Abstract
Hypobaric hypoxia (HH), a pathophysiological condition caused by an ascent to a high altitude. It occurs due to a deficiency of oxygen at the tissue level. Saliva is an advantageous biological sample because of easy, low cost and minimally invasive sample collection and processing. Although few reports have documented the effect of long term exposure to hypobaric hypoxia on salivary proteome but short term proteome based study remains uninvestigated. In this study, age-matched male SD rats were exposed to simulated hypobaric hypoxia (~ 25,000 ft, 7620 m) for 1 day (HD1), 3 days (HD3) and 7 days (HD7) followed by iTRAQ based LCMS/MS comparison with normoxic controls. Oxidative stress being the main event of hypobaric hypoxia suggested an initial increase in levels of oxidative stress parameters such as reactive oxygen species (ROS) and lipid peroxidation; and decrease in antioxidant enzymes such as the activity of catalase and reduced glutathione. Based on iTRAQ, out of 67 differentially expressed proteins, 36 (22 up-regulated and 14 down-regulated), 45 (24 up-regulated and 21 down-regulated), and 45 (19 up-regulated and 26 down-regulated) were modulated in HD1, HD3, and HD7 group respectively. Few proteins such as BPI fold-containing family A member 2, cystatin, and carbonic anhydrase 1 showed many folds differential expression in exposed groups as compared to normoxia. Through Ingenuity Pathways analysis, we have observed glucocorticoid receptor signaling and MSP-RON pathway to be most significantly affected. Glucocorticoid receptor signaling and MSP-RON signalling being the most significant pathways involved modulated proteins such as annexin 1, hsp90, keratin, actin-beta, and kallikrein 1. These proteins were found to aid in acclimatization and survival during hypobaric hypoxia. Taken together, this study provided the proof of concept for footprints of hypobaric hypoxia-mediated events during short term exposure through rat salivary proteome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypobaric hypoxia (HH) is characterized by deprived oxygen at tissue level due to the reduced atmospheric partial pressure of oxygen. Multiple clinical manifestations, such as shortness of breath, headache, dizziness, tachycardia, acute mountain sickness (AMS), pulmonary and cerebral edema (HAPE and HACE), are caused by oxidative stress related to hypobaric hypoxia (Hackett and Roach 2004; San et al. 2013; Sharp and Bernaudin 2004; Wilson et al. 2009). Various researchers have suggested the usage of SD rats for understanding human patho-physiologies and diseases (Gumprecht et al. 1993; Wu et al. 2004; Reaven 1991; Cai et al. 2006; Jin et al. 2008; Ramos et al. 2003; Patlolla et al. 2009) and hypobaric hypoxia using different biological samples such as plasma, serum, and tissues (Ma et al. 2015; Ou and Smith 1983; Underwood et al. 1998; Yang et al. 2012; Yuan et al. 2008; Kumar et al. 2014; Anamika Gangwar et al. 2019). Also, rat and human share common protein networks in relation to hypobaric hypoxia as suggested in recent meta-analytical evidence (Paul et al. 2017a). Thus, the SD rat model was selected for studying the effects of hypobaric hypoxia.
Saliva being a source of broad-spectrum of biomolecules (mainly proteins, lipids, hormones, and nucleic acids that originated from various local/systemic sources) holds promising future among diagnostic samples (Hu et al. 2006; Denny et al. 2008). It is a clear slightly acidic biological fluid secreted from major (parotid, submandibular and sublingual glands) and minor salivary glands (labial, buccal, lingual, and palatal glands) (Yoshizawa et al. 2013; Malathi et al. 2016; Lamy et al. 2010). Saliva is an advantageous biological sample because of easy, low cost and minimally invasive sample collection and processing (Yoshizawa et al. 2013; Campo et al. 2006; Chiappin et al. 2007; Kaczor-Urbanowicz et al. 2017; Schafer et al. 2014). In the context of saliva as a biological sample, in vivo model has been used for understanding modulations behind various non-pathological conditions such as psychosocial stress and pathophysiological conditions such as periodontal disease, Sjogren’s syndrome, diabetes, Chagas disease, etc. (Nayar et al. 2016; Alves et al. 1994; Colaianna et al. 2013; Martinez et al. 1979; Nakamura-Kiyama et al. 2014). In terms of hypobaric hypoxia, previous reports suggested decreased secretory capacity and histological structure of rat salivary glands under the effect of chronic hypoxia (Elverdin et al. 1995; Scott and Gradwell 1989). Also, a study suggested three months of chronic continuous hypoxia exposure to rats resulted in lower gum density, alveolar bone loss, and decreased antioxidant capacity that indicated a deleterious effect on oral health (Terrizzi et al. 2016).
Due to inadequate research on rat saliva proteome during short term exposure to hypobaric hypoxia, it was encouraged to analyze salivary proteome in response to hypobaric hypoxia through proteomics approach in an in vivo model for identifying multiple molecular signatures involved in different systemic processes. In this study, age-matched male SD rats were divided into four groups such as normoxic controls (N) and rats exposed to hypobaric hypoxia (~ 25,000 ft, 7620 m) for a short duration of 1 day (HD1), 3 days (HD3) and 7 days (HD7). After exposure, saliva was collected from the experimental groups and biochemical parameters were performed. Following this, the saliva samples from each experimental group were labeled with iTRAQ reagent and processed for LC–MS/MS. Then, differentially expressed proteins in the exposed groups as compared to normoxic controls were subjected to IPA software to identify relevant canonical pathways.
Materials and methods
All the chemicals unless specified were obtained from Sigma-Aldrich, USA.
Collection of whole rat saliva
Experimental animals used were age-matched male Sprague–Dawley rats weighing 200–230 g housed in polypropylene cages with paddy husk as substratum at 25 ± 5 ˚C with humidity at 50 ± 5% and a day-night cycle of 12 h each. Approval for animal procedures and experimental protocols were affirmed by Institutional Animal Ethics Committee (Authorization Number: 27/1999/CPCSEA) according to the standards set forth in the Guide for the Care and Use of Laboratory Animals (National Academy of Science, Washington, D.C.).
Twenty-four rats (n = 24) were randomly divided into four groups of six rats each, first normoxic controls (animals without simulated hypobaric hypoxia exposure; 500 ft), HD1 (animals exposed to simulated hypobaric hypoxia equivalent to 25,000 ft for 1 day), HD3 (animals exposed to simulated hypobaric hypoxia equivalent to 25,000 ft for 3 days) and HD7 (animals exposed to simulated hypobaric hypoxia equivalent to 25,000 ft for 7 days). Animals received a pre-exposure at 15,000 ft for 10 h followed by 1 h exposure at 500 ft prior to exposure of 25,000 ft for 24 h (Paul et al. 2017b).
Hypobaric hypoxia exposure and sample collection
Hypobaric Hypoxia exposure simulation was performed in custom-designed hypobaric hypoxia simulation chamber (7 star systems, Delhi, India) with a constant temperature and humidity of 25 ± 5 ˚C and 50 ± 5%, respectively and an ascent rate of 589 m/min during the exposure. The airflow of 2 L/min was maintained in the chamber and food and water was provided to the animals inside the chamber. Immediately after completion of the exposure, individual rat saliva secretion was induced with an intraperitoneal injection of pilocarpine and collected by micropipette from the mouth followed by centrifugation at 16,000×g for 5 min at 4 °C to remove particulate matter (Lamy et al. 2010; Costa et al. 2008). Protease inhibitor cocktail (Cat # P8340, Sigma, USA) was added to prevent protease activity. All the samples were stored in -800C until further use.
Estimation of oxidative stress parameters
Reactive oxygen species (ROS) estimation
In saliva, estimation of Reactive Oxygen Species (ROS) levels was performed using a fluorescent dye 2′, 7′-Dichlorofluorescin diacetate (DCFDA) (Cat. No. D6883). In the presence of intracellular reactive oxygen species, the dye enters through the cell membrane and cleaves into 2, 7-dichlorofluorescein by the action of intracellular esterase enzymes and produces fluorescence. The generated fluorescence is directly proportional to the reactive oxygen species levels (Wang and Joseph 1999). In brief, 150 µl of undiluted saliva along with 5 µl of 2 mM Dichlorofluorescin diacetate was incubated for 40 min at room temperature in amber tubes in dark. Following incubation, 2 ml of PBS was added to the tubes and 200 µl of each sample was added to 96-well plate (PBS as blank). Fluorescence was measured at excitation and emission wavelengths of 485 nm and 531 nm respectively using an ELISA plate reader (Synergy H1 microplate reader, BioTek USA).
Lipid peroxidation estimation using thiobarbituric acid reactive substances (TBARS) estimation
Thiobarbituric acid reactive substances (TBARS) assay was performed to estimate lipid peroxidation in saliva. It was measured directly using QuantiChrom™ TBARS Assay Kit (Cat. No. DTBA-100, Bioassays Systems, CA, USA) as suggested by the manufacturer’s protocol. In short, standards and samples were prepared by diluting with milli-Q and precipitating with ice-cold 10% TCA respectively. Following this, TBA was added to the diluted standards and saliva supernatant and incubated at 100 ℃ for 1 h in a water bath. The mixtures were then allowed to cool to RT. Afterwards, 200 µl of the mixtures were poured onto 96-well plate (TBA as blank) and the absorbance was measured at 535 nm using a spectrophotometer (EON Biotech, USA).
Measurement of antioxidant status
Estimation of reduced glutathione
In saliva samples, levels of reduced glutathione were measured by microplate assay kit (Cat. No. CS0260) as suggested by the manufacturer. In short, standards were reconstituted and serially diluted with milli-Q and 5% 5-sulfosalicylic acid (SSA) respectively. Standards and samples along with working mixture (assay buffer, enzyme and 5, 5-dithiobis-2-nitrobenzoic acid (DTNB) solution) were incubated for 5 min at RT followed by addition of NADPH solution to each well. Kinetic, as well as end-point absorbance, were recorded starting from 0 to 5 min (1 min interval) at 412 nm using an ELISA plate reader (EON Biotech, USA).
Estimation of catalase activity
In saliva, the activity of catalase enzyme was estimated using EnzyChromTM Catalase Assay Kit (Cat No. ECAT-100, Bioassay systems, USA) according to the manufacturer’s instructions. In brief, 4.8 mM H2O2 was used to prepare standards. 10 µl of the standards or samples along with 90 µl of 50 µM H2O2 substrate were added onto the microplate and incubated for 30 min at RT. Further, the plate was incubated with 100 µl of detection reagent for 10 min at RT and the absorbance was recorded at 570 nm using a microplate reader (EON Biotech, USA).
Measurement of nitric oxide (NO) metabolites
Measurement of nitric oxide (NO) metabolites such as nitrate and nitrite was performed in saliva samples by a fluorometric assay using Oxiselect nitric oxide assay Kit (Cat No. STA-801, Cell Biolabs, CA, USA) according to manufacturer’s instructions. In short, standards were diluted with milliq and samples were filtered using zeba spin desalting columns (Cat No. 89890, Thermo Fischer Scientific, USA) as per the manufacturer’s protocol prior to NO estimation. 50 µl of standards or samples along with 50 µl of reaction mixture containing assay buffer and enzyme cofactor was added onto the microtiter plate and incubated for 1 h at RT on a rocking platform in dark. 20 µl of DAN fluorometric probe was then added to the wells and the plate was incubated for 10 min followed by incubation with 10 µl of stop solution for another 10 min. Fluorescence was recorded at excitation and emission wavelengths of 362 nm and 440 nm respectively using a spectrophotometer (Synergy H1 microplate reader, BioTek USA).
High throughput proteomics using LC–MS/MS
Sample preparation
100 μg of protein from saliva samples (n = 6 from each group) was dispensed in 30 µl of dissolution buffer and processed for LC–MS/MS using iTRAQ kit (Cat No. 4352135, ABSciex, USA). Processing includes three steps: (a) denaturation using 1 µl of denaturant, (b) reduction by incubation with 2 µl of reducing agent at 60 °C for 1 h and (c) alkylation using 1 µl of cysteine blocking agent followed by overnight in-solution trypsin digestion using trypsin singles proteomics grade (Cat no. T7575). The digested peptides were then labeled with iTRAQ reagents (activated with isopropanol) and incubated for 2 h at RT. SCX cartridge (5 micron, 300 Å bead from ABSciex, USA) was used to fractionate samples into fractions eluted at 35 mM, 50 mM, 75 mM, 100 mM, 125 mM, 150 mM, 250 mM, 350 mM, 500 mM ammonium formate buffer (ammonium formate, 30% v/v ACN and 0.1% formic acid; pH = 2.9) and got analyzed individually on quadrupole-TOF hybrid mass (Triple TOF 5600 & 6600, Sciex USA) spectrometer coupled to an EksigentNanoLC-Ultra 2D plus system.
Mass spectrometry (LC–MS/MS)
Each sample fraction was poured onto a trap column (200 µm × 0.5 mm) and desalted at flow rate 2 µl/min for 45 min followed by peptides separation using a nano-C18 column (75 µm × 15 cm) through a gradient method with buffer A (99.9% LC–MS water + 0.1% formic acid) and buffer B (99.9% acetonitrile + 0.1% formic Acid). Data were obtained in an Information dependent acquisition mode with MS settings such as nebulizing gas of 25; a curtain gas of 25; an Ion spray voltage of 2400 V and heater interface temperature of 130 °C followed by TOFMS scan in the mass range of 400–1600 m/z with an accumulation time of 250 ms and, the MS/MS product ion scan in the mass range of 100–1800 m/z for 70 ms with a total cycle time of 2.05 s approximately. Parent ions with abundance > 150 cps and with a charge state of + 2 to + 5 were selected for MS/MS fragmentation followed by exclusion of its mass and isotopes for 3 s. Further, MS/MS spectra were obtained with an adjust collision energy setting with high sensitivity mode when using iTRAQ reagent. Abundance Ratios > 1.5 were classified as up-regulated, < 0.67 were classified as down-regulated. Ratios from 1.5–0.67 were considered moderate to no changes. The experiment was performed in triplicates.
Pathway analysis of proteomics data
Results obtained from high throughput LC–MS/MS were sorted based on FDR < 1 (false discovery rate) and p value < 0.05. Selected proteins with their respective abundance ratios were analyzed using a network analysis tool, Ingenuity Pathway analysis (IPA, Qiagen) with an inbuilt statistical analysis package. Fold change ≥ 1.5 was set as a cutoff for various programs such as canonical pathways, disease networks and functions, and protein networks. These networks played an essential role in suggesting key changes in cellular events, biochemical processes, and molecular cascades. To predict the directionality of the cellular event, a significant positive and negative z-score was used. Top canonical pathways and molecular events were selected using minimum p value criteria.
Results
Redox signaling including oxidative parameters and antioxidant status
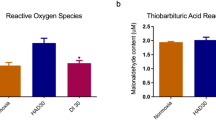
The main event of hypobaric hypoxia is oxidative stress, thus, we analyzed the levels of oxidative stress parameters. In oxidative stress parameters, the levels of reactive oxygen species (ROS) and lipid peroxidation were measured in rat saliva samples. The levels of ROS were found to be increased in the HD1 group followed by a decrease in HD7 group as compared to normoxic controls (Fig. 1a). The lipid peroxidation levels were found to be normalized in HD1 group, increased in HD3 group followed by a slight decrease in HD7 group as compared to normoxic controls (Fig. 1b).
Modulations in oxidative stress parameters in rat saliva. a Levels of reactive oxygen species were measured in rat saliva showing an increase in the levels of ROS at HD1 and HD3 groups in rat saliva as compared to N. b Lipid peroxides levels were measured and observed to be increased at HD3 followed by a decreased value in HD7 in rat saliva as compared to N. Results are expressed as Mean concentration ± SEM. Mean was calculated from three separate experiments (***represents p < 0.001 with respect to N)
Antioxidants provide protection to the damage caused by oxidative stress, thus, various antioxidants such as the activity of catalase and reduced glutathione were observed in rat saliva. Catalase activity was not found to be modulated in the HD1 group followed by an increase from HD3 group to HD7 group as compared to normoxic controls (Fig. 2a). The levels of reduced glutathione also showed different patterns such as the levels were decreased from normoxic controls to HD1 group which then increases till the HD7 group (Fig. 2b).
Antioxidant status in response to hypobaric hypoxia. a Catalase activity was measured in rat saliva showing an increase in the levels of catalase activity at HD3 and HD7 groups as compared to N. b Levels of reduced glutathione were measured and observed to be decreased at HD1 followed by an increased value in HD3 till HD7 in rat saliva as compared to N. Results are expressed as Mean concentration ± SEM. Mean was calculated from three separate experiments (*represents p < 0.05, **represents p < 0.01 and ***represents p < 0.001 with respect to N)
Nitrogen oxides are circulating vasoactive molecules that enable greater blood flow and oxygen delivery through vasodilation, thus, the levels of NOx were measured in rat saliva samples. The levels of NOx were found to be increased during hypoxia from normoxic controls to HD1 group and then decreased at HD7 group (Fig. 3).
Biochemical estimation of total nitrate + nitrite (NOx). Levels showing an increase in the levels of NO from N to HD3 group followed by a decreased value in HD7 groups as compared to N. Results are expressed as Mean concentration ± SEM. Mean was calculated from three separate experiments (***represents p < 0.001 with respect to N)
Quantitative rat saliva proteome analysis using LC–MS/MS
Following the investigation of rat salivary proteome using iTRAQ labeled LC–MS/MS analysis, a huge list of proteins with quantification was obtained from LC–MS/MS to understand the casual events occurring in hypobaric hypoxia by simulated high altitude. Sixty-seven proteins were differentially expressed in the experimental groups as compared to normoxia (Table 1).
From 67 proteins, 36 (22 up-regulated and 14 down-regulated), 45 (24 up-regulated and 21 down-regulated), and 45 (19 up-regulated and 26 down-regulated) proteins were differentially expressed in HD1, HD3 and HD7 group respectively (Fig. 4a). 23 proteins were common between all the three groups while 5 proteins were common between HD1 and HD3 groups, 10 proteins were common between HD3 and HD7 and 5 proteins were common between HD1 and HD7 groups (Fig. 4b). Based on iTRAQ data, proteins such as BPI fold-containing family A member 2, cystatin and carbonic anhydrase 1 showed many folds differential expression in exposed groups as compared to normoxia. Upon pathway analysis through Ingenuity Pathway Analysis (IPA) software, we observed Glucocorticoid receptor signaling to be the most significant pathways based on p-value (Fig. 4c).
Overview of rat salivary proteome along with affected pathways. a The total number of up-regulated (red) and down-regulated (green) proteins along with normalized proteins (blue) identified in HD1, HD3, and HD7 groups during LCMS/MS (iTRAQ labeled) analysis of rat saliva. Abundance ratio value greater than 1.5 fold was considered up-regulation while value lesser than 0.67 was considered as down-regulation. b Venn diagram of overlapping up- and down-regulated proteins among the proteins in HD1(blue), HD3 (yellow) and HD7 (green) groups using Oliveros, J.C. (2007–2015) Venny. An interactive tool for comparing lists with Venn's diagrams. https://bioinfogp.cnb.csic.es/tools/venny/index.html. c Ingenuity pathway analysis (IPA) of rat salivary proteins revealing Glucocorticoid receptor signaling to be the most significant pathways in HD1, HD3 and HD7 groups based on p value < 0.001
Further, the glucocorticoid receptor signaling pathway was investigated with overlaid differentially expressed proteins (Fig. 5a). The proteins involved were annexin 1, heat shock protein 90, and keratin. Their fold change values were represented in the form of a graph (Fig. 5b).
In glucocorticoid receptor signaling, annexin 1, also known as lipocortin 1 (possess anti-inflammatory properties) was observed to be decreased from normoxia to HD7 group. Another protein, hsp90, a chaperone protein was found to be normalized in HD1 followed by a decreased expression in HD3 and HD7 groups. Fold change values of different types of keratin cytoskeletal proteins were taken into account through IPA and it was observed that in HD1 group, KRT16 and KRT13 showed decreased expression; KRT14 and KRT4 were normalized; and KRT1 and KRT76 showed increased expression as compared to normoxia. In HD3 and HD7 groups, all types of keratin showed decreased expression as compared to normoxia.
Another important pathway observed was MSP-RON signaling. This pathway was significantly modulated in the HD1 group and investigated further for exploring significant proteins having differential expression in the exposure groups as compared to normoxia (Fig. 6). Two of the proteins were found to be involved in this pathway such as actin and kallikrein 1. In terms of expression, actin showed decreased expression in HD1 and HD7 while it was normalized at the HD3 group of exposure. And, kallikrein 1 showed increased expression from normoxia to HD3 group followed by a decrease in HD7 group.
Discussion
Hypobaric hypoxia is known to be associated with oxidative stress and leads to various clinical manifestations such as acute mountain sickness (AMS), high altitude pulmonary edema (HAPE) and high altitude cerebral edema (HACE) and non-clinical conditions such as acclimatization and adaptation. Proteomic markers have been studied during these pathophysiological and non-pathological conditions indicating a strong relationship between apparent changes and underlying molecular mechanisms (Ahmad et al. 2011, 2013, 2014; Jefferson et al. 2004; Magalhaes et al. 2005; Padhy et al. 2013; Sharma et al. 2013; Vij et al. 2005; Gaur et al. 2018; Jain et al. 2018, 2019). Previously suggested, oxidative stress involves the activation of inflammatory pathways that were found to be associated with various conditions such as cancer, cardiovascular, neurological, and pulmonary pathologies (Reuter et al. 2010). The differential expression of proteins holds promise for the assessment of underlying mechanisms based on putative protein markers, but, most of the proteins detected earlier were either systemic or specific to particular tissues and the detection methods were invasive. Also studies focussed on the effect of hypobaric hypoxia before seven days are limited. Therefore, the focus shifted to a non-invasive approach wherein saliva samples could be utilized using an animal model.
In this study, an important aspect, redox homeostasis, a key process in hypoxia has been explored in saliva through estimation of oxidative stress parameters and anti-oxidants levels using biochemical assays in the experimental groups from N to HD7. Reactive oxygen species were found to be highest in the HD1 group. Also, lipid peroxides were estimated and observed to be increased at HD3 followed by normalization at HD7. Another marker, nitric oxide, responsible for vasodilation during hypoxia, was increased from HD1 to HD3 followed by normalization at HD7. Antioxidant enzymes such as catalase and reduced glutathione showed opposite patterns to oxidative stress indicators. Earlier reports suggested oxidative stress occurs due to the imbalance between prooxidants and antioxidants which have been proved by our study also (Coimbra-Costa et al. 2017; Debevec et al. 2017).
Also, we have found the maximum number of differentially expressed proteins in HD3 and HD7 groups. Based on iTRAQ data, proteins such as BPI fold-containing family A member 2, cystatin and carbonic anhydrase 1 showed many folds differential expression in exposed groups as compared to normoxia. BPI fold-containing family A member 2 is a protein highly up-regulated in the exposure groups as compared to normoxic controls. Earlier researchers suggested this protein as an early biomarker of acute kidney injury (Honore et al. 2018; Kota et al. 2017). Increased expression of other proteins closely linked to cystatin and carbonic anhydrase 1 have already been proved by our previous studies in response to hypobaric hypoxia in the human model (Jain et al. 2018, 2020).
Upon IPA, the most significantly modulated pathway was glucocorticoid receptor signalling. In glucocorticoid receptor signalling pathway, major differentially modulated proteins were annexin 1, heat shock protein 90, and keratin. Previously, researchers suggested that inhibition of glucocorticoid receptors may provide protection to HH induced memory impairment (Baitharu et al. 2013). In terms of differentially expressed proteins, annexin 1, an immunomodulatory and anti-inflammatory protein plays a role as homeostatic protein that regulates essential transcription factors and miRNAs. It has been observed to be a clinical marker for gingivitis in pregnant women, coronary heart disease (Hassan et al. 2018; Sarndahl et al. 2010). Annexin A1 has also been found to be a potential marker and therapeutic agent for asthma using plasma as a biological fluid (Lee et al. 2018). Another protein, hsp90, a cytoprotective chaperone was recently recommended as a potential biochemical marker for screening of individuals susceptible or tolerant to acute hypobaric hypoxia (Jain et al. 2013). This molecular chaperone facilitates protein maturation, stabilization of aggregation-prone proteins, quality control of misfolded proteins, and assists in keeping proteins in activation-competent conformations. Many of the proteins chaperoned by Hsp90 (Hsp90 clients) are essential for the progression of various diseases, including cancer, Alzheimer's disease, and other neurodegenerative diseases, as well as viral and bacterial infections (Zuehlke et al. 2018). Also, it helps in mediating differential hypoxic tolerance by enhancing the ability to sustain survival under hypobaric hypoxia (Jain et al. 2014). Previous studies also demonstrated that plasma Hsp90α protein levels are useful as a diagnostic biomarker in lung cancer and acute lymphoblastic leukemia (ALL) (Shi et al. 2014; Milani et al. 2015). A study by St. Pierre Schneider B et. al. suggested, the third set of proteins; keratins, expressed in the differentiated epithelial layers of skin, oral cavity and squamous stomach involved in epithelial homeostasis have shown to be down-regulated in hypobaric hypoxia caused by long-distance air travel (Schneider et al. 2013; Sequeira et al. 2018). Also, Srikant Ambatipudi et. al. suggested down-regulation of krt76 is linked to oral carcinogenesis (Ambatipudi et al. 2013).
Another significant pathway observed was MSP-RON signalling. Earlier researchers have recommended the MSP-RON pathway as a therapeutic target for a variety of cancers because of its role in the pathogenesis of cancer. This pathway has been extensively studied in various experimental model systems (Yao et al. 2013). In MSP-RON signalling, two proteins such as actin and kallikrein 1 were differentially expressed in exposure groups as compared to normoxic controls. Previously, actin, a cytoskeletal protein has been observed to be down-regulated during hypobaric hypoxia and HAPE (Hernandez et al. 2013; Mairbaurl et al. 1985; Peinado et al. 2014). A recent study by Li K et. al. suggested over-expression of kallikrein 1, a serine protease may be closely linked to hypertension, inflammation, obesity and other gastric injuries associated with high altitude-induced polycythemia (Li et al. 2017).
Our observations also coincide with the earlier reports that suggested these pathways and involved proteins may have a role in regulating/modulating the hypoxic response and may help in the proteome based assessment of hypobaric hypoxia.
Conclusion
In conclusion, this study provided proof of concept for understanding hypobaric hypoxia-mediated events through rat salivary proteome. Glucocorticoid receptor signaling and MSP-RON signalling are the most significant pathways involving modulated proteins such as annexin 1, hsp90, keratin, actin-beta, and kallikrein 1. These proteins were found to aid in acclimatization and survival during hypobaric hypoxia.
Abbreviations
- iTRAQ:
-
Isobaric tags for relative and absolute quantification
- LCMS/MS:
-
Liquid chromatography based mass spectrometry
- HH:
-
Hypobaric hypoxia
References
Ahmad Y, Shukla D, Garg I, Sharma NK, Saxena S, Malhotra V, Bhargava K (2011) Identification of haptoglobin and apolipoprotein AI as biomarkers for high altitude pulmonary edema. Funct Integr Genom 11:407
Ahmad Y, Sharma NK, Garg I, Ahmad MF, Sharma M, Bhargava K (2013) An insight into the changes in human plasma proteome on adaptation to hypobaric hypoxia. PLoS ONE 8:e67548
Ahmad Y, Sharma NK, Ahmad MF, Sharma M, Garg I, Bhargava K (2014) Proteomic identification of novel differentiation plasma protein markers in hypobaric hypoxia-induced rat model. PLoS ONE 9:e98027
Alves JB, Alves MS, Naito Y (1994) Induction of synthesis of the rat cystatin S protein by the submandibular gland during the acute phase of experimental Chagas disease. Mem Inst Oswaldo Cruz 89:81–85
Ambatipudi S, Bhosale PG, Heath E, Pandey M, Kumar G, Kane S, Patil A, Maru GB, Desai RS, Watt FM, Mahimkar MB (2013) Downregulation of keratin 76 expression during oral carcinogenesis of human, hamster and mouse. PLoS ONE 8:e70688
Anamika Gangwar SJ, Paul S, Ahmad Y, Bhargava K (2019) Biofluids in hypobaric hypoxia: best possible use, investigative strategies and putative markers. J Proteins Proteom 10:191–206
Baitharu I, Deep SN, Jain V, Prasad D, Ilavazhagan G (2013) Inhibition of glucocorticoid receptors ameliorates hypobaric hypoxia induced memory impairment in rat. Behav Brain Res 240:76–86
Cai X, Wong YF, Zhou H, Xie Y, Liu ZQ, Jiang ZH, Bian ZX, Xu HX, Liu L (2006) The comparative study of Sprague-Dawley and Lewis rats in adjuvant-induced arthritis. Naunyn Schmiedebergs Arch Pharmacol 373:140–147
Campo J, Perea MA, del Romero J, Cano J, Hernando V, Bascones A (2006) Oral transmission of HIV, reality or fiction? An update. Oral Dis 12:219–228
Chiappin S, Antonelli G, Gatti R, De Palo EF (2007) Saliva specimen: a new laboratory tool for diagnostic and basic investigation. Clin Chim Acta 383:30–40
Coimbra-Costa D, Alva N, Duran M, Carbonell T, Rama R (2017) Oxidative stress and apoptosis after acute respiratory hypoxia and reoxygenation in rat brain. Redox Biol 12:216–225
Colaianna M, Schiavone S, Zotti M, Tucci P, Morgese MG, Backdahl L, Holmdahl R, Krause KH, Cuomo V, Trabace L (2013) Neuroendocrine profile in a rat model of psychosocial stress: relation to oxidative stress. Antioxid Redox Signal 18:1385–1399
da Costa G, Lamy E, Capelae SF, Andersen J, Sales BE, Coelho AV (2008) Salivary amylase induction by tannin-enriched diets as a possible countermeasure against tannins. J Chem Ecol 34:376–387
Debevec T, Millet GP, Pialoux V (2017) Hypoxia-induced oxidative stress modulation with physical activity. Front Physiol 8:84
Denny P, Hagen FK, Hardt M, Liao L, Yan W, Arellanno M, Bassilian S, Bedi GS, Boontheung P, Cociorva D, Delahunty CM, Denny T, Dunsmore J, Faull KF, Gilligan J, Gonzalez-Begne M, Halgand F, Hall SC, Han X, Henson B, Hewel J, Hu S, Jeffrey S, Jiang J, Loo JA, Ogorzalek Loo RR, Malamud D, Melvin JE, Miroshnychenko O, Navazesh M, Niles R, Park SK, Prakobphol A, Ramachandran P, Richert M, Robinson S, Sondej M, Souda P, Sullivan MA, Takashima J, Than S, Wang J, Whitelegge JP, Witkowska HE, Wolinsky L, Xie Y, Xu T, Yu W, Ytterberg J, Wong DT, Yates JR, Fisher SJ (2008) The proteomes of human parotid and submandibular/sublingual gland salivas collected as the ductal secretions. J Proteome Res 7:1994–2006
Elverdin JC, Chiarenza AP, Frid AB, Giglio MJ (1995) Effects of chronic hypoxia on the secretory responses of rat salivary glands. Arch Oral Biol 40:459–462
Gaur P, Saini S, Vats P, Kumar B (2018) Regulation, signalling and functions of hormonal peptides in pulmonary vascular remodelling during hypoxia. Endocrine 59:466–480
Gumprecht LA, Long CR, Soper KA, Smith PF, Haschek-Hock WM, Keenan KP (1993) The early effects of dietary restriction on the pathogenesis of chronic renal disease in Sprague-Dawley rats at 12 months. Toxicol Pathol 21:528–537
Hackett PH, Roach RC (2004) High altitude cerebral edema. High Alt Med Biol 5:136–146
Hassan MN, Belibasakis GN, Gumus P, Ozturk VO, Emingil G, Bostanci N (2018) Annexin-1 as a salivary biomarker for gingivitis during pregnancy. J Periodontol 89:875–882
Hernandez R, Blanco S, Peragon J, Pedrosa JA, Peinado MA (2013) Hypobaric hypoxia and reoxygenation induce proteomic profile changes in the rat brain cortex. Neuromol Med 15:82–94
Honore PM, De Bels D, Spapen HD (2018) BPI fold-containing family a member 2 as a biomarker of acute kidney injury-close but no (clinical) cigar? Ann Transl Med 6:191
Hu S, Loo JA, Wong DT (2006) Human body fluid proteome analysis. Proteomics 6:6326–6353
Jain K, Suryakumar G, Prasad R, Ganju L (2013) Upregulation of cytoprotective defense mechanisms and hypoxia-responsive proteins imparts tolerance to acute hypobaric hypoxia. High Alt Med Biol 14:65–77
Jain K, Suryakumar G, Ganju L, Singh SB (2014) Differential hypoxic tolerance is mediated by activation of heat shock response and nitric oxide pathway. Cell Stress Chaperones 19:801–812
Jain S, Ahmad Y, Bhargava K (2018) Salivary proteome patterns of individuals exposed to high altitude. Arch Oral Biol 96:104–112
Jain S, Paul S, Meena RN, Gangwar A, Panjwani U, Ahmad Y, Bhargava K (2019) Saliva panel of protein candidates: a comprehensive study for assessing high altitude acclimatization. Nitric Oxide 95:1–11
Jain S, Paul S, Meena RN, Gangwar A, Panjwani U, Ahmad Y, Bhargava K (2020) Saliva panel of protein candidates: a comprehensive study for assessing high altitude acclimatization. Nitric Oxide 95:1–11
Jefferson JA, Simoni J, Escudero E, Hurtado ME, Swenson ER, Wesson DE, Schreiner GF, Schoene RB, Johnson RJ, Hurtado A (2004) Increased oxidative stress following acute and chronic high altitude exposure. High Alt Med Biol 5:61–69
Jin F, Wu Q, Lu YF, Gong QH, Shi JS (2008) Neuroprotective effect of resveratrol on 6-OHDA-induced Parkinson's disease in rats. Eur J Pharmacol 600:78–82
Kaczor-Urbanowicz KE, Martin Carreras-Presas C, Aro K, Tu M, Garcia-Godoy F, Wong DT (2017) Saliva diagnostics - Current views and directions. Exp Biol Med (Maywood) 242:459–472
Kota SK, Pernicone E, Leaf DE, Stillman IE, Waikar SS, Kota SB (2017) BPI fold-containing family a member 2/parotid secretory protein is an early biomarker of AKI. J Am Soc Nephrol 28:3473–3478
Kumar S, Sharma P, Bansal A, Sharma PC, Aggarwal KK (2014) Hypobaric hypoxia-mediated protein expression in plasma of susceptible & tolerant rats. Indian J Med Res 140:756–765
Lamy E, Graca G, da Costa G, Franco C, Baptista ES, Coelho AV (2010) Changes in mouse whole saliva soluble proteome induced by tannin-enriched diet. Proteome Sci 8:65
Lee SH, Lee PH, Kim BG, Seo HJ, Baek AR, Park JS, Lee JH, Park SW, Kim DJ, Park CS, Jang AS (2018) Annexin A1 in plasma from patients with bronchial asthma: its association with lung function. BMC Pulm Med 18:1
Li K, Gesang L, Dan Z, Gusang L (2017) Transcriptome reveals the overexpression of a kallikrein gene cluster (KLK1/3/7/8/12) in the Tibetans with high altitude-associated polycythemia. Int J Mol Med 39:287–296
Ma TT, Wang Y, Zhou XL, Jiang H, Guo R, Jia LN, Chang H, Gao Y, Yao XY, Gao ZM, Pan L (2015) Research on rat models of hypobaric hypoxia-induced pulmonary hypertension. Eur Rev Med Pharmacol Sci 19:3723–3730
Magalhaes J, Ascensao A, Soares JM, Ferreira R, Neuparth MJ, Marques F, Duarte JA (2005) Acute and severe hypobaric hypoxia increases oxidative stress and impairs mitochondrial function in mouse skeletal muscle. J Appl Physiol 99:1247–1253
Mairbaurl H, Schwobel F, Hoschele S, Maggiorini M, Gibbs S, Swenson ER, Bartsch P (2003) Altered ion transporter expression in bronchial epithelium in mountaineers with high-altitude pulmonary edema. J Appl Physiol 95:1843–1850
Malathi ER, Aravindha Babu N, Jimson S (2016) Saliva as a diagnostic tool. Biomed Pharmacol J 9:867
Martinez JR, Martinez AM, Garrett L, Korman P (1979) Chronically reserpinized rat as a model for cystic fibrosis: Na+ transport inhibitory effect in submaxillary saliva. Pediatr Res 13:1156–1159
Milani M, Laranjeira AB, de Vasconcellos JF, Brandalise SR, Nowill AE, Yunes JA (2015) Plasma Hsp90 level as a marker of early acute lymphoblastic leukemia engraftment and progression in mice. PLoS ONE 10:e0129298
Nakamura-Kiyama M, Ono K, Masuda W, Hitomi S, Matsuo K, Usui M, Nakashima K, Yokota M, Inenaga K (2014) Changes of salivary functions in experimental periodontitis model rats. Arch Oral Biol 59:125–132
Nayar G, Gauna A, Chukkapalli S, Velsko I, Kesavalu L, Cha S (2016) Polymicrobial infection alter inflammatory microRNA in rat salivary glands during periodontal disease. Anaerobe 38:70–75
Ou LC, Smith RP (1983) Probable strain differences of rats in susceptibilities and cardiopulmonary responses to chronic hypoxia. Respir Physiol 53:367–377
Padhy G, Sethy NK, Ganju L, Bhargava K (2013) Abundance of plasma antioxidant proteins confers tolerance to acute hypobaric hypoxia exposure. High Alt Med Biol 14:289–297
Patlolla AK, Barnes C, Yedjou C, Velma VR, Tchounwou PB (2009) Oxidative stress, DNA damage, and antioxidant enzyme activity induced by hexavalent chromium in Sprague-Dawley rats. Environ Toxicol 24:66–73
Paul S, Bhargava K, Ahmad Y (2017a) The meta-analytical paradigm in an in silico hybrid: Pathways and networks perturbed during exposure to varying degrees of hypobaric hypoxia. Proteom Clin Appl. https://doi.org/10.1002/prca.201600160
Paul S, Gangwar A, Bhargava K, Ahmad Y (2017b) STAT3-RXR-Nrf2 activates systemic redox and energy homeostasis upon steep decline in pO2 gradient. Redox Biol 14:423–438
Peinado MA, Hernandez R, Peragon J, Ovelleiro D, Pedrosa JA, Blanco S (2014) Proteomic characterization of nitrated cell targets after hypobaric hypoxia and reoxygenation in rat brain. J Proteomics 109:309–321
Ramos EJ, Xu Y, Romanova I, Middleton F, Chen C, Quinn R, Inui A, Das U, Meguid MM (2003) Is obesity an inflammatory disease? Surgery 134:329–335
Reaven GM (1991) Insulin resistance, hyperinsulinemia, hypertriglyceridemia, and hypertension. Parallels between human disease and rodent models. Diabetes Care 14:195–202
Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB (2010) Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med 49:1603–1616
San T, Polat S, Cingi C, Eskiizmir G, Oghan F, Cakir B (2013) Effects of high altitude on sleep and respiratory system and theirs adaptations. Sci World J 2013:241569
Sarndahl E, Bergstrom I, Nijm J, Forslund T, Perretti M, Jonasson L (2010) Enhanced neutrophil expression of annexin-1 in coronary artery disease. Metabolism 59:433–440
Schafer CA, Schafer JJ, Yakob M, Lima P, Camargo P, Wong DT (2014) Saliva diagnostics: utilizing oral fluids to determine health status. Monogr Oral Sci 24:88–98
Schneider PB, Moonie S, Fulkerson ND, Nicholas J, Bammler T, Voss JG (2013) Simulated flight, muscle genetics, and inflammatory indicators in mice. Aviat Space Environ Med 84:840–844
Scott J, Gradwell E (1989) A quantitative study of the effects of chronic hypoxia on the histological structure of the rat major salivary glands. Arch Oral Biol 34:315–319
Sequeira I, Neves JF, Carrero D, Peng Q, Palasz N, Liakath-Ali K, Lord GM, Morgan PR, Lombardi G, Watt FM (2018) Immunomodulatory role of Keratin 76 in oral and gastric cancer. Nat Commun 9:3437
Sharma NK, Sethy NK, Bhargava K (2013) Comparative proteome analysis reveals differential regulation of glycolytic and antioxidant enzymes in cortex and hippocampus exposed to short-term hypobaric hypoxia. J Proteom 79:277–298
Sharp FR, Bernaudin M (2004) HIF1 and oxygen sensing in the brain. Nat Rev Neurosci 5:437–448
Shi Y, Liu X, Lou J, Han X, Zhang L, Wang Q, Li B, Dong M, Zhang Y (2014) Plasma levels of heat shock protein 90 alpha associated with lung cancer development and treatment responses. Clin Cancer Res 20:6016–6022
Terrizzi AR, Fernandez-Solari J, Lee CM, Conti MI, Martinez MP (2016) Deleterious effect of chronic continuous hypoxia on oral health. Arch Oral Biol 72:1–7
Underwood DC, Bochnowicz S, Osborn RR, Louden CS, Hart TK, Ohlstein EH, Hay DW (1998) Chronic hypoxia-induced cardiopulmonary changes in three rat strains: inhibition by the endothelin receptor antagonist SB 217242. J Cardiovasc Pharmacol 31(Suppl 1):S453–S455
Vij AG, Dutta R, Satija NK (2005) Acclimatization to oxidative stress at high altitude. High Alt Med Biol 6:301–310
Wang H, Joseph JA (1999) Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med 27:612–616
Wilson MH, Newman S, Imray CH (2009) The cerebral effects of ascent to high altitudes. Lancet Neurol 8:175–191
Wu LY, Juan CC, Ho LT, Hsu YP, Hwang LS (2004) Effect of green tea supplementation on insulin sensitivity in Sprague-Dawley rats. J Agric Food Chem 52:643–648
Yang T, Huang QY, Shan FB, Guan LB, Cai MC (2012) Effects of acute hypobaric hypoxia and exhaustive exercise on AMP-activated protein kinase phosphorylation in rat skeletal muscle. Sheng Li Xue Bao 64:193–198
Yao HP, Zhou YQ, Zhang R, Wang MH (2013) MSP-RON signalling in cancer: pathogenesis and therapeutic potential. Nat Rev Cancer 13:466–481
Yoshizawa JM, Schafer CA, Schafer JJ, Farrell JJ, Paster BJ, Wong DT (2013) Salivary biomarkers: toward future clinical and diagnostic utilities. Clin Microbiol Rev 26:781–791
Yuan F, Guo Z, Xu Y, Wang X, Bu HM, Zhong N, Zhang Y, Zhou ZN (2008) Comparison of the effects of chronic intermittent hypobaric hypoxia and continuous hypobaric hypoxia on hemodynamics in rats. Sheng Li Xue Bao 60:687–694
Zuehlke AD, Moses MA, Neckers L (2018) Heat shock protein 90: its inhibition and function. Philos Trans R Soc Lond B Biol Sci. https://doi.org/10.1098/rstb.2016.0527
Acknowledgements
This work was supported by grants (DIP-263) from Defence Research and Development Organization (DRDO), Ministry of Defence, Government of India. Authors would like to acknowledge Central Instrumentation Facility, University of Delhi south campus, Delhi for LC-MS/MS facility. Shikha Jain is recipient of Senior Research Fellowship from University Grants Commission (UGC). Subhojit Paul is recipient of Senior Research Fellowship from Council of Scientific and Industrial Research (CSIR). Anamika Gangwar is recipient of Senior Research Fellowship from Department of Science and Technology (DST-INSPIRE).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments by YA and KB. Experiments were performed by SJ, SP and AG. Representative figures were designed by SJ. Manuscript was written by SJ. Manuscript was critically evaluated by YA and KB.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest in this work.
Rights and permissions
About this article
Cite this article
Jain, S., Paul, S., Gangwar, A. et al. Quantitative proteomics reveal an altered pattern of protein expression in saliva of hypobaric hypoxia-induced rat model. J Proteins Proteom 11, 81–92 (2020). https://doi.org/10.1007/s42485-020-00037-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42485-020-00037-8