Abstract
The responses to arsenic (As) and curcumin (Cur) were examined in Vigna radiata L. (Mung bean) seedlings in order to evaluate the role of Cur in reducing the effects of As stress. To aim this, As, Cur and their interactions were investigated on growth, biochemical, cytological and histochemical traits in the plant seedlings. The findings demonstrated that As stress causes chromosomal abnormalities such as C-mitosis, Laggard chromosome, clumped metaphase, and Bridge in Anaphase. Additionally, some cells' nucleoli did not vanish during metaphase. The morphological traits like germination percentage and seedling growth were altered with a simultaneous aggravation in amylase and antioxidant activity under As stress. The application of Cur significantly improved morphological traits, and antioxidant activity. Chromosomal aberrations, mitotic index, oxidative stress marker, and histochemical parameters were also altered in the interaction study of As and Cur. The investigation revealed that the consequences of As-induced phytotoxicity and chromosomal aberrations in Vigna radiata seedlings were ameliorated by Cur by perking up the antioxidative enzymes and augmenting amylase activity in a dose-dependent manner.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic (As) is one of the fastest dissipating toxic contaminants in earth’s crust (Cooper et al. 2020). Arsenic occurs in the environment as an organic, inorganic, and gaseous forms and its contamination seriously menaces the quality and safety of agricultural products and its sustainable development (Zeeshan et al. 2021). Consumption or exposure to more than safe levels of As contaminated food for humans causes bronchiectasis, cardiac coronary diseases, damaged intellectual function, diabetes, gangrene, skin, and lung cancers, etc. (Singh et al. 2015). The growth and productivity of crops are sternly affected by As stress through alteration of physiological processes and disruption of osmotic potential. The excessive ROS provoked under As stress causes membrane lipid injury, altered carbohydrate metabolism, damaged proteins and nucleic acids, reduced water, and nutrient uptake that result phytotoxic effects like cell dysfunction and death in plants (Alvarenga et al. 2020). The genotoxic effects of this chemical mutagen are induced by DNA damages or chromosomal aberrations that lead to reduced mitotic index in plants (Hani et al. 2020). Gupta et al. (2020) reported on the phytotoxic and genotoxic effects of As in Vicia faba and it was evident through significant increases in hydrogen peroxide (H2O2), malondialdehyde (MDA) levels and carbonyl groups in root and shoot of V. faba. The cyto-genotoxic effects of As stress were seen through decreased mitotic index (MI), relative abnormality rate (RAR) as well as other chromosomal abnormalities along with micronuclei in root meristematic cells of V. faba. Picchi et al. (2021) demonstrated the phytotoxic effects of As contaminated soils in Cannabis sativa L. and Brassica juncea L and the effects were seen in antioxidant enzymatic activities and photosynthetic performance that lead to a reduction in biomass by 50 and 25% in C. sativa and B. juncea. González-Moscoso et al. (2022) also reported the phytotoxic effects of As on tomato plants as evidenced by reduced growth, reduced number of leaves, decreased photosynthetic pigments, yield, enzymatic and non-enzymatic antioxidant contents of tomato plants. To counteract the toxicological effects of As stress, plants are furnished with a complex network of defense systems including antioxidant defense systems that protect their cellular system from harmful effects. Hasanuzzaman et al. (2020) reported that plants activate their antioxidant defense system to alleviate the adverse effects of oxidative stress or ROS generated under metal stress, and it varies among plant species, concentration and duration of stress. Supplementations of various compounds are reported to boost the antioxidant defense system of plants to cope up with As stress. The study of Asgher et al. (2021) described that the application of an adequate amount of H2O2 modulates the expression and activity of antioxidant enzymes and protects the photosynthetic activity from As stress. They reported that the activities of antioxidant enzymes like ascorbate peroxidase (APX), superoxide dismutase (SOD) and glutathione reductase (GR) were elevated by the application of H2O2 on As stressed plants compared to the control plants.
Curcumin or diferuloylmethane is a polyphenol extracted from the rhizome of the Turmeric (Curcuma longa) plant, known for its high antioxidative property (Tang et al. 2021). Several pharmacological and clinical studies have been conducted on curcumin over time because it decreases oxidative stress by inhibiting peroxidation of lipids and neutralizing hydrolyzed and superoxidized radicals. Vigna radiata or Mung bean is one of the major legumes of Asia that is well known for its rich constituents of carbohydrate, proteins, dietary fiber, minerals, and vitamins (Mekkara et al. 2021). This legume has antioxidative, anticancerous, anti-inflammatory, hypolipidemic, nutraceutical and prebiotic properties and can be used as a meat alternative for vegetarians as it is a very rich source of protein. V. radiata has the potential for nitrogen fixation capability, fast growth, soil reinforcement, and prevention of soil erosion, etc. In India, mung bean is consumed almost in every house mainly as Prasad or as a potent source of protein (Katiyar et al. 2020). The purpose of this study was to evaluate the phytotoxic and genotoxic effects of As on growth, biochemical and cytological traits of V. radiata (Mung) seedlings and its amelioration by the antioxidative property of Cur. Our work is the first to report As induced chromosomal alleviating property of Cur in V. radiata seedlings.
Material and methods
From a local, certified shop, Mung bean seeds were purchased. Surface-sterilization was done with 0.1% (w/v) HgCl2 solution and then rinsed thrice with distilled water. Sodium arsenate heptahydrate (Na2HAsO4·7H2O) solution was used as the source of As. The test solution consists of two concentrations of As (25, 50 µM), two concentrations of Cur (10 and 25 µM), and an interaction of As and Cur i.e. 50 µM As + 10 µM Cur and 50 µM As + 25 µM Cur. The sterilized seeds were kept in test solutions for one hour in the shaker. After one hour, the seeds were washed with distilled water and set for germination on filter paper in Petri dishes containing the test solution for 48 h. The filter paper was previously soaked in water and each petri dish contained 20 seeds sprayed with 3 mL of test solutions. Otherwise in control, 3 ml of distilled water was used. Based on the literature study on seed priming and optimization of our work, we have used 3 ml of test solutions in treated seeds and distilled water was used in control. The plates were kept at 28 ± 1 °C in the incubator. After 48 h, sampling was done to determine growth, cytological, and biochemical parameters.
Ten randomly selected seedlings were taken from each petri dish to measure plumule, radical length, and fresh mass. The length of plumule and radical was measured using thread and scale. Radicle and plumule elongation percentage was calculated by dividing the value of desired concentration from the compared concentration, after that subtracting that value with the compared concentration and finally multiplying it with 100. For dry mass estimation, plumule and radical were wrapped separately with aluminum foil and were dried in the oven at 80∘ C for 48 h. Histochemical parameters were analyzed to detect membrane lipid injury and can be analyzed by using the Schiff reagent. For cytological parameters, Acetocarmine squash preparation was made by following the method of Sharma and Sharma (1980). The alpha-amylase activity and lipid peroxidation was determined by following the method of Swain and Dekker (1966) and Heath and Packer (1968). The superoxide dismutase (SOD) activity was assayed using the Giannopolitis and Reis (1977) method. Catalase (CAT) and GPX activity was measured according to the method of Chance and Maehly (1955). In addition to this, the method of Griffith (1980) was followed to determine Glutathione reductase (GR) activity.
Statistical analysis
The data obtained were analyzed by using a one-way analysis of variance (ANOVA) (P ≤ 0.01). Fisher’s least significant difference (LSD) method at P ≤ 0.05 was used to compare the difference between the means of every assay. Mean ± standard error of three experiments are represented by each bar.
Results
The result of our present study revealed varied results of germination percentage under 25 µM As, 50 µM As, 10 µM Cur, 25 µM Cur, and in their interactions [50 µM As + (10 + 25 µM Cur)], which are represented in Table 1. Germination percentage was lowest in 50 µM As treatment (84%) relative to the control (98%). Moreover, 50 µM As treatment reduced plumule and radicle length of seedlings by 59.67% and 82.40% respectively compared to the control. The plumule and radicle of 50 µM As + 10 µM cur interactions elongated by 40.32% and 105.26% concerning 50 µM As. The fresh and dry mass of the plumule and radicle followed the same hierarchy with the length being lowest at 50 µM As and highest at 25 µM Cur (Table 1 and Fig. 1).
Changes in germinating mung seeds (Vigna radiata) subjected to Arsenic (As) stress, curcumin (CUR) and their interaction. A 0 µM (Controlled), B 25 µM As, C 50 µM As, D 10 µM Cur, E 25 µM Cur, F 50 µM As + 10 µM Cur, G 50 µM As + 25 µM Cur. A bar with an asterisk (*) represents significant difference from the control (p < 0.05)
The effect of different concentrations of As, Cur and their interactions on the mitotic index (MI) in V. radiata has represented in Table 2. With respect to 50 µM As treatment, the mitotic index of 50 µM As + 10 µM Cur interactions escalated by 30.87%. Our results showed that the application of Cur improved the MI. The genotoxic effect of As cause chromosomal abnormalities in V. radiata seedlings. In our present study, chromosomal aberrations were progressively elevated with the increase of As treatments by 7% and 11.33% in 25 µM and 50 µM As treatments. No chromosomal aberrations and nuclear abnormalities were observed at the control and Cur treatments. As treatment-induced different types of chromosomal aberrations like clumped mitosis, c-mitosis, chromosomal bridges, laggard chromosomes, binucleated cells, etc.in different frequencies and are given in Table 2 and Fig. 2. In addition to this, our results showed that in some cells under 50 µM As treatment, nucleolus did not disappear during metaphase cell division. The structures of normal chromosomes are given in Fig. 3. Lipid peroxidation in plant roots can be visualized with Schiff’s reagent by the appearance of pink coloration that indicated the accumulation of lipid peroxidation products (malondialdehyde). The results related to the histochemical analysis of V. radiata roots under As stress is depicted in Fig. 4. Oxidation of membrane lipids or pink coloration occurred in every As treatment and was especially pronounced in 50 µM As treated plant root tips (Fig. 4Dd, Ee). Moreover, the As treated roots appeared hard, thick and shorter. Accumulation of malondialdehyde (MDA) was least significant in control and Cur treated plants (Fig. 4Aa, Bb. However, in the roots exposed to the interaction of As and Cur, staining was comparatively weaker than the 50 µM As treatment revealing the ability of Cur to downregulate membrane damage under As stress conditions (Fig. 4Ff, Gg.
Chromosomal aberrations were found at different stages of the cell cycle on root tip cells of mung (Vigna radiata) under As stress. A Cmitosis, B laggard chromosome, C clumped metaphase, D nucleolus did not disappear in metaphase, E unequal division of chromosome at anaphase, F multiple bridges in anaphase, G bridge in anaphase, H binucleated cell
Histochemical studies of roots of germinating mung seeds (Vigna radiata) subjected to As stress, Cur, and their interactions were observed under the simple microscope (a–g) and compound microscope (A–G). A a—Control, B b—10 µM Cur, C c—25 µM Cur, D d—25 µM As, E e—50 µM As, F f—50 µM As + 10 µM Cur, G g—50 µM As + 25 µM Cur
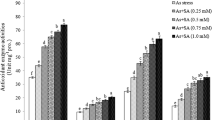
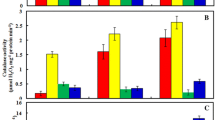
An elevated level of alpha-amylase activity was observed in V. radiata seeds with an increasing value of Cur by 2.08% at 10 µM and 21.73% at 25 µM relative to the control as shown in Fig. 5. It signifies the positive role of Cur in endospermic starch digestion and better seed germination of V. radiata seeds. The alpha-amylase activity was lowest at 50 µM As (39.13%) treatment in comparison with control. It increased by 19.42% in 50 µM As + 25 µM Cur interactions in comparison with 50 µM As treatment. The lipid peroxidation product or MDA has escalated progressively with the increase of As indicating the severe damaging effect of As on membrane lipids (Fig. 6). In our present study, almost all the antioxidant enzymes are slightly turned on under moderate As concentration (25 µM As) but gradually decreased at 50 µM As concentration. In our experiment, the SOD activity of plumule declines under As stress (25, 50 µM As) over control, 10 µM cur, 25 µM cur and in their interactions. But the radicle of 25 µM As showed the same SOD activity as the control whereas it significantly declines at 50 µM As treatments. The remarkable increase in SOD activity was observed both in plumule, and radicle in the interaction study of 50 µM As + 25 µM Cur in comparison with 50 µM (Fig. 7). Catalase (CAT) can abolish H2O2 by degenerating it into water H2O and O2. The CAT activity has increased at a low concentration of As (25 µM) by 22.30% in plumule relative to the control, signifying the As-induced increase in CAT activity and aforesaid total soluble protein content as a defense mechanism under moderate As stress. However, at 50 µM As treatment, the CAT activity reduced, and that could be due to the inactivation of the enzyme by the higher accumulation of H2O2 resulting from quenching of O2− by SOD (Fig. 7). The GPX activity of different concentrations were found in the series of 25 µM cur > 10 µM cur > control > 50 µM As + 10 µM Cur > 50 µM As + 25 µM Cur > 25 µM As > 50 µM As and are represented in Fig. 8. Glutathione reductase (GR) activity of both plumule and radicle slightly escalated at 25 µM As stress compared to control which may be due to augmentation of GR activity under As-inflicted oxidative stress. But under 50 µM As, GR activity of both plumule and radicle declined compared to the control that might be due to the generation of excessive ROS (Fig. 8). Application of Cur boosts GR activity which is crucial for detoxification of ROS, and improved defense against oxidative stress under As stress.
Discussions
Arsenic pollution is one of the major threats to all living organisms that may interfere with the growth and development of plants by altering the structure of chromosomes (aberration) resulting in changes in biochemical and morphological parameters. In this study, we have provided insight into how Cur regulates As stress to furnish protection to V. radiata seedlings. Higher concentration of As stress resulted in a decrease in the overall growth of plants, feasibly by inducing chromosomal aberrations, disturbing the antioxidative defense system and increasing H2O2 and MDA contents. However, Cur exposure provides protection and helps in the growth and development of V. radiata seedlings against As toxicity. It is reported that As exerts negative effects on plants by interacting with their chromosomal structures, disrupting their signalling mechanism and cell division that leads to changes in morphological and biochemical traits. It generates chromosomal aberrations by hampering the normal cell cycle (prophase, metaphase, anaphase, and telophase) of plants by damaging nucleic acids, and inhibiting DNA repair systems by binding to the thiol group. Sharma (2012) reported that metalloids decreased root turgor pressure, reduced cyclin and Cdk production which are crucial for regulating the cell cycle and lead to inhibition of cell enlargement and generate abnormalities in cell cycles. The degree of As stress was seen with the induced levels of chromosomal aberrations. The interactions of 50 µM As + 10 µM Cur showed fewer chromosomal aberrations than other respective concentrations of As, Cur and their interactions. Arsenic-treated chromosomal aberrations also had been reported in Allium cepa and V. faba roots (Gupta et al. 2018, 2020). But Cur-dependent mitigation of As toxicity by analyzing cytological parameters in V. radiata seedlings has not been reported yet. Our result is as per the study on animal model (mice) by Liju et al. (2021) in which Cur mitigated chromosomal aberrations in mice.
The progressive reduction of mitotic index (MI) with an increase in As concentration might be due to the reduction of cell division, and decreased extensions of the cell cycle under As stress. Our result is following the study on Allium sativum by Chatterjee and Chatterjee (2021) under As stress. Several studies demonstrated that chromosomal aberrations have been related to MI, and seedling length. Inhibition of mitosis due to the generation of chromosomal aberrations might be the reason for suppressing the elongation of cells by As that lead to the reduction of plant growth (Aidid and Okamoto 1992). The result is per the study by Tiwari and Rao, (2010) that reported the As-induced genotoxicity ameliorating property of Cur.
The first physiological process affected by As stress is observed in seed germination due to improper uptake of water and nutrients (Chandrakar et al. 2017; Yadu et al. 2019). The results of our present study showed gradual inhibition of germination rate with an increase in As concentration relative to the control. Similar outcomes were reported in Oryza sativa by Mridha et al. (2021) and in Lepidium sativum by Nouri and Haddioui (2021) under As stress. The decrease in moisture content of the seed might have inhibited the germination rate under As stress. Azad et al. (2021) demonstrated that seed germination is influenced by the reduction in the water potential gradient between seeds and their surrounding media. The application of Cur at lower concentrations alleviated the reduction of germination rate under As stress. These results are per the study of Upadhyaya et al. (2014). In the current study, the increase of germination percentage under As stress by Cur treatment suggests enhanced moisture content and proper hydration of the seeds that facilitated germination. The fundamental consequences of metalloid toxicity include inhibition of germination rate, decreased growth, root elongation, and reduced biomass accumulation as reported by Chandrakar et al. (2016). It may be due to the maximum use of accessible energy for the production of stress-linked crucial compounds (e.g. antioxidants, phytochelatins, etc.), enhanced leakage of cellular constituents, water loss, and protein degradation (Chandrakar et al. 2018; Agnihotri and Seth 2016). The concentration-dependent declines of plumule and radicle length suggested the correlation between seedling lengths with As concentration. The pronounced toxicity of As stress on radicle over plumule may be attributed to less translocation of As to plumule and more accumulation of As on radicle and it indicates the enhanced lipid peroxidation resulted from high oxidative stress under As stress (Singh et al. 2007). Wang et al. (2004) also reported exclusion mechanism adoption in which the metals are accumulated in roots (radicle) inhibiting their transport to the shoots (plumule). Our result following the study on wheat by Kumar et al. (2021) under As stress. Nevertheless, Cur treatment especially at 10 µM, improved all measured growth attributes under As stress because Cur is a potent antioxidant that prevents protein and membrane lipid degradation due to oxidative damage caused by excessive ROS (Mekkara et al. 2021).
Histochemical analysis with Schiff’s reagent represents a useful tool for the indirect detection of membrane lipid injury. Gilbert and Martin (2015) described that Schiff’s reagent produces a magenta or purple-colored Schiff base or imine by reacting with aldehydes originating from lipid peroxides downstream of reactive oxygen species. Arsenic treatment increased the lipid peroxidation of V. radiata seedling roots, with the strongest effects induced by 50 µM As treatment. In this treatment, strong and widespread histochemical staining was observed signifying the profound damage of the cell membrane lipids under As stress conditions in a dose-dependent manner. This demonstrated the increased production of free radicles under As stress in V. radiata roots. However, Cur-treated root tips weakly showed histochemical staining in comparison with the control. Interestingly, the interactions of As and Cur showed significantly faint staining compared to the 50 µM As treatment. Moreover, in the interaction study, the roots appeared thinner and longer than both of the As treatments. The result of our present study showed concentration-dependent declination of alpha-amylase activity with As concentration that may be contributed by the reduction in soluble sugars, and proteins (raw materials of structural components) under As stress that might diminish the growth of developing axis in germinating seed. The reduction of alpha-amylase activity under As stress is in agreement with the study on Oryza sativa (Choudhury et al. 2010), and mung (Ismail 2012). Our result is also in accordance with the study on medicinal pumpkins under salinity stress by Farsaraei et al. (2021). The application of Cur enhanced alpha-amylase activity at 25 µM concentration and also the interactions of 50 µM As + 25 µM Cur show a slight increase of alpha-amylase activity over As stress (25 and 50 µM), and interactions of 50 µM As + 10 µM Cur. The feasible mechanism for increasing alpha-amylase activity by Cur under As stress can be explained that Cur augments soluble sugars and proteins contents and thereby, increased the alpha-amylase activity under As stress.
Lipid peroxidation is considered a biomarker for the evaluation of oxidative damage to the membrane by ROS. In the current study, the degree of As stress can be seen with the increased levels of lipid peroxidation product or malondialdehyde (MDA) suggesting cell membrane damage due to exaggerated oxidative stress. The result of our study is following the study on Camellia sinensis by Li et al. (2021), and the study on barley and maize by AbdElgawad et al. (2021). The application of Cur (especially 25 µM) significantly reduced lipid peroxidation levels but the interactions of 50 µM As + 10 µM Cur showed better results in downregulating lipid peroxidation level over 50 µM As + 25 µM Cur interactions. Further, decreased MDA content under Cur supplementation might be closely associated with cellular ROS level under As stress. Our result is in agreement with the study on [Vigna radiata (L) Wilczek] by Upadhyaya et al. (2014) and on an animal model (rats) by Ahmadabady et al. (2021). Following the substantial increase in MDA under As stress, the result of our study demonstrated that increased levels of MDA were correlated with As-mediated oxidative burst in V. radiata seedlings, which indicated severe membrane damage by As stress.
Upon As stress, plants stimulate an efficient antioxidative defense system to cope with ROS and evade oxidative damages (Blokhina and Fagerstedt 2010). But depletion in antioxidant enzyme activities indicated oxidative injury and their insufficiency to counteract the As-induced oxidative stress (Yadu et al. 2018; Chandrakar et al. 2017; Hasanuzzaman and Fujita 2013). The first line of defense against reactive oxygen species (ROS) is established by the enzymatic antioxidant, super oxide dismutase (SOD) which scavenges the radical superoxide (O2−) to hydrogen peroxide (H2O2) and oxygen (O2) (Thounaojam et al. 2012). The decrease in SOD activity under 50 µM As may be due to the reduction in essential micronutrient constituents of SOD-like manganese (Mn), copper (Cu), and iron (Fe) under As stress as reported by Farnese et al. (2014). A similar result of reduced SOD activity under As stress was shown by the experiment of Katiyar et al. (2020). However, Cur’s treatment increased in SOD activity, which implied that Cur provided a safeguard against ROS under As stress. In addition to this, moderate stress (25 µM As) condition elevated CAT activity but at 50 µM As stress, its activity was downregulated which may be due to the induction of excessive oxidative stress. The result of our study following the study on wheat (Triticum sativum) under As stress by Sil and Biswas (2020). Reduction of GPX activity was more prominent in radicle particularly in 50 µM As stress. A decrease in GPX activity implies a higher accumulation of H2O2 and indicates abortive detoxification that leads to increased ROS-induced damages (Sil and Biswas 2020). Moreover, As induced growth retardation was reported to be more in roots than shoots (Ahmad et al. 2020). Similar results were shown in the study on Tomato plants by González-Moscoso et al. (2022). Some studies suggested that elevated activities of GPX conferred tolerance to heavy metal stress. The result of our present study revealed that the enhanced activities of GPX due to Cur treatment might be due to GSH-dependent peroxide scavenging that led to reduce oxidative damage. Glutathione reductase (GR) is a flavoprotein which involves in the detoxification of ROS, contributes to the pertinent functioning of the antioxidant systems, maintenance of a higher GSH to GSSG ratio, and phytochelatin synthesis in plants (Thounaojam et al. 2012). GR activity was enhanced under moderate As stress (25 µM) but its activity decreased at 50 µM As stress revealing the exaggerated generation of ROS under high As stress. Arsenic stress-induced reduction of GR activity was also reported in Brassica juncea by Ahmad et al. (2021) and in V. radiata by Sadeghipour and Monem (2021).
Abatement in plant growth under stress conditions has been regarded as an effect of decreased photosynthetic content and altered biochemical parameters in plants. The result of our study revealed the reduced activity of SOD, CAT, GPX, and GR of both plumule and radicle (especially radicle) under excessive (50 µM) As stress might be due to destruction of antioxidative defense mechanism under high As stress (Yan et al. 2021), Which thereby hindered the ROS detoxification mechanism and subsequently increased the oxidative stress. Sharma (2012) mentioned that As binds to thiol groups of antioxidant enzymes and directly affects biochemical reactions and reduced the overall growth of the plant. These findings suggested that Cur sturdily provides tolerance towards As stress in V. radiata seedlings. Moreover, the present study revealed that the elevation in growth occurred due to enhanced stimulation in antioxidative activity and reduced chromosomal aberrations and lipid peroxidation by Cur, which ultimately resulted in improved growth, biochemical, and histochemical traits in V. radiata seedlings.
Conclusion
In Vigna radiata L. (Mung), As stress significantly reduced germination, length of plumule, radicle, biomass, mitotic index, and amylase activity. Arsenic toxicity hampered water uptake or moisture content of seeds, increased lipid peroxidation and induced different types of chromosomal aberrations that may lead to the reduction of germination percentage, shortening of plumule, and radical length. The application of Cur alleviated As stress in the plant, which was revealed by the cytological, physiochemical, and antioxidative responses of the seedlings. The beneficial property of Cur may overcome the phytotoxic and genotoxic effects of As stress and help in the sustainable development of agriculture in a dose-dependent manner.
Data availability statement
The data that support this study are available in the article.
References
AbdElgawad H, Zinta G, Abuelsoud W, Hassan YM, Alkhalifah DH, Hozzein WN, Zrieq R, Beemster GT, Schoenaers S (2021) An actinomycete strain of Nocardiopsislucentensis reduces arsenic toxicity in barley and maize. J Hazard Mater 417:126055
Agnihotri A, Seth CS (2016) Exogenously applied nitrate improves the photosynthetic performance and nitrogen metabolism in tomato (Solanum lycopersicum L. cv Pusa Rohini) under arsenic (V) toxicity. Physiol Mol Biol Plants 22:341–349
Ahmad P, Alyemeni MN, Al-Huqail AA, Alqahtani MA, Wijaya L, Ashraf M, Kaya C, Bajguz A (2020) Zinc oxide nanoparticles application alleviates arsenic (As) toxicity in soybean plants by restricting the uptake of as and modulating key biochemical attributes antioxidant enzymes, ascorbate-glutathione cycle and glyoxalase system. Plants 9:825
Ahmad A, Khan WU, Shah AA, Yasin NA, Naz S, Ali A, Tahir A, Batool AI (2021) Synergistic effects of nitric oxide and silicon on promoting plant growth, oxidative stress tolerance and reduction of arsenic uptake in Brassica juncea. Chemosphere 262:128384
Ahmadabady S, Beheshti F, Shahidpour F, Khordad E, Hosseini M (2021) A protective effect of curcumin on cardiovascular oxidative stress indicators in systemic inflammation induced by lipopolysaccharide in rats. Biochem Biophys Rep 25:100908
Aidid SB, Okamoto H (1992) Effects of lead, cadmium and zinc on the electric membrane potential at the xylem/symplast interface and cell elongation of Impatiens balsamina. Environ Exp Bot 32:439–448
Alvarenga IF, Dos Santos FE, Silveira GL, Andrade-Vieira LF, Martins GC, Guilherme LR (2020) Investigating arsenic toxicity in tropical soils: a cell cycle and DNA fragmentation approach. Sci Total Environ 698:134272
Asgher M, Ahmed S, Sehar Z, Gautam H, Gandhi SG, Khan NA (2021) Hydrogen peroxide modulates activity and expression of antioxidant enzymes and protects photosynthetic activity from arsenic damage in rice (Oryza sativa L.). J Hazard Mater 401:123365
Azad N, Rezayian M, Hassanpour H, Niknam V, Ebrahimzadeh H (2021) Physiological mechanism of salicylic acid in Mentha pulegium L. under salinity and drought stress. Rev Bras Bot 44:359–369
Blokhina O, Fagerstedt KV (2010) Oxygen deprivation, metabolic adaptations and oxidative stress. Waterlogging Signal Toler Plants 119–147
Chance B, Maehly AC (1955) Assay of catalases and peroxidases. In: Colowick SP (eds) Methods in enzymology, vol 2. Academic Press, New York, pp 764–775
Chandrakar V, Dubey A, Keshavkant S (2016) Modulation of antioxidant enzymes by salicylic acid in arsenic exposed Glycine max L. J Soil Sci Plant Nutr 16:662–676
Chandrakar V, Yadu B, Meena RK, Dubey A, Keshavkant S (2017) Arsenic-induced genotoxic responses and their amelioration by diphenylene iodonium, 24-epibrassinolide and proline in Glycine max L. Plant Physiol Biochem 112:74–86
Chandrakar V, Pandey N, Keshavkant S (2018) Plant responses to arsenic toxicity: morphology and physiology. In: Mechanisms of arsenic toxicity and tolerance in plants. Springer, Singapore, pp 27–28
Chatterjee S, Chatterjee S (2021) Phytoremediation of arsenic using Allium sativum L. as a model system. In: Spatial modeling and assessment of environmental contaminants. Springer, Cham, pp 121–136
Choudhury B, Mitra S, Biswas AK (2010) Regulation of sugar metabolism in rice (Oryza sativa L.) seedlings under arsenate toxicity and its improvement by phosphate. Physiol Mol Biol Plants 16:59–68
Cooper AM, Felix D, Alcantara F, Zaslavsky I, Work A, Watson PL, Pezzoli K, Yu Q, Zhu D, Scavo AJ, Zarabi Y (2020) Monitoring and mitigation of toxic heavy metals and arsenic accumulation in food crops: a case study of an urban community garden. Plant Direct 4:e00198
Farnese FS, Oliveira JA, Gusman GS, Leão GA, Silveira NM, Silva PM, Ribeiro C, Cambraia J (2014) Effects of adding nitroprusside on arsenic stressed response of Pistia stratiotes L. under hydroponic conditions. Int J Phytoremediat 16:123–137
Farsaraei S, Mehdizadeh L, Moghaddam M (2021) Seed priming with putrescine alleviated salinity stress during germination and seedling growth of medicinal pumpkin. J Soil Sci Plant Nutr 22:1–1
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol 59:309–314
Gilbert JC, Martin SF (2015) Experimental organic chemistry: a miniscale & microscale approach. Cengage Learning
González-Moscoso M, Juárez-Maldonado A, Cadenas-Pliego G, Meza-Figueroa D, SenGupta B, Martinez-Villegas N (2022) Silicon nanoparticles decrease arsenic translocation and mitigate phytotoxicity in tomato plants. Environ Sci Pollut Res 29:1–17
Griffith OW (1980) Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106:207–212
Gupta K, Mishra K, Srivastava S, Kumar A (2018) Cytotoxic assessment of chromium and arsenic using chromosomal behavior of root meristem in Allium cepa L. Bull Environ Contam Toxicol 100:803–808
Gupta K, Srivastava A, Srivastava S, Kumar A (2020) Phyto-genotoxicity of arsenic contaminated soil from Lakhimpur Kheri, India on Vicia faba L. Chemosphere 241:125063
Hani U, Mansoor S, Hassan M, Farheen J (2020) Genotoxicity of heavy metals on mung bean (Vigna radiata) seedlings and its alleviation by priming with their lower concentrations. Cytologia 85:239–244
Hasanuzzaman MA, Fujita HM (2013) Exogenous sodium nitroprusside alleviates arsenic-induced oxidative stress in wheat seedlings by enhancing antioxidant defense and glyoxalase system. Ecotoxicol 22:584–596
Hasanuzzaman M, Bhuyan MB, Zulfiqar F, Raza A, Mohsin SM, Mahmud JA, Fujita M, Fotopoulos V (2020) Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator. Antioxidants 9:681
Heath RL, Packer LJ (1968) Photo peroxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Ismail GS (2012) Protective role of nitric oxide against arsenic-induced damages in germinating mung bean seeds. Acta Physiol Plant 34:1303–1311
Katiyar P, Yadu B, Korram J, Satnami ML, Kumar M, Keshavkant S (2020) Titanium nanoparticles attenuates arsenic toxicity by up-regulating expressions of defensive genes in Vigna radiata L. J Environ Sci 92:18–27
Kumar J, Kumar S, Mishra S, Singh AK (2021) Role of zinc oxide nanoparticles in alleviating arsenic mediated stress in early growth stages of wheat. J Environ Biol 42:518–523
Li X, Ahammed GJ, Zhang XN, Zhang L, Yan P, Zhang LP, Fu JY, Han WY (2021) Melatonin-mediated regulation of anthocyanin biosynthesis and antioxidant defense confer tolerance to arsenic stress in Camellia sinensis L. J Hazard Mater 403:123922
Liju VB, Thomas A, Sivadasan SD, Kuttan R, Maliakel B, Im K (2021) Amelioration of radiation-induced damages in mice by curcuminoids: the role of bioavailability. Nutr Cancer 73:617–629
MekkaraNikarthilSudhakaran S, Bukkan DS (2021) A review on nutritional composition, antinutritional components and health benefits of green gram (Vigna radiata (L.) Wilczek). J Food Biochem 45:e13743
Mridha D, Paul I, De A, Ray I, Das A, Joardar M, Chowdhury NR, Bhadoria PB, Roychowdhury T (2021) Rice seed (IR64) priming with potassium humate for improvement of seed germination, seedling growth and antioxidant defense system under arsenic stress. Ecotoxicol Environ Saf 219:112313
Nouri M, Haddioui A (2021) Improving seed germination and seedling growth of Lepidium sativum with different priming methods under arsenic stress. Acta Ecol Sin 41:64–71
Picchi C, Giorgetti L, Morelli E, Landi M, Rosellini I, Grifoni M, Franchi E, Petruzzelli G, Barbafieri M (2021) Cannabis sativa L. and Brassica juncea L. grown on arsenic-contaminated industrial soil: potentiality and limitation for phytoremediation. Environ Sci Pollut Res 22:1–6
Sadeghipour O, Monem R (2021) Improving arsenic toxicity tolerance in mung bean [Vigna radiata (L.) Wilczek] by salicylic acid application. Vegetos 13:1–8
Sharma I (2012) Arsenic induced oxidative stress in plants. Biologia 67:447–453
Sharma AK, Sharma A (1980) Chromosome techniques. Theory and practice, 3rd edn., Butterworths, London
Sil P, Biswas AK (2020) Silicon nutrition modulates arsenic-inflicted oxidative overload and thiol metabolism in wheat (Triticum aestivum L.) seedlings. Environ Sci Pollut Res 27:45209–45224
Singh HP, Batish DR, Kohli RK, Arora K (2007) Arsenic-induced root growth inhibition in mung bean (Phaseolus aureus Roxb.) is due to oxidative stress resulting from enhanced lipid peroxidation. Plant Growth Regul 53:65–73
Singh AP, Dixit G, Mishra S, Dwivedi S, Tiwari M, Mallick S, Pandey V, Trivedi PK, Chakrabarty D, Tripathi RD (2015) Salicylic acid modulates arsenic toxicity by reducing its root to shoot translocation in rice (Oryza sativa L.). Front Plant Sci 6:340
Swain RR, Dekker EE (1966) Seed germination studies I. Purification and properties of an α-amylase from the cotyledons of germinating peas. Biochim Biophys Acta Enzymol Biol Oxid 122:75–86
Tang W, Du M, Zhang S, Jiang H (2021) Therapeutic effect of curcumin on oral diseases: a literature review. Phytother Res 35:2287–2295
Thounaojam TC, Panda P, Mazumdar P, Kumar D, Sharma GD, Sahoo L, Sanjib P (2012) Excess copper induced oxidative stress and response of antioxidants in rice. Plant Physiol Biochem 53:33–39
Tiwari H, Rao MV (2010) Curcumin supplementation protects from genotoxic effects of arsenic and fluoride. Food Chem Toxicol 48:1234–1238
Upadhyaya H, Shome S, Roy D, Bhattacharya MK (2014) Arsenic induced changes in growth and physiological responses in Vigna radiata seedling: effect of curcumin interaction. Am J Plant Sci 5:3609
Wang SH, Yang ZM, Yang H, Lu B, Li SQ, Lu YP (2004) Copper-induced stress and antioxidative responses in roots of Brassica juncea L. BBAS 1:45
Yadu B, Chandrakar V, Korram J, Satnami ML, Kumar M, Keshavkant S (2018) Silver nanoparticle modulates gene expressions, glyoxalase system and oxidative stress markers in fluoride stressed Cajanus cajan L. J Hazard Mater 353:44–52
Yadu B, Chandrakar V, Tamboli R, Keshavkant S (2019) Dimethylthiourea antagonizes oxidative responses by upregulating expressions of pyrroline-5-carboxylate synthetase and antioxidant genes under arsenic stress. Int J Environ Sci Technol 16:8401–8410
Yan S, Wu F, Zhou S, Yang J, Tang X, Ye W (2021) Zinc oxide nanoparticles alleviate the arsenic toxicity and decrease the accumulation of arsenic in rice (Oryza sativa L.). BMC Plant Biol 21:1–1
Zeeshan M, Hu YX, Iqbal A, Salam A, Liu YX, Muhammad I, Ahmad S, Khan AH, Hale B, Wu HY, Zhou XB (2021) Amelioration of AsV toxicity by concurrent application of ZnO-NPs and Se-NPs is associated with differential regulation of photosynthetic indexes, antioxidant pool and osmolytes content in soybean seedling. Ecotoxicol Environ Saf 225:112738
Funding
This research did not receive any specific funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khan, Z., Thounaojam, T.C., Rajkumari, J.D. et al. Arsenic induced chromosomal aberrations, biochemical and morphological changes in Vigna radiata L. (Mung bean) seedlings and its amelioration by Curcumin. Vegetos 37, 1185–1194 (2024). https://doi.org/10.1007/s42535-023-00655-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42535-023-00655-9