Abstract
An efficient in vitro propagation method is developed for Artocarpus lakoocha Roxb. through direct shoot regeneration from cotyledonary node region of seedlings developed from immature seeds. The immature seeds were germinated on Murashige and Skoog (MS) medium having different concentrations of either 6-benzyl aminopurine (BA) or kinetin or Thidiazuron and subsequently, the cotyledonary node with primary shoot was transferred to MS medium without any growth regulator for shoot multiplication. Maximum (7.23 ± 0.46) shoots regenerated when immature seeds were cultured for 21 days on MS medium having 4.44 µM BA for germination. For rooting, shoots were pulse treated for 48 h with different concentrations of Indole-3-acetic acid (IAA) or Indole-3-butyric acid (IBA) followed by transfer on agar gelled MS basal medium. About 94.45% shoots rooted on pulse treatment with 5.0 µM IBA. Plantlets obtained after rooting were hardened and acclimatized into soil with 85–90% survival. The plantlets established into soil had similar vegetative morphology to the mother plant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Artocarpus lakoocha Roxb. of family Moraceae, native to humid sub-Himalayan region of India, South China, South-East Asia (Jagtap and Bapat 2010) is an economically useful evergreen recalcitrant fruit tree. Tree has wide applications in folk medicine and timber industry. The hardwood, which is comparable to teak wood, known as lakuch, is durable for under water and outdoor constructions. Fruit pulp is believed to function as liver tonic and the seed contains artocarpins which exhibit haemagglutination activity (Jagtap and Bapat 2010; Wongkham et al. 1995). Leaf, stem, bark and fruit extracts contain useful bioactive compounds which possess several biological activities such as antibacterial, antiviral, antifungal, antiplatelet, antitubercular and cytotoxic (Jagtap and Bapat 2010). The propagation of this plant is limited to seed. However, once seeds are taken from fruits, they quickly lose viability (within in a few days). Conventional propagation is not successful for multiplication of A. lakoocha (Joshee et al. 1991). Population of this plant is declining gradually due to their high demand and low rate of propagation and to meet the demand, an adequate and continuous supply of this plant is essential. Micropropagation is a proven method for mass multiplication and establishment of new plantations (Moharana et al. 2017; Pandey et al. 2006; Singh et al. 2001). The multiplication of fruit crops using in vitro culture techniques has considerable potential for improvement and multiplication of economically useful fruit trees that have been under cultivation for many generations (Giri et al. 2004; Singh et al. 2004). Although, a considerable number of economically useful plant species are regenerated through in vitro culture, yet the regeneration of tree species is still limited (Tripathi and Kumari 2010; Singh et al. 2004). Moreover, at present the yield of most fruits is low and is not up to the reach of working classes of the developing world because commercially, the cultivation of fruits is still in infancy (Singh et al. 2004).
Despite being economically useful, A. lakoocha tissue culture has not received attention so far, and except a preliminary report of Rahman and Amin (1995) on plant regeneration from seedling node no in vitro studies have been undertaken on this plant. Several limitations, such as, the viability of seeds for a very short duration and microbial infection of the germinated seeds, were observed during the plant regeneration by adopting the earlier established protocol of this tree. Thus, to optimize in vitro regeneration of A. lakoocha, the present study was undertaken to examine the potential of immature seeds to respond under in vitro conditions with the possibility of developing a protocol for the clonal multiplication.
Materials and methods
Plant material and surface sterilization
The ripe and fully developed unripe immature fruits were collected from the tree growing at Chandra Shekhar Azad Agriculture and Technology University campus, Kanpur, India. Collected fruits were washed thoroughly under running tap water. The washed fruits were surface sterilized by dipping in 1.0% (v/v) sodium hypochlorite solution for about 15 min, followed by washing with autoclaved double distilled water. The seeds from both ripe and unripe fruits were dissected out aseptically and dipped separately in 70% alcohol for 30 s followed by washing three times with autoclaved double distilled water.
Shoot induction
The surface sterilized immature and mature seeds were cultured for 3 weeks on 0.8% (w/v) agar gelled Murashige and Skoog medium (Murashige and Skoog 1962) supplemented with different concentrations (0.44, 4.44, 13.3 and 20.0 µM) of 6-benzyl aminopurine (BA) or Kinetin (kn; 0.46, 4.65, 13.95 and 23.25 µM) or Thidiazuron (TDZ; 0.004, 0.045, 0.45 and 4.50 µM) alone. The seedlings developed from the mature seeds were contaminated and died while those from immature seeds were survived and used for culture establishment. The cotyledonary node along with primary shoot without shoot tip was dissected out from 3 weeks old seedling and transferred either on MS basal medium (pulse treatment) or on MS basal medium supplemented with similar concentration of growth regulator in which immature seeds were germinated (continuous treatment). To optimize culture duration of immature seeds on germination medium, the seeds were cultured for different days (0, 7, 14, 21, 28 and 35 days) on agar gelled MS medium supplemented with 4.44 µM BA and subsequently, the cotyledonary node along with primary shoot without shoot tip was dissected out from seedlings developed in each treatment and cultured on agar gelled MS basal medium having 3% (w/v) sucrose. All the cultures were observed daily and data for number of explants showing shoot bud initiation, average number of regenerated shoots and shoot length were recorded after 4 weeks of culture of explants. To maintain the continuous shoot proliferating culture the elongated shoots were removed and the mother explants along with small shoots were transferred on the fresh medium with same composition.
In each experiment, MS medium was used for culture and the medium pH was maintained to 5.8 ± 0.02 before autoclaving for 15 min at 121 °C. All the explants were cultured in 25 × 150 mm glass culture tubes and 10 ml medium was poured in each culture tube. The cultures were incubated at 25 ± 2 °C and under a 16 h photoperiod with light intensity 25 µmol m−2 s−1 provided by cool white fluorescent tube lights.
Rooting of microshoots and acclimatization of plantlets
The shoots longer than 2 cm were selected and cultured for root induction both under continuous as well as 24 or 48 h pulse treatment with different concentrations (0.5, 5.0 and 15.0 µM) of either indole 3-acetic acid (IAA) or indole 3-butyric acid (IBA). The pulse treated shoots were subsequently transferred to agar gelled MS basal medium. All the cultures were kept under 16 h photoperiod inside the culture room. The number of rooted shoots, number of roots per shoots and length of roots were recorded after 30 days of transfer of shoots to MS basal medium. The shoots having well developed roots were washed carefully with tap water and transplanted into thermocoal pots (8 cm long and 6 cm diameter) filled with autoclaved garden soil and sand mixture in 1:3 (V:V) ratio. The transplanted plants were covered for 3 weeks with glass beaker and kept at 25–30 °C inside the laboratory. The plants were illuminated with white fluorescent tube lights with 25 µmol m−2 s−1 light intensity. On alternate day transplanted plants were irrigated with tap water. After 3 weeks the transplanted plants were exposed to sunlight for a short duration and gradually time to exposure under sunlight was increased up to full day. After acclimatization under sunlight the glass beaker was removed gradually and the acclimatized plants were transferred to pots containing only garden soil and the percent survival of transplanted plants was recorded after 2 weeks.
Experimental design and statistical analysis
The experiments were performed using a completely randomized design. All the experiments were repeated three times and in an experiment each treatment was consisted with twelve replications. One way Analysis of Variance test (ANOVA) was used for data analysis and comparisons between the mean values of treatments were performed using the least significant difference (LSD) test (Snedecor and Cochran 1989).
Results and discussion
Shoot multiplication
This study demonstrates an in vitro regeneration protocol in a recalcitrant fruit tree A. lakoocha from cotyledonary node region of immature seeds. Addition of cytokinin was necessary for seed germination and both mature and immature seeds were cultured for germination but seedlings developed from mature seeds did not survive due to exudation of phenolics and contamination after germination. However, cotyledonary node along with primary shoot excised from seedlings of immature seeds were successfully cultured for shoot multiplication studies, showing that immature seeds could overcome problems associated with culture establishment from tissues or explants of seedlings of mature seeds in A. lakoocha. The immature seeds have also been proved to be an efficient explant for in vitro plant regeneration in Lawsonia inermis (Moharana et al. 2017) Murraya koeniggi (Rani et al. 2012).
Each cytokinin tested was effective in shoot induction but the shoot proliferation was highly dependent on type, concentration and duration of cytokinin used into the medium. In comparison to BA and Kinetin, TDZ could induce shoots from cultured cotyledonary nodes at a very low concentration (Table 1). This agrees with the earlier studies on woody species that shoot multiplication can be induced using low concentrations of TDZ (Huetteman and Preece 1993; Pandey et al. 2006). Among three cytokinins used in this study, BA was more effective for shoot induction and multiplication from cotyledonary node region than Kn and TDZ and among various concentrations of BA tested maximum shoots were produced on medium having 4.44 µM BA. Similar observations were noticed in other fruit trees such as Morus (Choudhary et al. 2015), Malus spp. (Lu et al. 2015), Artocarpus heterophyllus (Kamal et al. 2018). This study also demonstrated that shoot multiplication and shoot growth was slow, when immature seeds were germinated on medium having cytokinin, and cotyledonary node along with primary shoot from such seedlings were cultured on medium fortified with cytokinin (Table 1; Fig. 1a, b). However, a significant increase in shoot multiplication was noticed on culture of such explants on medium devoid of any cytokinin (Table 2; Fig. 1c), showing continuous presence of cytokinin was not favourable for in vitro regeneration in A. lakoocha. Hence, to optimize culture duration of immature seeds on medium with cytokinin, the seeds were germinated by culturing for different days (0, 7, 14, 21, 28 and 35) on medium having 4.44 µM BA and subsequently the cotyledonary nodes with primary shoot from seedlings were transferred for shoot proliferation on medium devoid of growth regulator. Shoot multiplication and shoot growth increased with increase in duration of culture period of seeds on medium with BA and maximum shoots (7.23 ± 0.46) were produced on 21 days of culture period of seeds on BA containing medium. The multiplication rate decreased significantly with further increase in culture period of seeds with BA (Table 3). Thus, showing that an optimum period of culture into cytokinin was necessary for profuse shoot multiplication and this result is in consistent with the earlier report of Dendrocalamus strictus (Singh et al. 2001; Singh et al. 2014). A continuous proliferating culture could be maintained by culturing the mother explant along with smaller shoots on same medium after removal of elongated shoots (Fig. 1d). Subculturing of mother explants after removal of elongated shoots to the fresh shoot induction medium have also reported for high shoot multiplication in Crateva adansoni (Sharma et al. 2003), Spondias mangifera (Tripathi and Kumari 2010).
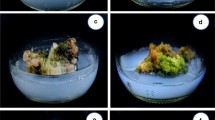
Micropropagation from immature seeds of A. lakoocha.a Initiation of shoot bud from cultured explants. b Cotyledonary node region of immature seed after 4 weeks of culture on medium containing 4.44 µM BA. c Elongated shoots on MS basal medium initially cultured for 3 weeks on 4.44 µM BA d: Shoot multiplication from the mother explant after 4 weeks of subculture. e The shoots rooted on 48 h pulse treatment with 5.0 µM IBA followed by subsequent transfer to MS basal medium. f Three month old well acclimatized plants of A. lakoocha
Rooting of shoots
The shoots with two to three nodes and longer than 2 cm, excised from the proliferating shoot cultures were cultured for root induction. Root induction failed on continuous culture of shoots in agar gelled MS basal and medium with different concentrations of IBA or IAA. However, the shoots could be rooted within 2 weeks, when cultured for 48 h pulse treatment either with IBA or IAA, subsequently transferred to MS basal medium (Table 4). Rahman and Amin (1995) reported rooting of shoots regenerated from seedling node explants of A. lakoocha on 1 week of culture on semisolid medium with IBA followed by transfer to MS basal medium. The auxin pulse treatment to induce rooting in microshoots is also reported in other woody species such as Terminalia arjuna (Pandey and Jaiswal 2002), Garcinia indica (Chabukswar and Deodhar 2005). In this study, among the two auxin examined for root induction, IBA was more efficient in comparison to IAA. The efficacy of IBA over IAA for rooting of in vitro regenerated shoots was also observed earlier in Dendrocalamus strictus (Singh et al. 2001), Embelia ribes (Dhavala and Rathore 2010). All the concentrations of IBA tested were effective in root induction but percent rooting of shoots was significantly low (12%) on 0.5 µM IBA pulse treatment, while with 5.0 µM IBA pulse treatment about 94% shoots rooted and root initiation was noted within 8–10 days (Table 4; Fig. 1e). The in vitro multiplied plantlets were hardened and acclimatized successfully in the soil and of the transplanted plantlets about 85–90% plants survived after acclimatization into soil (Fig. 1f). The soil transplanted plantlets did not show variation and were morphologically uniform.
In conclusion, the present study demonstrates the possibility for mass propagation of Artocarpus lakoocha by immature seed culture and this approach could overcome the regeneration barrier associated with mature seeds, seedling and tissue from the mature plant. All the plants established in soil showed homogeneity without any morphological evidence of somaclonal variation. Studies will be carried out in future for production of medicinally useful compounds from in vitro cultures. To the best of our knowledge, this is the first report in Artocarpus lakoocha where, immature seeds could be successfully used for in vitro regeneration.
References
Chabukswar MM, Deodhar MA (2005) Rooting and hardening of in vitro plantlets of Garcinia indica Chois. Indian J Biotech 4:409–413
Choudhary R, Chaudhury R, Malik SK (2015) Development of an efficient regeneration and rapid clonal multiplication protocol for three different Morus species using dormant buds as explants. J Hortic Sci Biotech 90:245–253
Dhavala A, Rathore TS (2010) Micropropagation of Embelia ribes Burm f. through proliferation of adult plant axillary shoots. Vitro Cell Dev Biol Plant 46:180–191
Giri CC, Shyamkumar B, Anjaneyulu C (2004) Progress in tissue culture, genetic transformation and application of biotechnology to trees: an overview. Trees Struct Funct 18:115–135
Huetteman CA, Preece JE (1993) Thidiazuron: a potent cytokinin for woody plant tissue culture. Plant Cell Tiss Org Cult 33:105–119
Jagtap UB, Bapat VA (2010) Artocarpus: a review of its traditional uses, phytochemistry and pharmacology. J Ethnopharmacol 129:142–166
Joshee N, Bastola DR, Agrawal VP, Yadav AK (1991) Lakoocha: A multipurpose tree of warm climate. In: Whipkey A (ed) Janick J. Trends in new crops and uses ASHS Press, Alexandria, pp 405–406
Kamal NHM, Aziz MA, Kadzimin S, Rashid AA (2018) In vitro mass multiplication of Artocarpus heterophyllus Lam var. Tekam yellow. Pertanika J Trop Agric Sci 41:1289–1314
Lu YF, Zhang LR, Wang YR, Yao YC (2015) Establishment of an efficient in vitro plantlet regeneration system from leaf explants of ornamental crabapple (Malus spp.). J Hortic Sci Biotech 90:585–592
Moharana A, Das A, Subudhi E, Naik SK, Durga P, Barik DP (2017) High frequency shoot proliferation from cotyledonary node of Lawsonia inermis L. and validation of their molecular finger printing. J Crop Sci Biotech 21:405–416
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Pandey S, Jaiswal VS (2002) Micropropagation of Terminalia arjuna Roxb. from cotyledonary nodes. Indian J Expt Biol 10:950–952
Pandey S, Singh M, Jaiswal U, Jaiswal VS (2006) Shoot initiation and multiplication from a mature tree of Terminalia arjuna. In Vitro Cell Dev Biol – Plant 42: 389-393.
Rahman MA, Amin MN (1995) High frequency in vitro plantlets regeneration from an endangered tree- Artocarpus lakoocha Roxb. Plant Tiss Cult 5:137–145
Rani U, Sharma MM, Ismail N, Batra A (2012) In vitro plant regeneration from immature seeds of Murraya koeniggi (L.) Spreng. Indian J Biotech 11:108–110
Sharma PK, Tyagi P, Sharma KC, Kothari SL (2003) Clonal micropropagation of Crateva adansoni (DC) Prodr: a multipurpose tree. Vitro Cell Dev Biol Plant 39:156–160
Singh M, Jaiswal U, Jaiswal VS (2001) Thidiazuron-induced shoot multiplication and plant regeneration in bamboo (Dendrocalamus strictus Nees.). J Plant Biochem Biotech 10:133–137
Singh M, Jaiswal U, Jaiswal VS (2004) In vitro regeneration and improvement in tropical fruit trees: an assessment. In: Srivastava PS, Narula A, Srivastava S (eds) Plant biotechnology and molecular markers. Springer, Dordrecht, pp 228–243
Singh M, Jaiswal VS, Jaiswal U (2014) Thidiazuron-induced anatomical changes and shoot morphogenesis in Dendrocalamus strictus Nees. Can J Pure Appl Sci 8:2901–2904
Snedecor GW, Cochran WG (1989) Statistical methods. Iowa State University Press, Ames
Tripathi M, Kumari N (2010) Micropropagation of tropical fruit tree Spondias mangifera Willd. through direct organogenesis. Acta Physiol Plant 32:1011–1015
Wongkham S, Wongkham C, Boonsiri P, Simasathiansophon S, Trisonthi C, Atisook K (1995) Isolectins from seeds of Artocarpus lakoocha. Phytochem 40:1331–1334
Acknowledgements
Financial assistance vide letter no. F. No. 33-468/2007 (SR) by University Grants Commission, New Delhi, India to MS is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Singh, M., Bhatti, S. & Verma, S.K. Improved plant regeneration method of Artocarpus lakoocha Roxb. from immature seeds. Vegetos 32, 269–274 (2019). https://doi.org/10.1007/s42535-019-00041-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42535-019-00041-4