franklin MR (2003) Hepatotoxicity: toxic effects on the liver. In: Williams PL, James RC, Roberts SM (eds) Principles of toxicology: environmental and industrial applications, 2nd edn. John Wiley & Sons, Inc., New York, pp 111–128
Suresh R, Tomy S, Ujwala TK, Celine S, Padma VM (2015) Antidiabetic activity of petroleum ether extract of triumfetta rhomboidea on streptozotocin induced diabetic rat model. World J Pharm Pharmaceut Sci 4(10):822–828
Wang X, Zhang W, Wang Y, Peng D et al (2010) Acute and sub-chronic oral toxicological evaluations of quinocetone in Wistar rats. Reg Toxic Pharmacol 58:421–427
Funding
The authors declare no financial support was received from any public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceptualization and supervision: Iyere Osolase Onoagbe; Data computation: Emmanuel Sina Akintimehin; Materials and Data collection: Emmanuel Sina Akintimehin; Manuscript drafting, revision of final version for submission: Emmanuel Sina Akintimehin and Iyere Osolase Onoagbe.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests with any internal or external entities in conducting this research.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Akintimehin, E.S., Onoagbe, I.O. Acute and sub-chronic toxicity studies of aqueous and ethanol extracts of Triumfetta rhomboidea (Tiliaceae) Leaf in healthy albino rats. Vegetos (2023). https://doi.org/10.1007/s42535-023-00762-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42535-023-00762-7