ABSTRACT
Bacillus licheniformis strain JS has been shown to produce antimicrobial protein (AMP) using wheat bran as carbon and nitrogen source. Monitoring of AMP production revealed maximum after 72 h and extracted optimally at 70% saturation of ammonium sulphate. The extracted protein was further purified by ion exchange column chromatography which was eluted at 0.3 M NaCl concentration. The 16 kDa purified antimicrobial protein shows more activity against Gram-positive bacteria Bacillus cereus as compared to other bacteria. Further, combinatorial effect of AMP with antibiotics showed increased efficiency of Kanamycin, Neomycin and Streptomycin. So, the AMP extracted from B. licheniformis strain JS could be promising to increase the efficiency of present antibiotics against antibiotic-resistant pathogens. Thus, this study could be useful to design new therapeutic strategies to treat infectious diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The alarming increase in antibiotic-resistant bacterial infections is a serious threat to humans and animals worldwide. In this respect, antimicrobial peptides (AMPs) are potential therapeutic tools, because of their rapid and specific killing activity against pathogens. So far, more than 800 AMPs have been isolated from different sources such as humans, animals, plants, insects and bacteria (Reddy et al. 2004). The AMPs are classified as ribosomal and non-ribosomal peptides. The ribosomal AMPs are also called as bacteriocins consist of only 19 to 37 amino acids, where as large peptide with molecular weight 90,000 Da (Joerger 2003). So far, the peptides exhibiting antimicrobial properties are small, amphipathic and cationic in nature, whereas very few reports on anionic peptides are found (Lai et al. 2002; Cytrynska et al. 2007; Akeel et al. 2017). Recently, several reports have suggested alternative mechanisms of AMPs on multiple targets, but specifically known for its interactions with bacterial cell membrane for permeabilization (Zhang et al. 2001; Jenssen et al. 2006). Anionic antibacterial peptides kill bacterial cells by causing precipitation of cytoplasmic proteins and intracellular content flocculation (Brogden et al. 1996, 2003).
The Bacillus genus has been known for its diversified characteristics. It has potential to produce antibiotics since for 50 years. The production of antimicrobial peptides has been described for many Bacillus species like B. thuringiensis (Paik et al. 1997), B. subtilis (Zheng et al. 1999), B. cereus (Bizani and Brandelli 2002) and B. licheniformis (Cladera-Olivera et al. 2004). Among the different species of Bacillus, the B. licheniformis has been widely accepted for its industrial applications (de Boer et al. 1994).
The novel antimicrobial peptides could be an option for the control of infectious diseases caused by the antibiotic resistant microorganisms. Thus, to understand its effectiveness, it is very important to isolate microorganisms producing AMPs and study its characteristics, and its mechanism of action against particular bacteria. Present work deals with the purification and characterization of antimicrobial peptide from B. licheniformis strain JS.
Materials and methods
Screening of AMP producers
The isolation of antimicrobial peptide producer organism was carried out by screening of various available cultures such as Bacillus licheniformis strain JS, Bacillus megaterium, Bacillus thuringiensis, and Bacillus subtilis. The B. licheniformis strain JS was found to produce antimicrobial compound, so it was selected for further studies.
Production of antimicrobial protein
The Czapek dox medium containing NaNO3 0.3 gm, K2HPO4 0.1 gm, MgSO4.7H2O and KCl 0.05 gm, FeSO4.7H2O 0.001 gm in 100 ml distilled water with Wheat bran as carbon source was used for production of antimicrobial peptide from B. licheniformis strain JS. The fresh culture of B. licheniformis strain JS was inoculated into production media. The flask was then incubated at 37 °C for 96 h in static condition. The antimicrobial activity of AMP was determined by antimicrobial assay.
Extraction of antimicrobial protein
After incubation the broth was centrifuged at 8000 rpm for 15 min at 4 °C and the supernatant was collected. Further the supernatant was used for extraction of Antimicrobial peptides by the following methods.
Method I—70% Ammonium sulphate was added to supernatant with constant stirring and kept overnight in refrigerator at 4 °C. Then, the mixture was centrifuged at 8000 rpm for 20 min at 4 °C and the precipitate was collected. Then, this precipitate was dissolved in 25 mM phosphate buffer having pH 7.0 and dialysis was carried out in same buffer.
Method II—after incubation period, the broth was centrifuged and collected supernatant was subjected to cold acetone precipitation (60%). In this, cold supernatant and chilled acetone were mixed with constant stirring and kept in deep freeze at − 15 °C for overnight. After overnight precipitation, the mixture was centrifuged at 8000 rpm for 20 min at 4 °C and precipitate was collected. The collected precipitate was then dissolved in 25 mM phosphate buffer and dialysis was carried out in same buffer.
Purification of antimicrobial protein
The column used for chromatography was packed by activated DEAE cellulose. Then, the flow rate was adjusted as 5 ml min−1 and dialyzed sample loaded on to the column. The protein was eluted by 0.1–1.0 M NaCl gradient solutions. The eluted fractions were checked for the protein content by using Lowry method and standard graph was obtained by Bovine Serum Albumin as standard protein. Then high protein containing fractions were dialyzed and used for antimicrobial activity.
Antimicrobial assay
Test organism such as Gram-positive B. subtilis and B. cereus, and Gram-negative Salmonella typhimurium and Shigella dysenteriae were spread on the nutrient agar plates. The wells were prepared, and in each well, 50 μl sample was added. The plates were kept for diffusion in refrigerator for 10 min. After diffusion, plates were incubated for 24 h at 37° and observed for the zone of inhibition.
Effect of temperature and trypsin digestion on antimicrobial activity
To study the effect of temperature, AMP was kept at different ranges of temperature such as 10–100 °C for 60 min. The effect of trypsin digestion on antimicrobial peptide was studied by adding 1 ml protein into 0.5 ml digestion buffer containing trypsin with concentration 1 μg/μl. Then, this mixture was incubated for 3 h at 37 °C and then kept at 95 °C for 5 min for inhibition of trypsin. After trypsin action, AMP was checked for antimicrobial activity.
Molecular weight determination
The fraction collected after ion exchange column chromatography showing antimicrobial activity was checked for purity by SDS-PAGE. The antimicrobial peptide band was visualized by Coomassie brilliant blue staining. The molecular weight of antimicrobial peptide was determined by comparison with standard molecular marker proteins (Phosphorylase b 98 kDa, Bovine Serum Albumin 66 kDa, Ovalalbumin 43 kDa, Carbonic Anhydrase 29 kDa, Soyabean Trypsin Inhibitor 20 kDa).
Combinatorial effect of AMP and Antibiotics
The combinatorial effect of AMP and different class of antibiotics (Penicillin, Kanamycin, Neomycin, Gentamicin and Streptomycin) were studied. The agar diffusion method was used to check efficiency of combined effect of AMP with different antibiotics. In this, test organisms such as B. cereus and S. typhimurium were spread on the nutrient agar plates and three wells on each plate were prepared. Then these three wells were added with AMP, respective antibiotic and mixture of AMP + antibiotic and plates were kept for diffusion at 4 °C for 10 min. After diffusion, plates were incubated at 37 °C for 24 h and observed for zone of inhibition.
Statistical Analysis
Statistical analysis was carried out using Graph Pad software (GraphPad InStat version 3.00, GraphPad Software, San Diego California USA). Results obtained were the mean of three or more determinants. Analysis of variance was carried out on all data at p < 0.05.
Results
Screening and production of AMP
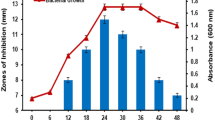
The different species of Bacillus were screened for the production of antimicrobial peptide. It was seen that among B. licheniformis strain JS, B. megaterium, B. thuringiensis, and B. subtilis, the B. licheniformis has the ability to produce AMP. So, it was selected for the further studies on AMP. B. licheniformis strain JS was found to produce antimicrobial peptide in Czapek dox medium with wheat bran as sole source of carbon. From Fig. 1, the maximum antimicrobial activity was found after 72 h in broth and further incubation leads to decrease in antimicrobial activity. AMP production was found enhanced at 37 °C at static condition than at shaking conditions, whereas most of reports suggest more production at shaking conditions.
Purification of antimicrobial peptide
The extracellular AMP produced by B. licheniformis strain JS in medium was precipitated by ammonium sulphate and acetone precipitation methods. The 70% saturation of ammonium sulphate was found optimum for the precipitation of AMP. However, AMP extracted by 60% acetone precipitation was less active as compared to ammonium sulphate. Hence, extracted AMP by ammonium sulphate precipitation was further purified by the ion exchange column chromatography. Various proteins were eluted from the column at NaCl gradient between 0.1 and 1.0 M, but AMP was eluted at 0.3 M NaCl concentration.
Antimicrobial activity of AMP
The antimicrobial peptide produced by B. licheniformis in crude form was tested against variety of Gram-positive (B. cereus, B. subtilis) and Gram-negative (S. dysenteriae, S. typhimurium) bacteria. The extracted AMP showed significant activity against Gram-positive and Gram-negative pathogens. The purified AMP was found more active than the crude AMP. The dilution study revealed that the AMP at 50 μl (100 μg/ml) shows maximum zone of inhibition as compared to 75 µl (100 μg/ml) and 100 µl (100 μg/ml) against Gram-positive (B. cereus) organisms (Fig. 2). In case of Gram-negative (S. dysenteriae) organism, the zone of inhibition was maximum at 100 µl (100 μg/ml).
Effect of temperature and trypsin digestion
In the study of effect of temperature on the antimicrobial activity of AMP, it was seen that the antimicrobial activity was 100% at temperature range between 10 and 90 °C (Table 1). This confirms that the antimicrobial peptide produced by the B. licheniformis strain JS is having thermotolerant activity. The trypsin digestion study reveals that the AMP retains its 100% activity.
Molecular weight determination
The AMP purified by ion exchange column chromatography analyzed by SDS-PAGE is shown in Fig. 3. The molecular mass of purified antimicrobial peptide was found as 16 kDa. The presence of single band indicates the complete purification by ion exchange column chromatography.
Combinatorial effect of AMP and antibiotics
The AMP extracted from B. licheniformis strain JS has shown very good inhibitory effect against different pathogenic bacteria. It was also found that when this AMP was mixed with antibiotics such as Kanamycin, Neomycin and Streptomycin, the efficiency of these antibiotics increased up to twofold, whereas no effect was observed in case of Penicillin antibiotic (Table 2).
Discussion
The B. licheniformis strain JS isolated from mushroom bed left over after the production of oyster mushroom (Pleurotus sajor-caju) which has been reported earlier for production of thermophilic chitinase (Waghmare and Ghosh 2010). In this study, we had first time reported the production of antimicrobial protein from B. licheniformis strain JS. So far, different strains of B. licheniformis have been reported for the production of antimicrobial compounds (Cladera-Olivera et al. 2004; Callow and Work 1952; Prave et al. 1972). It is essential that organism must be provided with optimal growth conditions to increase AMPs production. The B. licheniformis strain JS has produced maximum AMPs after 72 h of incubation period using wheat bran, whereas Cladera-Olivera et al.2004 reported the maximum production of AMPs after 15 h from B. licheniformis strain P40 using Brain Heart infusion medium. Thus, our study shows that the production of AMP is economically feasible from B. licheniformis strain JS when wheat bran is used as a sole source of carbon.
The 70% saturation of ammonium sulphate was found optimum for the precipitation of AMP, whereas Sirtori et al. (2006) reported that even 50% saturation of ammonium sulphate is also suitable for extraction. Among ammonium sulphate and acetone precipitation, AMP extracted by ammonium sulphate showed more antimicrobial activity. This suggests that ammonium sulphate extraction method is more significant method than the acetone precipitation. The extracted AMP purified by DEAE-Cellulose in the present study produced by Bacillus sp. Similarly, Motta et al. (2007) also reported the purification of antimicrobial peptide by DEAE-Sepharose. This result shows the binding affinity of purified peptide with DEAE indicating anionic nature of peptide. The single band on the SDS-PAGE indicates that the antimicrobial protein secreted by the B. licheniformis strain JS could be purified by subsequent steps such as ammonium sulphate extraction and DEAE-Cellulose ion exchange chromatography. AMP produced by B. licheniformis strain JS is more active in less concentration against Gram positive same as that of antibacterial peptide extracted from different species of Bacillus. The broad spectrum nature of AMP isolated from B. licheniformis strain JS showed antimicrobial activity against Gram-negative organism but at lesser extent than the Gram positive similar to the antimicrobial peptide extracted from B. licheniformis strain P40 (Teixeira et al. 2009).
AMP produced from B. licheniformis strain JS is thermolerant similar to earlier report on Bacillus sp. (Cladera-Olivera et al. 2004; Teixeira et al. 2009). The antimicrobial activity of AMP did not affect even after the action of trypsin at higher temperature. It indicates that AMP is resistant to action of trypsin. So, this property of AMP makes it applicable for the administration through digestive system. Generally, the AMPs produced by the bacteria are < 10 kDa (Reddy et al. 2004), but the AMP produced by B. licheniformis strain JS having large molecular weight, i.e., 16 kDa. Similarly, few reports suggest that certain bacterial genus have ability to synthesize antimicrobial proteins of high molecular weight such as 30 kDa from P. aeruginosa JU-Ch 1 (Grewal et al. 2014), Lactobacillus rhamnosus 231 (Ambalam et al. 2009), Clavibacter michiganensis subsp. michiganensis (Liu et al. 2013) and B. subtilis ATCC 21331 (Aishah et al. 2014). The combinatorial effect of AMP with antibiotics showed increase in the efficiency of Kanamycin, Neomycin and Streptomycin antibiotics. So, it could be because of AMPs produced by B. licheniformis may facilitate entry of these antibiotics inside the pathogens and increase their efficiency. This suggests that the AMPs extracted from B. licheniformis formulated with Kanamycin, Neomycin and Streptomycin could be useful to control antibiotic resistant pathogens.
Conclusion
The present work concludes the ability of B. licheniformis strain JS to produce a novel 16 kDa antimicrobial protein. It shows stability in the presence of trypsin at high temperature. The purified peptide also increases the efficiency of Kanamycin, Neomycin and Streptomycin. Hence, the isolated AMP would be promising for the development of new therapeutics in the treatment of infectious diseases.
References
Aishah AWN, Hanina MN, Asyikin IIN, Shahril MH, Salina MR, Maryam MR, Jalil AKA, Rosfarizan M (2014) Antimicrobial protein produced by Bacillus subtilis ATCC 21332 in the presence of Allium sativum. Asian Pacific J Trop Dis 4:245
Akeel R, Mateen A, Syed R, Alyousef AA, Shaik MR (2017) Screening, purification and characterization of anionic antimicrobial proteins from Foeniculum vulgare. Molecules 22:602–611
Ambalam PS, Prajapati JB, Dave JM, Nair BM, Ljungh ASA, Vyas BRM (2009) Isolation and characterization of antimicrobial proteins produced by a potential probiotic strain of human Lactobacillus rhamnosus231 and its effect on selected human pathogens and food spoilage organisms. Microb Ecol Health D 21:211–220
Bizani D, Brandelli A (2002) Characterization of a bacteriocin produced by a newly isolated Bacillus sp. strain 8A. J Appl Microbiol 93:512–519
Brogden KA, De Lucca AJ, Bland J, Elliott S (1996) Isolation of an ovine pulmonary surfactant-associated anionic peptide bactericidal for Pasteurella haemolytica. Proc Natl Acad Sci USA 93:412–417
Brogden KA, Ackermann M, McCray PB, Tack BF (2003) Antimicrobial peptides in animals and their role in host defences. Int J Antimicrob Agents 22:465–478
Callow RK, Work TS (1952) Antibiotic peptides from Bacillus licheniformis, Licheniformins A, B and C. Biochem J 51:558–567
Cladera-Olivera F, Caron GR, Brandelli A (2004) Bacteriocin-like substance production by Bacillus licheniformis strain P40. Lett Appl Microbiol 38:251–256
Cytrynska M, Mak P, Zdybicka-Barabas A, Suder P, Jakubowicz T (2007) Purification and characterization of eight peptides from Galleria mellonella immune hemolymph. Peptides 28:533–546
de Boer AS, Priest F, Diderichsen B (1994) On the industrial use of Bacillus licheniformis: a review. Appl Microbiol Biot 40:595–598
Grewal S, Bhagat M, Vakhlu J (2014) Antimicrobial protein produced by Pseudomonas aeruginosa JU-Ch 1, with a broad spectrum of antimicrobial activity. J Biocatal Agri Biotech 3:332–337
Jenssen H, Hamill P, Hancock RE (2006) Peptide antimicrobial agents. Clin Microbiol Rev 19:491–511
Joerger RD (2003) Alternatives to antibiotics: bacteriocins, antimicrobial peptides and bacteriophages. Poultry Sci 82:640–647
Lai R, Liu H, Lee WH, Zhang Y (2002) An anionic antimicrobial peptide from toad Bombina maxima. Biochem Biophys Res Commun 295:796–799
Liu Z, Holtsmark PMI, Skaugen M, Eijsink VGH, Brurberg MB (2013) New type of antimicrobial protein produced by the plant pathogen Clavibacter michiganensis subsp. michiganensis. Appl Environ Microbiol 79:5721–5727
Motta AS, Lorenzini DM, Brandelli A (2007) Purification and partial characterization of an antimicrobial peptide produced by a novel Bacillus sp. isolated from the Amazon Basin. Curr Microbiol 54:282–286
Paik HD, Bae SS, Park SH, Pan JG (1997) Identification and partial characterization of tochicin, a bacteriocin produced by Bacillus thuringiensis subsp. tochigiensis. J Ind Microbiol Biot 19:294–298
Prave P, Sukatsch D, Vertsy L (1972) Proticin, A new phosphorus containing antibiotic. J Antibiot 1:1–3
Reddy KVR, Yedery RD, Aranha C (2004) Antimicrobial peptides: premises and promises. Int J Antimicrob Ag 24:536–547
Sirtori LR, Cladera-Olivera F, Lorenzini DM, Tsai SM, Brandelli A (2006) Purification and partial characterization of an antimicrobial peptide produced by Bacillus sp. strain P45, a bacterium from the Amazon basin fish Piaractus mesopotamicus. J Gen Appl Microbiol 52:357–363
Teixeira ML, Cladera-Olivera F, dos Santos J, Brandelli A (2009) Purification and characterization of a peptide from Bacillus licheniformis showing dual antimicrobial and emulsifying activities. Food Res Int 42:63–68
Waghmare SR, Ghosh JS (2010) Chitobiose production by using novel thermostable chitinase from Bacillus licheniformis strain JS isolated from a mushroom bed. Carbohydr Res 345:2630–2635
Zhang L, Rozek A, Hancock RE (2001) Interaction of cationic antimicrobial peptides with model membranes. J Biol Chem 276:35714–35722
Zheng G, Yan LZ, Vederas JC, Suber P (1999) Genes of the sbo-alb locus of Bacillus subtilis are required for production of the antilisterial bacteriocin subtilosin. J Bacteriol 181:7346–7355
Acknowledgement
Authors are very much thankful to Department of Science and Technology, Government of India, New Delhi, for financial support under DST PURSE-II scheme. Authors are also grateful to Department of Microbiology, Shivaji University, Kolhapur, India for providing laboratory facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have declared that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Waghmare, S.R., Randive, S.A., Jadhav, D.B. et al. Production of novel antimicrobial protein from Bacillus licheniformis strain JS and its application against antibiotic-resistant pathogens. J Proteins Proteom 10, 17–22 (2019). https://doi.org/10.1007/s42485-018-00002-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42485-018-00002-6